Abstract
Objectives
The purpose of this study was to examine the association of allergic rhinitis with air pollutant concentrations using the follow-up data of elementary school children in Ulsan, Korea.
Methods
All students of four elementary schools in Ulsan, South Korea were surveyed at two-year intervals. The survey used data collected five times, over a nine-year period from June 2009 to April 2018. The questionnaire used in the survey was a modified version of the ISAAC (International society of asthma and allergy of children) questionnaire. A skin prick test (SPT) was performed with 24 standard antigens. To estimate the levels of exposure to outdoor air pollution, the concentrations of sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), carbon monoxide (CO), and particulate matter 10 μm or less in diameter (PM10) were used. As a dependent variable, a history of allergic rhinitis diagnosed by a doctor during the last 1-year was considered. Logistic regression analysis was used to select variables suitable for the statistical model. The selected variables were then used to assess their association with the dependent variable using the generalized estimation equation.
Results
Among environmental factors, SO2 was associated with a high risk and PM10 was associated with a low risk of allergic rhinitis. The risk of allergic rhinitis from living in a house built within the last year was high, and the risk from living in a multi-family house or apartment was higher than that from living in a segregated house. History of allergic diseases in the family was a high-risk factor for allergic rhinitis. There was a relationship between a history of bronchiolitis at less than 2 years of age and a high risk of allergic rhinitis. Boys were at a higher risk than girls.
Conclusion
From the follow-up data of elementary school students in Ulsan, Korea, the concentration of SO2, which is an indicator of the degree of industrialization, was related to the prevalence of allergic rhinitis. Among all the risk factors, history of allergic disease in the parents was the most important factor, and the study reconfirmed the results of the previous studies.
Introduction
According to a report by the World Allergy Organization in 2013, approximately 20% of the global population has an allergic disease such as asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis [1]. A recent systematic review of the prevalence rates of allergic diseases in Korea over the past 30 years found continuous increases in the rates of allergic rhinitis, allergic conjunctivitis, atopic dermatitis, and food allergy, although there was no distinct trend in the prevalence of asthma [2]. A notable observation was that the prevalence of allergic rhinitis in children has continuously increased in recent decades [2–4].
Numerous previous studies on the causes of allergic diseases have focused on natural antigens such as house dust mites [5–7], dogs or cats [8, 9], and cockroaches [10], as well as on personal factors such as history of vaccination, use of antibiotics, and changes in lifestyle [11, 12]. A study has reported that the byproducts of diesel combustion might act as allergic sensitizers [13], while others have reported that people living in regions with severe air pollution due to industrialization showed lower sensitization to allergens [14]. The health effects of air pollutants remain unclear, as myriad chemical substances are developed and released each year. Thus, further studies on air pollutants are needed.
In the European Union guidelines on the testing and management of air quality, the following substances were set as targets to be controlled in consideration of their negative impacts on the human body and plants: O3, NO2, SO2, CO, and PM10 [15]. However, the results of studies on air pollutants vary significantly depending on the number of subjects, study design, target substance, measuring device, duration of measurement, region of measurement, and the methods [16, 17]. For example, one previous study examined the correlation between the concentration of SO2 and the risk of allergic rhinitis in elementary school students (odds ratio: 1.30) [18], while another study determined the correlation between SO2, CO, and NO2 concentrations and the prevalence of allergic rhinitis [19]. There is even a case in which the same study obtained inconsistent results depending on how subjects were grouped; specifically, an analysis of all subjects showed a correlation between NO2 concentration and increased prevalence of allergic rhinitis but an analysis of subgroups defined by the region of air pollution showed a correlation with reduced prevalence [20]. Most previous studies on this topic were cross-sectional, and had limitations related to study design. Thus, there is a need for a cohort study that analyzes patients over time.
Ulsan is a large metropolitan city located on the south-eastern coast of South Korea, with a population of approximately 1.1 million (as of 2019). Urban areas of Ulsan are close to national industrial complexes located along the labyrinthine coast, including petrochemical complex, car manufacturers, and shipbuilders [21]. According to the Annual Report of Air Quality in Korea 2017, the average level of SO2 in Ulsan is considerably higher than that in non-urban areas in South Korea and is notably higher than that in other metropolitan cities; for example, since 2000, in two years (2001 and 2004) Ulsan was the only city in South Korea in which SO2 levels exceeded the threshold level in the annual record (July 2017, 9 ppb) [22]. In addition, recent studies have shown a significant increase in the prevalence of allergic rhinitis in children in Ulsan [23]. There is growing concern in Ulsan about the effects of air pollutants on allergic rhinitis in areas with high air pollution due to the national industrial complexes.
Thus, in the present work we examined the association of allergic rhinitis with air pollutant concentrations, mainly SO2, using the follow-up data of elementary school children in Ulsan, Korea.
Materials and methods
Study design
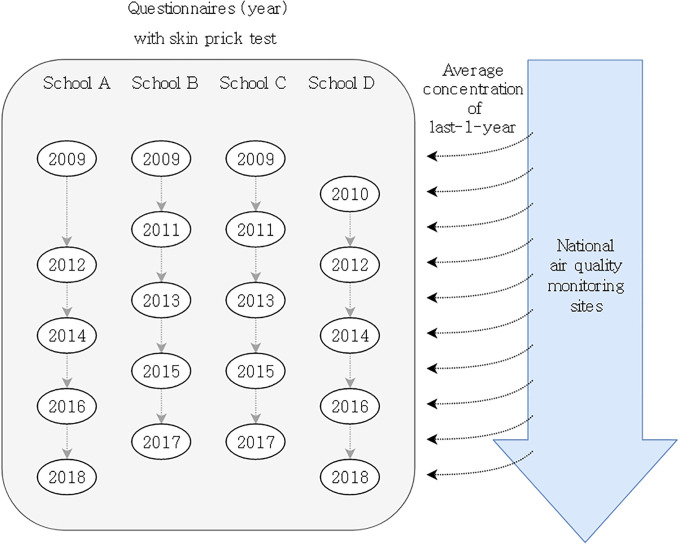
All data were from survey questionnaires, the results of the skin prick test (SPT, an objective indicator of allergic sensitization), and measurements of air pollutants (Fig 1). The overall study design is presented in Fig 1. Detailed description on Fig 1 were provided in subsections of ‘Study area’ and ‘Data collection’.
Fig 1. Study design.
School A: Suburban residential, B: Near industrial area, C: Coastal residential, D: Central urban.
Study area
The average temperature and wind speed in Ulsan were 14.1°C and 2.1 m/s, and the temperature is relatively higher than that in the other regions (1981–2010; the top 12%-tile and 38%-tile, respectively, among the 73 national monitoring sites managed by the Korea Meteorological Administration). In contrast, the average relative humidity was markedly lower at 64.2% (the low 7%-tile), which uniquely distinguishes this region from others [24]. North-westerly winds are predominant throughout the year, while the frequency of easterly and south-westerly winds increases during spring and summer.
O3 concentration in Ulsan has shown a rising trend as that in other cities [25]. In the case of volatile organic compounds, the level is higher in regions with industrial complexes than that in residential regions, clearly demonstrating the impact of the industrial activities in Ulsan [26]. Considering the environmental characteristics such as large-scale industrial facilities located in the coastal areas and the urban areas nearby regions, the surrounding mountains and frequent wind from the sea, it is likely that a large proportion of the urban population are frequently exposed to pollutants from the industrial complexes and city traffic [21].
