Abstract
Cerebral ischemia causes tissue death owing to occlusion of the cerebral blood vessels, and cerebral ischemia activates mitogen-activated protein kinase (MAPK) and induces secretion of pro-inflammatory cytokines. Adenosine A2A receptor agonist, polydeoxyribonucleotide (PDRN), suppresses the secretion of pro-inflammatory cytokines and exhibits anti-inflammatory effect. In the current study, the therapeutic effect of PDRN on cerebral ischemia was evaluated using gerbils. For the induction of cerebral ischemia, the common carotid arteries were exposed, and then aneurysm clips were used to occlude the common carotid arteries bilaterally for 7 minutes. In the PDRN-treated groups, the gerbils were injected intraperitoneally with 0.3 mL of saline containing 8 mg/kg PDRN, per a day for 7 days following cerebral ischemia induction. In order to confirm the participation of the adenosine A2A receptor in the effects mediated by PDRN, 8 mg/kg 7-dimethyl-1-propargylxanthine (DMPX), adenosine A2A receptor antagonist, was treated with PDRN. In the current study, induction of ischemia enhanced the levels of pro-inflammatory cytokines and increased phosphorylation of MAPK signaling factors in the hippocampus and basolateral amygdala. However, treatment with PDRN ameliorated short-term memory impairment by suppressing the production of pro-inflammatory cytokines and inactivation of MAPK signaling factors in cerebral ischemia. Furthermore, PDRN treatment enhanced the concentration of cyclic adenosine-3,5’-monophosphate (cAMP) as well as phosphorylation of cAMP response element-binding protein (p-CREB). Co-treatment of DMPX and PDRN attenuated the therapeutic effect of PDRN on cerebral ischemia. Based on these findings, PDRN may be developed as the primary treatment in cerebral ischemia.
1. Introduction
Cerebral ischemia, also known as stroke, is an acute disease induced by insufficient blood supply to the brain, which can result in severe complications and a high mortality rate [1, 2]. Currently, there is no effective drug therapy for acute ischemic stroke other than intravenous or intraarterial thrombolysis. However, as the time scope for treatment with a thrombolytic agent is narrow, the utility of thrombolytic agents is very limited [3, 4].
After an ischemic stroke, the development of lesions is generally associated with severity of inflammatory reaction [5]. During stroke, inflammation-inducing mediators are created, and inflammation serves to exacerbate the symptoms and progress ischemic stroke [6]. Inflammation exacerbates ischemic damage through the secretion of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 [7].
Cerebral ischemia activates mitogen-activated protein kinase (MAPK), and MAPK activation plays a selective role in determining neuronal survival or death [8]. Activation of MAPK functions primarily as a mediator for cell survival or apoptosis through phosphorylation of intracellular enzymes, transcription factors and cytoplasmic proteins [9, 10]. Extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38 kinase are included in the MAPK system, and the MAPK signaling pathway regulates expressions of inflammatory cytokines and apoptosis factors during stroke. Therefore, this MAPK signaling pathway can be a therapeutic target when developing appropriate therapeutic agents [11, 12]. Thus, studies aimed at regulating inflammation through the MAPK system in ischemic brain tissue may provide a basis for discovering new therapeutic agents targeting stroke patients.
Adenosine and its receptors are essential neuromodulators playing important roles in various pathophysiological conditions. Adenosine A1, A2A, A2B and A3A are included in the adenosine receptors, and the adenosine receptors are expressed in inflammatory cells and immune cells. Among them, stimulation of A2A has been reported to reduce the secretion of pro-inflammatory cytokines in various neurological disease conditions [13, 14]. The physiological role of adenosine A2A receptor-induced BDNF production was demonstrated through synapse formation from immature and mature neurons, as well as protecting neurons from excitatory toxicity and increasing neurite expansion [15].
The adenosine A2A receptor agonist, polydeoxyribonucleotide (PDRN), exhibits an anti-inflammatory effect by inhibiting pro-inflammatory cytokine production. PDRN is also known to inhibit apoptosis in several disease states such as gastric ulcers, acute lung injury, and osteoarthritis [16–18]. Despite the excellent pharmacological effects of PDRN, few studies have evaluated the efficacy of PDRN in cerebral ischemia.
In the current study, the effect of PDRN on short-term memory and inflammation in the hippocampus after induction of transient global ischemia was evaluated using gerbils. For the experiment, the step-down avoidance task was conducted for short-term memory, and the concentrations of TNF-α, IL-1β and cyclic adenosine-3’,5’-monophosphate (cAMP) were analyzed by enzyme-linked immunoassay (ELISA). In addition, the expression of neuronal nuclei (NeuN) was determined by immunohistochemical analysis, and the levels of adenosine A2A receptor, TNF-α, IL-1β, ERK, JNK, p38, cAMP response element-binding protein (CREB) and protein kinases A (PKA) were analyzed by western blotting.
2. Materials and methods
2.1. Animals and classification
The male adult Mongolian gerbils, weighing 50 ± 2 g (15 weeks old), were bred in the controlled temperature (23 ± 2°C) and lighting (08:00 to 20:00 h) conditions, and food and water were provided freely. The gerbils were randomly classified into the four groups such as sham-operation group, cerebral ischemia-induced group, cerebral ischemia-induced and PDRN-treated group, cerebral ischemia-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX)-treated group (n = 10 in each group).
