Abstract
Background
Patients with chronic obstructive pulmonary disease (COPD) are highly susceptible from respiratory exacerbations from viral respiratory tract infections. However, it is unclear whether they are at increased risk of COVID-19 pneumonia or COVID-19-related mortality. We aimed to determine whether COPD is a risk factor for adverse COVID-19 outcomes including hospitalization, severe COVID-19, or death.
Methods
Following the PRISMA guidelines, we performed a systematic review of COVID-19 clinical studies published between November 1st, 2019 and January 28th, 2021 (PROSPERO ID: CRD42020191491). We included studies that quantified the number of COPD patients, and reported at least one of the following outcomes stratified by COPD status: hospitalization; severe COVID-19; ICU admission; mechanical ventilation; acute respiratory distress syndrome; or mortality. We meta-analyzed the results of individual studies to determine the odds ratio (OR) of these outcomes in patients with COPD compared to those without COPD.
Findings
Fifty-nine studies met the inclusion criteria, and underwent data extraction. Most studies were retrospective cohort studies/case series of hospitalized patients. Only four studies examined the effects of COPD on COVID-19 outcomes as their primary endpoint. In aggregate, COPD was associated with increased odds of hospitalization (OR 4.23, 95% confidence interval [CI] 3.65–4.90), ICU admission (OR 1.35, 95% CI 1.02–1.78), and mortality (OR 2.47, 95% CI 2.18–2.79).
Interpretation
Having a clinical diagnosis of COPD significantly increases the odds of poor clinical outcomes in patients with COVID-19. COPD patients should thus be considered a high-risk group, and targeted for preventative measures and aggressive treatment for COVID-19 including vaccination.
Keywords: COVID-19, COPD, Meta-analysis, mortality
Research in context.
Evidence before this study
Some studies have suggested that patients with chronic obstructive pulmonary disease (COPD) are at increased risk of poor outcomes in COVID-19, including mortality. Previous systematic reviews on this topic included small numbers of studies mainly from a single region, with heterogeneous outcome definitions and potentially overlapping patient populations. We conducted a systematic review of the literature published between November 1st 2019 and January 28th 2021, using search terms [(chronic obstructive pulmonary disease OR COPD) AND (COVID-19 outcomes)].
Added value of this study
This is the largest systematic review to date on the effects of COPD on the risk of poor COVID-19 outcomes, covering a large number of studies across multiple continents. COPD significantly increases the risk of hospitalization, ICU admissions, and mortality.
Implications of all the available evidence
Patients with COPD have a higher risk of poor outcomes from COVID-19 than those without COPD; they should be strongly encouraged to take aggressive preventative measures and should be prioritized for COVID-19 vaccination.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], [2], [3], which is responsible for the current global health crisis. The clinical presentation of COVID-19 is diverse. Some patients are asymptomatic or mildly symptomatic; while others progress rapidly to severe pneumonia, respiratory failure, multi-organ disease and even death [2,4]. To date, over 110 million individuals have contracted SARS-CoV-2 worldwide, of which more than 2.5 million have succumbed to the disease [5].
Chronic obstructive pulmonary disease (COPD) is a progressive disorder that is characterized by persistent low-grade lung inflammation and airflow obstruction [6]. There is no clear evidence that COPD increases COVID-19 susceptibility independent of other established risk factors. However, there is a growing concern that COPD may be a risk factor for poor clinical outcomes in established COVID-19. While the mechanisms for this are not well known, it is now well established that angiotensin converting enzyme-2 (ACE-2), which is the receptor responsible for SARS-CoV-2 entry into cells [2,7,8], is up-regulated in the small airway epithelium and alveoli of individuals with COPD [9,10]. Patients with COPD are also known to have impaired innate and adaptative immune responses and demonstrate delayed clearance of respiratory viruses [11,12]. Together, these factors may facilitate the propagation of SARS-CoV-2 in the lungs of COPD patients, leading to rapid clinical deterioration and progression to severe COVID-19.
A number of investigators have conducted reviews on the relationship between COPD and COVID-19 outcomes [13], [14], [15], [16]. However, these reviews included small numbers of studies that were overwhelmingly from China and published in the early phase of the pandemic. Regional differences in populations, health care resources, and local protocols may limit the generalisability of these results to the rest of the world. Many of the reviewed studies did not differentiate COPD from other chronic lung diseases, and hospitalisation was not considered as an outcome. Critically, many of the studies included in the meta-analyses reported on patients from the same hospitals and time periods, meaning that some patients may have been inadvertently analysed more than once, thus biasing the results.
We therefore conducted a comprehensive, up-to-date systematic review and a meta-analysis of published literature to determine whether COPD is a risk factor for poor outcomes in COVID-19, focusing on the specific endpoints of hospitalization, severe disease, and mortality.
2. Methods
2.1. Search strategy and selection criteria
This systematic review was performed according to the recommendations of the Preferred Reporting in the Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17] and has been prospectively registered with Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42020191491).
We performed an initial digital search of MedRxiv, Google Scholar, Pubmed and Ovid Medline of all COVID-19 clinical studies that were published between November 1st, 2019 and August 31st, 2020. A broad search strategy was adopted using the keywords: (chronic obstructive pulmonary disease or COPD) and (COVID-19 outcomes). We also searched the bibliographies of any relevant reviews. We included any clinical study (randomized clinical trials, cohort studies, cross sectional studies, case reports and series, and case-control studies) that reported the number of patients with COPD (as deemed by the investigators), and specified at least one of the following clinical outcomes stratified by COPD status: hospitalization status; severe COVID-19 (by any definition, differentiated from non-severe disease); intensive care unit (ICU) admission status; invasive mechanical ventilation; acute respiratory distress syndrome (ARDS); or mortality. We excluded studies that did not specify the number of patients with COPD as a comorbidity, that reported only COPD patients (and no comparator group), or that did not enumerate the clinical outcomes according to COPD status. Three reviewers (FVG, LHC and AB) independently assessed full text articles for inclusion. On January 28th, 2021, we updated the search to identify any recently-published studies and replaced any pre-print studies with the published versions; pre-print studies that had not been published in a peer-reviewed journal by this date were subsequently excluded.
We assessed the risk of bias for each of the included studies using the Clinical Advances through Research and Information Translation (CLARITY) group tool to assess risk of bias in cohort studies [18]. This eight-item tool was chosen on the assumption that most studies would be observational (non-randomised) and that, regardless of the study's original intent, our desired analysis would conform to a cohort study design with COPD as the “exposure”. Two reviewers (FVG and SM) performed the assessments independently, with any disagreement resolved by discussion. The bias assessment was made at an outcome level where appropriate. Three reviewers (FVG, LHC and AB) independently extracted data on patient characteristics and clinical outcomes, with any discrepancies resolved by iteration. Where this was not possible or the discrepancy could not be resolved (five studies), an independent referee (CC, blinded to the other reviewers’ assessments) reviewed the paper in question and extracted the relevant data. In instances where the selected publication reported COPD patients only as a percentage of the total population, we estimated the number based on the given percentage (three studies). In instances where the age of the total study population was not reported, the mean age was estimated based on the reported means of subgroups.
