Abstract
Objectives
This article presents findings from a large prospective examination of Canadian medical cannabis patients, with a focus on the impacts of cannabis on prescription opioid use and quality of life over a 6-month period.
Methods
The Tilray Observational Patient Study took place at 21 medical clinics throughout Canada. This analysis includes 1,145 patients who had at least one postbaseline visit, with follow-up at 1, 3, and 6 months. Instruments included a comprehensive cannabis use inventory, the World Health Organization Quality of Life Short Form (WHOQOL-BREF), and a detailed prescription drug questionnaire.
Results
Participants were 57.6% female, with a median age of 52 years. Baseline opioid use was reported by 28% of participants, dropping to 11% at 6 months. Daily opioid use went from 152 mg morphine milligram equivalent (MME) at baseline to 32.2 mg MME at 6 months, a 78% reduction in mean opioid dosage. Similar reductions were also seen in the other four primary prescription drug classes identified by participants, and statistically significant improvements were reported in all four domains of the WHOQOL-BREF.
Conclusions
This study provides an individual-level perspective of cannabis substitution for opioids and other prescription drugs, as well as associated improvement in quality of life over 6 months. The high rate of cannabis use for chronic pain and the subsequent reductions in opioid use suggest that cannabis may play a harm reduction role in the opioid overdose crisis, potentially improving the quality of life of patients and overall public health.
Keywords: Cannabis, Pain, Opioids, Substitution, Quality of Life, Harm Reduction
Background
Cannabis is widely used around the world, and it is associated with both personal and social benefits as well as harms [1–3]. Possible benefits include a wide range of medical applications [4, 5]. Evidence from randomized trials supports the therapeutic use of cannabis-based medicines in the treatment of chronic pain, pediatric epilepsy, and other conditions [6–12]. Nevertheless, cannabis use is not without potential harms. Chronic use has been associated with potential cognitive deficits, and the psychoactive effects of use and associated impairment can lead to increased personal health and public safety risks associated with driving [1, 13–15].
Although the evidence in regard to associations between cannabis smoking and cancer is inconclusive and contradictory [16–21], research suggests that those who smoke cannabis regularly may be at increased risk of bronchial issues [19, 20].
A growing body of evidence suggests that cannabis use can lead to a reduction in the use of prescription drugs, alcohol, tobacco, and illicit substances such as cocaine, heroin, and other opioids [22–25], suggesting that cannabis-related social harms and benefits should be examined in the context of the public health and safety effects of other regulated and unregulated substances. In light of the significant impacts of the opioid overdose crisis in Canada and around the world [26–29], research examining the potential impact of cannabis on opioid use may be of particular importance.
Ecological studies examining population-level changes in opioid use and associated morbidity and mortality following the introduction of more liberal access regulations to medical and nonmedical cannabis have found contradictory results. Although a significant number of studies have found evidence of reduced prescription opioid prescriptions and use associated with the passing of state-level medical cannabis or nonmedical adult cannabis access programs [24, 25, 30], a large population-level study by Shover et al. [31] extended a previous study by Bachhuber et al. [32] that found that between 1999 and 2010, states with medical cannabis laws experienced slower increases in opioid analgesic overdose mortality, with a mean reduction in overdoses of 24.8% per 100,000 people following the introduction of such laws. The new analysis confirmed the earlier Bachhuber et al. [32] results but also included data from 2010 to 2017, finding that the trend ultimately reversed itself, and that states that passed medical cannabis laws experienced a 22.7% increase in deaths [31]. There may be public health and policy reasons unrelated to cannabis policy that explain this change in opioid use and mortality (such as the shift in opioid users from licit prescription opioids to an illicit supply contaminated with fentanyl and carfentanyl), but the biggest difficulty in interpreting these findings is the inability to determine causality in population-level studies.
A large number of observational, individual-level studies of medical cannabis in Canada and the United States, Israel, and other jurisdictions have found evidence suggesting that cannabis reduces the use of opioids and other prescription drugs, particularly in patients affected by chronic pain [22, 23, 25, 33–37]. However, a 4-year observational study in Australia comparing 157 cannabis users with 1,047 nonusers concluded that there was no evidence that cannabis improved patient outcomes [38]. Nevertheless, as most participants were not using cannabis under the supervision of a physician and the source of cannabis was neither legal nor regulated for quality or consistency, it is difficult to draw conclusions from this study. Subsequently, a systematic review of medical cannabis for the reduction of opioid dosage in the treatment of noncancer pain that included 7,222 participants found that 32–59.3% reported substituting cannabis for opioids, resulting in a 64–75% reduction in opioid dosage when combined with cannabis, although methodological issues with the studies and an associated high risk of bias negated any causal inference [39]. Additionally, individual-level prospective studies focused on problematic opioid use have found that daily cannabis use was associated with slower rates of injection drug initiation among street youth [40] and swifter rates of injection cessation in people who inject drugs in Vancouver [40] and that cannabis use may lead to reduced opioid withdrawal and improved treatment outcomes for those enrolled in pharmacological opioid replacement therapy (ORT) [41–43].
Although the balance of evidence suggests that cannabis may play a role in reducing the use and associated impacts of opioids on public and personal health, it is apparent that more robust prospective studies with a focus on the individual-level impacts of medical cannabis on prescription substance use and associated outcomes could help clarify some of these ongoing questions and contradictions.
Medical Cannabis in Canada
Canada’s federal medical cannabis program is governed by the Cannabis Act and its supporting regulations [44]. These regulations allow patients, with the support of a physician or nurse practitioner, to register with authorized licensed producers (LPs) to access a tightly regulated supply of medical cannabis flower and extract products (oils, capsules, vape pens or cartridges, edibles, topical creams, and so on) that are shipped via mail order. Under the Cannabis Act, a medical recommendation for cannabis is not product specific but instead requires health care practitioners to state the maximum amount of cannabis a patient is authorized to use per day, in grams. Patients then order cannabis products of their choice up to the gram limit (or equivalent amounts of extract products as determined by conversion formulas established by the LP), trying different products until they find one or more that they feel may be relieving their symptoms and/or improving their well-being. The nonspecific nature of the cannabis recommendations by health care practitioners presents a unique opportunity to observe patient patterns of medical cannabis use in a naturalistic setting.
The objectives of this prospective observational study are to identify the primary characteristics of a large national cohort of medical cannabis patients in Canada, including detailed patterns of cannabis use; to assess the impacts of regulated, physician-supervised medical cannabis access on prescription substance use and quality of life; and to analyze key variables potentially [45] associated with changes in prescription drug use and quality of life over 6 months.
Methods
The Tilray Observational Patient Study was a national, multisite, prospective examination of the impact of medical cannabis use on quality of life and prescription drug use among federally authorized Canadian medical cannabis patients. The study used a pretest and posttest repeated-measures design, with data gathered at baseline, 1 month, 3 months, and 6 months.
The study was ethics reviewed and approved by Advarra (formerly Institutional Review Board Services) on January 22, 2016, the University of Victoria on April 7, 2016, and the Health Research Ethics Board of Alberta October 3, 2016. Patient recruitment took place at 21 medical clinics across five provinces in Canada, with a total of 2,055 patients completing a baseline visit. Of those, 1,145 participants who had completed at least one follow-up as of October 15, 2018, were included in this analysis, thereby ensuring that all study participants had occasion to try medical cannabis.