This experimental procedure was approved by the Institutional Animal Care and Use Committee of Kyung Hee University and received the following approval number (KHUASP[SE]-17-071). The experimental procedures were conducted in good faith in accordance with the guidelines for animal care from the National Institutes of Health and the Korean Institute of Medical Sciences. The gerbils underwent sufficient anesthesia with Zoletil 50® (Vibac Laboratories, Carros, France) to performed surgery or sacrifice. No gerbils died or were euthanized before the end of the experiment.
2.2. Transient global ischemia induction
Transient global ischemia was surgically made in the same manner as described above [19, 20]. A bilateral neck incision was made after anesthetizing the gerbils by Zoletil 50® (10 mg/kg; Vibac Laboratories). Then two common carotid arteries were exposed and closed using surgical clips for 7 minutes. By a Homeothermic Blanket Control Unit (Harvard Apparatus, Massachusetts, MA, USA) that wrapped around the head and body, the temperature of the head and body was maintained at 36 ± 0.5°C during the course of operation. After 7 minutes of closing, the surgical clips were removed and cerebral blood flow was allowed to reflow. Local brain blood flow on either side of the forebrain was determined using a BLF21D laser Doppler flowmeter (Transonic Systems Inc., New York, NY, USA). To prevent hypothermia, the gerbils were observed for an additional 4 hours after recovery. The animals from the sham-operation group were managed in a similar manner, but both common carotid arteries were not closed during the neck operation.
2.3. Treatment
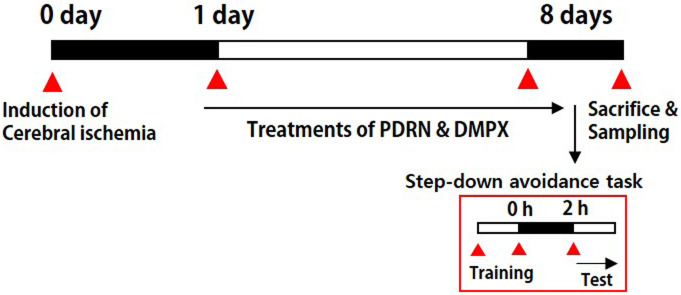
The gerbils from the PDRN-treated groups were injected intraperitoneally with 0.3 mL of normal saline (0.9%) containing 8 mg/kg of PDRN (Rejuvenex®, PharmaResearch Products, Pangyo, Korea), one time per a day continued for 7 days, started a day after operation. Based on preliminary data and previous studies, the PDRN concentration considered to be the most effective was used in this experiment [17, 21]. Additionally, in order to confirm that the adenosine A2A receptor is involved in the effect mediated by PDRN, 8 mg/kg DMPX (Sigma Chemical Co., St. Louis, MO, USA), an adenosine A2A receptor antagonist, was simultaneously administered with PDRN. In the sham-operation group and in the cerebral ischemia-induced group, the gerbils received 0.3 mL of normal saline without drugs according to the same timetable. The experiment schedule is shown in Fig 1.
Fig 1. Experimental schedule.
2.4. Step-down avoidance task
In the same manner as described above [22, 23], the step-down avoidance task was performed to determine the short-term memory. Eight days after induction of cerebral ischemia, the gerbils implemented a step-down avoidance task. Each gerbil was placed on a 7 × 25 cm platform at height of 2.5 cm, and the platform faced a grid of parallel steel bars (45 × 25 cm) with a diameter of 0.1 cm and a spacing of 1 cm. Going down the grid during training, the animal immediately took the out of the box after receiving a 0.3 mA foot shock for 2 seconds. After 2 hours of training period, the latency of each gerbil was determined. During the test time, the gerbils were placed back on the platform, and the latency time was defined as the time until the animal descended and placed all four feet on the grid. A delay of latency time more than 180 seconds was also calculated as 180 seconds.
2.5. Tissues preparation
On the 8th day after ischemia induction, immediately after measuring the latency of the step-down avoidance task, the animals were sacrificed. After anesthetizing the gerbils using the Zoletil 50® (10 mg/kg, ip; Vibac Laboratories), heart puncture was used to collect blood, left at room temperature for 1 hour and centrifugation was performed at 3,000 rpm for 20 minutes to obtain serum. After blood sampling, 50 mM phosphate-buffered saline (PBS) was transcardially perfused, and then fixed with a solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). After removing the brains, the brains were with the same fixative solution and then transferred to 30% sucrose solution to prevent freezing. Thereafter, 40-μm thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany), and an average of 10 sections was obtained in the CA1 region of each gerbil.
2.6. Pro-inflammatory cytokines and cAMP concentrations
Serum and hippocampus levels of pro-inflammatory cytokines (TNF-α and IL-1β) and cAMP concentrations were determined using enzyme-linked immunosorbent assay (ELISA). Enzyme immunoassay kits were used to detect the concentrations of TNF-α, IL-1β and cAMP in accordance with the manufacturer’s instructions (Abcam, Cambridge, UK), in the same manner as described above (n = 3 in each group) [17].