2.2. Data analysis
We performed a meta-analysis, using the metafor (2.1-0) [19] package in R (3.6.0) programming language, to determine the risk of hospitalization for COVID-19, risk of severe COVID-19, and risk of mortality in COPD patients compared to non-COPD patients. In each of the meta-analyses, we fitted a random effects model using the Restricted Maximum Likelihood (REML) estimator with measure "OR" (odds ratio) and all default values, which was calculated based on the data provided in the selected studies. We assessed for evidence of publication bias using funnel plots, and for heterogeneity of data across the included studies using Cochran's Q test and I2 value. Where there was significant heterogeneity, a stepwise removal of the studies was employed until the heterogeneity p-value of > 0.05 was achieved. Since we only had access to summary-level data, we were unable to adjust our models for potential confounders. However, to assess whether age or male sex (both established risk factors for poor outcomes in COVID-19) accounted for the observed between-study heterogeneity, we performed meta-regressions of the ORs with reported mean (or median) age and percentage of male participants as factors.
2.3. Role of the funding source
There was no direct funding source for this work. All authors had full access to the data. CWTY, FVG and DDS verified the underlying data, and all authors take responsibility for the submission.
3. Results
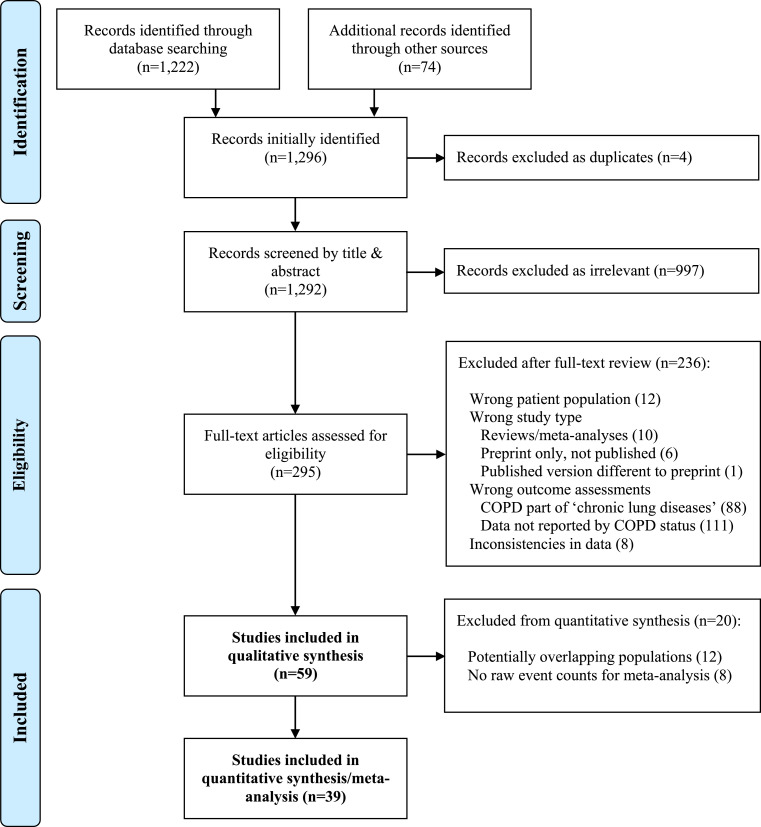
From an initial screening of 1,292 papers, we carefully reviewed the full text of 295 papers for eligibility (Fig. 1). We excluded 236 papers, due mainly to the fact that they did not report at least one of the prespecified clinical outcomes for COPD patients (n = 111) or that COPD was combined with other lung diseases (n = 88). Six studies initially identified in preprint were subsequently excluded since they had not been peer-reviewed and published by 28th January 2021; additionally, one study that was initially included based on preprint was subsequently removed since the peer-reviewed version did not meet our study's inclusion criteria. In total, 59 studies [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78] were selected for the qualitative synthesis, of which 39[[23], [24], [25], [26],[30], [31], [32], [33], [34],[37], [38], [39], [40], [41], [42],44,45,[47], [48], [49], [50], [51], [52], [53], [54],57,[59], [60], [61], [62], [63], [64], [65], [66], [67], [68]] were utilized for the quantitative synthesis (meta-analysis).
Fig. 1.
PRISMA flow chart for the systematic review.
We screened 1,292 records identified by digital search of MedRxiv, Google Scholar, Pubmed and Ovid Medline for COVID-19 clinical studies that were published between November 1st, 2019 and January 28th, 2021. We excluded any preprint papers that were initially identified but not peer-reviewed and published by 28th January, 2021. In total, 59 studies were selected for the qualitative synthesis, of which 39 were utilized for quantitative synthesis (meta-analysis).
The selected studies represented reports from 16 different countries with the highest representation from China and the United States. A summary of the included studies is presented in Table 1. The majority of the included studies were retrospective. Many were described by their respective authors as cohort studies, although these were often better described as case series. We determined that three studies had a case-control design [22,28,73]. Six studies[32,41,43,54,69,70] appeared to be prospective in nature, although the time course of enrolment and data collection was ambiguous for at least three of these studies [32,41,69]. The study settings and data sources varied from large community-based registries, general hospital wards, dedicated COVID-19 units, and ICUs. The number of COVID-19 patients included in each study ranged from 32 (a single-centre case series from India) [20] to 331,298 (a nationwide COVID-19 registry from Mexico) [60]. Of the total 698,042 confirmed COVID-19 patients reported by the studies, 14,913 (2.1%) had COPD as a comorbidity. Not surprisingly, only one study used a standard definition of COPD (a case-control study from China specifically investigating COPD as a risk factor for severe COVID-19) [73]: in almost all studies, COPD status was determined from the medical record (with or without confirmed respiratory physician diagnosis), and no studies confirmed the diagnosis by spirometry. The effect of using a clinical definition of COPD, compared to more objective criteria, is difficult to know since it would include both over- and underdiagnosis. Nevertheless, we made sure to include only studies specifying COPD as opposed to undifferentiated chronic respiratory disease.
Table 1.
Data extracted from the included studies.