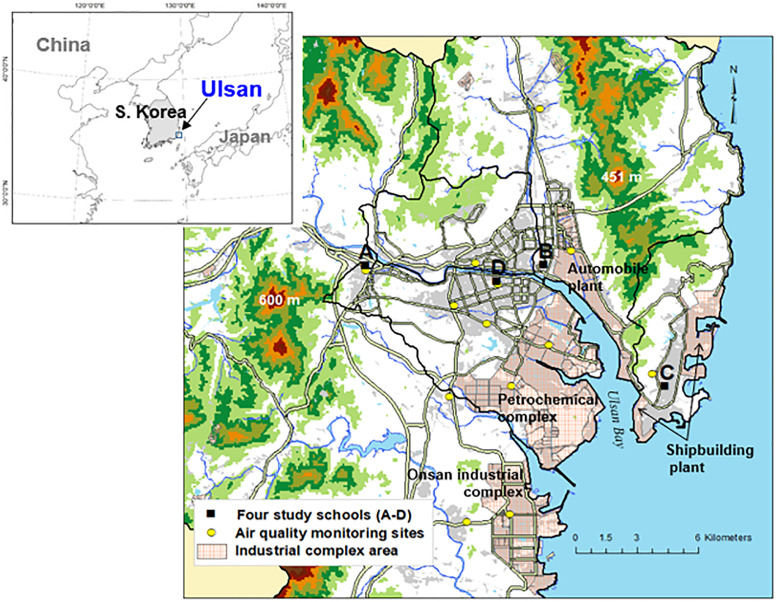
Among the locations in the region of Ulsan that show varying atmospheric characteristics, four elementary schools were selected (Fig 2) [21]. The data for this study were collected from the students of all years at the four schools. The location of the first school (A) had the characteristics of a suburban, residential area, and was relatively far away from the city center and the industrial complex. The second school (B) was located close to the industrial complex with the probability of being under a significant influence of air pollutants released from the large industrial complex of the Ulsan region. A global car manufacturer was found across the 8-lane road, and across the river was the petrochemical complex. The location of the third school (C) had the characteristics of a coastal and residential area, as it was near the sea with a lower temperature and higher wind speed than in the urban area [27]. The fourth school (D) was in the central urban area with multipurpose buildings including shopping quarters and residential areas. Table 1 summarizes the characteristics of each elementary school.
Fig 2. Geographical features in Ulsan and the location of the study schools (A-D) and air quality monitoring sites (AQMS).
Light red shaded regions are industrial area. Gray shaded regions and thick lines indicate built-up areas and main roads, respectively.
Table 1. Characteristics of the four examined schools during 2019.
| Suburban residential (A) | Near industrial (B) | Coastal residential (C) | Central urban (D) | |
|---|---|---|---|---|
| Students per grade | 100 | 140 | 90 | 180 |
| Year when built | 2000 | 2003 | 2004 | 1991 |
| Floors | 6 | 6 | 5 | 5 |
| Playground | + | + | + | + |
| Gymnasium | + | + | + | + |
School A-D: see Fig 2.
While the four schools did not significantly vary in terms of the overall size or the presence of a playing field or an indoor gym, the elementary school in the central urban area was built before the other schools.
Data collection
Survey questionnaire
The survey questionnaire was administered to all the students of all years at the four selected elementary schools. The survey has been conducted by the Environment Health Care Center of Ulsan University Hospital since June 2009 until 2019. Each year, the survey was conducted at two schools; thus, each school underwent the survey at two-year intervals.
In this study, the data collected five times, over a nine-year period up to Apr 15, 2018, were used. To apply the same air pollution dataset to the student group who conducted the survey at the same period, the questionnaire was sent to the parents through the school to be filled in and sent back within one week.
The questionnaire in the survey contained the key questions suggested by the International Society of Asthma and Allergy of Children (ISAAC) as well as various questions regarding the basic personal information (age, sex, etc.), family history of allergy, socio-economic indicators (education level of patients, family income, etc.), indoor and outdoor environmental data (smoking, distance from home to the road, odor, air pollutants, ventilation, having mold, etc.). The response to the questionnaire was computerized by a trained investigator at the Environment Health Care Center of Ulsan University Hospital.
To detect the internal inconsistency for each round of the survey, the questionnaires were rechecked for cases where the recorded response was not from among the multiple choices provided or was in contradiction to the response from another questions, and the responses of such cases were recorded again.
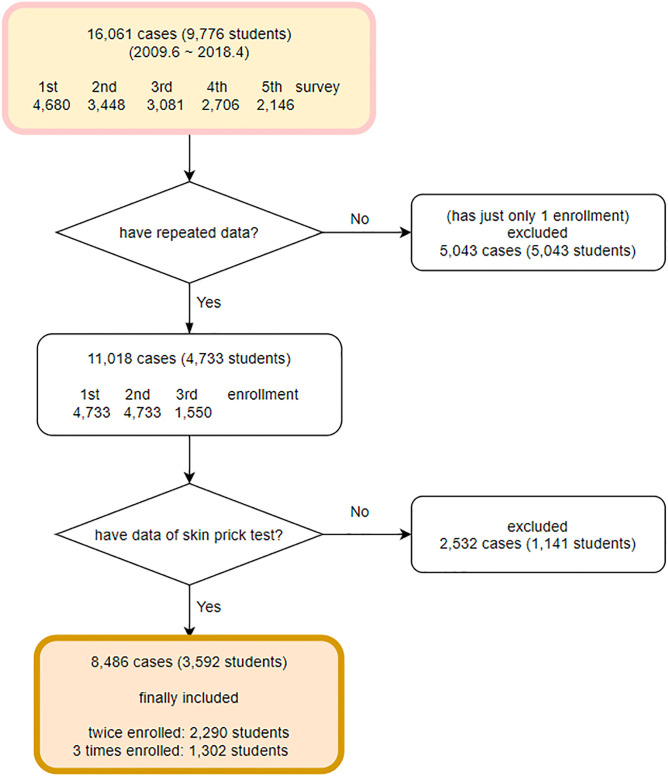
The students whose data of repeated measurements was available were selected, thus providing the data of 11,018 cases, while the data of 5,043 students with only a single measurement were excluded. The 11,018 cases comprised the responses of 4,733 students who participated in the survey up to two or three times. Among 4,733 students, 1,141 students who never conducted SPT were excluded in this study. After all, total 8,486 cases of 3,592 students were used in analysis. The data were categorized based on the time of survey of each student, and the result showed that 3,592 students had participated in the first and second surveys, while 1,302 students had participated up to the third survey (Fig 3) The cases in which an error was found to be revised accounted for 1.72% of all the follow-up data, including 112 cases of errors in recording and 78 cases of recording in a wrong order.
Fig 3. Case selection.
Surveys were conducted 1~3 times per student and data of 16,061 cases were collected from 9,776 students in all. The number of participating students and the response rate for the five rounds of the survey were as follows, from 1 to 5 respectively: 4,680 (83.9%), 3,448 (87.8%), 3,081 (89.3%), 2,706 (92.7%), and 2,146 (74.6%).
The general characteristics of the included and excluded students were examined (Table 2). Compared to the included students, the excluded students were from higher levels of the school year at the time of survey, and had higher age, height, and weight. This is presumed to be due to the overall participation rate, as the students who had participated in the survey during lower school years continued to participate in the higher years, while those who had already been in year 5 or 6 at the time of their first survey were excluded from the follow-up. Thus, students excluded from the follow-up showed a lower mean of the number of survey and a higher mean of the school year. In addition, the one-year diagnosis of major diseases generally showed no significant difference, except for allergic conjunctivitis.