2.7. Western blot analysis
According to the same manner as described above [24, 25], analysis of western blotting was conducted (n = 4 in each group). Priority, approximately 30 mg of hippocampal tissues were extracted using 100 mg/mL of lysis buffer. The tissues were homogenized using a lysis buffer consisting of 1 mM PMSF, 1 mM EGTA, 1 mM Na2VO4, 1.5 mM MgCl2·6H2O, 50 mM Tris-HCl (pH 8.0), 100 mM NaF, 150 mM NaCl, 1% Triton X-100 and 10% glycerol, and then this homogeneous mixture was centrifuged at 14,000 rpm for 30 minutes. Concentration of protein was detected by a colorimetric protein analysis kit (Bio-Rad, Hercules, CA, USA). Protein 30 μg was separated from each sample on SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The antibodies for rabbit CREB antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), phosphorylated (p)-CREB antibody (1:1,000; Santa Cruz Biotechnology), rabbit PKA antibody (1:1,000; Santa Cruz Biotechnology), p-PKA antibody (1:1,000; Santa Cruz Biotechnology), rabbit ERK antibody (1:2,000; Cell Signaling Technology, Danvers, USA), rabbit p-ERK antibody (1:2,000; Cell Signaling Technology), rabbit JNK antibody (1:2,000; Cell Signaling Technology), rabbit p-JNK antibody (1:2,000; Cell Signaling Technology), rabbit p38 antibody (1:2,000; Cell Signaling Technology), rabbit p-p38 antibody, (1:2,000; Cell Signaling Technology), mouse TNF-α antibody (1:1,000; Santa Cruz Biotechnology), mouse IL-1β antibody (1:1,000; Santa Cruz Biotechnology), mouse adenosine A2A receptor antibody (1:1,000; Santa Cruz Biotechnology), and β-actin antibody (1:1,000; Santa Cruz Biotechnology) used were as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibodies (1:2,000; Vector Laboratories, Burlingame, CA, USA) for β-actin, TNF-α, IL-1β, adenosine A2A receptor and horseradish peroxidase-conjugated anti-rabbit antibodies (1:3,000; Vector Laboratories) for p-CREP, CREB, p-PKA, PKA, p-ERK, ERK, p-JNK, JNK, p-p38, p38 were used as the secondary antibodies.
The entire experimental step was carried out under normal laboratory conditions, except for membrane transfer performed at 4°C using pre-cooled buffer with cold pack. An enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology) was used for band measurements. Each sample was loaded twice, and the number of samples was 4 per group.
2.8. Immunohistochemistry for NeuN
Immunohistochemistry for NeuN was conducted in the same manner as described above [26, 27]. The sections were treated with mouse anti-NeuN antibody (1:500; Abcam, Cambridge, MA, USA) overnight, and then treated with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for an additional 1 hour. Secondary antibody was amplified using the Vector Elite ABC kit® (1:100; Vector Laboratories), and then 0.03% 3,3′-diaminobenzidine was used to show antibody-biotin-avidin-peroxidase complexes. The sections were then mounted on gelatin-coated slides, air-dried at room temperature overnight, and finally Permount® (Fisher Scientific, New Jersey, NJ, USA) was used to mount the coverslips.
2.9. Data analysis
The area of the hippocampal CA1 region on each slide was observed by optical microscope (Olympus, Tokyo, Japan), and the number of NeuN-positive cells was calculated using the Image-Pro® plus computer-assisted image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA). The number of NeuN-positive cells was calculated using the following equation: N = Nv × Vref. N is the total number of NeuN-positive cells of the CA1 region, which is counted by multiplying the NeuN-positive cell numerical density Nv by the reference volume (mm3) Vref. Nv is the average numerical density of NeuN-positive cells and it is calculated based on the sum of counts within the CA1 region of each section and the volume of the CA1 region contained in each section. Vref is calculated according to Cavalieri’s method as follows [28, 29]: Vref = a × t × s. In this equation, a is the average area of the CA1 cell layer, t represents the average thickness (40 μm) of the microtome section, and s indicates the total number of sections through the reference volume.
In order to compare the relative levels of protein expressions, the bands were detected densitometrically by Molecular AnalystTM version 1.4.1 (Bio-Rad, Hercules). For relative quantification, a random value of 1.00 was given to the results of the sham-operation group (western blotting). One-way ANOVA with Duncan’s post-hoc test was used for data analysis, and the results are presented as mean ± standard error of the mean. For statistical analysis, the value of P < 0.05 was determined to be statistically significant.
3. Results
3.1. Changes of cerebral blood flow
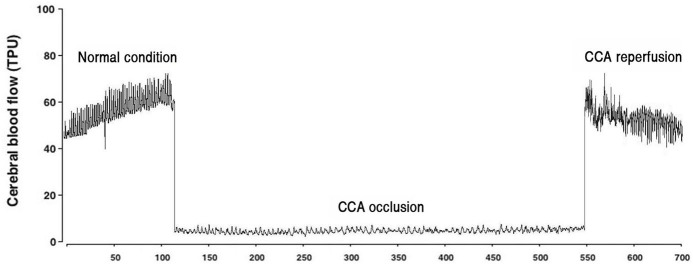
The flow of brain blood during carotid artery occlusion and reperfusion is shown in Fig 2. Occlusion of the carotid artery reduced brain blood flow and increased brain blood flow during reperfusion.
Fig 2. Brain blood flow during occlusion and reperfusion of both common carotid arteries.