Clinics and participating physicians were identified and trained in the administration of the study by the principal investigator (PI), and all clinic-based co-investigators underwent online training in human research ethics via the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans Course on Research Ethics (TCPS 2: CORE), providing a certificate of completion as proof. Data were gathered digitally using an iPad loaned to participating physicians for the course of the study, and all instruments and data were gathered and stored on REDCap, an electronic data capture system that is compliant with the Health Insurance Portability and Accountability Act and the Personal Information Protection and Electronic Documents Act, meaning that it has been found to adequately safeguard the privacy of digital information gathered via this system.
Participants were federally authorized, English-speaking, medical cannabis patients aged 18 years and older with the capacity to consent for themselves who received a new cannabis recommendation from a participating physician, “agreed” to the informed consent form (ICF), and subsequently registered with Tilray to obtain their medical cannabis products. As compensation for their time, participants received a $25 credit toward their medical cannabis costs after completing each subsequent set of surveys at 1, 3, and 6 months.
Measures
This study was composed of validated and novel instruments made up of multiple-choice questions—including single and multiple answers—ratings, visual assessment scales, Likert scales, and matrix and drop-down menu questions. Many questions also included an “other” option and provided a box for a short textual response if this option was selected.
Demographic data such as age, gender, marital status, education level, employment status, and geographic location (province and postal code) were self-reported and gathered via multiple-choice questions informed by past longitudinal and cross-sectional surveys by the PI and others [23, 46, 47]. To evaluate the patterns of use between new and existing medical cannabis users, a measure of patient experience with cannabis was developed that allowed for a comparison of baseline characteristics and outcome variables between cannabis-naive and non-naive participants, with naive patients defined as those who had used cannabis four times or less in the previous 12 months. Additionally, the study included three instruments: the World Health Organization Quality of Life Short Form (WHOQOL-BREF) [48], a cannabis use survey (CUS), and a prescription drug questionnaire (PDQ).
The WHOQOL-BREF is a 26-item questionnaire derived from data collected using the WHOQOL-100. It produces scores for four domains related to quality of life: physical health, psychological health, social relationships, and environment. It also includes one facet on overall quality of life and general health. It has been found to be a sound, cross-culturally valid assessment of quality of life [49].
The CUS is a 17-question self-administered patient questionnaire designed to gather cross-sectional and/or prospective information on medical cannabis patient primary conditions, symptoms, and patterns of medical cannabis use. This includes amounts used per day, per week, and per use session (in incremental gram amounts via a drop-down list); primary methods of use (via a drop-down list of common methods of use and an “other” text box option); and cannabis varietal preferences (via drop-down menu options of sativa, indica, hybrid—all of which were subclassified as “high THC” [tetrahydrocannabinol] for binary analysis—and high cannabidiol [CBD] or “other” with a text box option). For the primary condition, patients could check only one box from a list of 17 common conditions associated with medical cannabis use, and an “other” text box allowed patients to enter any condition not listed. For primary symptoms, patients could check multiple boxes from a list of 13 common symptoms associated with medical cannabis use, as well as “other” prompting a text box. Additionally, this survey included questions specific to Tilray chemovars and medical cannabis products, and versions of this questionnaire were included in the 2017 Tilray Patient Survey and the Medical Cannabis in Older Patients Study.
The PDQ was designed to produce an accurate inventory of current prescription drug use by patients and was completed by physicians and/or medical clinic staff in cooperation with the patient during a scheduled medical visit. It gathers information on daily and nondaily prescription drug use in milligrams per dose and doses per day or week (where applicable) and has an autofill function connected to the National Drug Data File (NDDF), a US-based national prescription drug database, to ensure that consistent generic prescription drug names are used across participants to facilitate the analysis of prescription drug use over 6 months.
The ICF, demographic data, and first two measures were self-administered by the patient, and the PDQ was filled out by the physician and/or clinic staff. This battery was administered at four different time periods: at baseline after a patient had received a medical cannabis recommendation from the participating physician and then at 1 month, 3 months, and 6 months.
Data Analysis
Analysis was focused on 1,145 patients enrolled on or before October 15, 2018, who had completed at least one postbaseline visit. For demographics, summary statistics were presented by mean, median, standard deviation (SD), and interquartile range (IQR) and then additionally divided into naive and non-naive participants. For the quality of life analysis, the four domains of the WHOQOL-BREF were summarized at each study visit, as well as the change from baseline at each visit. Change scores were based on patients who had both the baseline visit and the indicated follow-up visit. Mixed-effects linear regression was used to model the time trend of the four domains of the WHOQOL-BREF over the 6-month period for all patients and to model different levels of demographic variables (e.g., past cannabis usage, age, primary illness or symptoms).
Changes in prescription drug use included descriptive summaries of the number and percentage of patients who used each medication, stratified by baseline use of the particular medication and by patient characteristics. Formal analyses were only considered for opioids, non-opioid pain medications, antidepressants, antiseizure drugs, and benzodiazepines, as there were few patients who used the other medication types in any study visits (i.e., sleep aids or muscle relaxants [n=68, 5.9%], stimulants [n=10, 0.9%], antiemetics [n=35, 3.1%], and antipsychotics [n=34, 3.0%]).
To assess the dosage and usage frequency data among those who used the medication at baseline, milligrams per dose was first converted to milligrams per day by multiplying milligrams per dose by the frequency per day. For opioids, dosages were converted to the morphine milligram equivalent (MME) [50]. For non-opioid pain medications, antidepressants, antiseizure drugs, and benzodiazepines, the observed dosage was divided by its defined daily dose (DDD) to facilitate the summary of dosage data across patients [51]. DDD is defined by the World Health Organization (WHO) as “the assumed average maintenance dose per day for a drug used for its main indication in adults” [52]; therefore, the converted dosage would be interpreted as the number of DDDs used per day. As a sensitivity analysis of the MME data, this analysis was also repeated for opioids.
Given the substantial reduction in the proportion of patients using these medications during follow-up, mixed-effects logistic regression was used to examine the change in likelihood of using a particular medication, resulting in an odds ratio (OR) for using the medication at each visit relative to baseline. The change in dosage (milligrams per day) and usage frequency (times per day) was also formally assessed over time. Because very few participants who did not use medication at baseline went on to initiate use postbaseline, only those who used the medication at baseline were included in the analysis of dosage and frequency. Due to the large number of patients who stopped using prescription medication post-baseline, there was an excessive amount of “zero” responses; therefore, quantile regression was done separately for each study visit to examine the median change in dosage and frequency relative to baseline. A pharmacoeconomic analysis of the changes in monthly prescription drug costs for the top five medication classes reported by participants (opioid and non-opioid pain medications, antidepressants, benzodiazepines, and antiseizure drugs) was conducted by obtaining the price of medications via drug formularies used by publicly funded insurance programs in British Columbia and Ontario and then calculating the 30-day cost as the drug unit price multiplied by daily dosage and 30 days. The same was done with cannabis-related costs, using the assumption of a mean cost of $9 per gram.
Finally, in light of the high lost to follow-up (LTFU) rate, a sensitivity analysis was conducted that compared the WHOQOL-BREF at each visit between patients who dropped out after that particular visit and patients who remained in the study to assess the potential role that poor outcomes may have had on retention.
All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC). All statistical tests were two sided, with significance levels of 0.05 unless otherwise indicated.
Results
Of the 1,145 patients who were enrolled on or before October 15, 2018, and who completed at least one postbaseline visit, 1,067 completed a 1-month visit (M1=93.2%), 764 completed a 3-month visit (M3=66.7%), and 419 of the remaining 1,011 completed a 6-month visit (M6=41.4%).