Table 2. General characteristics of the excluded and included students.
| Excluded students (n = 6,184) | Included students (n = 3,592) | P-value† | |
|---|---|---|---|
| Number of Survey * | 2.38 ± 1.61 | 2.76 ± 1.27 | < 0.001 |
| School year * | 3.72 ± 1.87 | 3.57 ± 1.63 | < 0.001 |
| Male:Female | 1: 0.95 | 1: 0.97 | 0.305 |
| Age * | 9.18 ± 1.92 | 9.08 ± 1.71 | 0.003 |
| Height * | 138.27 ± 13.10 | 136.74 ± 11.79 | < 0.001 |
| Weight * | 35.28 ± 13.80 | 33.96 ± 10.17 | < 0.001 |
| Asthma‡ | 1.8% | 2.0% | 0.380 |
| Allergic rhinitis‡ | 26.8% | 27.7% | 0.280 |
| Atopic dermatitis‡ | 12.4% | 11.6% | 0.150 |
| Allergic conjunctivitis *‡ | 11.8% | 13.0% | 0.043 |
| Food allergy‡ | 2.2% | 2.6% | 0.170 |
†: p-value by student’s t test or chi-squared test (categorical variables).
*: p-value < 0.05.
‡: history of diagnosis by the doctor in the last one year.
After explanation of the survey, parents of all participants were provided written informed consent prior to participation. This survey was approved by the Institutional Review Board of the Ulsan University Hospital (approval no. UUH 2009-09-061).
Skin prick test
SPT was performed as an objective indicator for determining the allergic sensitization of a student. The standard antigens used in the SPT were as follows: negative control (physiological saline), positive control (histamine), American mite, European mite, mold mite, cockroach, ragweed, Asiatic plantain, willow, mugwort, Japanese hop, Japanese alder, dog, cat, indoor mold, outdoor mold, Koji mold, Japanese white birch, oak, pine, white goosefoot, maple, shrimp, flour, milk, and egg. A student was categorized as SPT positive if he or she showed at least one positive reaction to a minimum of one antigen among the 24 standard antigens listed above.
Concentration of air pollutants
This study used the monthly average data from 13 air quality monitoring sites (AQMS) managed by the National Institute of Environmental Research (NIER) and the Community Multiscale Quality (CMAQ) model (version 5.0.1) [28, 29] predictions to estimate the environmental exposure of participants to air pollutants (Fig 2).
Assuming that the students resided in an area close to their respective elementary schools and spent most of their time at either school or home, the average air pollutant exposure level of the students at each school was estimated based on the data of air pollutant concentrations collected from the monitoring site close to the school.
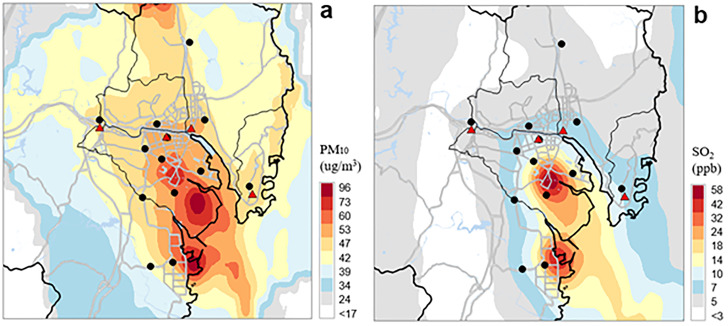
The CMAQ model was run for one year of 2014, in the middle of study period, in a nested mode at 27, 9, 3, and 1 km horizontal grid dimensions; The fine-scale innermost domain is the target area of the study that covers Ulsan and the surrounding area, which is the same as one used in Bang et al.(2018)’s study [30]. The detailed CMAQ-ready meteorological and emission inputs, initial and boundary conditions, and physical and chemical options used in our CMAQ modeling were described in Oh et al. (2021)’s study [31]. The hourly 1-km gridded concentrations predicted by the CMAQ model were averaged on monthly basis and then were blended with ambient monitored data from 13 air quality monitoring sites in Ulsan (AQMS) (Fig 2). This fusion process combines the inverse distance weighting (IDW) method for spatial monitored data interpolation with spatial scaling using gridded CMAQ predictions [31, 32]. For example, Fig 4 shows the fused annual mean concentrations of PM10 and SO2 for 2014. Adjusted concentrations by fusing monitoring data with CMAQ prediction can provide more reliable concentration information at four school areas because there can be significant in concentrations differences between school area and monitoring site. We assumed these fused gridded concentrations at school areas as exposure estimation for participants. Several previous studies have used this fusion technique to improve the CMAQ predictions for air pollution exposure estimation [31–34].
Fig 4. Horizontal variations of annual mean (a) PM10 and (b) SO2 concentrations predicted by fusing CMAQ predictions (1 km grid spacing) with monitored concentrations from air quality monitoring sites (AQMS) for 2014.
Black dots and red triangles indicate AQMS and four study schools, respectively.
The result of the air pollutant measurements is presented in Table 3, for each round of the survey and the schools.
Table 3. The mean annual concentration of air pollutants of the last one year of the survey, classified by schools.
| School † | Date of survey (year. month) | NO2 (ppb) | SO2 (ppb) | O3 (ppb) | CO (ppb) | PM10 (μg/m3) |
|---|---|---|---|---|---|---|
| A | 9.07 | 24.9 | 6.3 | 34.1 | 457.3 | 50.4 |
| 12.03 | 30.3 | 4.5 | 30.1 | 515.9 | 43.5 | |
| 14.05 | 34.4 | 6.4 | 40.8 | 517.2 | 45.1 | |
| 16.04 | 27.2 | 4.3 | 42 | 458.3 | 38 | |
| 18.04 | 29.8 | 4.1 | 41.4 | 541.5 | 38.2 | |
| B | 9.07 | 22.1 | 4.7 | 39.3 | 497.1 | 50.3 |
| 11.03 | 23.4 | 5.4 | 37.9 | 485.4 | 51 | |
| 13.03 | 23.5 | 5.1 | 42.3 | 544.8 | 49.6 | |
| 15.04 | 23 | 5 | 41 | 508.5 | 49.8 | |
| 17.04 | 21.7 | 4.4 | 44.9 | 506.3 | 40.5 | |
| C | 9.07 | 19.3 | 7.6 | 34.2 | 466.8 | 45.1 |
| 11.03 | 18.9 | 8 | 36 | 526 | 45.7 | |
| 13.03 | 19.4 | 8.8 | 42.5 | 511.8 | 48.6 | |
| 15.04 | 18.9 | 7.6 | 41.6 | 566.5 | 46.4 | |
| 17.04 | 17.4 | 6.7 | 40.8 | 484.2 | 42.8 | |
| D | 10.03 | 32.8 | 6.1 | 35.8 | 380 | 48.8 |
| 12.03 | 23.2 | 6 | 33.7 | 503 | 45.5 | |
| 14.04 | 25 | 8.5 | 40.1 | 534.7 | 45.5 | |
| 16.04 | 22.6 | 7.1 | 40.2 | 495 | 42.8 | |
| 18.04 | 22.6 | 6.5 | 43 | 600.5 | 39.7 |
SO2: sulfur dioxide, NO2: nitrogen dioxide, O3, ozone, CO: carbon monoxide, PM10: particulate matter 10 μm or less in diameter.
† School A: Suburban residential, B: Near industrial, C: Coastal residential, D: Central urban.
Statistical analysis
Among the six allergic diseases, this study focused on the analysis of allergic rhinitis that showed the highest prevalence. The prevalence indicator was the question “Have you been diagnosed with allergic rhinitis by a doctor in the past 12 months?” and the response was used to define the dependent variable of one-year diagnosis of allergic rhinitis. The indicator based on the diagnosis by a doctor to prevent the interference due to the subjective assessment of allergic rhinitis, is considered as a suitable indicator to identify the corresponding prevalence.