3.2. Changes of short-term memory latency and NeuN-positive cell number in the CA 1 region
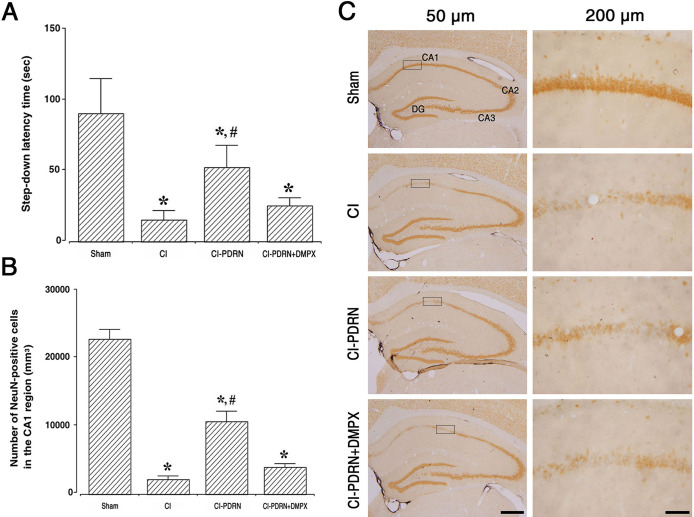
Fig 3A shows the results of the latency in the step-down avoidance task. Following the induction of cerebral ischemic damage, short-term memory was impaired (P < 0.05), and treatment with PDRN ameliorated this ischemia-induced memory impairment (P < 0.05). The co-administration of PDRN and DMPX failed to increase the latency observed with PDRN in cerebral ischemia.
Fig 3. Changes in short-term memory and neuronal survival in the CA1 region.
A. Latency of the step-down avoidance task in each group. B. Number of NeuN-positive cells in each group. C. Photomicrographs of NeuN-positive cells in the hippocampal CA1 region. The scale bar represents 50 μm (left) and 200 μm (right). (□) Area of magnification at 200 μm in CA1 region. Sham, sham-operation group; CI, cerebral ischemia-induced group; CI-PDRN, cerebral ischemia-induced and polydeoxyribonucleotide (PDRN)-treated group; CI-PDRN+DMPX, cerebral ischemia-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX)-treated group. * indicates P < 0.05 compared with the sham-operation group. # indicates P < 0.05 compared with the cerebral ischemia-induced group.
Fig 3B and 3C are the photomicrographs of NeuN-positive cells in the hippocampal CA1 region. Cerebral ischemic damage decreased the number of NeuN-positive cells, indicating reduced neuronal survival in the hippocampal CA1 region (P < 0.05), and treatment with PDRN improved neuronal survival in cerebral ischemia (P < 0.05). The co-administration of PDRN and DMPX failed to enhance the neuronal survival observed with PDRN in cerebral ischemia.
3.3. Changes of pro-inflammatory cytokine expression
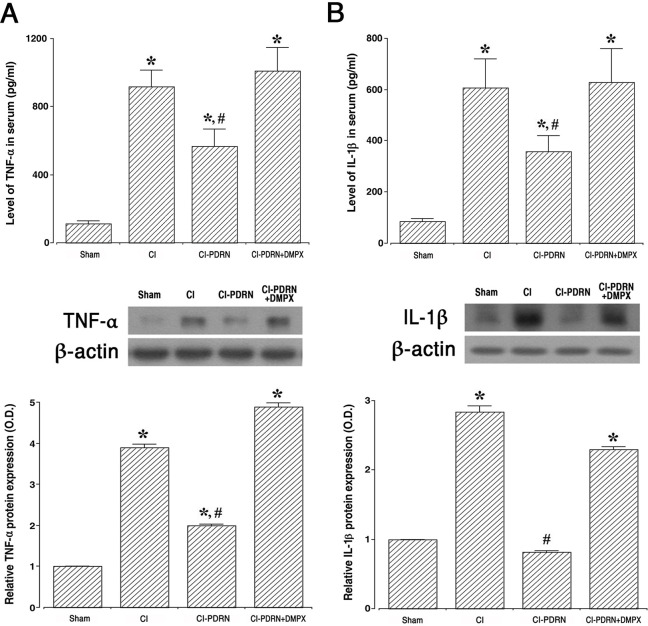
To determine whether PDRN improves cerebral ischemia, ELISA and western blot analysis were performed by examining the effect on PDRN on production of pro-inflammatory cytokines, TNF-α (Fig 4A) and IL-1β (Fig 4B). Following cerebral ischemic damage, TNF-α and IL-1β expressions were enhanced in the serum and hippocampus (P < 0.05), and treatment with PDRN suppressed the expressions of TNF-α and IL-1β (P < 0.05). The co-administration of PDRN and DMPX failed to decrease TNF-α and IL-1β expressions observed with PDRN in cerebral ischemia.
Fig 4. Altered expression of pro-inflammatory cytokines in the serum and hippocampus.
A-upper. Concentration of tumor necrosis factor-α (TNF-α) in the serum. A-lower. The relative level of TNF-α in the hippocampus. B-upper. Concentration of interleukin (IL)-1β in the serum. B-lower. The relative level of IL-1β in the hippocampus. Sham, sham-operation group; CI, cerebral ischemia-induced group; CI-PDRN, cerebral ischemia-induced and polydeoxyribonucleotide (PDRN)-treated group; CI-PDRN+DMPX, cerebral ischemia-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX)-treated group. * indicates P < 0.05 compared with the sham-operation group. # indicates P < 0.05 compared with the cerebral ischemia-induced group.
