Introduction
The innate immune system is essential for recognizing foreign invaders and engaging a cellular and physiological response to control infection. Most human pathogenic fungi are opportunistic and take advantage of immune system defects. The 2 pathogens responsible for the majority of fungal infections in humans, Candida albicans and Aspergillus fumigatus, are no exception. One of the major components of the innate immune system that has critical roles in fighting microbial infections, including fungal infections, is the inflammasome [1,2]. Inflammasomes are multimeric protein platforms that lead to inflammatory caspase-1 activation, which in turn drives proteolytic maturation of proinflammatory cytokines IL-1β and IL-18. Processing of IL-1β and IL-18 could also be inflammasome-independent during infections due to host intrinsic proteases, and this has been extensively reviewed elsewhere [3]. Active caspase-1 also parallelly induces the cleavage and activation of gasdermin D (GSDMD), which forms pores in the plasma membrane, leading to a lytic form of inflammatory cell death called pyroptosis and facilitating the extracellular release of IL-1β and IL-18 [4,5]. To date, multiple innate sensors with the potential to assemble inflammasome complexes have been identified, including the nucleotide-binding domain and leucine-rich repeat receptors (NLRs) NLRP1, NLRC4, and NLRP3 as well as absent in melanoma 2 (AIM2) and pyrin [1].
Inflammasomes are activated when the sensor recognizes pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). In the context of fungal infection, polysaccharides, which are the major component of the fungal cell wall, serve as a large source of fungal PAMPs. Cell wall-purified zymosan and mannan were the first fungal ligands shown to activate the canonical NLRP3 inflammasome [6]. While several fungal species are now known to be capable of activating inflammasomes, including C. albicans, A. fumigatus, Cryptococcus neoformans, Paracoccidioides brasiliensis, Malassezia spp., and Microsporum canis, among others [7–13], the full range of fungal PAMPs responsible for inflammasome activation remains a key topic of study for several of these pathogens.
Growing understanding of the physiological importance of inflammasomes in antimicrobial defense has resulted in the rapid exploration of fungi-induced inflammasome activation [6,14,15]. Initial studies focused on the role of the IL-1 family cytokines IL-1β and IL-1α in restricting the fungal pathogen C. albicans during systemic infection have provided convincing evidence for the role of inflammasomes in antifungal immunity [14]. Similarly, mice lacking IL-1β and/or IL-18, the 2 inflammasome-dependent effector cytokines, are susceptible to aspergillosis [15]. Here, we focus on the molecular mechanisms of inflammasome activation and its importance for host defense in the context of the 2 most deadly fungi in humans: C. albicans, a dimorphic yeast, and A. fumigatus, a filamentous fungus.
Candida albicans
Molecular mechanisms of inflammasome regulation
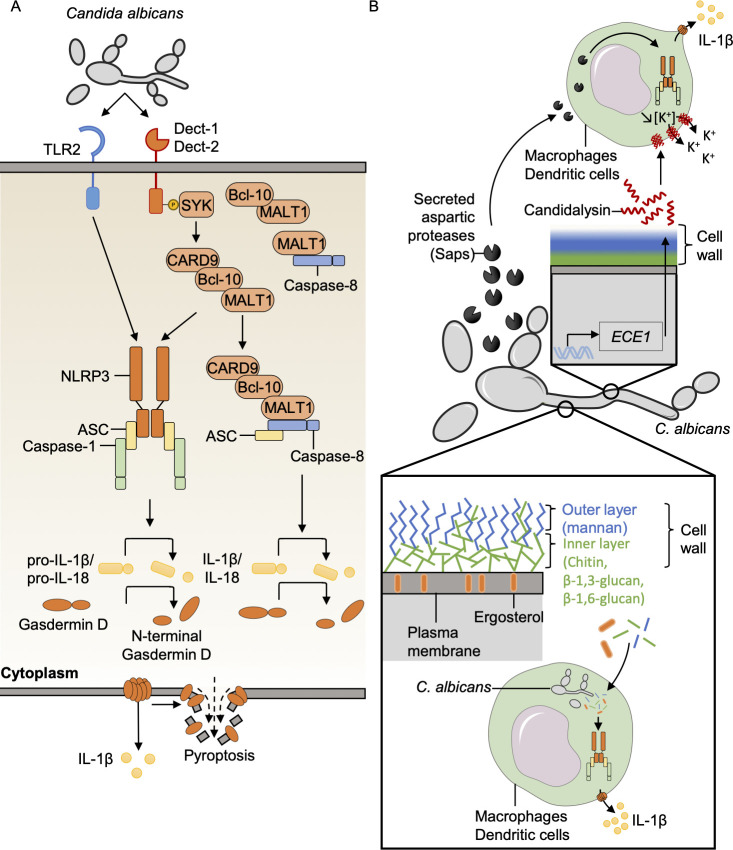
In vitro, C. albicans induces caspase-1 cleavage and IL-1β release in an NLRP3– and caspase-1– dependent manner in macrophages and dendritic cells (DCs) (Fig 1A) [16–19]. Fungi-induced inflammasome activation also requires the C-type lectin receptor (CLR) pathway. In particular, the Dectin-1-SYK-CARD9 signaling axis is critical to activate caspase-1, leading to the release of IL-1β and IL-18 (Fig 1A) [16,18,20–25]. Both Toll-like receptor (TLR) and CLR innate immune pathways contribute to fungal recognition and the cellular signaling necessary for priming and activation of the NLRP3 inflammasome (Fig 1A) [22]. However, priming with an exogenous ligand does not rescue inflammasome activation in Dectin1−/− bone marrow-derived DCs (BMDCs), suggesting a priming-independent role for the CLR pathway in inflammasome activation during Candida infection [26].