Table 1 highlights the primary patient baseline characteristics. Study participants were 57.6% female (n=659) and well educated, with 55.8% (n=639) reporting at least a college certificate or higher. The median age was 52 (IQR, 38.0–62), and 56.1% (n=642) were married or equivalent. Additionally, cannabis-naive patients included a significantly greater proportion of women (61.4% vs 51.8%; P=0.002) and a greater proportion of married people (61.8% vs 47%; P<0.001) and on average were significantly older (56.0; IQR, 45.0–66.0) compared with non-naive patients (43.0; IQR, 33.0–54.0) (P<0.001) (Table 1).
Table 1.
Patient baseline characteristics of 1,145 participants and comparison of cannabis-naive with non-naive participants
| Characteristic | All (N=1,145) | Naive (n=691) | Non-Naive (n=438) | P Value |
|---|---|---|---|---|
| Gender, n (%) | 0.002 | |||
| Male | 485 (42.4) | 266 (38.5) | 211 (48.2) | |
| Female | 659 (57.6) | 424 (61.4) | 227 (51.8) | |
| Other | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| Education, n (%) | 0.991 | |||
| High school or lower | 506 (44.2) | 310 (44.9) | 190 (43.4) | |
| College or higher | 639 (55.8) | 381 (55.1) | 248 (56.6) | |
| Marital status, n (%) | <0.001 | |||
| Single, divorced, widowed, or separated | 503 (43.9) | 264 (38.2) | 232 (53.0) | |
| Married or living as married | 642 (56.1) | 427 (61.8) | 206 (47.0) | |
| Age | <0.001 | |||
| Mean, n (%) | 51.2 (15.4) | 55.6 (14.9) | 44.3 (13.5) | |
| Median (IQR) | 52.0 (38.0, 62.0) | 56.0 (45.0, 66.0) | 43.0 (33.0, 54.0) | |
| Range | 18–95 | 19–95 | 18–88 |
IQR = interquartile range.
Table 2 highlights the primary conditions and symptoms reported by participants. Chronic pain was the most common condition (68.8%; n=787), followed by anxiety disorder (9.5%; n=109), arthritis (7.1%; n=81), insomnia (5.0%; n=57), and headache (2.2%; n=25), and these top five conditions accounted for 92.5% of participants (n=1,059). Similarly, the top five primary symptoms cited by patients involved pain, insomnia, or mental health conditions: chronic pain (79.9%; n=915), insomnia (33.5%; n=384); anxiety (28.6%; n=327), depression (19.1%; n=219), and stress (19.1%; n=219). The naive group had a greater proportion of patients with pain symptoms, whereas the non-naive group, in contrast, had a greater proportion of patients with mental health conditions, insomnia, appetite loss or nausea, or gastrointestinal symptoms (P<0.001; Table 2).
Table 2.
Primary conditions and symptoms of 1,145 participants and comparison of cannabis-naive with non-naive participants
| Variable | All (N=1,145) | Naive (n=691) | Non-Naive (n=438) | P Value |
|---|---|---|---|---|
| Primary illness or medical condition you currently treat with medical cannabis | n (%) | n (%) | n (%) | |
| Unknown | 1 | 1 | 0 | |
| Anxiety disorder | 109 (9.5) | 31 (4.5) | 74 (16.9) | <0.001 |
| Arthritis | 81 (7.1) | 53 (7.7) | 27 (6.2) | 0.333 |
| Brain injury | 3 (0.3) | 3 (0.4) | 0 (0.0) | 0.287 |
| Cancer or leukemia | 17 (1.5) | 10 (1.4) | 7 (1.6) | 0.841 |
| Chronic pain | 787 (68.8) | 523 (75.8) | 257 (58.7) | <0.001 |
| Crohn’s disease | 6 (0.5) | 2 (0.3) | 4 (0.9) | 0.215 |
| Diabetes | 2 (0.2) | 2 (0.3) | 0 (0.0) | 0.525 |
| Eating disorder | 2 (0.2) | 0 (0.0) | 2 (0.5) | 0.151 |
| Epilepsy | 5 (0.4) | 4 (0.6) | 1 (0.2) | 0.654 |
| Gastrointestinal disorder | 6 (0.5) | 2 (0.3) | 4 (0.9) | 0.215 |
| Headache | 25 (2.2) | 15 (2.2) | 10 (2.3) | 0.903 |
| Insomnia | 57 (5.0) | 26 (3.8) | 28 (6.4) | 0.044 |
| Movement disorder | 9 (0.8) | 9 (1.3) | 0 (0.0) | 0.016 |
| Osteoporosis | 1 (0.1) | 0 (0.0) | 1 (0.2) | 0.388 |
| Psychiatric or mental health disorder | 4 (0.3) | 1 (0.1) | 3 (0.7) | 0.305 |
| PTSD | 8 (0.7) | 2 (0.3) | 6 (1.4) | 0.062 |
| Seizures | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1.000 |
| Other | 21 (1.8) | 6 (0.9) | 14 (3.2) | 0.004 |
| Primary symptoms you currently treat with medical cannabis | ||||
| Anxiety | 327 (28.6) | 124 (17.9) | 189 (43.2) | <0.001 |
| Appetite loss | 105 (9.2) | 24 (3.5) | 79 (18.0) | <0.001 |
| Chronic pain | 915 (79.9) | 581 (84.1) | 325 (74.2) | <0.001 |
| Depression | 219 (19.1) | 82 (11.9) | 129 (29.5) | <0.001 |
| Gastrointestinal disorder | 60 (5.2) | 26 (3.8) | 32 (7.3) | 0.009 |
| Headache | 166 (14.5) | 67 (9.7) | 98 (22.4) | <0.001 |
| Insomnia | 384 (33.5) | 189 (27.4) | 189 (43.2) | <0.001 |
| Intraocular eye pressure | 14 (1.2) | 11 (1.6) | 3 (0.7) | 0.180 |
| Memory loss | 43 (3.8) | 22 (3.2) | 17 (3.9) | 0.532 |
| Nausea | 95 (8.3) | 33 (4.8) | 60 (13.7) | <0.001 |
| Seizures | 11 (1.0) | 6 (0.9) | 5 (1.1) | 0.649 |
| Spasms | 118 (10.3) | 76 (11.0) | 39 (8.9) | 0.257 |
| Stress | 219 (19.1) | 75 (10.9) | 133 (30.4) | <0.001 |
| Other | 24 (2.1) | 12 (1.7) | 12 (2.7) | 0.255 |
PTSD = posttraumatic stress disorder.