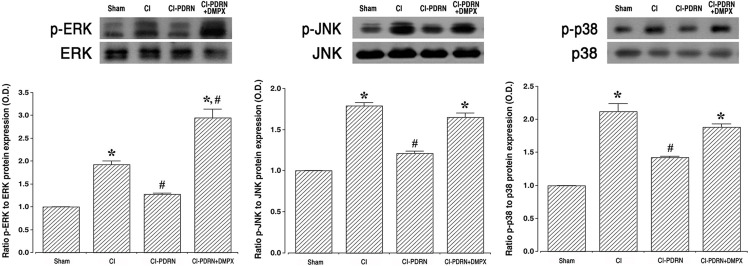
3.4. Changes of phosphorylation of MAPK cascade
To determine whether cerebral ischemia is improved by PDRN, the effect of PDRN on MAPK phosphorylation was investigated using western blotting (Fig 5). Induction of cerebral ischemia promoted MAPK cascade phosphorylation, such as ERK, JNK and p38 (P < 0.05). Interestingly, treatment with PDRN more enhanced the phosphorylation of ERK, JNK and p38 (P < 0.05). The co-administration of PDRN and DMPX failed to further enhance ERK, JN and p38 phosphorylation observed with PDRN in cerebral ischemia.
Fig 5. Changes in the mitogen-activated protein kinase (MAPK) cascade in the hippocampus.
Left. Ratio of phosphorylated extracellular signal-regulated kinases (p-ERK) to ERK. Middle. Ratio of phosphorylated c-Jun NH2-terminal kinases (p-JNK) to JNK. Right. Ratio of phosphorylated p38 kinase (p-p38) to p38 in the hippocampus. Sham, sham-operation group; CI, cerebral ischemia-induced group; CI-PDRN, cerebral ischemia-induced and polydeoxyribonucleotide (PDRN)-treated group; CI-PDRN+DMPX, cerebral ischemia-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX)-treated group. * indicates P < 0.05 compared with the sham-operation group. # indicates P < 0.05 compared with the cerebral ischemia-induced group.
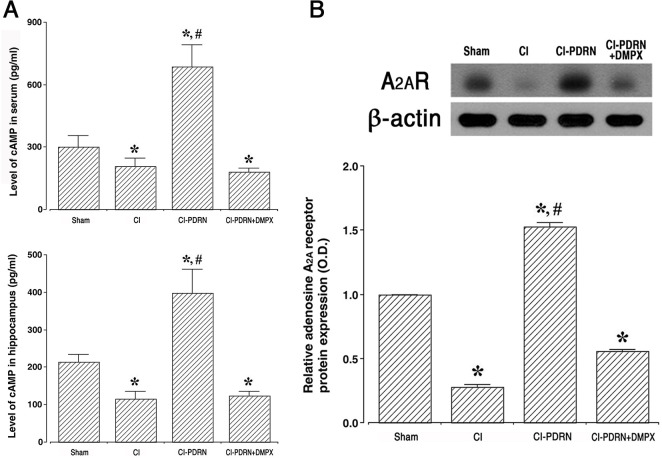
3.5. Changes of cAMP concentration and adenosine A2A receptor expression
The cAMP concentration in the serum and hippocampus and adenosine A2A receptor expression in the hippocampus are shown in Fig 6. Induction of cerebral ischemia decreased the levels of cAMP concentration and adenosine A2A receptor expression (P < 0.05), and treatment with PDRN improved the levels of cAMP concentration and adenosine A2A receptor expression (P < 0.05). The co-administration of PDRN and DMPX failed to increase the levels of cAMP concentration and adenosine A2A receptor expression observed with PDRN in cerebral ischemia.
Fig 6. Changes in cAMP concentration and adenosine A2A receptor expression.
A-upper. Concentration of cAMP in serum. A-lower. Concentration of cAMP in the hippocampus. B. The relative expression of the adenosine A2A receptor in the hippocampus. Sham, sham-operation group; CI, cerebral ischemia-induced group; CI-PDRN, cerebral ischemia-induced and polydeoxyribonucleotide (PDRN)-treated group; CI-PDRN+DMPX, cerebral ischemia-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX)-treated group. * indicates P < 0.05 compared with the sham-operation group. # indicates P < 0.05 compared with the cerebral ischemia-induced group.
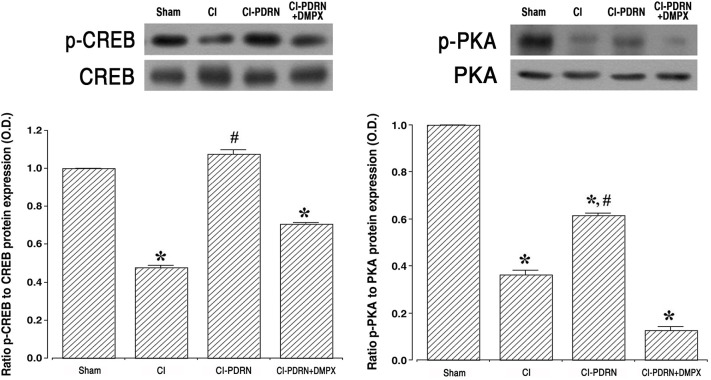
3.6. Changes of ratio in p-CREB vs CREB and p-PKA vs PKA
Western blot analysis was used to measure the relative expressions of p-CREB vs CREB and p-PKA vs PKA (Fig 7). Induction of cerebral ischemia decreased the ratio of p-CREB vs CREB and the ratio of p-PKA vs PKA when compared with the sham-operation group (P < 0.05). Treatment with PDRN increased the ratio of p-CREB vs CREB and ratio of p-PKA vs PKA in the hippocampus (P < 0.05). The co-administration of PDRN and DMPX failed to enhance the phosphorylation of CREB and PKA observed with PDRN in cerebral ischemia.