| Authors | Country | Study type | Study setting | Study period | Total partici-pants, n (% male) | Age, years† | COPD patients, n | COPD status determination | Main reported outcomes/analyses | Outcomes used for meta-analysis‡ |
|---|---|---|---|---|---|---|---|---|---|---|
| Aggarwal et al [20] | India | Retrospective case series | Hospital inpatients – designated COVID-19 facility (single centre) | April 10–30, 2020 | 32 (59) | 54 (46, 60) | 5 | Extracted from medical record | Primary composite endpoint of admission to ICU/mechanical ventilation/death | none |
| Argenziano et.al [21] | USA | Retrospective cohort study | Hospital inpatients (multi-centre) & ERs | March 1 and April 6 2020 (followed til April 30 2020) | 1000 (59.6) | 63 | 66 | Extracted from medical record | Clinical course of the COVID-19 patients across the emergency department, hospital wards, and intensive care units. | H,S |
| Atkins et al [22] | England | Case-control study | Community based registry (UK biobank) | March 16–April 26, 2020 | 507 (61) | 74±5 | 62 | Participant reports of doctor-diagnosed disease | Predictors of COVID-19 PCR positive | none |
| Attaway et.al [23] | USA | Retrospective cohort study | Hospital system database (multi-centre) | March 8 and May 13, 2020 | 2527 (48) | 61 | 164 | Self-report, then confirmed by physician diagnosis in medical record | Effect of COPD on hospitalization and COVID-19 outcomes | H,S,M |
| Auld et al [24] | USA | Retrospective cohort study | ICU patients (multi-centre) | March 6–April 17, 2020 | 217 (55) | 64 (54, 73) | 21 | Extracted from medical record | Descriptive, with focus on mortality rate | M |
| Azoulay et al [25] | France | Retrospective cohort study | ICU patients (multi-centre) | February 21–April 24, 2020 (status recorded May 15) | 376 (77) | 66 (53, 68) | 20 | Extracted from medical record | Predictors of 28-day mortality | M |
| Barman et.al [26] | Turkey | Retrospective cohort study | Hospital inpatients (multi-centre) | March 20 and April 20, 2020 | 607 (55) | 62.5 ± 14.3 | 73 | Extracted from medical record | Prognostic importance of myocardial damage in patients hospitalized with COVID-19 | M |
| Bello-Chavolla et al [27] | Mexico | Cross-sectional study | Nationwide COVID-19 case registry | All PCR-positive cases up to June 3, 2020 | 101,238 (56) | Data not reported | 1,990 | From health information uploaded to the registry by personnel from healthcare facilities | Predictors of mortality | noneb |
| Bravi et al [28] | Italy | Retrospective case-control study | Laboratory database of PCR positive cases across two provinces | PCR-positive cases at April 2 (Ferrara province)/April 24 (Pescara province) | 1,603 (47) | 58±21 | 97 | Extracted from linked hospital discharge abstracts, queried from January 1, 2015 to date of COVID-19 diagnosis | Predictors of severe/lethal COVID-19, including the treatment with ACEi/ARB medications | H,M |
| Buckner et al [29] | USA | Retrospective case series | Hospital inpatients (multi-centre) | March 2–March 26, 2020 | 105 (50) | 69 (23, 97) | 11 | Extracted from medical record | Severe COVID-19 composite endpoint of ICU admission or death | none |
| Calmes et.al [30] | Belgium | Retrospective cohort study | Hospital inpatients (single centre) | March 18 and April 17, 2020 | 596 (49) (calculated) | 59 | 46 | Extracted from medical record | Understanding if obstructive diseases increase the chance of ICU admission and death among COVID-19 patients. | S,M |
| Caraballo et al [31] | USA | Cross-sectional study | Patients in Yale Heart Failure Registry | Data queried up to April 16, 2020 | 206 (45) | 78 (65, 87) | 67 | Documented as a comorbidity in the registry | Prevalence and predictors of COVID-19; predictors of early outcome (ICU/intubation/death) | M |
| Cen et al [32] | China | (Prospective?) cohort study | Hospital inpatients (multi-centre) with mild disease at admission | From February 10, 2020 (followed for 28 days after admission) | 1,007 (49) | 61 (49, 68) | 46 | Extracted from medical record | Predictors of disease progression (conversion from mild/moderate to severe or critical stage, or death) | M |
| Ciardullo et al [33] | Italy | Retrospective cohort study | Hospital inpatient (single centre) | February 22–May 15, 2020 | 373 (65) | 72±14 | 39 | Extracted from medical record | In-hospital mortality, including diabetes as a predictor | M |
| Cui et.al [34] | China | Retrospective cohort study | Hospital inpatients (single centre) | January 14 and March 9, 2020 | 836 (52.5) | 64 | 48 | Extracted from medical record | Predictors of mortality | Md |
| Feng Y et al [35] | China | Retrospective cohort study | Hospital inpatients (multi-centre) | January 1–February 15, 2020 (followed til 21 March) | 476 (57) | 53 (40, 64) | 22 | Extracted from medical record | Discharge from hospital, or death | nonea |
| Giannouchos T et al [36] | Mexico | Cross-sectional study | COVID-19 case registry (Mexican Ministry of Health) | All registry patients up to May 31, 2020 | 89,756 (56) | 46±16 | 1,773 | From health information uploaded to the registry by personnel from healthcare facilities | Positive cases among suspected cases; hospitalization; “adverse outcome” (intubation/ICU/death) | noneb |
| Goyal et al [37] | USA | Retrospective case series | Hospital inpatients (multi-centre) | March 3–27, 2020 | 393 (61) | 62 (49, 74) | 20 | Extracted from medical record | Descriptive, with focus on rate of invasive mechanical ventilation | Sc |
| Grasselli.G et al [38] | Italy | Retrospective case series | ICU patients (single centre) | February 20–April 22, 2020 (followed til May 30, 2020) | 3,988 (80) | 63 (56, 69) | 93 | Extracted from medical record | Predictors of mortality | M |
| Guan W J et al [39] | China | Retrospective case series | Hospital inpatients (multi-centre) | December 11, 2019–January 31, 2020 | 1,590 (57) | 49±16 | 24 | Patient self-report upon admission | Predictors of a composite endpoint of ICU admission/invasive ventilation/death | S,Ma |
| Gupta R et.al [40] | USA | Retrospective cohort study | Hospital inpatients (single centre) | March 2–April 23, 2020 | 529 (54) | 70 | 36 | Extracted from medical record | Predictors of mortality | M |
| Gupta S et.al [41] | USA | Prospective cohort study | ICU patients (multi-centre) | March 4-April 4, 2020 (followed til June 4 2020) | 2215 (64.8) | 60.5 | 173 | Extracted from medical record | Predictors of mortality | M |
| Hansen et.al [42] | Denmark | Retrospective cohort | Hospital inpatients (multi- centre) and outpatient | February 1-July 10, 2020 | 5104 (47) | 54.6 | 432 | Extracted from health registry. | Effect of COPD and asthma on composite outcome of severe COVID-19, ICU, death | S,M |
| Huang C et al [43] | China | Prospective case series | Hospital inpatients – designated COVID-19 facility (single centre) | December 16, 2019–January 2, 2020 | 41 (73) | 49 (41, 58) | 1 | Extracted from medical record, hospital admission report, patients or families if required | Descriptive, focus on rate of ICU care | nonea |
| Islam et.al [44] | Bangladesh | Retrospective cohort study | Hospital inpatients (single centre) | May 2020 | 1016 (64.1) | 37 (28,49) | 85 | Extracted from medical record | Predictors of morbidity and mortality among COVID-19 patients | M |
| Israelsen et al [45] | Denmark | Retrospective case series | Hospital inpatients (single centre) | March 10–April 23, 2020 | 175 (49) | 71 (55, 81) | 11 | Extracted from medical record | Descriptive, focus on rate of ICU care | S |
| Itelman et al [46] | Israel | Retrospective cohort study | Hospital inpatients –coronavirus unit and ICU (single centre) | February–April 10 2020 | 162 (65) | 52±20 | 2 | Extracted from medical record | Descriptive, focus on COVID-19 severity | none |
| Jalili et al [47] | Iran | Retrospective cohort study | Hospital inpatients in a nation-wide COVID-19 registry | February 20–April 20, 2020 | 28,981 (56) | 57±18 | 683 | From health information uploaded to the registry by individual hospitals | Descriptive, focus on mortality rate | M |
| Javanian et al [48] | Iran | Retrospective cohort study | Hospital inpatients (multi-centre) | February 25–March 12, 2020 (followed til March 17, 2020) | 100 (51) | 60±14 | 12 | Extracted from medical record | Predictors of in-hospital mortality | M |
| Jiang et.al [49] | China | Retrospective cohort study | ICU patients (single centre) | January 30 to March 8, 2020 (followed til April 10 2020) | 281 (51) (calculate) | 70 | 38 | Extracted from medical record | Predictors of 28-day mortality | Me |