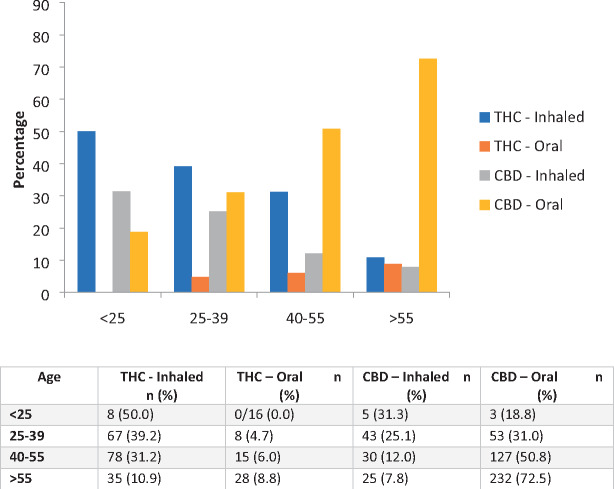
Table 3 highlights methods of baseline cannabis use as well as changes in use over 6 months. In regard to cannabis use, flower use was reported by 38.3% of participants at baseline (n=438), increasing to 93.6% (n=392) by M6. Mean flower cannabis use per week at M1 was 6.2 g (SD=6.2), increasing to 6.9 g at M6 (SD=6.5) or just below 1 g per day, therefore remaining quite stable over the first 5 months of use. Despite the prevalence of flower use, 51.3% (n=201) of participants reported that oral ingestion was their primary method of use at M6, which was greater than inhalation via smoking (joint + pipe + bong = 24.5%; n=96) or vaporization (22.9%; n=90) combined. Further analysis revealed a significant linear association between older age and a preference for oral ingestion of CBD, with participants below 25 years of age citing a significant preference for the inhalation of THC, which was gradually replaced with oral ingestion of CBD in older participants, resulting in very little inhalation of THC in patients over 55 years old (P<0.001; Figure 1). There were also significant differences between THC and CBD preferences by primary condition, with those affected by chronic pain conditions citing a preference for CBD products, whereas those affected by mental health issues or insomnia cited a significant preference for THC products (P<0.001).
Table 3.
Primary method of use over 6 months for 1,145 participants
| Primary method of use, n (%) | Baseline | M1 | M3 | M6 |
|---|---|---|---|---|
| n=438* | n=1,067 | n=764 | n=419 | |
| Unknown | 1 | 88 | 61 | 27 |
| Vaporizer—cannabis flowers or bud | 85 (19.5) | 140 (14.3) | 125 (17.8) | 86 (21.9) |
| Vaporizer or nail—cannabis extracts | 8 (1.8) | 10 (1.0) | 7 (1.0) | 4 (1.0) |
| Joint | 206 (47.1) | 172 (17.6) | 106 (15.1) | 72 (18.4) |
| Oral ingestion | 72 (16.5) | 588 (60.1) | 401 (57.0) | 201 (51.3) |
| Pipe | 27 (6.2) | 30 (3.1) | 23 (3.3) | 11 (2.8) |
| Water pipe or bong | 39 (8.9) | 29 (3.0) | 27 (3.8) | 13 (3.3) |
| Topical ingestion | 0 (0.0) | 9 (0.9) | 7 (1.0) | 2 (0.5) |
| Other | 0 (0.0) | 1 (0.1) | 7 (1.0) | 3 (0.8) |
M1 = month 1; M3 = month 3; M6 = month 6.
Baseline number of participants reporting primary methods of use is lower due to the high number of naive users who would not have answered this question.
Figure 1.
Age-related preferences for THC vs CBD and oral vs inhaled cannabis products in 1,145 participants. THC = tetrahydrocannabinol; CBD = cannabidiol.
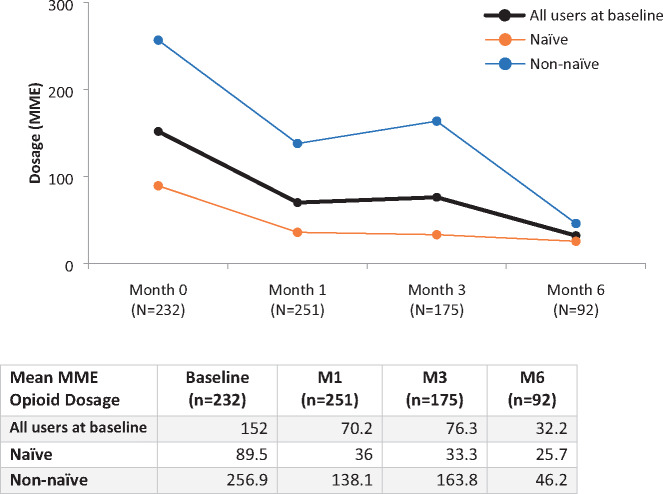
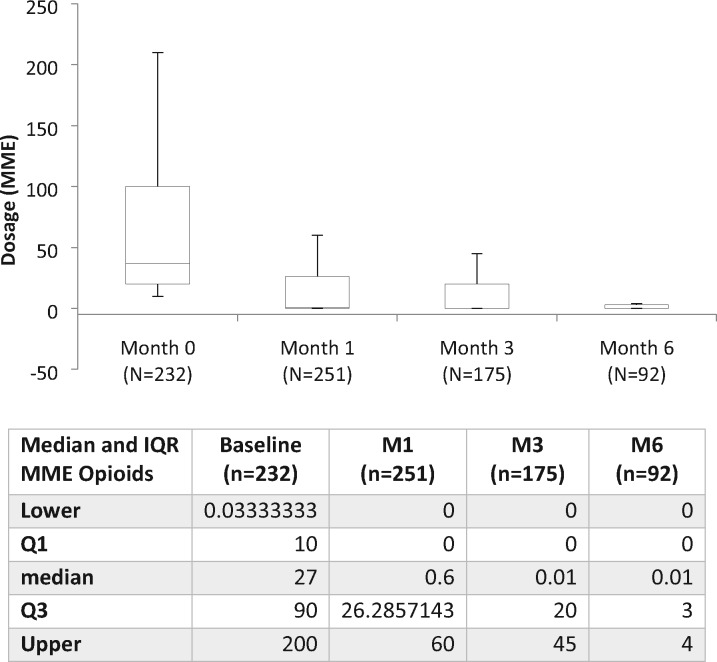
In regard to prescription drug use, baseline opioid use was reported by 28.1% (n=313) of participants, declining to 11.3% (n=47) of participants at M6. As a result, the OR of using opioids at M6 was 0.07 relative to baseline (95% confidence interval [CI], 0.04–0.12; P<0.001). Significant declines in actual opioid doses were also observed: the mean MME daily dose at baseline was 152 mg (SD=387.4), declining to MME 32.2 mg (SD=116.1) at M6, a 78% reduction over 6 months (Figure 2). Notably, the most significant change was in those reporting previous experience with cannabis, who went from a mean MME of 256.9 mg at baseline to 46.2 mg per day at M6 compared with naive users, who saw a more modest decline from 89.5 to 25.7 mg MME. The changes in median MME opioid use were also significant, with participants reporting 27 mg MME at baseline (IQR, 10.7–98.0), declining to 0.0 mg per day MME at M6 (IQR, 0.0–4.5) (Figure 3).
Figure 2.
Changes in mean MME opioid dosage over 6 months in naive and non-naive cannabis users. MME = morphine milligram equivalent.
Figure 3.
Changes in median MME opioid dosage over 6 months (IQR). MME = morphine milligram equivalent; IQR = interquartile range.