Fig 1. Mechanisms of C. albicans-induced inflammasome activation.
(A) C. albicans is recognized by TLRs and Dect receptors to mediate efficient priming and inflammasome activation. The CLR pathway is activated via the adaptor protein SYK, and the downstream supramolecular complex CARD9–Bcl-10–MALT1 drives the recruitment of caspase-8/ASC and/or NLRP3/ASC/caspase-1 to cleave gasdermin D and process and release IL-1β. (B) PAMPs and toxins from C. albicans that can activate the inflammasome have been identified. The C. albicans Saps mediate inflammasome activation. During germination of C. albicans, ECE1 regulates candidalysin toxin production and secretion; candidalysin forms pores in the host cell membrane and induces potassium efflux to cause canonical NLRP3 inflammasome activation. The cell wall, composed of an inner layer (Chitin/β-1,3-glucan/β-1,6-glucan) and an outer layer (mannan), participates in inflammasome activation. ASC, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; Bcl-10, B-cell lymphoma/leukemia 10; CARD9, caspase activation and recruitment domain-containing protein 9; CLR, C-type lectin receptor; Dect, Dectin; IL-1β, interleukin 1 beta; IL-18, interleukin 18; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; NLRP3, nucleotide-binding domain and leucine-rich repeat family pyrin domain-containing 3; PAMP, pathogen-associated molecular pattern; Saps, secreted aspartic proteases; SYK, spleen tyrosine kinase; TLR, Toll-like receptor.
Dectin-1 signaling during treatment with the fungal PAMP curdlan in vitro induces the formation of a Dectin-1/SYK downstream complex composed of CARD9, Bcl-10, MALT1, and caspase-8 [20]. Caspase-8 is then necessary to recruit and interact with ASC and process pro–IL-1β (Fig 1A) [20]. Moreover, before fungal recognition and inflammasome activation, MALT1 constitutively forms 2 independent complexes, one with Bcl-10 and one with caspase-8; whether these constitutive complexes have specific function requires further investigation (Fig 1A) [20]. Recently, the innate immune sensor Z-DNA binding protein 1 (ZBP1) has been shown to be a fungal sensor during C. albicans infection to activate the inflammasome and PANoptosis, a unique inflammatory programmed cell death pathway regulated by the PANoptosome complex (Fig 2) [27]. The Zα2 domain of ZBP1 is crucial for triggering the activation of cell death pathways, but the fungal ligand remains unknown [27].
Fig 2. Graphical representation of PANoptosome/PANoptosis.
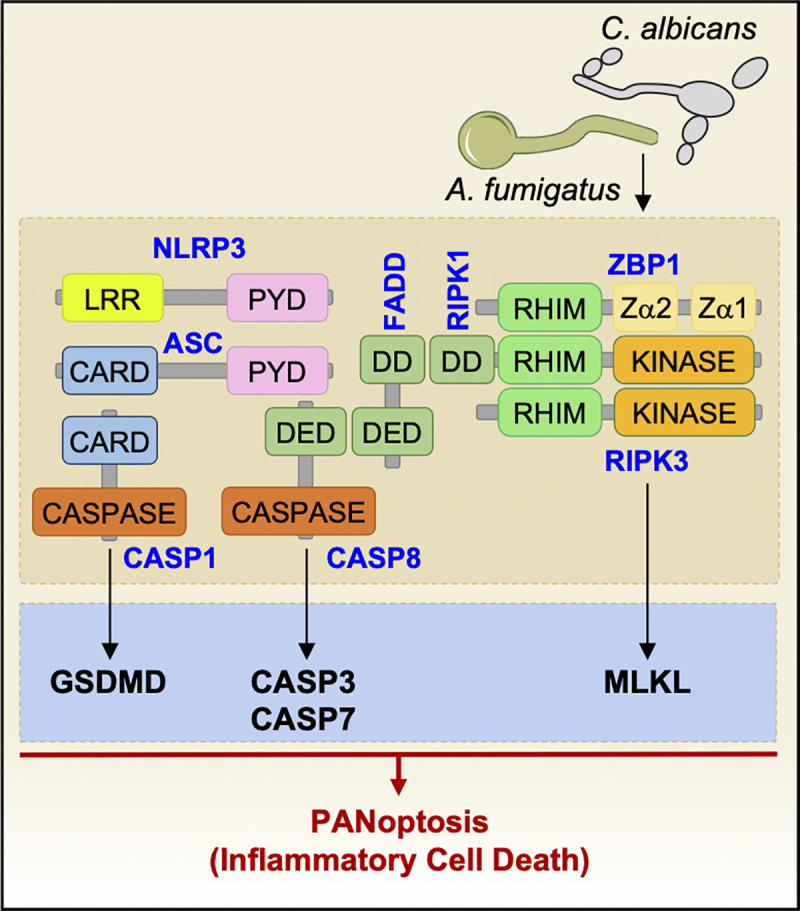
ZBP1 senses C. albicans and A. fumigatus infection to mediate PANoptosome (ZBP1-RIPK1-RIPK3-FADD-CASP8) complex formation and drive PANoptosis, inflammatory cell death characterized by NLRP3/CASP1 activation (pyroptosis), CASP3/CASP7 activation (apoptosis), and MLKL activation (necroptosis). ASC, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; CARD, caspase activation and recruitment domain; CASP, caspase; DD, death domain; DED, death effector domain; FADD, fas-associated death domain protein; GSDMD, gasdermin D; LRR, leucine-rich repeat; MLKL, mixed lineage kinase domain-like pseudokinase; NLRP3, nucleotide-binding domain and leucine-rich repeat family pyrin domain-containing 3; PYD, pyrin domain; RHIM, receptor-interacting protein homotypic interaction motif; RIPK, receptor-interacting serine/threonine kinase; ZBP1, Z-DNA-binding protein 1.