| Jimenez et.al [50] | Spain | Retrospective case series | Hospital inpatients (single centre) | March 1 to May 28, 2020 | 1549 (57.5) | 69 | 211 | Extracted from medical record | Predominantly descriptive; predictors of COVID-19 complications | S,M |
| Kalyanaraman Marcello et.al [51] | USA | Retrospective cohort study | All patients tested in a public health system | March 5 and April 9, 2020 (followed til April 16, 2020) | 13442 (56) | 52.7 | 284 | Extracted from medical record | Understanding disparities in COVID-19 outcomes | H,M |
| Kim et.al [52] | South Korea | Retrospective cohort study | Nationwide COVID-19 case registry | Data queried up to April 30, 2020 | 2959 (40) | Data not reported | 28 | From national based data base | Risk factors for severe COVID-19 | S |
| Lagi et al [53] | Italy | Retrospective cohort study | Hospital inpatients (single centre) | February 25–March 26, 2020 | 84 (65) | 62 (51,72) | 5 | Extracted from medical record | Descriptive, focus on need for ICU, and impact of an on-ward intervention | S |
| Lanini et.al [54] | Italy | Longitudinal cohort study | Hospital inpatients (single centre) | January 29 to March 28, 2020 | 379 (72.03) | 61.67 | 49 | Extracted based on standardised forms provided at the point of care to patients | Identify prognostic clinical biomarkers associated with mortality/survival of patients with COVID-19 | Mf |
| Li K. et al [55] | China | Retrospective cohort study | Hospital inpatients – quarantine unit (single centre) | January 31–March 5, 2020 (followed til March 25, 2020) | 102 (58) | 57 (45,70) | 2 | Extracted from medical record | Early radiographic change as a predictor of mortality | noned |
| Liu W et al [56] | China | Retrospective case series | Hospital inpatients (multi-centre) | December 30, 2019–January 15, 2020 (follow til 2 weeks post-hospitalization) | 78 (50) | 38 (33, 57) | 2 | Not specified | Predictors of disease progression | none |
| Ludwig et.al [57] | Germany | Retrospective observational study | Anonymized national healthcare claims data | Feb 17-July 21, 2020. | 2343 (54) | 62 | 306 | Extracted based on the statutory health insurance claims data | Descriptive, with focus on ICU admission compared to influenza | S |
| Mancilla-Galindo et.al [58] | Mexico | Retrospective cohort study | COVID-19 case registry (Mexican Ministry of Health) | February 28-May 30, 2020 | 83779 (56.6) | 46.3 | 1695 | Extracted from medical record available through the data platform of the Federal Government of Mexico | Predictors of mortality | noneb |
| Mohamed et.al [59] | USA | Retrospective case series | Hospital inpatients (multi-centre) | March 28-April 16, 2020 | 7624 (54.6) | Data not reported | 198 | Extracted from the Mount Sinai Data Warehouse | Descriptive only, focus on chronic kidney disease and mortality | Mc |
| Parra-Bracamonte et.al [60] | Mexico | Retrospective cohort study | National based registry (Epidemiologic Surveillance Source of Respiratory Viral Diseases) | January 13–July 17, 2020 | 331,298 (53.8) | 44 | 5458 | Extracted from national based registry | Risk factors for mortality | H,Mb |
| Rica R et al [61] | Spain | Retrospective cohort study | Hospital inpatients (single centre) | March 15–31, 2020 | 48 (67) | 66±14 | 5 | Self-reported by patients at hospital triage | Predictors of need for ICU care | S |
| Salacup G et al [62] | USA | Retrospective case series | Hospital inpatients (single centre) | March 1–April 24, 2020 | 242 (51) | 66±15 | 30 | Extracted from medical record | Predictors of in-hospital mortality | M |
| Shah et.al [63] | USA | Retrospective cohort study | Community based registry | March 2–May 6, 2020 | 522 (41.8) | 63 | 47 | Extracted from medical record | Predictors of mortality | M |
| Smith A et al [64] | USA | Retrospective study | Hospital inpatients (multi-centre) | March 1–April 22, 2020 | 346 (56) | Mean 67 | 58 | Extracted from medical record | Predictors of in-hospital mortality | M |
| Song.J et al [65] | China | Retrospective cohort study | Hospital inpatients – designated COVID-19 facility (single centre) | February 1–March 6, 2020 | 961 (52) | 63 (49-70) | 21 | Extracted from medical record | Predictors of mortality in patients with COPD and COVID-19 | M |
| Suleyman G et al [66] | USA | Retrospective case series | Cases from hospitals/emergency departments (multi-centre) | March 9–27, 2020 (clinical outcomes followed for 30 days) | 463 (44) | 58±17 | 49 | Extracted from medical record | Predictors of need for ICU care and 30-day mortality | H,S |
| Tomlins J et al [67] | England | Retrospective case series | Hospital inpatients (single centre) | March 10–30 (final day of follow up on April 6th) | 95 (63) | 75 (59, 82) | 10 | Not clear | Descriptive, focus on in-hospital mortality | M |
| van Gerwen et.al [68] | USA | Retrospective cohort study | State based registry | March 1–April 1, 2020 (followed til May 13, 2020) | 3703 (55.3) | 56.8 ± 18.2 | 172 | Extracted from medical record | Predictors of need for hospitalization, mechanical ventilation and all‐cause mortality | Hc |
| Violi F et al [69] | Italy | (Prospective?) cohort study | Hospital inpatients – designated COVID-19 facilities (multi-centre) | March–April 2020 | 319 (61) | Survivors, 66±18; non-survivors, 77±11 | 41 | Not clear | Predictors of mortality, focus on serum albumin level as a predictor | none f |
| Wang D et al [70] | China | Prospective case series | Hospital inpatients – designated COVID-19 facility (single centre) | January 1–28, 2020 (follow-up til February 3, 2020) | 138 (54) | 56 (42, 68) | 4 | Extracted from medical record | Descriptive, focus on need for ICU care | none |
| Wang L et.al [71] | China | Retrospective cohort study | Hospital inpatients (single centre) | January 31–February 5, 2020 | 213 (44.6) | 62 (51-69) | 10 | Extracted from medical record | Predictors of death, with focus on coagulation laboratory parameters | nonee |
| Wang Y et al [72] | China | Retrospective case series | ICU patients | January 1–February 10, 2020 | 110 (44) | Data not reported | 6 | Extracted from medical record | Predictors of severe COVID-19 pneumonia | nonea |
| Wu.F et al [73] | China | Retrospective case-control study | Inpatient and outpatient COVID-19 cases (multi-centre) | December 11, 2019–February 20, 2020 | 443 (60) | Data not reported | 38 | Previously diagnosed by a respiratory physician based on post-bronchodilator FEV1/FVC <0.7 and symptoms | COPD as a predictor of a composite endpoint of ICU admission/invasive ventilation/death | S,M |
| Yang X et al [74] | China | Retrospective cohort study | Critically ill COVID-19 inpatients (single centre) | December 24, 2019–January 26, 2020 (follow-up til February 9, 2020) | 52 (67) | 60±13 | 4 | Extracted from medical record | Predictors of 28-day mortality after ICU admission | nonea |
| Zhang G et al [75] | China | Retrospective case series | Hospital inpatients (single centre) | January 2–February 10, 2020 | 221 (49) | 55 (39, 66.6) | 6 | Extracted from medical record | Descriptive, focus on severe COVID-19 | none |
| Zhang J et al [76] | China | Retrospective cohort study | Hospital inpatients – designated COVID-19 facility (single centre) | January 16–February 3, 2020 | 140 (51) | 57 (25, 87) | 2 | Extracted from medical record | Descriptive, focus on severe COVID-19 | none |
| Zheng F et al [77] | China | Retrospective cohort study | Hospital inpatients (single centre) | January 17–February 7, 2020 | 161 (50) | 45 (34, 57) | 6 | Not clear | Descriptive, focus on severe COVID-19 | none |
| Zhou F et al [78] | China | Retrospective cohort study | Hospital inpatients (multi-centre) | December 29, 2019–January 31, 2020 | 191 (62) | 56 (46, 67) | 6 | Extracted from medical record | Predictors of in-hospital mortality | nonea |
| Totals | 698,042 | 14,913 |
Abbreviations: PCR, polymerase chain reaction; ICU, intensive care unit; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; ARDS, acute respiratory distress syndrome; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity. †age presented as mean±SD or median (interquartile range) unless otherwise specified. ‡outcomes analyzed were hospitalization (H), severe COVID-19 (S), and mortality (M). Superscript letters represents groups of potentially overlapping populations, with only the largest study in each group analyzed
Guan et al
Parra-Bracamonte et al
each study used for separate outcomes
Cui et al
Jiang et al
Lanini et al.