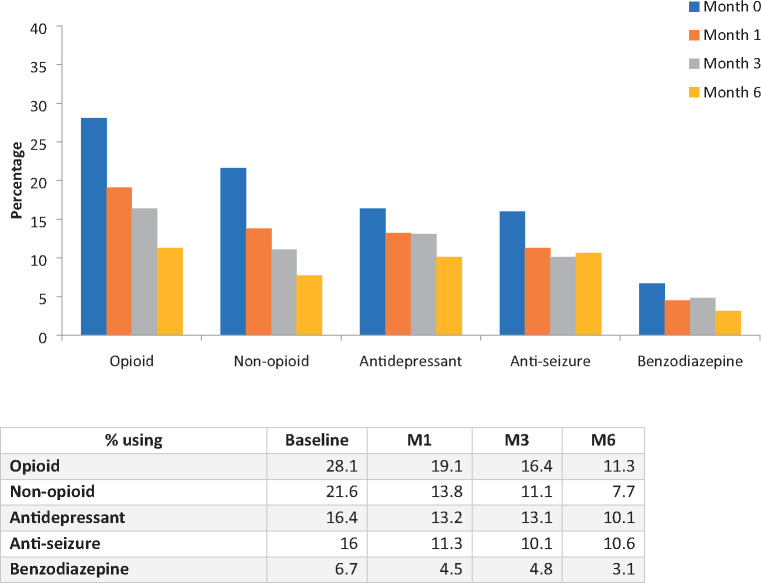
Similar statistically significant declines in prescription drug use between baseline and M6 were also found in the four other primary prescription drug classes reported by participants. Over 6 months, the percentage of patients using non-opioid pain medications went from 21.6% (n=241) to 7.7% (n=32), use of antidepressants declined from 16.4% (n=183) to 10.1% (n=42), use of antiseizure medications went from 16% (n=178) to 10.6% (n=44), and benzodiazepine use decreased from 6.7% of participants at baseline (n=75) to 3.1% at M6 (Figure 4).
Figure 4.
Percentage of patients using prescription medications from baseline to 6 months.
As a result of these sharp declines in use, the odds of using these medications at M6 relative to baseline declined significantly for non-opioid pain medications (OR = 0.12; 95% CI, 0.07–0.20; P<0.001), antidepressants (OR = 0.17; 95% CI, 0.09–0.30; P<0.001), antiseizure medications (OR = 0.11; 95% CI, 0.06–0.22; P<0.001), and benzodiazepines (OR = 0.33; 95% CI, 0.17–0.65; P=0.001).
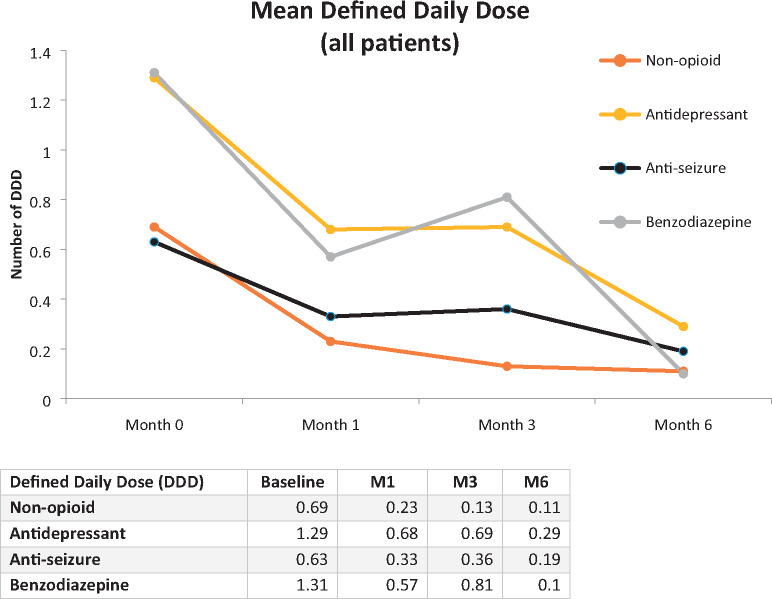
Additionally, the DDD decreased significantly between baseline and M6. For non-opioid pain medications, the mean DDD dropped from 0.69 (SD=0.75) to 0.11 (SD=0.34) at M6; for antidepressants, from 1.29 (SD=0.92) to 0.29 (SD=0.55); for antiseizure medications, from 0.63 (SD=0.52) to 0.19 (SD=0.39); and for benzodiazepines, from 1.31 (SD=2.76) to 0.10 (SD=0.33) (Figure 5). Results from the pharmacoeconomic analysis suggest that mean 30-day prescription drug costs for patients reporting baseline use of one or more of the five primary drug classes fell from $106.00 (SD=348.5) at baseline to $18.40 (SD=54.1) at M6, an 83% decline. However, when comparing the change in combined prescription drug and medical cannabis costs between baseline and M6, mean 30-day costs increased slightly from $238.80 (SD=425.4) to $275.10 (SD=255.4), suggesting that although there may be additional costs associated with the regulated use of medical cannabis under the care of a physician or nurse practitioner, some of these costs could be mitigated by subsequent reductions in prescription drug costs.
Figure 5.
Changes in mean defined daily dose of prescription medications from baseline to month 6.
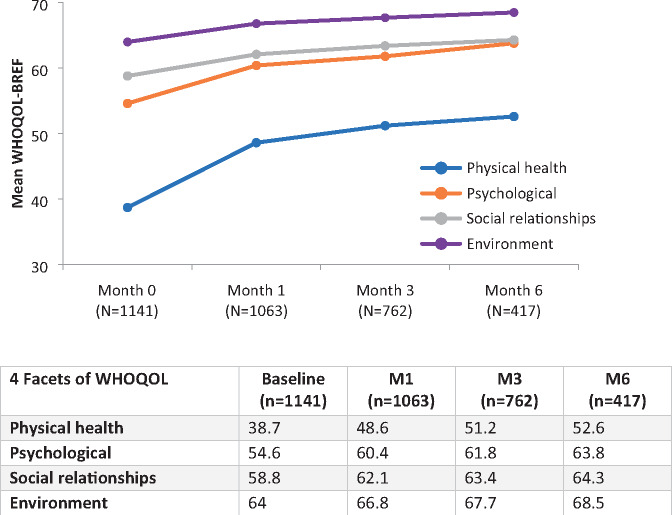
With regard to quality of life, statistically significant improvements were noted in the mean scores for the four domains of the WHOQOL-BREF at all follow-up visits relative to baseline, with the biggest changes seen in physical health (13.9 points [36% increase]; 95% CI, 11.7–15.0) and psychological health (9.2 points [17% increase]; 95% CI, 6.6–9.7) (Figure 6).
Figure 6.
Mean quality of life changes over 6 months as measured by the WHOQOL-BREF. WHOQOL-BREF = World Health Organization Quality of Life Short Form.
In general, there was no consistent difference in the magnitude of the change in quality of life across subgroups defined by demographic variables, and the magnitude of these changes was generally similar between the naive and non-naive patients for all study visits and domains of the WHOQOL-BREF (all P values for homogeneity greater than or equal to 0.058). However, greater improvements in psychological health were found among participants with mental health issues (11.85 points; 95% CI, 7.99–15.71) compared with those with pain issues (7.86 points; 95% CI, 6.52–9.21) (P≤0.009 at M1 and M3; P=0.056 at M6).
Spearman correlation analysis was conducted using the changes in quality of life and changes in medication dosage relative to baseline to examine the potential relationship between reductions in prescription drug use and improvements in quality of life, but no strong evidence was found to suggest a consistent relationship between these variables over time.
Finally, the results of the sensitivity analysis comparing the WHOQOL-BREF results at each visit between patients who dropped out after that particular visit and patients who remained in the study showed no statistically significant differences in quality of life outcomes between these two groups (Table A1).
Discussion
This study adds to the growing body of evidence that medical cannabis use is associated with reductions in the use of prescription drugs [23–25, 33, 34, 39, 53] and improvements in quality of life [54, 55]. Due to its prospective individual-level design, detailed gathering of both cannabis and prescription drug use data, and diversity of patient characteristics and medical conditions in this large cohort, these findings provide a more granular understanding of the variables associated with reductions in prescription drug use and improvements in quality of life associated with medical cannabis in a number of different contexts.