Several components of C. albicans have been shown to induce inflammasome activation. The C. albicans secreted aspartic proteases (Saps), Sap2 and Sap6, induce canonical activation of the NLRP3 inflammasome (Fig 1B) [28,29]. Fungal strains lacking Saps induce significantly less neutrophil influx and IL-1β release [30]. Candidalysin, a cytolytic peptidic toxin secreted from the hyphal form of C. albicans, induces cell damage and death and canonical NLRP3 inflammasome activation [31–35]. Candidalysin forms pores in the plasma membrane of macrophages and DCs to drive potassium efflux-mediated inflammasome activation [31]. A fungal strain lacking ECE1 (ece1Δ/Δ), which is defective in the production of candidalysin, causes reduced IL-1β release in human macrophages but not in murine cells [31]. However, purified candidalysin acts similarly on both human and mouse cells [31]. Endocytosis of the toxin is required to induce potassium efflux and subsequent NLRP3 inflammasome activation, but candidalysin induces pyroptosis in a caspase-1–independent manner [31]. Interestingly, the ece1Δ/Δ strain induces significantly less IL-1β release compared to the parental strain, which results in a failure to control the fungal burden during central nervous system infection with the mutant in mice [36].
In addition to the secreted molecules that mediate inflammasome activation, C. albicans cell wall components are also important drivers. C. albicans hyphae have been reported to contribute to inflammasome activation [17]. However, other studies showed hyphal switching is not essential [26,37]. Indeed, it has been shown that β-(1,3)-glucan present on the yeast form in the inner layer of the cell wall can activate the inflammasome (Fig 1B) [6,38,38–40], but fungal mutants deficient in glucan exposure are still able to induce pyroptosis [41]. The cell surface localization of ergosterol is also critical for pyroptosis, and the presence of O-mannoproteins is required for efficient exposition of ergosterol on the C. albicans surface (Fig 1B) [42]. The importance of hyphal switching remains to be fully established. Overall, C. albicans induces inflammasome activation through both secreted and cell surface molecules, which has important implications for a protective host response.
C. albicans mediating cell death
C. albicans induces GSDMD cleavage and pyroptosis in bone marrow-derived macrophages (BMDMs) (Fig 1A) [27,38,41]. Interestingly, C. albicans induces 2 sequential types of cell death. During the initial stage of infection, the yeast predominantly drives pyroptotic cell death downstream of caspase-1 [38]. Then, a second phase of caspase-1–independent cell death occurs [38,41]. It is proposed that in 2 concomitant mechanisms, hyphae physically rupture the cell membrane while fungal growth consumes the glucose from the host cells that is necessary for them to survive, finally permitting the escape of C. albicans from macrophages [38,41,43]. Furthermore, C. albicans induces PANoptosis, characterized by caspase-1 activation (pyroptosis), caspase-8, caspase-3, and caspase-7 activation (apoptosis), and MLKL phosphorylation (necroptosis), in a ZBP1-dependent manner [27].
Inflammasomes in host resistance to C. albicans
Mice lacking the inflammasome components NLRP3, ASC, and caspase-1 are highly susceptible to systemic candidiasis [16,18,24]. In addition to NLRP3, NLRC4 has also been shown to play antifungal roles in the stromal compartment during mucosal infection [16,44]. During vulvovaginal candidiasis, the NLRP3 inflammasome is required to control C. albicans dissemination [30,45]. However, in an oral mucosal model, NLRC4 is necessary to dampen the detrimental proinflammatory effect of NLRP3 activation. NLRC4 is activated in response to IL-22 and secretes the anti-inflammatory cytokine IL-1ra to protect against detrimental inflammation induced by NLRP3 [45]. Whether intrinsic C. albicans or microbiota pattern components regulate and activate NLRC4 during fungal mucosal infection requires further investigation. C. albicans also triggers inflammasome activation and bioactivation of IL-1β in microglial cells, which in turn promotes CXCL1-neutrophil–mediated control of the fungal infection [36]. Additionally, inflammasome activation in microglia requires the CARD9–Bcl-10–MALT1 complex downstream of C-type lectins [36,46]. Overall, it is clear that inflammasome activation plays an important role in host defense against C. albicans infection and that balancing this activation is critical to prevent hyperinflammation.