To determine the explanatory variables within a suitable model for describing the dependent variables, logistic regression analysis was performed from the cross-sectional analysis. We performed logistic regression analysis with a backward stepwise likelihood ratio entry method, which selected the following explanatory variables: air concentration of SO2, air concentration of PM10, response to the SPT, sex, age, BMI, family history of allergic diseases, education level of father, history of bronchiolitis, type of house, age of house, use of a humidifier, use of an air-conditioner, ever moved to a newly built or remodeled house, and ever had a pet. Then the concentrations of NO2, O3, and CO were added to the explanatory variables to investigate the effects of air pollutant concentrations. Finally, using the repeated measurement data, the association of allergic rhinitis with explanatory variables including air pollutants was evaluated by calculation of odds ratios (ORs) using a multivariate generalized estimating equation (GEE). To check the influence of all variables suitable in the model, variables without statistical significance were excluded in GEE.
For all statistical analyses, SPSS Statistics 21.0 (SPSS Inc., Chicago, IL, USA) was used, with the significance level set to p <0.05.
Results
With the variables selected via the logistic regression analysis as the explanatory variables, the GEE analysis was performed for the prevalence of allergic rhinitis using the data of one-year diagnosis, and the results are presented in Table 4.
Table 4. Factors related to prevalence of allergic rhinitis by last-1-year diagnosis by multivariate generalized estimating equation. (n = 3452).
| Variable | OR | 95% CI of OR | p-value |
|---|---|---|---|
| NO2 (ppb) | 1.002 | 0.987–1.017 | 0.816 |
| SO2 (ppb)* | 1.056 | 1.006–1.109 | 0.028 |
| O3 (ppb) | 1.006 | 0.990–1.023 | 0.447 |
| CO (ppb) | 1.000 | 0.999–1.001 | 0.926 |
| PM10 (μg/m3)* | 0.979 | 0.962–0.997 | 0.021 |
| Age (year) | 0.967 | 0.933–1.002 | 0.064 |
| BMI (kg/m2)* | 1.021 | 1.005–1.038 | 0.008 |
| Sex (female vs. male)* | 0.796 | 0.696–0.911 | 0.001 |
| SPT (positive vs. negative)* | 1.828 | 1.589–2.104 | < 0.001 |
| Father’s allergy history* (yes vs. no) | 1.956 | 1.718–2.227 | < 0.001 |
| Mother’s allergy history* (yes vs. no) | 2.390 | 2.100–2.721 | < 0.001 |
| Sibling’s allergy history* (yes vs. no) | 1.508 | 1.323–1.720 | < 0.001 |
| History of bronchiolitis under 2 years of age* (yes vs. no) | 1.338 | 1.126–1.591 | 0.001 |
| Type of house | |||
| Segregated house | reference | ||
| Multi-family* | 1.731 | 1.174–2.554 | 0.006 |
| Apartment* | 1.625 | 1.164–2.269 | 0.004 |
| Age of the house | |||
| < 1 year | reference | ||
| 1–4 years* | 0.658 | 0.434–0.998 | 0.049 |
| 5–9 years* | 0.568 | 0.381–0.847 | 0.006 |
| 10 years or over* | 0.626 | 0.423–0.927 | 0.019 |
| Humidifier (yes vs. no)* | 1.305 | 1.143–1.490 | < 0.001 |
| Air-conditioner (yes vs. no)* | 1.486 | 1.212–1.821 | < 0.001 |
| Moved to new home (yes vs. no) | 0.981 | 0.863–1.114 | 0.763 |
| Pets (ever vs. never)* | 0.842 | 0.726–0.975 | 0.022 |
The results indicated a significant positive correlation between the prevalence of allergic rhinitis and the concentration of SO2 among the analyzed air pollutants (OR 1.056), whereas a significant inverse correlation with the concentration of PM10 (OR 0.979) was found. No correlation was found for other air pollutants. A positive result of the SPT was significantly correlated with allergic rhinitis (OR 1.828). The risk of allergic rhinitis was significantly high if a parent or sibling showed a history of allergic disease, and a history of allergic disease in the mother was shown to be the most influential variable (OR 2.390). The risk of allergic rhinitis was high in the student who lived in a house built within the past year, and it was higher in those who lived in multi-family building or apartment than in a segregated house. The risk was significantly higher in male students than in female students, and in the students who had a history of bronchiolitis at less than two years of age. The risk of allergic rhinitis was also higher in the student with higher BMI. The use of a humidifier or air conditioner was also found to be associated with an increased risk of allergic rhinitis. The history of living with a pet was correlated with a lower risk of allergic rhinitis.
Discussion
The health hazards caused by exposure to air pollution are particularly concerning in children, as their immune system and lung development are not adequately mature against the air pollutants; Hence, the level of hazard is much higher than in adults [35]. In addition, as children spend considerably more time outdoors than adults do, they are more susceptible to air pollution [36].
This study focused on determining the association of allergic rhinitis with air pollutants, among the various potential factors. The results showed a significant association between the prevalence of allergic rhinitis and the concentrations of SO2 and PM10, among the air pollutants investigated.
This study showed that SO2 concentration was associated with an increased risk of allergic rhinitis with an OR of 1.056 (1.006–1.109), a significant finding that confirmed a partial influence of the industrial complex on the prevalence of allergic rhinitis. SO2 is produced from organic decomposition or volcanic eruption in nature, while it is mostly considered to be a combustion byproduct of organic fuels such as petroleum in industrial areas, and serves as an indicator of the level of urbanization or industrialization [37].
Industrial facilities, including factories, a power plant, and oil refineries, are the main sources of SOx emissions in Ulsan (Fig 2), and emissions from the petrochemical complex are predominant throughout the year [38]. Additionally, ship transportation in the coastal area near a shipbuilding plant is a significant source of SOx emission that can influence ambient SO2 concentrations at most regions near the coast. This explains the higher atmospheric SO2 concentrations at Schools C and D than at School B under certain wind conditions, although School B is closest to the industrial complex (an automobile plant). The predominantly westerly winds in Ulsan throughout the year explain why the SO2 concentration at school C (the western-most school) was highest among the four schools (Table 3, Fig 2). Differences in SO2 concentrations at the four Schools can also be affected by uncertainties in SOx emission input from CMAQ modeling and differences in the estimated periods of SO2 exposure.
In previous studies on the relationship between SO2 and the prevalence of allergic rhinitis, OR in the range of 1.14–1.43 was reported in a cross-sectional study conducted by Hwang et al. among 32,143 elementary and middle school students in Taiwan [19], while Kim et al. conducted a cross-sectional study among 4,545 elementary school students in Korea and reported an OR of 1.30 [18]. In the study by Kim et al. [18], the geographic locations of the elementary schools were categorized into hometown zone, traffic related zone, and complex source zone. In the hometown and traffic related zones, no significant correlation with SO2 was found, while in the regions close to the industrial complex, a significant correlation between SO2 and allergic rhinitis was observed. The measured values of SO2 were reported to be 4 ppb for the hometown and traffic related zones and 5–7 ppb for the complex source zone. Based on the findings in the present study wherein the SO2 concentration was in the range of 4.1–8.5 ppb, we can consider the possibility that an environment with SO2 concentration above a certain level leads to allergic rhinitis in school-age children. In a meta-analysis of nine studies that investigated the relationship between allergic rhinitis and SO2 concentration during the period Jan 2000 –Feb 2018, the finding from the data of 46,392 children showed an OR of 1.085 [39]. Among the nine reviewed studies including four cohort studies, no study reported a significant correlation between the prevalence of allergic rhinitis and SO2 [40–43]. The statistically significant correlation between SO2 and allergic rhinitis in the present study is thus significant in confirming the findings of the previous cross-sectional studies as well as in detecting the rare statistical significance in the follow-up data.