The novel findings that significant substitution for prescription drugs occurred and that quality of life improved whether patients were new to medical cannabis (naive) or experienced users (non-naive) suggests that access to a regulated, quality-controlled source of cannabis with known dosages could play a part in reducing prescription substance use and improving quality of life above and beyond the use of unregulated cannabis of unknown quality, safety, and potency. The finding that cannabis use did not increase significantly over a 6-month period is encouraging from both a therapeutic and a public health perspective and adds to the growing body of evidence suggesting that although patients appear to develop a tolerance to some of the side effects of cannabis-based medicines, they do not seem to develop a tolerance to many of the primary therapeutic effects [56–58].
Research suggests that older patients are among the fastest-growing demographics reporting medical cannabis use [59, 60], and with a median age of 52 years, this cohort certainly reflects this trend. The finding of significant age-related differences in patterns of use—with younger patients preferring to inhale high-THC cannabis and older patients preferring oral ingestion of CBD extract products—may be due to a number of factors, including the following:
Younger individuals are more likely to use cannabis recreationally [61, 62], and therefore younger patients may have a preference for products that can also lead to impairment.
Older patients may be more concerned about the risks of impairment, including potential falls, and therefore may find orally ingested, high-CBD products more predictable and less likely to lead to impairment than inhaled high-THC products.
Conditions associated with aging (such as arthritis, cancer, gastrointestinal tract issues, and even trauma, depression, and anxiety) may respond better to orally ingested CBD than inhaled THC.
Oils and capsules may seem more like traditional pharmaceutical drugs than cannabis in flower form and therefore may appeal to an older, more conservative demographic.
Regardless why older patients use medical cannabis differently than younger patients, at this time very little is known about the impacts of medical cannabis on conditions related to aging and on older populations, and more research on this patient demographic is warranted.
The reductions in prescription drug use and related costs associated with medical cannabis observed in this study have significant public and personal health implications. In light of the current opioid overdose crisis affecting North America and many other parts of the world [26–29], this study adds to a growing body of individual-level observational research suggesting that medical cannabis can lead to reductions in opioid use in patient populations [23, 33, 36, 63]—particularly in the treatment of chronic pain [35, 37]—and could therefore play a harm reduction role in addressing the opioid overdose crisis around the globe. However, from a medical standpoint, the data suggesting significant reductions in the use of antiseizure drugs may be a cause for concern if this was not done as part of a deliberate tapering program guided by the health care provider (HCP). Both these cases suggest that health care practitioners should be aware of potential changes in pharmaceutical drug use following the prescription of cannabis-based medicines and that health care practitioners may in fact see opportunities to introduce a deliberate tapering strategy for medications that may have a high rate of side effects, dependence, or mortality. These positive effects could also be enhanced by providing cost coverage for medical cannabis via Canada’s private insurers and/or provincial public health systems, similar to the coverage of potentially more dangerous prescription drugs. Results from the pharmacoeconomic analysis showing that 30-day prescription drug costs declined by 83% over 6 months while quality of life improved significantly during the same period may help inform such considerations. In light of these findings, studies should be conducted using validated pharmacoeconomic measures and taking into consideration the impact of medical cannabis on doctor and emergency department visits, hospitalizations, and other aspects of health care utility.
This study has several strengths and limitations. This was a convenience sample recruited at 21 medical clinics throughout five Canadian provinces, and this sample may not be representative of the general Canadian medical cannabis patient population. As many of the clinics specialize in the treatment of chronic pain, patients affected by chronic pain may be overrepresented in this cohort; however, our results are consistent with those of other large observational studies of cannabis patients conducted in Canada and other jurisdictions [33, 34, 64, 65]. Data regarding the use of cannabis were self-reported by patients, and the study did not benefit from biological drug detection to confirm use or nonuse of cannabis, resulting in a potential recall bias. Additionally, participants could have used other sources of cannabis in addition to those provided by Tilray, which could bias results. Perhaps most significantly, as participants were compensated with Tilray credits to assist with covering the cost of medical cannabis, there is the possibility of a retention bias in this population. In response to this concern, a sensitivity analysis was conducted comparing changes in WHOQOL-BREF results for participants lost to follow-up with those that remained in the study and found no significant differences, suggesting that LTFU was not likely associated with study outcomes. Furthermore, recruitment and data gathering for this study took place during a time period when Canada formally legalized the nonmedical use of cannabis for adults (legalization officially began on October 17, 2018), which may have affected retention in longitudinal studies examining the medically supervised use of cannabis by patients. In order to address the high LTFU rate, mixed-effects regression—which assumes missing at random data—was used to provide unbiased results. Finally, in light of the high LTFU rate in this study and in other Canadian prospective medical cannabis studies [12], a formal survival analysis of this population was conducted to identify potential baseline participant characteristics associated with retention that will be the focus of a separate publication and that could inform recruitment and retention strategies in future longitudinal cannabis studies. Future studies could also consider including control groups and the tracking of adverse events to better understand variables that may be affecting retention and other outcomes in prospective medical cannabis studies. However, these limitations are counterbalanced by several methodological strengths, including the large number of participants (to the best of our knowledge, this is the largest national prospective survey of Canadian medical cannabis patients to date), longitudinal pretest and posttest repeated-measures design, and data entry of prescription drug use by physicians rather than relying on patient self-reporting.
Conclusions
With the goal of recruiting and following a highly heterogeneous population, the present study was not designed to assess overall changes in specific health conditions, and instead used quality of life as a proxy of changes in overall health and happiness. Nonetheless, the overall improvements in quality of life suggest that cannabis may be effective at treating a diverse number of conditions and symptoms and at subsequently reducing the use of prescription drugs as well as the associated costs and potential harms.
Although cannabis use is not without potential risks in certain individuals and/or vulnerable populations, if its medical use can provide a relatively safe and effective alternative to prescription drugs with far greater morbidity and mortality such as opioids and benzodiazepines, then perhaps cannabis should also be viewed as a potential harm reduction tool that may prove to be effective in addressing the opioid overdose crisis. Data from the present study could inform current and future opioid reduction strategies, particularly in patient populations addressing chronic pain, and certainly suggest that further observational and clinical studies examining the impact of cannabis on the use of prescription drugs and other substances are warranted.
Acknowledgments
We would like to thank all of the patients who shared their experiences and all of the health care providers and clinics for their commitment of time and resources toward this study. We would also like to thank Tilray for funding this study, Joel Singer and Terry Lee at the Centre for Health Evaluation and Outcome Sciences for their skilled support with the analysis of these data and Sunny Razzagh for assistance in recruiting study sites.
Appendix
Table A1.