Aspergillus fumigatus
Molecular mechanisms of inflammasome regulation
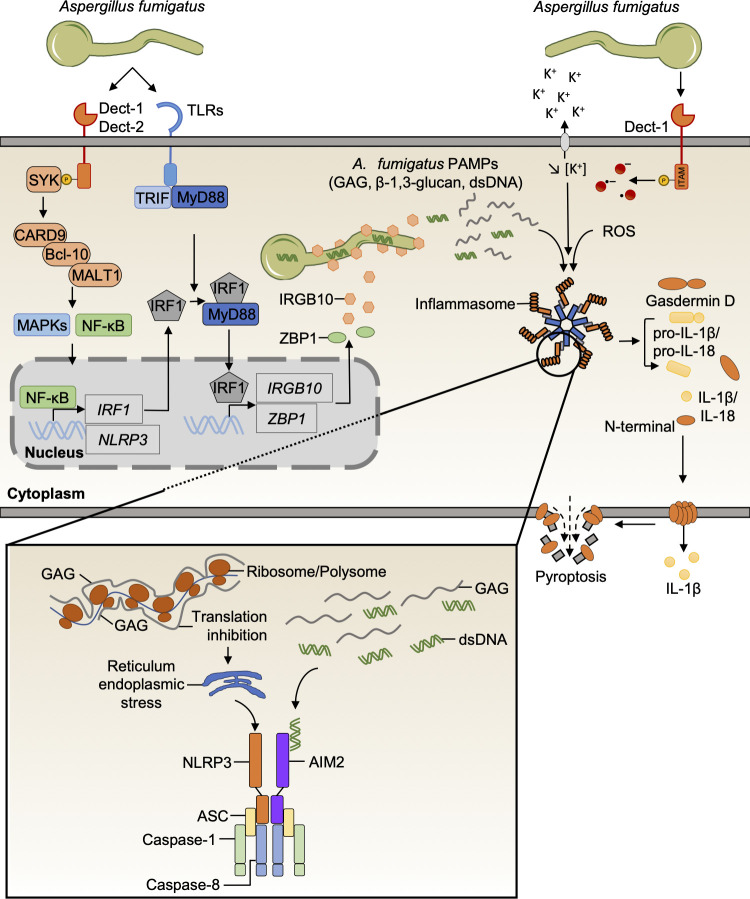
A. fumigatus is a filamentous fungus that triggers inflammasome activation in an NLRP3-dependent manner in macrophages and DCs [15,47]. Aspergillus-induced NLRP3 inflammasome activation in vitro is dependent on potassium efflux and reactive oxygen species (ROS) production, but not caspase-11 (Fig 3) [15,47]. However, unlike C. albicans, A. fumigatus requires the AIM2 receptor for an efficient inflammasome response in murine DCs [15]. Interestingly, NLRP3 and AIM2 are redundant and simultaneously present in the same inflammasome complex with the adaptor ASC, caspase-1, and also caspase-8 (Fig 3) [15,20]. Moreover, caspase-8 and FADD drive caspase-1 activation and IL-1β release in BMDCs [15].
Fig 3. Mechanisms of A. fumigatus-induced inflammasome activation.
A. fumigatus hyphae induce canonical inflammasome activation after recognition by the CLR and TLR pathways, which is dependent on ROS production and potassium efflux. SYK activation initiates the formation of a supramolecular CARD9–Bcl-10–MALT1 complex to drive MAPKs and NF-κB signaling and induce inflammasome priming. Parallelly, SYK regulates ROS production to mediate the canonical activation of the NLRP3 inflammasome. The NF-κB pathway regulates the expression of IRF1, and the TLR pathway mediates efficient activation of IRF1 after recognition of A. fumigatus via the adaptor molecules MyD88 and TRIF. IRF1 expression and activation induce IRGB10 and ZBP1 expression. IRGB10 targets the fungal cell surface and causes hyphae damage, inhibiting A. fumigatus growth and releasing GAG, β-1,3-glucan, and dsDNA to activate the inflammasome. GAG interacts with ribosomes to block translation, inducing ER stress and NLRP3 inflammasome activation, whereas A. fumigatus dsDNA is recognized by the AIM2 inflammasome. ASC, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain; Bcl-10, B-cell lymphoma/leukemia 10; CARD9, caspase activation and recruitment domain-containing protein 9; CLR, C-type lectin receptor; Dect, dectin; ER, endoplasmic reticulum; GAG, galactosaminogalactan; IL-1β, interleukin 1 beta; IL-18, interleukin 18; IRGB10, immunity-related GTPase B10; IRF1, interferon regulatory factor 1; ITAM, immunoreceptor tyrosine-based activation motif; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MAPKs, mitogen activated protein kinases; NF-κB, nuclear factor kappa B; NLRP3, nucleotide-binding domain and leucine-rich repeat family pyrin domain-containing 3; MyD88, myeloid differentiation primary response 88; PAMPs, pathogen-associated molecular patterns; ROS, reactive oxygen species; SYK, spleen tyrosine kinase; TLR, Toll-like receptor; TRIF, Toll/interleukin 1 receptor domain-containing adapter-inducing interferon-β; ZBP1, Z-DNA-binding protein 1.
In contrast to macrophages and DCs, in vitro, neutrophils require caspase-11 for caspase-1 activation and IL-1β release in response to A. fumigatus [15,48,49]. It will be interesting to investigate whether the caspase-11/gasdermin axis drives secondary NLRP3 inflammasome activation in neutrophils.
A. fumigatus is sensed by both TLRs and CLRs, which together orchestrate an effective innate immune response to this pathogen (Fig 3) [50]. Indeed, chemical inhibition or genetic deletion of the CLR pathway abrogates Aspergillus-induced caspase-1 activation and IL-1β secretion [20,40,47,49]. Activation of the CLR pathway induces the formation of a CARD9–Bcl-10–MALT1–caspase-8 complex for inflammasome activation [20,40]. Perturbation of this complex also directly affects NF-κB signaling and, hence, expression of the NLRP3 inflammasome (Fig 3) [40]. Future studies should help us understand priming-independent functions of this signalosome complex in promoting the activation of NLRP3–AIM2–ASC–caspase-1 inflammasome complexes in response to Aspergillus and other fungal pathogens.