Few previous studies investigated the relationship between allergic rhinitis and the level of PM10 in elementary school students. One study reported a significant relationship (OR = 1.14, 95% CI = 1.02–1.27) [20]. However, a meta-analysis of studies that used the ISAAC questionnaire reported an OR of about 1, with little influence and inconsistent directionality among studies [44]. A prospective cohort study of 3,049 schoolchildren showed no significant correlation between PM10 and asthma [45]. Another study showed that seasonal allergic rhinitis was not associated with PM10 but was associated with temperature [46].
Kang et al. investigated changes in allergic symptoms in 108 patients with allergies and 47 healthy subjects by comparing their 120-day symptom scores and found that none of the allergic symptoms correlated with daily PM10 changes. However, their data indicated that allergic symptoms were significantly aggravated by an increase in time outdoors and that temperature had a significant effect in both groups [47]. They concluded that their results were similar to those of a previous ISAAC study in Japan [45, 47]. There are few longitudinal studies of the link between PM10 and allergic rhinitis, and the results of Kang et al. are similar to those of the present study.
PM10 can act as carriers of allergen, aggravating allergic reactions, and smaller particles can enter the respiratory tract, breaching the mucosa [39, 48]. However, since it is difficult to identify the source or mechanism of the release of PM10, the interpretation of the result is challenging because PM10 considered the size of the dust particle rather than the characteristics of the components [39]. Few previous studies investigated the relationship between the composition of PMs and allergy. A longitudinal study found that total PM2.5 was not significantly associated with any asthmatic symptom or medication use. However, a detailed analysis of the composition of PM2.5 found that PM2.5 mass concentration coming from motor vehicles or road dust was consistently associated with increased likelihood of respiratory symptoms and inhaler use [49]. Another study of the effect of PM sources in children showed that oil had significant negative association with the symptom of wheezing [50].
The present study did not find the association between NO2 concentration with allergic rhinitis. The association between NO2, the biomarker of traffic-related air pollutants, with allergic rhinitis has been widely reported in adults and children in Asia and Europe [19, 51–58]. However, the association between NO2 and allergic rhinitis has not always been consistent. Ambient SO2 concentration was positively correlated with the prevalence of allergic rhinitis, but there was no correlation with other air pollutants such as PM10 and NO2 in Chinese adults [59]. De Marco et al. showed that NO2 was positively associated with allergic rhinitis in the Mediterranean region but not in the subcontinental region in Italy [60]. They argued that that result implies that the climate interacts with outdoor air pollutants. A Korean study on elementary school children showed a significant OR of 1.34 (1.17–1.53) in complex areas around the factory and an OR of 0.71 (0.54–0.91) in areas with heavy traffic [20]. Further prospective studies are needed.
For the analyses of other environmental factors, the risk of allergic rhinitis was significantly higher in the students living in a house built within the past year. This might be attributed to the building syndrome caused by the construction materials of a newly built house [61]. For example, a study of newly built houses in Japan reported that some volatile organic compounds (VOCs) and formaldehyde had significant dose-dependent relationships with nonspecific subjective symptoms that resembled “sick building syndrome” [62]. A study in Korea reported that the inner walls of many homes are covered with materials that emit air pollutants, including VOCs and formaldehyde, and this increased the risk for atopic dermatitis in children [63]. Huss-Marp et al. showed that exposure to VOCs damaged the epidermal barrier and worsened atopic dermatitis at concentrations commonly found in indoor environments [64].
The risk of allergic rhinitis in this study was higher in students staying in a multi-family building or in an apartment than in a segregated house. This might be attributed to the high humidity and moldiness observed in a multi-family building [65]. It could also be conjectured that living in a multi-family building increases the chances of exposure to cigarette smoke if there is a smoker among the neighbors.
Some studies found high risk of building syndrome in a newly built house, multi-family building, and public housing. These studies suggested that the age of each building, the ownership, and the characteristics of the residents should be examined in addition to living in a multi-family building [66–68]. Although the questionnaire in this study did not include any item on the ownership, the results could be interpreted in line with the previous studies. We also investigated the effect of the type of heating fuel used in each home, but this factor was not significant in the logistic regression, so was not included in the final analysis.
The present study found a significant correlation between the use of a humidifier or an air conditioner with the risk of allergic rhinitis, although history of living with a pet had a significantly lower risk. This may be because parents with a child who has allergic rhinitis may eschew pet ownership, but use an air conditioner or air purifier more frequently.
The interpretation becomes slightly more complicated in the case of use of humidifier. A study reported bacterial growth inside the humidifier [69], while another reported the problem of bacterial or fungal growth caused by the moisture on the inner wall, on continuous use of the humidifier [70]. However, another large-scale cohort study among young children found no association between the use of humidifier and either house mites or fungi [71]. For the correlation found in this study between the use of humidifier and the prevalence of allergic rhinitis, the causality cannot be precisely defined based solely on the results.
Among the non-environmental factors shown to have a correlation with the prevalence of allergic rhinitis, the one with the highest risk was the history of allergy in a parent or sibling. Among other variables, a significantly higher risk was associated with SPT positivity, history of bronchiolitis, female students than in male students, and BMI. For the family history of allergy, where the temporal relationship is clear and the conditions cannot be arbitrarily modified unlike in the case of the use of humidifier or air conditioner, the results might be interpreted as the cause-effect relationship. The factor that showed the highest OR was the history of allergy in the mother. The fact that the first and second factors with the highest OR were the history of allergy in the mother and father clearly indicates the presence of a genetic factor in allergic rhinitis. Numerous previous studies have reported the correlation between the history of allergy in a parent and the probability of an allergy in the child [72–76]. A recent study also showed a significant increase in the risk of allergic diseases including asthma, allergic rhinitis, and atopic dermatitis, if a parent had a history of allergy [77]. Research on the association between family history and allergic diseases has been widely conducted, and recent studies have shown the association of specific genetic polymorphism with the susceptibility to allergic rhinitis [78, 79].
The findings in this study also confirmed the high correlation between the SPT positivity and the prevalence of allergic rhinitis [80]. Though SPT cannot predict the severity of illness by stratifying the size of the skin-prick test result, SPT can be applied to support the diagnosis of allergic rhinitis [81]. Jung et al. suggested that SPT is the recommended first choice than serum IgE level measurement for detecting allergy to house-dust mites in patients <30 years old [82].
A history of bronchiolitis below the age of two years showed a higher risk of allergic rhinitis, which might be interpreted in two different ways based on the previous studies. First, there is a report on the correlation between an increased risk of asthma or allergic rhinitis, and the use of antibiotics during the early days after birth that alters the normal flora in the gastro-intestinal tract [83], based on which the correlation between the history of bronchiolitis and the prevalence of allergic rhinitis in this study can be explained. As another plausible explanation, a study reported an incidence of allergic disease caused by glutathione deficiency due to the reduced protective function of antioxidants in the respiratory mucosa because of the use of paracetamol (the most well-known product name is Tylenol®), a drug most commonly used to treat inflammation and fever [77, 84]. Ever since Aspirin® was reported to cause Reye’s syndrome, the use of paracetamol is highly likely as there are limited options for an antipyretic that can be used to reduce the fever in infant, which thus lends support for the finding in this study.