Fig 7. Changes in phosphorylated cAMP response element-binding protein (p-CREB) to CREB ratio and phosphorylated protein kinases A (p-PKA) to PKA ratio.
Left. Ratio of p-CREB to CREB in the hippocampus. Right. Ratio of p-PKA to PKA in the hippocampus. Sham, Sham-operation group; CI, cerebral ischemia-induced group; CI-PDRN, cerebral ischemia-induced and polydeoxyribonucleotide (PDRN)-treated group; CI-PDRN+DMPX, cerebral ischemia-induced and PDRN with 7-dimethyl-1-propargylxanthine (DMPX)-treated group. * indicates P < 0.05 compared with the sham-operation group. # indicates P < 0.05 compared with the cerebral ischemia-induced groups.
4. Discussion
Damage by ischemia particularly destroys pyramidal neurons in the hippocampal CA1 region, and ischemia induces apoptotic cell death in the hippocampal CA1 neurons [19, 30]. Pyramidal neurons are crucial for learning and memory, and appearance of passive avoidance memory impairment after ischemia is associated with damage in the neurons of the CA1 region [31, 32]. In the current study, the cerebral ischemic injury resulted in cell loss in the hippocampal CA1 neurons, and this neuronal cell loss reduced short-term memory when compared with the gerbils of the sham-operation group. The present findings are similar to previous studies showing that loss of neurons in the CA1 region caused short-term memory impairment [31, 33].
The ischemic brain exhibits inflammation characterized by the accumulation of inflammatory cells and mediators. Previous studies have suggested that induction of ischemic damage enhances neuronal cell loss owing to increased levels of inflammatory exudates and pro-inflammatory cytokines [6, 7, 34]. Furthermore, an increment of inflammatory cytokines in the brain acts as a major causes of neuronal cell loss and memory impairment [35, 36]. Based on current findings, enhanced secretion of TNF-α and IL-1β, pro-inflammatory cytokines, in the serum, hippocampus, and basolateral amygdala (Supplement 1 in S1 File) exacerbated the symptoms of ischemic injury. These results indicate that symptoms were worsened by excessive production of pro-inflammatory cytokines during cerebral ischemia. Inhibiting the secretion of pro-inflammatory cytokines is one of the important treatment strategies for the brain ischemic injury.
Most of cells involved in wound healing express the adenosine A2A receptor [17], and this adenosine A2A receptor is locates in several brain regions and modulates the pathophysiological response to ischemic stroke [14]. Agonists on adenosine A2A receptor have been reported to be useful for the treating of inflammatory diseases [14, 37]. In the previous studies, adenosine A2A receptor knockout mice exhibited symptoms of chronic cerebral ischemia such as working memory impairment, increased demyelination, glial proliferation, and increased pro-inflammatory cytokines [4, 38]. Reduced functional capacity of BDNF in adenosine A2A receptor knockout mice was associated with a decrease in hippocampal BDNF level, and pharmacological blockade of adenosine A2A receptors significantly reduced BDNF level in the hippocampus of normal mice [39]. These results indicated that tonic activation of adenosine A2A receptor is required for BDNF-induced potentiation of synaptic transmission and for sustaining a normal BDNF tone in the hippocampus [39]. The facilitating action of BDNF on hippocampal long-term potentiation is critically dependent on the presence of extracellular adenosine and activation of the A2A receptor through a cAMP/PKA-dependent mechanism [40]. Activation of the adenosine A2A receptor regulates BDNF production in rat cortical neurons, which provides neuroprotective action [15].
In the current study, PDRN treatment substantially suppressed the secretion of pro-inflammatory cytokines. Inflammation is a compensatory response to cellular and tissue damage caused by ischemia-reperfusion injury, and the inflammatory response is primarily regulated by the signaling pathway of the MAPK cascade [8, 12]. MAPK is essential for the regulation of various inflammatory mediators, and MAPK is a kind of kinases that regulate cellular response to external stress signals or inflammatory cytokines [2, 41]. It was demonstrated that the induction of ischemic damage activated phosphorylation of the MAPK cascade, and this activation of MAPK controls a wide range of cellular processes [2, 12]. Our current study found that phosphorylation of the MAPK cascade pathway was increased by ischemic damage and this phosphorylation of the MAPK cascade pathway was reduced by PDRN treatment.
The intracellular concentration of cAMP is increased by stimulation of the adenosine A2A receptor, and this increased cAMP concentration serves as a physiological inhibitor to function of inflammatory neutrophil [42]. Furthermore, adenosine A2A receptor activation promotes signal from the cAMP-PKA pathway and accelerates the level of CREB phosphorylation. Elevated cAMP concentration suppresses the level of phosphorylation of the MAPK cascade pathway in stimulated cells [42, 43]. Adenosine A2A receptor agonist, PDRN, has been proposed to have therapeutic potential in inflammatory diseases [16, 17]. In the current study, PDRN treatment increased the cAMP concentration in gerbils presenting cerebral ischemia, and this increased cAMP concentration inhibited phosphorylation of the MAPK cascade pathway, thereby inactivating the MAPK cascade pathway in the hippocampus and basolateral amygdala (Supplements 2–4 in S1 File).