Sensitivity analysis comparing WHOQOL-BREF outcomes between participants lost to follow-up and those who remained in the study at M3 and M6
| Has follow-up at M3? |
Has follow-up at M6? |
|||||
|---|---|---|---|---|---|---|
| Change from baseline to month 1 | No | Yes | P Value* | No | Yes | P Value* |
| WHOQOL—Physical Health | 0.270 | |||||
| n | 329 | 730 | 297 | 400 | 0.466 | |
| Mean (SD) | 10.9 (15.6) | 9.8 (14.3) | 12.3 (17.2) | 13.2 (15.2) | ||
| Median (IQR) | 10.7 (0.0, 21.4) | 7.1 (0.0, 17.9) | 10.7 (0.0, 25.0) | 10.7 (3.6, 21.4) | ||
| Range | –35.7 to 64.3 | –42.9 to 67.9 | –39.3 to 67.9 | –28.6 to 75.0 | ||
| WHOQOL—Psychological | 0.420 | 0.978 | ||||
| n | 329 | 730 | 297 | 400 | ||
| Mean (SD) | 6.3 (13.6) | 5.5 (14.0) | 7.3 (16.7) | 7.3 (13.6) | ||
| Median (IQR) | 4.2 (–4.2, 12.5) | 4.2 (–4.2, 12.5) | 4.2 (0.0, 16.7) | 4.2 (0.0, 14.6) | ||
| Range | –29.2 to 54.2 | –62.5 to 45.8 | –50.0 to 75.0 | –37.5 to 54.2 | ||
| WHOQOL—Social Relationships | 0.111 | 0.171 | ||||
| n | 329 | 730 | 297 | 400 | ||
| Mean (SD) | 4.6 (17.5) | 2.8 (16.5) | 5.4 (19.9) | 3.5 (17.2) | ||
| Median (IQR) | 0.0 (–8.3, 16.7) | 0.0 (–8.3, 8.3) | 0.0 (–8.3, 16.7) | 0.0 (–8.3, 16.7) | ||
| Range | –66.7 to 58.3 | –41.7 to 66.7 | –75.0 to 91.7 | –50.0 to 83.3 | ||
| WHOQOL—Environment | 0.175 | 0.409 | ||||
| n | 329 | 730 | 297 | 400 | ||
| Mean (SD) | 3.4 (11.4) | 2.4 (11.5) | 2.6 (14.2) | 3.4 (12.2) | ||
| Median (IQR) | 3.1 (–3.1, 9.4) | 0.0 (–3.1, 9.4) | 3.1 (–6.3, 12.5) | 3.1 (–3.1, 9.4) | ||
| Range | –28.1 to 43.8 | –46.9 to 50.0 | –50.0 to 50.0 | –28.1 to 53.1 | ||
WHOQOL-BREF = World Health Organization Quality of Life Short Form; SD = standard deviation; M3 = month 3; M6 = month 6; IQR = interquartile range.
P values based on t test.
Funding sources: This study was funded by Tilray, a federally authorized Canadian medical cannabis production and research company.
Conflicts of interest: Philippe Lucas is Vice President, Global Patient Research and Access, for Tilray, the funder of this study, and his compensation includes stock options for Tilray. Susan Boyd has no competing financial interests. M.-J. Milloy has no competing financial interests. He is supported by the US National Institutes of Health (U01-DA0251525), a New Investigator Award from the Canadian Institutes of Health Research, and a Scholar Award from the Michael Smith Foundation for Health Research. M.-J. Milloy is also the Canopy Growth professor of cannabis science at the University of British Columbia (UBC), a position created using unstructured arm’s-length gifts to the university from Canopy Growth Corporation, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. UBC has also received unstructured funding from National Green Biomed Ltd., an applicant to the Canadian federal government for a license to produce cannabis, to support M.-J. Milloy. Zach Walsh is the primary investigator in a Tilray-sponsored randomized clinical trial of medical cannabis and posttraumatic stress disorder and has also received research funding from DOJA, a licensed producer of cannabis; he receives no financial compensation from Tilray or DOJA. Zach Walsh is also a director of the Indigenous Bloom Corporation, which works to establish opportunities for Canadian Indigenous groups to engage in the cannabis industry. He has been compensated for his work with shares in Indigenous Bloom.
References
- 1. Fischer B, Jeffries V, Hall W, Room R, Goldner E, Rehm J.. Lower Risk Cannabis use Guidelines for Canada (LRCUG): A narrative review of evidence and recommendations. Can J Public Health 2011;102(5):324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grinspoon L. On the future of cannabis as medicine. Clin Trials 2007;2(2):13–5. [Google Scholar]
- 3. Wu L-TGhitza UEBatch BC, . et al. Substance use and mental diagnoses among adults with and without type 2 diabetes: Results from electronic health records data. Drug Alcohol Depend 2015;156:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nunberg H, Kilmer B, Pacula RL, Burgdorf JR.. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal 2011;4(1): (doi:10.2202/1941-2851.1017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med 2018;49:12–9. [DOI] [PubMed] [Google Scholar]
- 6. Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007;68(7):515–21. [DOI] [PubMed] [Google Scholar]
- 7. Abrams DI. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med 2018;49:7–11. [DOI] [PubMed] [Google Scholar]
- 8. Kowal MA, Hazekamp A, Grotenhermen F.. Review on clinical studies with cannabis and cannabinoids 2010-2014. Cannabinoids 2016;11(special issue):1–18. [Google Scholar]
- 9. McCoy B, Wang L, Zak M, et al. A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann Clin Transl Neurol 2018;5(9):1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag 2008;4(1):245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lynch ME, Ware MA.. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol 2015;10(2):293–301. [DOI] [PubMed] [Google Scholar]
- 12. Ware MAWang TShapiro S, . et al. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain 2015;16(12):1233–42. [DOI] [PubMed] [Google Scholar]
- 13. Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai L-H.. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007;447(7141):178–82. [DOI] [PubMed] [Google Scholar]
- 14. Brands BMann REWickens CM, . et al. Acute and residual effects of smoked cannabis: Impact on driving speed and lateral control, heart rate, and self-reported drug effects. Drug Alcohol Depend 2019;205:107641. [DOI] [PubMed] [Google Scholar]
- 15. Arkell TRLintzeris NKevin RC, . et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 2019;236(9):2713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aldington S, Harwood M, Cox B, et al. ; Cannabis and Respiratory Disease Research Group. Cannabis use and risk of lung cancer: A case-control study. Eur Respir J 2008;31(2):280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Callaghan RC, Allebeck P, Sidorchuk A.. Marijuana use and risk of lung cancer: A 40-year cohort study. Cancer Causes Control 2013;24(10):1811–20. [DOI] [PubMed] [Google Scholar]
- 18. Barbosa PCRStrassman RJda Silveira DX, . et al. Psychological and neuropsychological assessment of regular hoasca users. Compr Psychiatry 2016;71:95–105. [DOI] [PubMed] [Google Scholar]
- 19. Hashibe M, Morgenstern H, Cui Y, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: Results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2006;15(10):1829–34. [DOI] [PubMed] [Google Scholar]
- 20. Huang Y-HJ, Zhang Z-F, Tashkin DP, Feng B, Straif K, Hashibe M.. An epidemiologic review of marijuana and cancer: An update. Cancer Epidemiol Biomarkers Prev 2015;24(1):15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013;10(3):239–47. [DOI] [PubMed] [Google Scholar]
- 22. Bradford AC, Bradford WD, Abraham A, Bagwell Adams G. Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population. JAMA Intern Med 2018;178(5):667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lucas P, Baron EP, Jikomes N.. Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm Reduct J 2019;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi Y, Liang D, Bao Y, An R, Wallace MS, Grant I. Recreational marijuana legalization and prescription opioids received by Medicaid enrollees. Drug Alcohol Depend 2019;194:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen H, Hockenberry JM. Association of Medical and Adult-Use Marijuana Laws With Opioid Prescribing for Medicaid Enrollees. JAMA Intern Med 2018;178(5):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuczyńska K, Grzonkowski P, Kacprzak Ł, Zawilska JB. Abuse of fentanyl: An emerging problem to face. Forensic Sci Int 2018;289:207–14. [DOI] [PubMed] [Google Scholar]