This study had several limitations. First, we were unable to evaluate the exposure level of each individual student. Instead, we estimated the air pollutant exposure at each school based on average air pollutant concentrations at nearby monitoring sites. We believe these measurements meaningful because all students resided close to their schools, spent most of their time at school or home, and we provided more reliable adjusted concentrations at each school by fusing monitoring data with CMAQ predictions. Second, most values were based on questionnaire responses rather than objective measurements, so recall bias or misclassification bias is possible. Third, we used mean annual concentration of air pollutants of the last one year of the survey, but not changes of concentrations, seasonal changes, or short-term concentrations in the analysed period. However, uncertainties may be decreased because we used a diagnosis with allergic rhinitis by a doctor in the past 12 months as outcome variable. Finally, we did not include school-related factors, such as the age of the building, humidity and moldiness, building materials, and heating systems that vary among schools, because of the strong multicollinearity between measured air pollution and school-related variables.
The strength of the present study was that we examined a large population over a long period of time and there was little variation in the subjects, as the study investigated all students at the elementary schools. Hence, the association between air pollutants and the prevalence of allergic rhinitis could be verified.
In conclusion, the concentrations of SO2, which is indicator of the degree of industrialization, was related to the prevalence of allergic rhinitis. Among all the risk factors, history of allergic disease in the parents was the most important factor, and the study reconfirmed the results of the previous studies. Further research with the measurement of indoor and outdoor factors at each home and the schools is needed.
Supporting information
(SAV)
(SPS)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This research was supported by the Environmental Health Center funded by the Ministry of Environment, Republic of Korea KY received this fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pawankar R, Holgate S, Canonica G, Lockey RF, Blaiss M. WAO white book on allergy 2013 update. World Allergy Organization. 2013. [Google Scholar]
- 2.Kang SY, Song WJ, Cho SH, Chang YS. Time trends of the prevalence of allergic diseases in Korea: A systematic literature review. Asia Pac Allergy. 2018;8(1):e8. Epub 2018/02/10. 10.5415/apallergy.2018.8.e8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KS, Rha YH. Research on pediatric allergic rhinitis in Korea. Allergy, Asthma & Respiratory Disease. 2018;6(Suppl 1):S58–S65. [Google Scholar]
- 4.Hwang SH, Jung SY, Lim DH, Son BK, Kim JH, Yang J-M, et al. Epidemiology of allergic rhinitis in Korean children. Allergy, Asthma & Respiratory Disease. 2013;1(4):321–32. [Google Scholar]
- 5.Smith JM, Disney ME, Williams JD, Goels ZA. Clinical significance of skin reactions to mite extracts in children with asthma. Br Med J. 1969;2(5659):723–6. Epub 1969/06/21. 10.1136/bmj.2.5659.723 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke C, Aldons P. The nature of asthma in Brisbane. Clinical & Experimental Allergy. 1979;9(2):147–52. 10.1111/j.1365-2222.1979.tb01535.x [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto T, Johansson S, Ito K, Horiuchi Y. Atopic allergy in Japanese subjects: studies primarily with radioallergosorbent test. Journal of Allergy and Clinical Immunology. 1974;53(1):9–19. 10.1016/0091-6749(74)90094-3 [DOI] [PubMed] [Google Scholar]
- 8.Erwin EA, Rönmark E, Wickens K, Perzanowski MS, Barry D, Lundbäck B, et al. Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. Journal of allergy and clinical immunology. 2007;119(2):359–65. [DOI] [PubMed] [Google Scholar]
- 9.Rönmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundbäck B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. Journal of Allergy and Clinical Immunology. 2009;124(2):357–63. e15. 10.1016/j.jaci.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulett A, Dockhorn R. House dust, mite (D. farinae) and cockroach allergy in a midwestern population. Annals of allergy. 1979;42(3):160–5. [PubMed] [Google Scholar]
- 11.Peat J, Tovey E, Mellis C, Leeder S, Woolcock A. Importance of house dust mite and Alternaria allergens in childhood asthma: an epidemiological study in two climatic regions of Australia. Clinical & Experimental Allergy. 1993;23(10):812–20. 10.1111/j.1365-2222.1993.tb00258.x [DOI] [PubMed] [Google Scholar]
- 12.Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. Journal of Allergy and Clinical Immunology. 1999;103(1):1–10. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. Journal of allergy and clinical immunology. 1999;104(6):1183–8. 10.1016/s0091-6749(99)70011-4 [DOI] [PubMed] [Google Scholar]
- 14.Von Mutius E, Martinez FD, Fritzsch C, Nicolai T, Roell G, Thiemann H-H. Prevalence of asthma and atopy in two areas of West and East Germany. American journal of respiratory and critical care medicine. 1994;149(2):358–64. [DOI] [PubMed] [Google Scholar]
- 15.Directive C. 96/62/EC of 27 September 1996 on ambient air quality assessment and management. Official Journal L. 1996;296(21):11. [Google Scholar]
- 16.Gonzalez-Barcala F, Pertega S, Garnelo L, Castro T, Sampedro M, Lastres J, et al. Truck traffic related air pollution associated with asthma symptoms in young boys: a cross-sectional study. Public Health. 2013;127(3):275–81. 10.1016/j.puhe.2012.12.028 [DOI] [PubMed] [Google Scholar]
- 17.Liu M-M, Wang D, Zhao Y, Liu Y-Q, Huang M-M, Liu Y, et al. Effects of outdoor and indoor air pollution on respiratory health of Chinese children from 50 kindergartens. Journal of epidemiology. 2013;23(4):280–7. 10.2188/jea.je20120175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H-H, Lee C-S, Jeon J-M, Yu S-D, Lee C-W, Park J-H, et al. Analysis of the association between air pollution and allergic diseases exposure from nearby sources of ambient air pollution within elementary school zones in four Korean cities. Environmental Science and Pollution Research. 2013;20(7):4831–46. 10.1007/s11356-012-1358-2 [DOI] [PubMed] [Google Scholar]
- 19.Hwang B-F, Jaakkola JJ, Lee Y-L, Lin Y-C, Guo Y-lL. Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respiratory research. 2006;7(1):23. 10.1186/1465-9921-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HH, Lee CS, Yu SD, Lee JS, Chang JY, Jeon JM, et al. Near-road exposure and impact of air pollution on allergic diseases in elementary school children: a cross-sectional study. Yonsei medical journal. 2016;57(3):698–713. 10.3349/ymj.2016.57.3.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh I-B, Kim Y, Sim Cs, Lee Jh. Prevalence of Children’s Allergic Diseases in Ulsan: Local Differences and Environmental Risk Factors. J Environ Health Sci. 2012;38(6):472–81. [Google Scholar]
- 22.Ministry of Environment. ANNUAL REPORT OF AIR QUALITY IN KOREA 2017. 2018.
- 23.Lee J, Oh I, Kim M-h, Bang JH, Park SJ, Yun SH, et al. Change in the Prevalence of Allergic Diseases and its Association with Air Pollution in Major Cities of Korea-Population under 19 Years Old in Different Land-use Areas. Journal of Environmental Health Sciences. 2017;43(6):478–90. [Google Scholar]
- 24.Korea Meteorological Administration. Climatological Normals of Korea, 1981~2010 2011 ed. Seoul: Korea Meteorological Administration; 2011.
- 25.Ministry of Environment. ANNUAL REPORT OF AIR QUALITY IN KOREA 2011. 2012.
- 26.National Institute of Environmental Research. Research on Hazardous Air Pollutants (HAPs) in Ulsan Area. National Institute of Environmental Research; 2010.