The current study has revealed that PDRN treatment inhibits inflammation, improves neuronal cell survival, and prevents a decline in short-term memory in a brain ischemia animal model (S1 Graphic abstract). Co-administration of PDRN and adenosine A2A receptor antagonist DMPX attenuated the therapeutic effect of PDRN in cerebral ischemia. Based on these findings, PDRN may be developed as the primary treatment in cerebral ischemia.
Supporting information
(DOCX)
(TIF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
• Jin Hee Han • NRF-2017R1D1A1B03032827 • National Research Foundation of Korea • https://www.nrf.re.kr/ • Research fund support.
References
- 1.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004; 22(3–5):281–99. [PubMed] [Google Scholar]
- 2.Wang T, Wang F, Yu L, Li Z. Nobiletin alleviates cerebral ischemic-reperfusion injury via MAPK signaling pathway. Am J Transl Res. 2019; 11(9):5967–77. [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Qi Z, Luo Y, Hinchliffe T, Ding G, Xia Y, et al. Non-pharmaceutical therapies for stroke: mechanisms and clinical implications. Prog Neurobiol. 2014; 115(2):246–69. 10.1016/j.pneurobio.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedata F, Pugliese AM, Coppi E, Dettori I, Maraula G, Cellai L, et al. Adenosine A2A receptors modulate acute injury and neuroinflammation in brain ischemia. Mediators Inflamm. 2014; 2014:805198. 10.1155/2014/805198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltzschig HK, Eckle T. Ischemia and reperfusion-from mechanism to translation. Nat Med. 2011; 17(11):1391–401. 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong LL, Wang ZY, Han N, Zhuang XM, Wang ZZ, Li H, et al. Neutralization of chemokine-like factor 1, a novel C-C chemokine, protects against focal cerebral ischemia by inhibiting neutrophil infiltration via MAPK pathways in rats. J Neuroinflammation. 2014; 11:112. 10.1186/1742-2094-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010; 1(2):74–84. 10.1007/s12975-010-0023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovalska M, Kovalska L, Pavlikova M, Janickova M, Mikuskova K, Adamkov M, et al. Intracellular signaling MAPK pathway after cerebral ischemia-reperfusion injury. Neurochem Res. 2012; 37(7):1568–77. 10.1007/s11064-012-0752-y [DOI] [PubMed] [Google Scholar]
- 9.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012; 92(2):689–737. 10.1152/physrev.00028.2011 [DOI] [PubMed] [Google Scholar]
- 10.Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo C, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation. 2014; 11:167. 10.1186/s12974-014-0167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CH, Yen TL, Hsu CY, Thomas PA, Sheu JR, Jayakumar T. Multi-targeting andrographolide, a novel NF-κB inhibitor, as a potential therapeutic agent for stroke. Int J Mol Sci. 2017; 18(8):E1638. 10.3390/ijms18081638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng X, Xie W, Xu Q, Liang T, Xu X, Sun G, et al. Neuroprotective effects of radix scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules. 2018; 23(9):E2401. 10.3390/molecules23092401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melani A, Pugliese AM, Pedata F. Adenosine receptors in cerebral ischemia. Int Rev Neurobiol. 2014; 119:309–48. 10.1016/B978-0-12-801022-8.00013-1 [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Zeng X, Li G, Yang Q, Xu J, Zhang M, et al. Inactivation of endothelial adenosine A2A receptors protects mice from cerebral ischaemia-induced brain injury. Br J Pharmacol. 2019;176(13):2250–63. 10.1111/bph.14673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon SJ, Rhee SY, Ryu JH, Cheong JH, Kwon K, Yang SI, et al. Activation of adenosine A2A receptor up-regulates BDNF expression in rat primary cortical neurons. Neurochem Res. 2011; 36(12):2259–69. 10.1007/s11064-011-0550-y [DOI] [PubMed] [Google Scholar]
- 16.An J, Park SH, Ko IG, Jin JJ, Hwang L, Ji ES, et al. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced lung injury by inhibiting apoptotic cell death in rats. Int J Mol Sci. 2017; 18(9):E1847. 10.3390/ijms18091847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko IG, Kim SE, Jin JJ, Hwang L, Ji ES, Kim CJ, et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018; 203:12–9. 10.1016/j.lfs.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Yoon S, Kang JJ, Kim J, Park S, Kim JM. Efficacy and safety of intra-articular injections of hyaluronic acid combined with polydeoxyribonucleotide in the treatment of knee osteoarthritis. Ann Rehabil Med. 2019; 43(2):204–14. 10.5535/arm.2019.43.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS, et al. Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav. 2009;91(4):629–35. 10.1016/j.pbb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Ko IG, Kim SE, Hwang L, Jin JJ, Choi HH, et al. Aqueous extract of Cordyceps alleviates cerebral ischemia-induced short-term memory impairment in gerbils. J Exerc Rehabil. 2016; 12(2):69–78. 10.12965/jer.1632586.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon JW, Lee JI, Shin HP, Cha JM, Joo KR, Kim SH, et al. Adenosine A2A-receptor agonist polydeoxyribonucleotide promotes gastric ulcer healing in Mongolian gerbils. Animal Cells Syst. 2014; 18(6): 399–406. 10.1080/19768354.2014.983968. [DOI] [Google Scholar]