There was significant variation in the COVID-19 outcomes reported in the studies (Figure S1). There were no true general population studies that quantified whether COPD confers increased susceptibility to COVID-19 or poor clinical outcomes; the closest was a case-control study from the UK Biobank which determined the predictors of test positivity among COVID-19 PCR tests [22]. Most studies were conducted in a hospital setting, with only eight studies reporting on hospitalization versus outpatient cases.[21,23,28,36,51,60,66,68] For severe COVID-19, we noted marked variation in what the authors of those studies considered to be “severe” disease. For example, some authors defined severe COVID-19 as simply the need for supplemental oxygen therapy [46,53] whilst others used strict definitions based on clinical practice guidelines (e.g. National Health Commission of China Interim Guidelines on the Diagnosis and Treatment of COVID-19, various versions available at http://en.nhc.gov.cn/publications.html; WHO Clinical Management of COVID-19 Interim Guidance, available at https://www.who.int/publications/i/item/clinical-management-of-covid-19) [32,35,43,55,56,73,76,78]. Other studies used surrogate measures of severity, such as the need for ICU admission or mechanical ventilation. Two studies reported on the presence of ARDS [26,63]. We noted that, even when predefined criteria were used, how and when these determinations were made was often not specified by the authors.
Of note, even though all the studies reported their enrolment periods, a majority of them failed to specify when clinical data were collected from their enrolled patients. Critically, amongst the selected articles that were published at the beginning of the pandemic, there appeared to be some overlap in study locations and enrolment periods (Table S1). This is perhaps not surprising given the urgency with which data were being collected, reported and updated at that time. Subsequent comparison of the authors, site of the study and the date of recruitment and follow-up identified 22 studies with potentially overlapping populations [27,[34], [35], [36], [37],39,43,49,54,55,[58], [59], [60],68,69,71,72,74,78]. To avoid the double counting of patients we included only the largest (by participant numbers) of the potentially overlapping studies in the quantitative analysis.
We assessed the risk of bias in each study using the CLARITY group tool [18]. Heterogeneity in study design meant that some items on the tool were not necessarily appropriate for all studies, which likely contributed to an overall impression of high risk of bias (Table S2). Nevertheless, there were some consistent themes that suggested higher risk of bias in the included studies. For example, COPD status (Q2: “Can we be confident in the assessment of exposure?”) was most often extracted from the medical record, without specifying by whom and without a standardized data collection method. Prognostic factors (Q5: “Can we be confident in the assessment of the presence or absence of prognostic factors?”) such as age and sex were routinely recorded, but the determination of other important factors such as comorbidities was generally taken from the medical record without confirmation. Not surprisingly, co-interventions for COPD and non-COPD groups (Q8: “Were co-interventions similar between groups?”) were rarely specified. Of note, it was often not clear who determined disease severity (investigators, treating physicians, etc) (Q6: “Can we be confident in the assessment of outcome?”). Finally, a prominent potential source of bias for both severe COVID-19 and mortality outcomes was inadequate follow-up (Q7: “Was the follow up of cohorts adequate?”), since many studies included patients who were still in hospital at the time of ascertainment, without having met a definitive outcome (e.g. survival to discharge, or death).
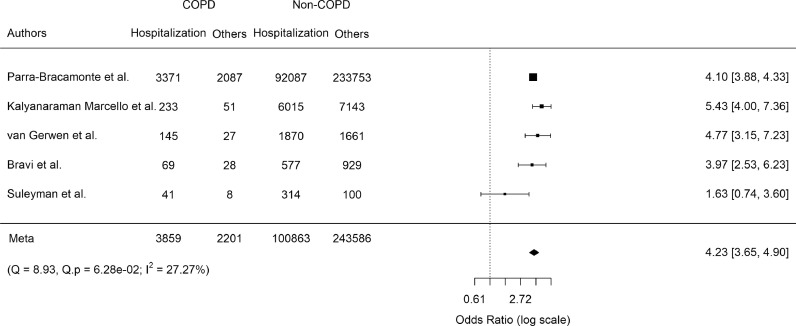
We quantified the association between a diagnosis of COPD and each of the three prespecified COVID-19 outcomes. For hospitalization, we evaluated only those studies that reported data on both outpatient and patient's hospital admissions. Among the seven studies identified, there was some asymmetry to the funnel plot, raising the possibility of publication bias (Figure S2). By far the largest of these studies was by Parra-Bracamonte et al [60], which was from a large healthcare registry in Mexico and included 331,298 patients with COVID-19 confirmed by a positive PCR test. COPD patients had increased odds of hospital admission (pooled odds ratio [OR] 3.12; 95% confidence interval [CI], 2.05–4.72; p < 0.0001), but there was severe heterogeneity (Cochran's Q 32.84, p < 0.0001; and I2 91.66%) (Figure S3). Following stepwise removal of the two studies [21,23] contributing the greatest to heterogeneity, the final pooled OR was 4.23 (95% CI 3.65–4.90, p < 0.0001) without evidence of residual heterogeneity (Fig. 2).
Fig. 2.
Pooled odds ratio of COVID-19-related hospitalization in COPD patients:
Only studies with no overlapping study populations were analyzed. Odds ratios [95% confidence intervals] for individual studies (squares and bars) and the pooled odds ratio [95% CI] (diamond). Results are following stepwise removal of two studies contributing to heterogeneity (Cochran's Q and I2 tests).
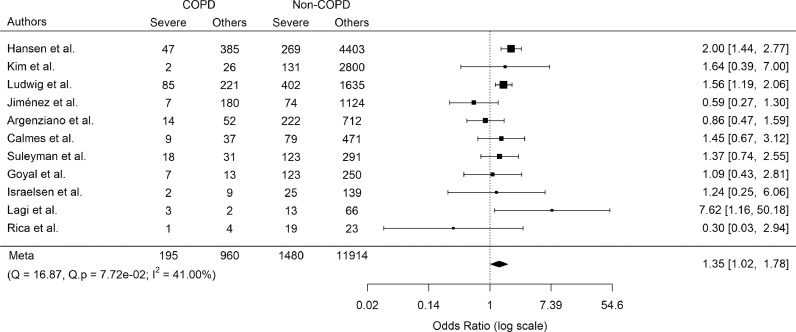
Since there was significant variation in how the studies described or defined severe COVID-19, the reviewers felt that it was inappropriate to include all studies in a meta-analysis for this outcome. For consistency, we chose ICU admission as a relatively objective surrogate marker of severe COVID-19. We identified 14 non-overlapping studies that reported on ICU admission of COVID-19 patients stratified by COPD status. There was no evidence of a significant publication bias (Figure S4). COPD as a comorbidity increased the odds of severe COVID-19 (pooled OR 1.78; 95% CI, 1.20–2.64; p = 0.004) with moderate heterogeneity of results across studies (Cochran's Q 45.03, p < 0.0001; and I2 79.52%) (Fig. S5). Following stepwise removal of the three studies [23,39,73] contributing the greatest to heterogeneity, the final pooled OR was 1.35 (95% CI, 1.02–1.78; p = 0.03; Fig. 3) without evidence of residual heterogeneity.