- 27. Manchikanti L. Reframing the Prevention Strategies of theOpioid Crisis: Focusing on Prescription Opioids,Fentanyl, and Heroin Epidemic. Pain Physician 2018;1(21;1):309–26. [PubMed] [Google Scholar]
- 28. Belzak L, Halverson J.. The opioid crisis in Canada: A national perspective. Health Promot Chronic Dis Prev Can 2018;38(6):224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu Rev Public Health 2015;36(1):559–74. [DOI] [PubMed] [Google Scholar]
- 30. Bradford AC, Bradford WD.. Medical marijuana laws reduce prescription medication use in Medicare Part D. Health Aff 2016;35(7):1230–6. [DOI] [PubMed] [Google Scholar]
- 31. Shover CL, Davis CS, Gordon SC, Humphreys K. Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proc Natl Acad Sci U S A 2019;116(26):12624–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical Cannabis Laws and Opioid Analgesic Overdose Mortality in the United States, 1999-2010. JAMA Intern Med 2014;174(10):1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boehnke KF, Scott JR, Litinas E, Sisley S, Williams DA, Clauw DJ.. Pills to pot: Observational analyses of cannabis substitution among medical cannabis users with chronic pain. J Pain 2019;20(7):830–41. [DOI] [PubMed] [Google Scholar]
- 34. Reiman A, Welty M, Solomon P.. Cannabis as a substitute for opioid-based pain medication: Patient self-report. Cannabis Cannabinoid Res 2017;2(1):160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sagy I, Bar-Lev Schleider L, Abu-Shakra M, Novack V.. Safety and efficacy of medical cannabis in fibromyalgia. J Clin Med 2019;8(6):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abuhasira R, Schleider B-L, Mechoulam R, Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Inter Med 2018;49:44–50. [DOI] [PubMed] [Google Scholar]
- 37. Safakish RKo GSalimpour V, . et al. Medical Cannabis for the Management of Pain and Quality of Life in Chronic Pain Patients: A Prospective Observational Study. Pain Med 2020;21(11):3073–86. [DOI] [PubMed] [Google Scholar]
- 38. Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: Findings from a 4-year prospective cohort study. Lancet Public Health 2018;3(7):e341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okusanya B, Asaolu IO, Ehiri JE, Kimaru LJ, Okechukwu A, Rosales C.. Medical cannabis for the reduction of opioid dosage in the treatment of non-cancer chronic pain: A systematic review. Clin Pharmacol 2020;9(1): (doi:10.1186/s13643-020-01425-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reddon H, DeBeck K, Socias ME, et al. Cannabis use is associated with lower rates of initiation of injection drug use among street-involved youth: A longitudinal analysis. Drug Alcohol Rev 2018;37(3):421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raby WN, Carpenter KM, Rothenberg J, et al. Intermittent marijuana use is associated with improved retention in naltrexone treatment for opiate-dependence. Am J Addict 2009;18(4):301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scavone JL, Sterling RC, Weinstein SP, Van Bockstaele EJ.. Impact of cannabis use during stabilization on methadone maintenance treatment. Am J Addict 2013;22(4):344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Socías ME, Wood E, Lake S, et al. High-intensity cannabis use is associated with retention in opioid agonist treatment: A longitudinal analysis. Addiction 2018;113(12):2250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannabis Act. 2019. Available at: https://laws-lois.justice.gc.ca/eng/acts/C-24.5/ (accessed August 2020).
- 45. Lucas P. Regulating compassion: An overview of Canada’s federal medical cannabis policy and practice. Harm Reduct J 2008;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: A survey of authorized medical cannabis patients. Int J Drug Policy 2017;42:30–5. [DOI] [PubMed] [Google Scholar]
- 47. Walsh Z, Callaway R, Belle-Isle L, et al. Cannabis for therapeutic purposes: Patient characteristics, access, and reasons for use. Int J Drug Policy 2013;24(6):511–6. [DOI] [PubMed] [Google Scholar]
- 48. Saxena S, , Carlson D, , Billington R. The WHO quality of life assessment instrument (WHOQOL-Bref): the importance of its items for cross-cultural research. Qual Life Res 2001;10(8):711–21. [DOI] [PubMed] [Google Scholar]
- 49. Saxena S, Carlson D, Billington R, Orley J.. The WHO quality of life assessment instrument (WHOQOL-Bref): The importance of its items for cross-cultural research. Qual Life Res 2001;10(8):711–21. [DOI] [PubMed] [Google Scholar]
- 50.US Centers for Medicare & Medicaid Services. Opioid oral morphine milligram equivalent (MME) conversion factors. 2017. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-April-2017.pdf (accessed February 2019).
- 51.WHO Collaboration Centre for Drug Statistics Methodology. ATC/DDD Index 2020. 2020. Available at: https://www.whocc.no/atc_ddd_index/ (accessed July 2019).
- 52.WHOCC - Definition and general considerations. Available at: http://www.whocc.no/ddd/definition_and_general_considera/ (accessed December 8,. 2020). [Google Scholar]
- 53. Chen X, Cowan A, Inan S, et al. Opioid-sparing effects of cannabinoids on morphine analgesia: Participation of CB1 and CB2 receptors. Br J Pharmacol 2019;176(17):3378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goldenberg M, Reid MW, IsHak WW, Danovitch I. The impact of cannabis and cannabinoids for medical conditions on health-related quality of life: A systematic review and meta-analysis. Drug and Alcohol Dependence 2017;174:80–90. [DOI] [PubMed] [Google Scholar]
- 55. Walsh Z, Hendricks PS, Smith S, et al. Hallucinogen use and intimate partner violence: Prospective evidence consistent with protective effects among men with histories of problematic substance use. J Psychopharmacol 2016;30(7):601–7. [DOI] [PubMed] [Google Scholar]
- 56. Russo EB, Guy GW, Robson PJ.. Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem Biodivers 2007;4(8):1729–43. [DOI] [PubMed] [Google Scholar]
- 57. St Pierre M, Russo EB, Walsh Z.. No evidence of altered reactivity to experimentally induced pain among regular cannabis users. Clin J Pain 2020;36(8):589–93. [DOI] [PubMed] [Google Scholar]
- 58. Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT.. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage 2013;46(2):207–18. [DOI] [PubMed] [Google Scholar]
- 59. Bobitt J, Kaskie BP, Arora K, Shannon K, Milavetz G.. Older persons and medical cannabis use. Innov Aging 2017;1(suppl 1):605–6. [Google Scholar]
- 60. Kaskie B, Ayyagari P, Milavetz G, Shane D, Arora K.. The increasing use of cannabis among older Americans: A public health crisis or viable policy alternative? Gerontologist 2017;57(6):1166–72. [DOI] [PubMed] [Google Scholar]
- 61. Farmer RF, Kosty DB, Seeley JR, et al. Natural course of cannabis use disorders. Psychol Med 2015;45(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. Vol H-41.; 2011. Available at: http://oas.sahmsa.gov/NSDUH/2k10NSDUH/2k10Results.pdf (accessed December 2020)
- 63. Bar-Lev Schleider LMechoulam RLederman V, . et al. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med 2018;49:37–43. [DOI] [PubMed] [Google Scholar]
- 64. Degenhardt LLintzeris NCampbell G, . et al. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 2015;147:144–50. [DOI] [PubMed] [Google Scholar]
- 65. Hazekamp A, Ware MA, Muller-Vahl K, Abrams D, Grotenhermen F.. The medicinal use of cannabis and cannabinoids—An international cross-sectional survey on administration forms. J Psychoactive Drugs 2013;45(3):199–210. [DOI] [PubMed] [Google Scholar]