- 22.Park JH, Kim SE, Jin JJ, Choi HS, Kim CJ, Ko IG. Pentoxifylline alleviates perinatal hypoxic-ischemia-induced short-term memory impairment by suppressing apoptosis in the hippocampus of rat pups. Int Neurourol J. 2016; 20(2):107–13. 10.5213/inj.1632532.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko IG, Kim CJ, Kim H. Treadmill exercise improves memory by up-regulating dopamine and down-regulating D2 dopamine receptor in traumatic brain injury rats. J Exerc Rehabil. 2019; 15(4):504–11. 10.12965/jer.1938316.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko IG, Kim SE, Hwang L, Jin JJ, Kim CJ, Kim BK, et al. Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats. J Exerc Rehabil. 2018; 14(3):327–34. 10.12965/jer.1836248.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin MS, Kim TW, Park SS, Ko IG, Kim CJ, Kim M, et al. Long-term surgical and chemical castration deteriorates memory function through downregulation of PKA/CREB/BDNF and c-Raf/MEK/ERK pathways in hippocampus. Int Neurourol J. 2019; 23(2):116–24. 10.5213/inj.1938103.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JW, Jung SY, Kim DY, Chung YR, Choi HH, Jeon JW, et al. PI3K-Akt-Wnt pathway is implicated in exercise-induced improvement of short-term memory in cerebral palsy rats. Int Neurourol J. 2018; 22(3):S156–64. 10.5213/inj.1836224.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JM, Ji ES, Kim TW, Kim CJ, Shin MS, Lim BV, et al. Treadmill exercise improves memory function by inhibiting hippocampal apoptosis in pilocarpine-induced epileptic rats. J Exerc Rehabil. 2018; 14(5):713–23. 10.12965/jer.36394.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997; 386(6624):493–5. 10.1038/386493a0 [DOI] [PubMed] [Google Scholar]
- 29.Hwang L, Ko IG, Jin JJ, Kim SH, Kim CJ, Chang B, et al. Dexmedetomidine ameliorates memory impairment in sleep-deprived mice. Anim Cells Syst (Seoul). 2019; 23(6):371–9. 10.1080/19768354.2019.1688185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991; 40(3):599‐636. 10.1016/0306-4522(91)90001-5 [DOI] [PubMed] [Google Scholar]
- 31.Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F, et al. Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides. 2015; 49:63–8. 10.1016/j.npep.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Cho CH, Byun HR, Jover-Mengual T, Pontarelli F, Dejesus C, Cho AR, et al. Gadd45b acts as neuroprotective effector in global ischemia-induced neuronal death. Int Neurourol J. 2019; 23(1):S11–21. 10.5213/inj.1938040.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamani M, Hassanshahi J, Soleimani M, Zamani F. Neuroprotective effect of olive oil in the hippocampus CA1 neurons following ischemia: Reperfusion in mice. J Neurosci Rural Pract. 2013; 4(2):164‐70. 10.4103/0976-3147.112753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012; 32(9):1677–98. 10.1038/jcbfm.2012.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brea D, Sobrino T, Ramos-Cabrer P, Castillo J. Inflammatory and neuroimmunomodulatory changes in acute cerebral ischemia. Cerebrovasc Dis. 2009; 27:S48–S64. 10.1159/000200441 [DOI] [PubMed] [Google Scholar]
- 36.Naderi Y, Sabetkasaei M, Parvardeh S, Moini Zanjani T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol. 2017; 28:214–22. 10.1097/FBP.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 37.Milne GR, Palmer TM. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal. 2011; 11:320–39. 10.1100/tsw.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adén U, Halldner L, Lagercrantz H, Dalmau I, Ledent C, Fredholm BB. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003; 34(3):739–44. 10.1161/01.STR.0000060204.67672.8B [DOI] [PubMed] [Google Scholar]
- 39.Tebano MT, Martire A, Potenza RL, Grò C, Pepponi R, Armida M, et al. Adenosine A2A receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem. 2008; 104(1):279–86. 10.1111/j.1471-4159.2007.05046.x [DOI] [PubMed] [Google Scholar]
- 40.Fontinha BM, Diógenes MJ, Ribeiro JA, Sebastião AM. Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology. 2008; 54(6):924–33. 10.1016/j.neuropharm.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 41.Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, et al. Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018; 55(2):1082–96. 10.1007/s12035-017-0394-9 [DOI] [PubMed] [Google Scholar]
- 42.Giambelluca MS, Pouliot M. Early tyrosine phosphorylation events following adenosine A2A receptor in human neutrophils: identification of regulated pathways. J Leukoc Biol. 2017; 102(3):829–36. 10.1189/jlb.2VMA1216-517R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chera S, Ghila L, Wenger Y, Galliot B. Injury-induced activation of the MAPK/CREB pathway triggers apoptosis-induced compensatory proliferation in hydra head regeneration. Dev Growth Differ. 2011; 53(2):186–201. 10.1111/j.1440-169X.2011.01250.x [DOI] [PubMed] [Google Scholar]