The interferon pathway is also essential for inflammasome activation and particularly for the expression of the transcription factor IRF1 and IRGB10 [51,52]. Fungi-induced CLR pathways drive the expression of IRF1, and cells lacking IRF1 are defective in inflammasome activation in response to A. fumigatus (Fig 3) [40,51,53]. IRF1 is activated during A. fumigatus recognition through the TLR pathway (Fig 3) [40,54]. During A. fumigatus infection, the immunity-related GTPase IRGB10 targets the fungal cell wall to facilitate the release of PAMPs that, in turn, drive inflammasome activation (Fig 3) [40]. Similarly, ZBP1, an IRF1-dependent protein, is also required for A. fumigatus-mediated inflammasome activation (Figs 2 and 3) [27]. However, the A. fumigatus ligand recognized by the sensor ZBP1 is unknown and requires further studies.
Galactosaminogalactan (GAG) was recently identified as a specific A. fumigatus PAMP that activates the NLRP3 inflammasome [55]. GAG is a polysaccharide present at the surface of the A. fumigatus cell wall and is secreted [56]. Mechanistically, the phagocytosed conidia grow and synthesize GAG. The galactosamine moiety then interacts with ribosomes by charge–charge interactions [55]. GAG immobilizes the ribosome and polysome to inhibit translation, which induces endoplasmic reticulum stress and triggers NLRP3 inflammasome activation [55]. β-(1,3)-glucan in the cytosol has also been shown to directly activate the inflammasome in macrophages [40], but the exact mechanism is unclear. Future studies should address whether there is a general mechanism for polysaccharide sensing and what role, if any, the biochemical properties of the specific polymers play in inducing inflammasome activation and/or priming. Further studies will allow for the discovery of new cytosolic sensors and inflammasome activation mechanisms.
Aspergillus mediating cell death
Pyroptosis has not been well characterized during A. fumigatus infection. The cleavage of GSDMD was recently identified in macrophages during A. fumigatus infection (Fig 3). Additionally, similar to what has been observed with C. albicans, A. fumigatus induces PANoptosis in a ZBP1-dependent manner (Fig 2) [27]. It has previously been assumed that hyphal growth induces cell death through physical damage, but it was recently demonstrated that A. fumigatus hyphae form a network through the bronchial epithelium which forms an actin gate and permits hyphae to pierce the plasma membrane without inducing cell death [57]. It will be interesting to determine if similar mechanisms exist in Aspergillus-infected macrophages and DCs.
Inflammasomes in host resistance to A. fumigatus
A. fumigatus often acts as a pulmonary pathogen which infects immunocompromised patients and induces varying pathologies, the most grave being invasive pulmonary aspergillosis (IPA) [58]. Loss of both NLRP3 and AIM2 inflammasome components in mice results in susceptibility to IPA [15,48]. Both inflammasomes are required in hematopoietic and stromal compartments to protect against fungal infection [15]. Although NLRC4 was shown to be protective in a mucosal model of C. albicans infection [44,45], its role during IPA is not known. IRGB10-deficient mice, which are defective in caspase-1 activation and IL-1β release, were also shown to be susceptible to infection [40]. Furthermore, A. fumigatus that overproduce GAG and enhance inflammasome activation elicit more protective responses from the host and improve survival compared with wild-type A. fumigatus, while a fungal strain that is deficient in GAG has increased virulence during systemic infection [55]. Similarly, in a model of colitis, an inflammatory disease where inflammasome activation preserves colon homeostasis, GAG treatment protects mice by inducing inflammasome activation and IL-18 release [55,59]. Collectively, these findings highlight the importance of GAG in inflammasome activation and in host protection during A. fumigatus infection.
Beyond canonical inflammasome activation, mice lacking caspase-11 do not control the fungal burden in an Aspergillus keratitis infection model or in IPA [48,49]. Moreover, mice lacking both caspase-1 and caspase-11 are more susceptible than caspase-1–deficient mice. Caspase-11 is required for inflammasome activation only in neutrophils in response to A. fumigatus [48,49]. Since neutrophils are essential to fight aspergillosis, it is important to further understand the role of caspase-11 in inflammasome-dependent and inflammasome-independent functions of neutrophils. Whether A. fumigatus activates caspase-11 through an LPS-independent mechanism or via host microbiome–mediated cell death needs to be explored further.
Discussion
The central role of inflammasomes in health and disease has triggered enormous interest in this area of research over the last decade and continues to entice scientists. However, study of the role and regulation of inflammasomes in fungal infections is still in its infancy, and several important questions remain unanswered. These unexplored areas include the role of interferons (IFNs) in inflammasome regulation during fungal infection. Type I IFN (IFN-I) acts as a central regulator of inflammasome activation in bacterial and viral infections [60], but its role in fungal infections is poorly understood and currently controversial [61]. The IFN-I receptor IFNAR1 is required for inflammasome activation in neutrophils infected with A. fumigatus [49], but no evidence has been found to support this function in macrophages or DCs. Additionally, during C. albicans infection, Saps mediate IFN-I secretion for caspase-11 activation and cooperate with NLRP3 inflammasome activation [62]. However, IFN-I has also been shown to inhibit inflammasome activation and IL-1β production in response to C. albicans [63]. It is not clear why opposing mechanism are observed. Is this phenomenon specific to C. albicans or general to a range of fungal pathogens? Is IFN-I required for caspase-11–mediated NLRP3 inflammasome activation during fungal infection? Further investigation into the role of IFNs in the regulation of inflammasomes during fungal infection is necessary.