Fig.3.
Pooled odds ratio of severe COVID-19 (ICU admission) in COPD patients:
Only studies with no overlapping study populations were analyzed. Odds ratios [95% confidence intervals] for individual studies (squares and bars) and the pooled odds ratio [95% CI] (diamond). Results are following stepwise removal of three studies contributing to heterogeneity (Cochran's Q and I2 tests).
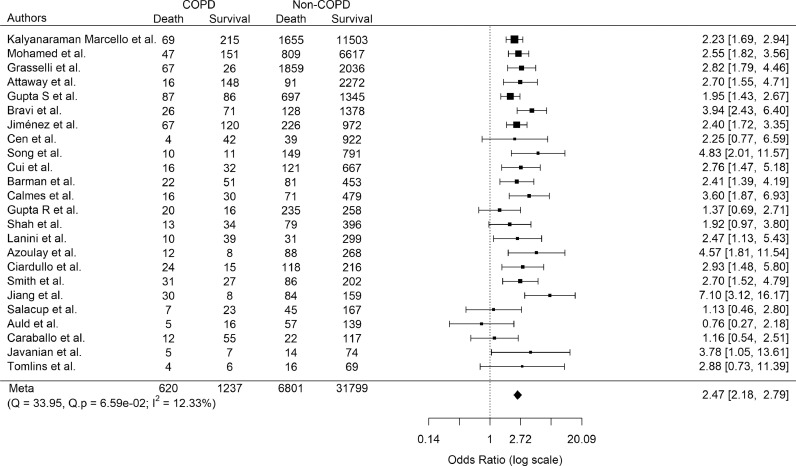
For mortality, this outcome was reported separately in 37 of the 59 included studies. Most of these did not specifically model the effects of COPD on mortality. 30 non-overlapping studies reported the vital status of patients according to COPD status and were therefore used in the meta-analysis. There was no suggestion of publication bias (Figure S6). COPD increased the odds of mortality (pooled OR 2.85; 95% CI 2.37–3.42; p < 0.0001; Fig. S7). However, there was severe heterogeneity of results across the studies (Cochran's Q 189.01, p < 0.0001; and I2 82.53%). Following stepwise removal of six papers [39,42,44,47,60,73] contributing the greatest amount to the heterogeneity, the pooled OR was 2.47 (95% CI, 2.18–2.79; p < 0.0001; Fig. 4) with no evidence of residual heterogeneity.
Fig. 4.
Pooled odds ratio of COVID-19-related mortality in COPD patients:
Only studies with no overlapping study populations were analyzed. Odds ratios [95% confidence intervals] for individual studies (squares and bars) and the pooled odds ratio [95% CI] (diamond). Results are following stepwise removal of six studies contributing greatest to the heterogeneity (Cochran's Q and I2 tests).
Finally, we examined whether age and sex of the study participants could explain the heterogeneity of results across studies, using meta-regression. Three studies [52,59,73] were not included in this analysis due to missing population level age statistics. The mean/median age and percentage of males of studies accounted for 0%, 56.38% and 21.34% of the heterogeneity for hospitalisation, severe COVID-19 and mortality, respectively (Table 2). Age alone accounted for 19.79%, 63.06%, and 28.21% of the heterogeneity for hospitalisation, severe COVID-19, and mortality, respectively (Figs. S8-S10). Increasing mean/median age of the study populations was associated with lower OR for each of the COVID-19 outcomes; these trends were significant for severe COVID-19 (p = 0.0024) and mortality (p = 0.0046) but not for hospitalisation (p = 0.11).
Table 2.
Meta-regression to evaluate the relationship of age and sex with COVID-19 outcomes.
| Variable | OR | 95% CI | P-value | Heterogeneity accounted (R2) |
|---|---|---|---|---|
| Hospitalization | ||||
| Mean/median age | 0.95 | [0.88, 1.02] | 0.14 | NA |
| Male % | 0.99 | [0.91, 1.09] | 0.89 | NA |
| Model | 3.02 | [1.97, 4.63] | NA | 0% |
| Severe COVID-19 | ||||
| Mean/median age | 0.92 | [0.86, 0.97] | 0.0053 | NA |
| Male % | 1.001 | [0.95, 1.05] | 0.97 | NA |
| Model | 1.47 | [1.07, 2.01] | NA | 56.38% |
| Mortality | ||||
| Mean/median age | 0.97 | [0.95, 0.99] | 0.0052 | NA |
| Male % | 1.004 | [0.98, 1.02] | 0.68 | NA |
| Model | 2.70 | [2.26, 3.21] | NA | 21.34% |
Abbreviations: OR, odds ratio; CI, confidence interval
4. Discussion
The most important finding in this systematic review was that COPD is a significant risk factor for hospitalization, ICU stay, as well as for mortality in patients with COVID-19, increasing the odds by up to 4-fold in a series of meta-analyses. The heterogeneity in the effects of COPD on these outcomes was not fully explained by differences in the average age or sex distribution of the study participants. Our results were derived from patients who already had COVID-19, and thus we cannot comment on whether COPD confers an increased risk of contracting SARS-CoV-2 infection or asymptomatic COVID-19. Indeed, it is quite probable that COPD patients are underrepresented among COVID-19 cases due to increased social distancing during the pandemic [79]. However, our results confirm that, after contracting SARS-CoV-2, COPD patients are at a high risk of progression to a poor clinical outcome.
To our knowledge, this is the largest and most comprehensive systematic review of the association between COPD and severe COVID-19 to date. Previous systematic reviews on this and similar topics[13], [14], [15], [16] did not include hospitalisation as an outcome, which is particularly important as health care resources become stretched during the pandemic. The maintenance of essential health services is a key strategic priority of the WHO COVID-19 response [80]. The previous systematic reviews were also overwhelmingly influenced by the early reports from China; this is important not only due to regional differences in patient populations, but also since the clinical management of COVID-19 has evolved rapidly to include treatments that were not recommended in the early stages. Our systematic review, of which more than half the included studies were from outside China and across four continents, confirm those early reports but are arguably more generalizable to the global community. A particular strength of our review is that we made sure not to meta-analyse the results from studies where there was a possibility of overlapping patient populations. We identified 22 such studies based on the authors, sites, and recruitment periods. Whilst not confirmed, it is plausible that duplicate reporting of study populations exists due to the rapid release and updating of clinical studies early in the pandemic. Excluding these potentially overlapping studies from the quantitative analysis avoided any double-counting of patients.
It was beyond the purview of the current study to explore the mechanisms by which COPD confers increased risk of poor COVID-19 outcomes. However, several possibilities exist. For example, poor lung function reserves in patients with COPD means that super-imposed COVID-19 pneumonia, acute respiratory distress syndrome (ARDS), or pulmonary vascular thrombo-embolic events that are observed in COVID-19 [81,82] may easily precipitate respiratory failure. Indeed, COPD patients are at a high risk of mortality from other respiratory infections such as influenza [83] and community-acquired pneumonia [84]. Another possibility is upregulation of the SARS-CoV-2 receptor, angiotensin converting enzyme-2 (ACE-2), in the airways [9] and lungs [10] of individuals with COPD. Over-expression of the virus receptor could allow faster spread of the virus into the distal airways and alveoli, facilitating progression from a relatively mild bronchitis or an upper respiratory tract infection to severe COVID-19 pneumonia. Additionally, COPD is associated with impaired innate immune responses to viruses [12]; defective interferon responses to SARS-CoV-2 have been associated with increased risk of severe COVID-19 [85]. although this has not yet been demonstrated in COPD patients. Finally, it is possible that the association is confounded by the presence of other risk factors for severe COVID-19 that are common in people with COPD, such as older age, cardiovascular diseases, hypertension and diabetes [86]. Few of the studies we reviewed analyzed the effects of COPD independent of these other known risk factors, and other important determinants of health outcomes such as race, socioeconomic status and access to healthcare were rarely considered.