- 27.Oh I, Bang J-H, Kim Y. Meteorological Characteristics in the Ulsan Metropolitan Region: Focus on Air Temperature and Winds. Journal of Korean Society for Atmospheric Environment. 2015;31(2):181–94. [Google Scholar]
- 28.Byun D, Ching J. Science Algorithms of the EPA Models-3 Community Multi-scale Air Quality (CMAQ) Modeling System, EPA Report. EPA/600/R-99/030, NERL, Research Triangle Park, NC, 1999.
- 29.Byun D, Schere KL. Review of the governing equations, computational algorithms, and other components of the Models-3 Community Multiscale Air Quality (CMAQ) modeling system. 2006.
- 30.Bang J-H, Oh I, Kim S, You S, Kim Y, Kwon H-J, et al. Modeling the effects of pollutant emissions from large industrial complexes on benzene, toluene, and xylene concentrations in urban areas. Environmental health and toxicology. 2017;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh I, Hwang M-K, Bang J-H, Yang W, Kim S, Lee K, et al. Comparison of different hybrid modeling methods to estimate intraurban NO2 concentrations. Atmospheric Environment. 2021;244:117907. [Google Scholar]
- 32.Timin B, Wesson K, Thurman J. Application of Model and Ambient Data Fusion Techniques to Predict Current and Future year PM2. 5 concentration in unmonitored areas. Air Pollution Modeling and Its Application XX Netherlands: Springer. 2010:175–9.
- 33.Chen G, Li J, Ying Q, Sherman S, Perkins N, Sundaram R, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Science of the total environment. 2014;485:563–74. 10.1016/j.scitotenv.2014.03.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. Estimating the national public health burden associated with exposure to ambient PM2. 5 and ozone. Risk Analysis: An International Journal. 2012;32(1):81–95. 10.1111/j.1539-6924.2011.01630.x [DOI] [PubMed] [Google Scholar]
- 35.Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113(Supplement 3):1037–43. [PubMed] [Google Scholar]
- 36.Moya J, Bearer CF, Etzel RA. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics. 2004;113(Supplement 3):996–1006. [PubMed] [Google Scholar]
- 37.Yang N, Zhang Z, Xue B, Ma J, Chen X, Lu C. Economic growth and pollution emission in China: Structural path analysis. Sustainability. 2018;10(7):2569. [Google Scholar]
- 38.Lee D-G, Lee Y-M, Jang K-W, Yoo C, Kang K-H, Lee J-H, et al. Korean national emissions inventory system and 2007 air pollutant emissions. Asian Journal of Atmospheric Environment. 2011;5(4):278–91. [Google Scholar]
- 39.Zou Q-Y, Shen Y, Ke X, Hong S-L, Kang H-Y. Exposure to air pollution and risk of prevalence of childhood allergic rhinitis: A meta-analysis. International journal of pediatric otorhinolaryngology. 2018;112:82–90. 10.1016/j.ijporl.2018.06.039 [DOI] [PubMed] [Google Scholar]
- 40.Chung H-Y, Hsieh C-J, Tseng C-C, Yiin L-M. Association between the first occurrence of allergic rhinitis in preschool children and air pollution in Taiwan. International Journal of Environmental Research and Public Health. 2016;13(3):268. 10.3390/ijerph13030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Huang C, Hu Y, Fu Q, Zou Z, Sun C, et al. Associations of gestational and early life exposures to ambient air pollution with childhood respiratory diseases in Shanghai, China: A retrospective cohort study. Environment international. 2016;92:284–93. 10.1016/j.envint.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 42.Deng Q, Lu C, Yu Y, Li Y, Sundell J, Norbäck D. Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Respiratory medicine. 2016;121:67–73. 10.1016/j.rmed.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 43.Wang I-J, Tung T-H, Tang C-S, Zhao Z-H. Allergens, air pollutants, and childhood allergic diseases. International journal of hygiene and environmental health. 2016;219(1):66–71. 10.1016/j.ijheh.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 44.Anderson HR, Ruggles R, Pandey KD, Kapetanakis V, Brunekreef B, Lai CK, et al. Ambient particulate pollution and the world-wide prevalence of asthma, rhinoconjunctivitis and eczema in children: Phase One of the International Study of Asthma and Allergies in Childhood (ISAAC). Occupational and environmental medicine. 2010;67(5):293–300. 10.1136/oem.2009.048785 [DOI] [PubMed] [Google Scholar]
- 45.Shima M, Nitta Y, Ando M, Adachi M. Effects of air pollution on the prevalence and incidence of asthma in children. Archives of Environmental Health: An International Journal. 2002;57(6):529–35. 10.1080/00039890209602084 [DOI] [PubMed] [Google Scholar]
- 46.Cabrera M, Subiza J. Association Between Seasonal Allergic Rhinitis and Air Pollution, Meteorological Factors, and Grass Pollen Counts in Madrid (1996 and 2009). Journal of investigational allergology & clinical immunology. 2018;29(5):371–7. [DOI] [PubMed] [Google Scholar]
- 47.Kang I, Ju Y, Jung J, Ko K, Oh D, Kim J, et al. The effect of PM10 on allergy symptoms in allergic rhinitis patients during spring season. International journal of environmental research and public health. 2015;12(1):735–45. 10.3390/ijerph120100735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devalia J, Rusznak C, Davies R. Air pollution in the 1990s-cause of increased respiratory disease? Respiratory medicine. 1994;88(4):241–4. 10.1016/0954-6111(94)90052-3 [DOI] [PubMed] [Google Scholar]
- 49.Gent JF, Koutrakis P, Belanger K, Triche E, Holford TR, Bracken MB, et al. Symptoms and medication use in children with asthma and traffic-related sources of fine particle pollution. Environ Health Perspect. 2009;117(7):1168–74. Epub 2009/08/06. 10.1289/ehp.0800335 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohr A, Habre R, Godbold J, Moshier E, Schachter E, Kattan M, et al. Asthma exacerbation is associated with particulate matter source factors in children in New York City. Air Quality, Atmosphere & Health. 2014;7. 10.1007/s11869-013-0230-y [DOI] [Google Scholar]
- 51.Cesaroni G, Badaloni C, Porta D, Forastiere F, Perucci CA. Comparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adults. Occup Environ Med. 2008;65(10):683–90. Epub 2008/01/22. 10.1136/oem.2007.037846 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai Y, Zijlema WL, Doiron D, Blangiardo M, Burton PR, Fortier I, et al. Ambient air pollution, traffic noise and adult asthma prevalence: a BioSHaRE approach. Eur Respir J. 2017;49(1). Epub 2016/11/09. 10.1183/13993003.02127-2015 . [DOI] [PubMed] [Google Scholar]
- 53.Jacquemin B, Sunyer J, Forsberg B, Aguilera I, Briggs D, García-Esteban R, et al. Home outdoor NO 2 and new onset of self-reported asthma in adults. Epidemiology. 2009:119–26. 10.1097/EDE.0b013e3181886e76 [DOI] [PubMed] [Google Scholar]
- 54.Chung HY, Hsieh CJ, Tseng CC, Yiin LM. Association between the First Occurrence of Allergic Rhinitis in Preschool Children and Air Pollution in Taiwan. Int J Environ Res Public Health. 2016;13(3). Epub 2016/03/02. 10.3390/ijerph13030268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krämer U, Koch T, Ranft U, Ring J, Behrendt H. Traffic-related air pollution is associated with atopy in children living in urban areas. Epidemiology. 2000;11(1):64–70. 10.1097/00001648-200001000-00014 [DOI] [PubMed] [Google Scholar]