The inflammasomes induced by fungi require caspase-8 in addition to caspase-1. Caspase-8 is recruited to the NLRP3 inflammasome complex and is necessary for pro–IL-1β processing [15,20,64]. Indeed, it has been observed that caspase-8 can process the pro-form of IL-1β independent of caspase-1 in a dectin-1–dependent manner [20]. Recently, the link between pyroptosis, apoptosis, and necroptosis (PANoptosis) has been highlighted [27,65]. It will be interesting to further study the supramolecular complex, the PANoptosome, formed in response to fungi to understand whether it is dependent on the C-type lectin pathway.
Interestingly, B lymphocytes (B cells), T cells, and T regulatory cells have a CARD11, Bcl-10, MALT1 (CBM) signalosome complex downstream of the B cell receptor (BCR) and T-cell receptor (TCR) that is similar to the complex formed in the CLR pathway necessary for fungal recognition and inflammasome activation [66]. Mutations causing inhibition or aberrant activation of the human CBM signalosome complex are associated with primary immunodeficiency diseases, cancers, and infectious diseases [66]. Because B cells and T cells express all of the inflammasome components [67–69], it will be interesting to study the interaction between CBM complex and inflammasome components in lymphocytes. These findings may complement the mechanisms of fungi-induced inflammasome activation observed in myeloid cells.
Additionally, recognition of bacterial and viral ligands in the cytosol by the inflammasome-forming innate sensors is well studied [60]; however, the fungal ligands triggering inflammasome activation are poorly characterized to date. β-(1,3)-glucan and mannan with ATP treatment are known to activate the canonical NLRP3 inflammasome, and recently, the presence of GAG or β-(1,3)-glucan in the cytosol was shown to directly activate NLRP3 in macrophages [6,40,55]. The mechanism of NLRP3 inflammasome activation has been elucidated for GAG but remains to be discovered for other fungal PAMPs.
Fungal polysaccharide-mediated inflammasome control has been found to be beneficial in multiple disease models. For example, GAG-mediated inflammasome activation is protective in a murine colitis model [55]. Additionally, β-(1,3)-glucan mediates reprogramming and training of innate immune cells [70], and β-(1,3)-glucan–reprogrammed macrophages have impaired canonical NLRP3 inflammasome activation [71]. When macrophages from patients with cryopyrin-associated autoinflammatory syndrome are treated with β-(1,3)-glucan, they have reduced caspase-1 activation and IL-1β release compared with untreated macrophages [71], which could be therapeutically beneficial for these patients. These finding should open new perspectives in studies focused on the ability of fungal polysaccharides to modulate inflammasome activation as potential therapeutic strategies. Indeed, the inflammasome can have both beneficial and detrimental roles, and the fine-tuning of its activity is crucial during disease to activate protective inflammatory cascades during C. albicans or A. fumigatus infections while avoiding detrimental inflammation [45,72,73]. Targeting inflammasome activity could have important clinical applications to enhance the immune response during fungal infection or to reduce its effects when needed. The clinical relevance of inflammasomes during fungal infection is still undercharacterized, and further clinical studies should help to decipher this role.
The role of inflammasomes during fungal infection is crucial in host resistance. Improving our understanding of this specific regulation may lead to the discovery of novel fungi-specific mechanisms distinct from bacteria or viruses and new drug targets to treat fungal infections, which remain difficult to eradicate.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
Work from the Kanneganti laboratory is supported by funding from the National Institutes of Health (grants AI101935, AI124346, AR056296, and CA253095 to T.-D.K.) and the American Lebanese Syrian Associated Charities (to T.-D.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Man SM, Kanneganti T-D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Veerdonk FL, Joosten LAB, Netea MG. The interplay between inflammasome activation and antifungal host defense. Immunol Rev. 2015;265: 172–180. 10.1111/imr.12280 [DOI] [PubMed] [Google Scholar]
- 3.Netea MG, van de Veerdonk FL, van der Meer JWM, Dinarello CA, Joosten LAB. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33: 49–77. 10.1146/annurev-immunol-032414-112306 [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 5.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 6.Lamkanfi M, Malireddi RKS, Kanneganti T-D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–81. 10.1074/jbc.M109.023689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C, et al. Internalized Cryptococcus neoformans activates the canonical caspase-1 and the noncanonical caspase-8 inflammasomes. J Immunol. 2015;195:4962–72. 10.4049/jimmunol.1500865 [DOI] [PubMed] [Google Scholar]
- 8.Kistowska M, Fenini G, Jankovic D, Feldmeyer L, Kerl K, Bosshard P, et al. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp Dermatol. 2014;23:884–9. 10.1111/exd.12552 [DOI] [PubMed] [Google Scholar]