There were limitations to this study. First, the diagnosis of COPD was based largely on self-report or physician diagnosis and not on spirometry or imaging measures, and the results from these studies cannot be seen as equivalent to those derived from more objective definitions of COPD. Given that COPD is grossly under-recognized and under-diagnosed across the world (especially in developing countries), there could have been significant misclassification of patients [87]. Any non-differential bias arising from diagnostic misclassification would have diluted the results toward the null value. Second, we did not have data on the treatment for COPD or COVID-19. As the standard treatment for COPD exacerbation is systemic corticosteroids [6], it is possible that individuals with COPD could have been preferentially given these medications. However, in view of the RECOVERY trial which demonstrated the unequivocal benefits of systemic dexamethasone in reducing mortality in patients receiving invasive mechanical ventilation by ∼36% and among those receiving oxygen without invasive mechanical ventilation by ∼18% [88], such preferential treatment of COPD patients with steroids would have reduced the point estimates. Thus, our findings may be an underestimate of the true impact of COPD on severe COVID-19 outcomes. Third, we did not have access to individual patient-level data and as such we could not adjust for important potential confounders such as age, sex, and smoking status. We therefore cannot conclude that COPD is a risk factor for poor COVID-19 outcomes independent of such factors, which is particularly relevant since both COPD and COVID-19 are highly associated with increased age and multi-comorbidity. Indeed, some studies that specifically enrolled COPD patients have found no effect of COPD on severe COVID-19 and related mortality after adjusting for age and sex [30,42]. We attempted to explore the effects of age and male sex on our findings using meta-regression; although this is an imprecise method for evaluating potential confounders, it suggested that the effects of COPD on these COVID-19 outcomes became less pronounced as the mean/median age of the study populations increased. COPD may therefore be a particularly relevant risk factor in a younger age group, but less so in the older age group where other age-associated risk factors are more important determinants of COVID-19 outcomes. The relationship between smoking and COVID-19 outcomes is currently being debated with some studies showing a positive relationship [13,16], while others suggesting a protective association [89].
In conclusion, COVID-19 patients with COPD demonstrate increased odds of being hospitalized, requiring ICU admission, and mortality compared to COVID-19 patients who do not have COPD. These data highlight the importance of public and personal health measures to protect patients with COPD from becoming infected with SARS-CoV-2 (e.g. with the use of well-fitting masks, social distancing and hand hygiene measures) and managing these patients (should they develop COVID-19) with aggressive systemic corticosteroids and other strategies to mitigate their excess risk for morbidity and mortality. These data also demonstrate COPD patients should be prioritized for immunization with COVID-19 vaccine(s).
Declaration of Competing Interest
SM reports personal fees from Novartis and Boehringer-Ingelheim, outside the submitted work. DDS reports grants and personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, and personal fees from Grifols outside the submitted work. FVG, CC, XL, CWTY, AT, LC, and AB have nothing to disclose.
Acknowledgments
Acknowledgements
The authors would like to thank Mr. Irving Ding and Mrs. Chun Hong Tao for their financial support of the St Paul's Foundation COVID-19 Response Fund. FVG, SM, and AT are supported by the MITACS Accelerate programme. DDS holds the De Lazzari Family Chair at HLI and is a Tier 1 Canada Research Chair in COPD.
Contributors
FVG, SM, LHC and AB collected the data; FVG, SM and XL analysed the data; CC, CWTY, AT and DDS reviewed and interpreted the results; CWTY managed the project; FVG wrote the first draft of the manuscript; all authors reviewed and edited the manuscript and approved the final draft; all authors had full access to the data; CWTY, FVG and DDS verified the underlying data.
Funding
There were no direct financial sponsors for the submitted work.
Data sharing statement
All underlying data analysed in this work were extracted from published materials. Full summary data will be made available immediately following publication to any investigators and for any purpose, upon reasonable request. Proposals should be made directly to the Principal Investigator, Dr. Don Sin (don.sin@hli.ubc.ca).
Footnotes
Funding: None.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100789.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu PP, Blet A, Smyth D, Li H. The Science Underlying COVID-19: implications for the Cardiovascular System. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 3.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns Hopkins University and Medical Center . 2021. Johns Hopkins coronavirus resource center.https://coronavirus.jhu.edu [online resource]. Available at. [last accessed 11 February] [Google Scholar]
- 6.Singh D, Agusti A, Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 7.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang J, Ye G, Shi K. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung JM, Yang CX, Tam A. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milne S, Yang CX, Timens W, Bosse Y, Sin DD. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir Med. 2020;8(6):e50. doi: 10.1016/S2213-2600(20)30224-1. -e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herr C, Beisswenger C, Hess C. Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64(2):144–149. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 12.Mallia P, Message SD, Gielen V. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183(6):734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alqahtani JS, Oyelade T, Aldhahir AM. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabbani G, Shariful Islam SM, Rahman MA. Pre-existing COPD is associated with an increased risk of mortality and severity in COVID-19: a rapid systematic review and meta-analysis. Expert Rev Respir Med. 2021:1–12. doi: 10.1080/17476348.2021.1866547. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: a systematic review and meta-analysis. Respir Med. 2020;171 doi: 10.1016/j.rmed.2020.106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarity Group at McMaster University . 2021. Tool to assess risk of bias in cohort studies.https://www.evidencepartners.com/wp-content/uploads/2017/09/Tool-to-Assess-Risk-of-Bias-in-Case-Control-Studies.pdf [online resource]. Available at. [last accessed 02 February] [Google Scholar]
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 20.Aggarwal A, Shrivastava A, Kumar A, Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Ass Phys India. 2020;68(7):19–26. [PubMed] [Google Scholar]
- 21.Argenziano MG, Bruce SL, Slater CL. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkins JL, Masoli JAH, Delgado J. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attaway AA, Zein J, Hatipoglu US. SARS-CoV-2 infection in the COPD population is associated with increased healthcare utilization: an analysis of Cleveland clinic's COVID-19 registry. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auld SC, Caridi-Scheible M, Blum JM. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48(9):e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azoulay E, Fartoukh M, Darmon M. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 2020;46(9):1714–1722. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barman HA, Atici A, Sahin I. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. 2020 doi: 10.1097/MCA.0000000000000914. [Published online 23 June] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bello-Chavolla OY, Gonzalez-Diaz A, Antonio-Villa NE. Unequal impact of structural health determinants and comorbidity on COVID-19 severity and lethality in older Mexican adults: Considerations beyond chronological aging. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa163. [Published online 29 June] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bravi F, Flacco ME, Carradori T. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckner FS, McCulloch DJ, Atluri V. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis. 2020;71(16):2167–2173. doi: 10.1093/cid/ciaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calmes D, Graff S, Maes N. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9(1):160–169. doi: 10.1016/j.jaip.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caraballo C, McCullough M, Fuery MA. COVID-19 infections and outcomes in a live registry of heart failure patients across an integrated health care system. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cen Y, Chen X, Shen Y. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. 2020;26(9):1242–1247. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciardullo S, Zerbini F, Perra S. Impact of diabetes on COVID-19-related in-hospital mortality: a retrospective study from Northern Italy. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui N, Yan R, Qin C, Zhao J. Clinical characteristics and immune responses of 137 deceased patients with COVID-19: a retrospective study. Front Cell Infect Microbiol. 