- 56.Lee YL, Shaw CK, Su HJ, Lai JS, Ko YC, Huang SL, et al. Climate, traffic-related air pollutants and allergic rhinitis prevalence in middle-school children in Taiwan. Eur Respir J. 2003;21(6):964–70. Epub 2003/06/12. 10.1183/09031936.03.00094602 . [DOI] [PubMed] [Google Scholar]
- 57.Lu C, Deng Q, Ou C, Liu W, Sundell J. Effects of ambient air pollution on allergic rhinitis among preschool children in Changsha, China. Chinese Science Bulletin. 2013;58(34):4252–8. 10.1007/s11434-013-5725-2 [DOI] [Google Scholar]
- 58.Wang J, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, et al. Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci Total Environ. 2021;751:141734. Epub 2020/09/04. 10.1016/j.scitotenv.2020.141734 . [DOI] [PubMed] [Google Scholar]
- 59.Wang XD, Zheng M, Lou H, Wang C, Zhang Y, Bo M, et al. An increased prevalence of self‐reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170–80. 10.1111/all.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Marco R, Poli A, Ferrari M, Accordini S, Giammanco G, Bugiani M, et al. The impact of climate and traffic‐related NO2 on the prevalence of asthma and allergic rhinitis in Italy. Clinical & Experimental Allergy. 2002;32(10):1405–12. [DOI] [PubMed] [Google Scholar]
- 61.Hahm MI, Chae Y, Kwon HJ, Kim J, Ahn K, Kim WK, et al. Do newly built homes affect rhinitis in children? The ISAAC phase III study in K orea. Allergy. 2014;69(4):479–87. 10.1111/all.12355 [DOI] [PubMed] [Google Scholar]
- 62.Kishi R, Yoshino H, Araki A, Saijo Y, Azuma K, Kawai T, et al. New scientific evidence-based public health guidelines and practical manual for prevention of sick house syndrome. Nihon eiseigaku zasshi Japanese journal of hygiene. 2018;73(2):116–29. [DOI] [PubMed] [Google Scholar]
- 63.Kim J, Kim H, Lim D, Lee Y-K, Kim JH. Effects of indoor air pollutants on atopic dermatitis. International journal of environmental research and public health. 2016;13(12):1220. 10.3390/ijerph13121220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huss‐Marp J, Eberlein‐König B, Breuer K, Mair S, Ansel A, Darsow U, et al. Influence of short‐term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clinical & Experimental Allergy. 2006;36(3):338–45. 10.1111/j.1365-2222.2006.02448.x [DOI] [PubMed] [Google Scholar]
- 65.Saijo Y, Nakagi Y, Ito T, Sugioka Y, Endo H, Yoshida T. Relation of dampness to sick building syndrome in Japanese public apartment houses. Environmental health and preventive medicine. 2009;14(1):26. 10.1007/s12199-008-0052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norlen U, Andersson K. An indoor climate survey of the Swedish housing stock (the ELIB study). Proceedings of indoor air. 1993;93:743–8. [Google Scholar]
- 67.Engvall K, Norrby C, Bandel J, Hult M, Norbäck D. Development of a multiple regression model to identify multi‐family residential buildings with a high prevalence of sick building syndrome (SBS). Indoor Air. 2000;10(2):101–10. 10.1034/j.1600-0668.2000.010002101.x [DOI] [PubMed] [Google Scholar]
- 68.Engvall K, Hult M, Corner R, Lampa E, Norbäck D, Emenius G. A new multiple regression model to identify multi-family houses with a high prevalence of sick building symptoms “SBS”, within the healthy sustainable house study in Stockholm (3H). International archives of occupational and environmental health. 2010;83(1):85. 10.1007/s00420-009-0447-0 [DOI] [PubMed] [Google Scholar]
- 69.Hamilton LA, Falkinham JO III. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. Journal of medical microbiology. 2018;67(10):1491–5. 10.1099/jmm.0.000822 [DOI] [PubMed] [Google Scholar]
- 70.Lee JH, Ahn KH, Yu IJ. Outbreak of bioaerosols with continuous use of humidifier in apartment room. Toxicological research. 2012;28(2):103. 10.5487/TR.2012.28.2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izadi N, Brar KK, Lanser BJ. Evaporative coolers are not associated with dust mite or mold sensitization in a large pediatric cohort. Annals of Allergy, Asthma & Immunology. 2018;120(5):542–3. [DOI] [PubMed] [Google Scholar]
- 72.Diepgen TL, Blettner M. Analysis of familial aggregation of atopic eczema and other atopic diseases by ODDS RATIO regression models. Journal of investigative dermatology. 1996;106(5):977–81. 10.1111/1523-1747.ep12338475 [DOI] [PubMed] [Google Scholar]
- 73.Nam SY, Yoon HS, Kim W. Prevalence of Allergic Disease in Kindergarten Age Children in Korea. Pediatric allergy and respiratory disease. 2005;15(4):439–45. [Google Scholar]
- 74.Peroni D, Piacentini G, Bodini A, Rigotti E, Pigozzi R, Boner A. Prevalence and risk factors for atopic dermatitis in preschool children. British Journal of Dermatology. 2008;158(3):539–43. 10.1111/j.1365-2133.2007.08344.x [DOI] [PubMed] [Google Scholar]
- 75.Kim HY, Jang EY, Sim JH, Kim JH, Chung Y, Hwang EM, et al. Effects of Family History on the Occurrence of Atopic Dermatitis in Infants. Pediatric allergy and respiratory disease. 2009;19(2):106–14. [Google Scholar]
- 76.Alsowaidi S, Abdulle A, Bernsen R, Zuberbier T. Allergic rhinitis and asthma: a large cross-sectional study in the United Arab Emirates. International archives of allergy and immunology. 2010;153(3):274–9. 10.1159/000314368 [DOI] [PubMed] [Google Scholar]
- 77.Chinratanapisit S, Suratannon N, Pacharn P, Sritipsukho P, Vichyanond P. Prevalence and risk factors of allergic rhinitis in children in Bangkok area. Asian Pacific journal of allergy and immunology. 2018. [DOI] [PubMed] [Google Scholar]
- 78.Chen S, Li Z, Zhou L, Zhang Y. Analysis on correlation between single nucleotide polymorphisms of vitamin D receptor gene with susceptibility to allergic rhinitis. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2019;33(5):402–6. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Li J, Zhao Y, Wang C, Zhang L. Identification of rare variants of allergic rhinitis based on whole genome sequencing and gene expression profiling: A preliminary investigation in four families. World Allergy Organization Journal. 2019;12(6):100038. 10.1016/j.waojou.2019.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al., editors. International consensus statement on allergy and rhinology: allergic rhinitis. International forum of allergy & rhinology; 2018: Wiley Online Library. [DOI] [PubMed]
- 81.Tatar EÇ, Sürenoğlu ÜA, Saylam G, Işık E, Özdek A, Korkmaz H. Is there any correlation between the results of skin-prick test and the severity of symptoms in allergic rhinitis? American journal of rhinology & allergy. 2012;26(1):e37–e9. 10.2500/ajra.2012.26.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung YG, Cho H-J, Park GY, Min J-Y, Kim HY, Dhong H-J, et al. Comparison of the skin-prick test and Phadia ImmunoCAP as tools to diagnose house-dust mite allergy. American journal of rhinology & allergy. 2010;24(3):226–9. 10.2500/ajra.2010.24.3459 [DOI] [PubMed] [Google Scholar]
- 83.Ni J, Friedman H, Boyd BC, McGurn A, Babinski P, Markossian T, et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC pediatrics. 2019;19(1):225. 10.1186/s12887-019-1594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eneli I, Sadri K, Camargo C Jr, Barr RG. Acetaminophen and the risk of asthma: the epidemiologic and pathophysiologic evidence. Chest. 2005;127(2):604–12. 10.1378/chest.127.2.604 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
(SPS)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.