- 9.Guo C, Chen M, Fa Z, Lu A, Fang W, Sun B, et al. Acapsular Cryptococcus neoformans activates the NLRP3 inflammasome. Microbes Infect. 2014;16:845–54. 10.1016/j.micinf.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 10.Tavares AH, Magalhães KG, Almeida RDN, Correa R, Burgel PH, Bocca AL. NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis. 2013;7:e2595. 10.1371/journal.pntd.0002595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao L, Zhang L, Li H, Chen W, Wang H, Wu S, et al. Pathogenic fungus Microsporum canis activates the NLRP3 inflammasome. Infect Immun. 2014;82:882–92. 10.1128/IAI.01097-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feriotti C, Bazan SB, Loures FV, Araújo EF, Costa TA, Calich VLG. Expression of dectin-1 and enhanced activation of NALP3 inflammasome are associated with resistance to paracoccidioidomycosis. Front Microbiol. 2015;6. 10.3389/fmicb.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amorim BC, Pereira-Latini AC, de Assis Golim M, Ruiz Júnior RL, Bok Yoo HH, Parreira de Arruda MS, et al. Enhanced expression of NLRP3 inflammasome components by monocytes of patients with pulmonary paracoccidioidomycosis is associated with smoking and intracellular hypoxemia. Microbes Infect. 2019. [cited 2019 Dec 9]. 10.1016/j.micinf.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JWM, Kullberg BJ. Endogenous interleukin (IL)–1α and IL-1β Are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193: 1419–1426. 10.1086/503363 [DOI] [PubMed] [Google Scholar]
- 15.Karki R, Man SM, Malireddi RKS, Gurung P, Vogel P, Lamkanfi M, et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17:357–68. 10.1016/j.chom.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–6. 10.1038/nature07965 [DOI] [PubMed] [Google Scholar]
- 17.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting Edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–81. 10.4049/jimmunol.0901323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–97. 10.1016/j.chom.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, et al. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal β-glucan. J Immunol. 2009;183:8061–7. 10.4049/jimmunol.0902477 [DOI] [PubMed] [Google Scholar]
- 20.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–54. 10.1038/ni.2222 [DOI] [PubMed] [Google Scholar]
- 21.Brown GD, Gordon S. A new receptor for β-glucans. Nature. 2001;413:36–7. 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- 22.Brown GD, Willment JA, Whitehead L. C-type lectins in immunity and homeostasis. Nat Rev Immunol. 2018;18:374–89. 10.1038/s41577-018-0004-8 [DOI] [PubMed] [Google Scholar]
- 23.Cheng S-C, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90:357–66. 10.1189/jlb.1210702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Veerdonk FL, Joosten LAB, Shaw PJ, Smeekens SP, Malireddi RKS, van der Meer JWM, et al. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur J Immunol. 2011;41:2260–8. 10.1002/eji.201041226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Veerdonk FL, Joosten LAB, Devesa I, Mora-Montes HM, Kanneganti T-D, Dinarello CA, et al. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1β production by the fungal pathogen Candida albicans. J Infect Dis. 2009;199:1087–96. 10.1086/597274 [DOI] [PubMed] [Google Scholar]
- 26.Ganesan S, Rathinam VAK, Bossaller L, Army K, Kaiser WJ, Mocarski ES, et al. Caspase-8 modulates dectin-1 and complement receptor 3–driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193:2519–30. 10.4049/jimmunol.1400276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S, et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem. 2020:jbc.RA120.015924. 10.1074/jbc.RA120.015924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrella D, Rachini A, Pandey N, Schild L, Netea M, Bistoni F, et al. The inflammatory response induced by aspartic proteases of Candida albicans Is independent of proteolytic activity. Infect Immun. 2010;78:4754–62. 10.1128/IAI.00789-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, et al. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol. 2013;43:679–92. 10.1002/eji.201242691 [DOI] [PubMed] [Google Scholar]
- 30.Bruno VM, Shetty AC, Yano J, Fidel PL, Noverr MC, Peters BM. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. MBio. 2015:6. 10.1128/mBio.00182-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasper L, König A, Koenig P-A, Gresnigt MS, Westman J, Drummond RA, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9:1–20. 10.1038/s41467-017-02088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogiers O, Frising UC, Kucharíková S, Jabra-Rizk MA, Loo G, van Dijck PV, et al. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. MBio. 2019;10:e02221–18. 10.1128/mBio.02221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–8. 10.1038/nature17625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naglik JR, Gaffen SL, Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol. 2019;52:100–9. 10.1016/j.mib.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowes DJ, Hevener KE, Peters BM. The second-generation anti-diabetic sulfonylureas inhibit Candida albicans and Candidalysin mediated activation of the NLRP3 inflammasome. Antimicrob Agents Chemother. 2019. [cited 9 Dec 2019]. 10.1128/AAC.01777-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond RA, Swamydas M, Oikonomou V, Zhai B, Dambuza IM, Schaefer BC, et al. CARD9 + microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20:559–70. 10.1038/s41590-019-0377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wellington M, Koselny K, Krysan DJ. Candida albicans morphogenesis is not required for macrophage interleukin 1β production. MBio. 2013:4. 10.1128/mBio.00433-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uwamahoro N, Verma-Gaur J, Shen H-H, Qu Y, Lewis R, Lu J, et al. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. MBio. 2014;5. 10.1128/mBio.00003-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camilli G, Eren E, Williams DL, Aimanianda V, Meunier E, Quintin J. Impaired phagocytosis directs human monocyte activation in response to fungal derived β-glucan particles. Eur J Immunol. 10.1002/eji.201747224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briard B, Karki R, Malireddi RKS, Bhattacharya A, Place DE, Mavuluri J, et al. Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat Microbiol. 2019;4:316–27. 10.1038/s41564-018-0298-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell. 2014;13:329–40. 10.1128/EC.00336-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koselny K, Mutlu N, Minard AY, Kumar A, Krysan DJ. Wellington M. A genome-wide screen of deletion mutants in the filamentous Saccharomyces cerevisiae background identifies ergosterol as a direct trigger of macrophage pyroptosis. MBio. 2018;9:e01204–18. 