2020;10(774) doi: 10.3389/fcimb.2020.595333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, Ling Y, Bai T. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannouchos TV, Sussman RA, Mier JM, Poulas K, Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases. Eur Respir J. 2020 doi: 10.1183/13993003.02144-2020. [Published online 1 August] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal P, Choi JJ, Pinheiro LC. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grasselli G, Greco M, Zanella A. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan WJ, Liang WH, Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta R, Agrawal R, Bukhari Z. Higher comorbidities and early death in hospitalized African-American patients with COVID-19. BMC Infect Dis. 2021;21(1):78. doi: 10.1186/s12879-021-05782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta S, Hayek SS, Wang W. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436–1446. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen ESH, Moeller AL, Backer V. Severe outcomes of COVID-19 among patients with COPD and asthma. ERJ Open Res. 2021;7(1) doi: 10.1183/23120541.00594-2020. 00594-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Islam MZ, Riaz BK, Islam A. Risk factors associated with morbidity and mortality outcomes of COVID-19 patients on the 28th day of the disease course: a retrospective cohort study in Bangladesh. Epidemiol Infect. 2020;148:e263. doi: 10.1017/S0950268820002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Israelsen SB, Kristiansen KT, Hindsberger B. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March-April 2020. Danish Medical Journal. 2020;67(6) [PubMed] [Google Scholar]
- 46.Itelman E, Wasserstrum Y, Segev A. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Israel Med Ass J. 2020;22(5):271–274. [PubMed] [Google Scholar]
- 47.Jalili M, Payandemehr P, Saghaei A, Sari HN, Safikhani H, Kolivand P. Characteristics and mortality of hospitalized patients with COVID-19 in Iran: a national retrospective cohort study. Ann Intern Med. 2021;174(1):125–127. doi: 10.7326/M20-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Javanian M, Bayani M, Shokri M, et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Romanian journal of internal medicine = Revue roumaine de medecine interne2020; 58(3): 161-7. doi:10.2478/rjim-2020-0013. [DOI] [PubMed]
- 49.Jiang Y, Abudurexiti S, An MM, Cao D, Wei J, Gong P. Risk factors associated with 28-day all-cause mortality in older severe COVID-19 patients in Wuhan, China: a retrospective observational study. Sci Rep. 2020;10(1):22369. doi: 10.1038/s41598-020-79508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jimenez E, Fontan-Vela M, Valencia J. Characteristics, complications and outcomes among 1549 patients hospitalised with COVID-19 in a secondary hospital in Madrid, Spain: a retrospective case series study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalyanaraman Marcello R, Dolle J, Grami S. Characteristics and outcomes of COVID-19 patients in New York City's public hospital system. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim S-R, Nam S-H, Kim Y-R. Risk factors on the progression to clinical outcomes of COVID-19 patients in South Korea: using national data. Int J Environ Res Pub Health. 2020;17(23):8847. doi: 10.3390/ijerph17238847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagi F, Piccica M, Graziani L. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 2020;25(17) doi: 10.2807/1560-7917.ES.2020.25.17.2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lanini S, Montaldo C, Nicastri E. COVID-19 disease-temporal analyses of complete blood count parameters over course of illness, and relationship to patient demographics and management outcomes in survivors and non-survivors: a longitudinal descriptive cohort study. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li K, Chen D, Chen S. Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res. 2020;21(1):146. doi: 10.1186/s12931-020-01411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu W, Tao ZW, Wang L. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ludwig M, Jacob J, Basedow F, Andersohn F, Walker J. Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany. Int J Infect Dis. 2021;103:316–322. doi: 10.1016/j.ijid.2020.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancilla-Galindo J, Vera-Zertuche JM, Navarro-Cruz AR. Development and validation of the patient history COVID-19 (PH-Covid19) scoring system: a multivariable prediction model of death in Mexican patients with COVID-19. Epidemiol Infect. 2020;148:e286. doi: 10.1017/S0950268820002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohamed NE, Benn EKT, Astha V. Association between chronic kidney disease and COVID-19-related mortality in New York. World J Urol. 2021 doi: 10.1007/s00345-020-03567-4. [Published online 23 January] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020;52:93. doi: 10.1016/j.annepidem.2020.08.005. -8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de la Rica R, Borges M, Aranda M. Low albumin levels are associated with poorer outcomes in a case series of COVID-19 patients in spain: a retrospective cohort study. Microorganisms. 2020;8(8):1106. doi: 10.3390/microorganisms8081106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salacup G, Lo KB, Gul F. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: a single tertiary center cohort. J Med Virol. 2020 doi: 10.1002/jmv.26252. [Published online 4 July] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah P, Owens J, Franklin J. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52(7):354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith AA, Fridling J, Ibrahim D, Porter PS., Jr. Identifying patients at greatest risk of mortality due to COVID-19: a new england perspective. West J Emerg Med. 2020;21(4):785–789. doi: 10.5811/westjem.2020.6.47957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song J, Zeng M, Wang H. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2020 doi: 10.1111/all.14517. [Published online 28 July] [DOI] [PubMed] [Google Scholar]
- 66.Suleyman G, Fadel RA, Malette KM. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomlins J, Hamilton F, Gunning S, Sheehy C, Moran E, MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J Infect. 2020;81(2):e59–e61. doi: 10.1016/j.jinf.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Gerwen M, Alsen M, Little C. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907–915. doi: 10.1002/jmv.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Violi F, Cangemi R, Romiti GF. Is albumin predictor of mortality in COVID-19? Antioxid Redox Signal. 2020 doi: 10.1089/ars.2020.8142. [Published online 12 June] [DOI] [PubMed] [Google Scholar]
- 70.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, He WB, Yu XM, Hu DL, Jiang H. Prolonged prothrombin time at admission predicts poor clinical outcome in COVID-19 patients. World J Clin Cases. 2020;8(19):4370–4379. doi: 10.12998/wjcc.v8.i19.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Zhou Y, Yang Z, Xia D, Hu Y, Geng S. Clinical characteristics of patients with severe pneumonia caused by the SARS-CoV-2 in Wuhan, China. Respiration. 2020;99(8):649–657. doi: 10.1159/000507940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu F, Zhou Y, Wang Z. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. J Thorac Dis. 2020;12(5):1811–1823. doi: 10.21037/jtd-20-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang G, Hu C, Luo L. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang JJ, Dong X, Cao YY. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 77.Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eu Rev Med Pharma Sci. 2020;24(6):3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 78.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. European Respir J. 2020;56(2) doi: 10.1183/13993003.02108-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organization. Maintaining essential health services: operational guidance for the COVID-19 context interim guidance [online resource]. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-essential-health-services-2020.1 [Accessed 02 February 2021]. 2020.
- 81.Ackermann M, Verleden SE, Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grasselli G, Tonetti T, Protti A. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulpuru S, Li L, Ye L. Effectiveness of influenza vaccination on hospitalizations and risk factors for severe outcomes in hospitalized patients with COPD. CHEST. 2019;155(1):69–78. doi: 10.1016/j.chest.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 84.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28(2):346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 85.Lee JS, Park S, Jeong HW. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 2020;5(49) doi: 10.1126/sciimmunol.abd1554. eabd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab Syndr. 2020;14(5):1133–1142. doi: 10.1016/j.dsx.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi: 10.1016/S2213-2600(17)30119-4. [DOI] [PubMed] [Google Scholar]
- 88.Horby P, Lim WS, Recovery Collaborative Group et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [Published online 18 July] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rossato M, Russo L, Mazzocut S, Di Vincenzo A, Fioretto P, Vettor R. Current smoking is not associated with COVID-19. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01290-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.