10.1128/mBio.01204-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucey TM, Verma J, Harrison PF, Snelgrove SL, Lo TL, Scherer AK, et al. Glucose homeostasis is important for immune cell viability during Candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27: 988–1006.e7. 10.1016/j.cmet.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, et al. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;e1002379:7. 10.1371/journal.ppat.1002379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borghi M, De Luca A, Puccetti M, Jaeger M, Mencacci A, Oikonomou V, et al. Pathogenic NLRP3 inflammasome activity during Candida infection is negatively regulated by IL-22 via activation of NLRC4 and IL-1Ra. Cell Host Microbe. 2015;18:198–209. 10.1016/j.chom.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 46.Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, et al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog. 2015;11:e1005293. 10.1371/journal.ppat.1005293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saïd-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE. 2010;5:e10008. 10.1371/journal.pone.0010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Man SM, Karki R, Briard B, Burton A, Gingras S, Pelletier S, et al. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci Rep. 2017;7:srep45126. 10.1038/s41598-017-00035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Abbondante S, Karmakar M, Carrion S de J, Che C, Hise AG, et al. Neutrophil caspase-11 Is required for cleavage of caspase-1 and secretion of IL-1β in Aspergillus fumigatus infection. J Immunol. 2018;201:2767–75. 10.4049/jimmunol.1701195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–88. 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 51.Man SM, Karki R, Malireddi RKS, Neale G, Vogel P, Yamamoto M, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16:467–75. 10.1038/ni.3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. Cell. 2016;167: 382–396.e17. 10.1016/j.cell.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuriakose T, Zheng M, Neale G, Kanneganti T-D. IRF1 is a transcriptional regulator of ZBP1 promoting NLRP3 inflammasome activation and cell death during influenza virus infection. J Immunol. 2018;200:1489–95. 10.4049/jimmunol.1701538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negishi H, Fujita Y, Yanai H, Sakaguchi S, Ouyang X, Shinohara M, et al. Evidence for licensing of IFN-γ-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. PNAS. 2006;103:15136–41. 10.1073/pnas.0607181103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Briard B, Fontaine T, Samir P, Place DE, Muszkieta L, Malireddi RKS, et al. Galactosaminogalactan activates the inflammasome to provide host protection. Nature. 2020:1–5. 10.1038/s41586-020-2996-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briard B, Muszkieta L, Latgé J-P, Fontaine T. Galactosaminogalactan of Aspergillus fumigatus, a bioactive fungal polymer. Mycologia. 2016;108:572–80. 10.3852/15-312 [DOI] [PubMed] [Google Scholar]
- 57.Fernandes J, Hamidi F, Leborgne R, Beau R, Castier Y, Mordant P, et al. Penetration of the human pulmonary epithelium by Aspergillus fumigatus hyphae. J Infect Dis. 2018;218:1306–13. 10.1093/infdis/jiy298 [DOI] [PubMed] [Google Scholar]
- 58.Latgé J-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 1999;12:310–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gresnigt MS, Bozza S, Becker KL, Joosten LAB, Abdollahi-Roodsaz S, van der Berg WB, et al. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of interleukin-1 receptor antagonist. PLoS Pathog. 2014;e1003936:10. 10.1371/journal.ppat.1003936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Man SM, Kanneganti T-D. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. 10.1111/imr.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. 10.1038/nri3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabrielli E, Pericolini E, Luciano E, Sabbatini S, Roselletti E, Perito S, et al. Induction of caspase-11 by aspartyl proteinases of Candida albicans and implication in promoting inflammatory response. Infect Immun. 2015;83:1940–8. 10.1128/IAI.02895-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–23. 10.1016/j.immuni.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 64.Gurung P, Anand PK, Malireddi RKS, Walle LV, Opdenbosch NV, Dillon CP, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–46. 10.4049/jimmunol.1302839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, et al. Identification of the PANoptosome: A molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10. 10.3389/fcimb.2020.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turvey SE, Durandy A, Fischer A, Fung S-Y, Geha RS, Gewies A, et al. The CARD11-BCL10-MALT1 (CBM) signalosome complex: Stepping into the limelight of human primary immunodeficiency. J Allergy Clin Immunol. 2014;134:276–84. 10.1016/j.jaci.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859–70. 10.1038/ni.3202 [DOI] [PubMed] [Google Scholar]
- 68.Ali MF, Dasari H, Van Keulen VP, Carmona EM. Canonical stimulation of the NLRP3 inflammasome by fungal antigens links innate and adaptive B-lymphocyte responses by modulating IL-1β and IgM production. Front Immunol. 2017;8. 10.3389/fimmu.2017.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Javanmard Khameneh H, Leong KWK, Mencarelli A, Vacca M, Mambwe B, Neo K, et al. The inflammasome adaptor ASC intrinsically limits CD4+ T-cell proliferation to help maintain intestinal homeostasis. Front Immunol. 2019:10. 10.3389/fimmu.2019.01566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Camilli G, Bohm M, Piffer AC, Lavenir R, Williams DL, Neven B, et al. β-Glucan–induced reprogramming of human macrophages inhibits NLRP3 inflammasome activation in cryopyrinopathies. J Clin Invest. 2020;130:4561–73. 10.1172/JCI134778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christgen S, Place DE, Kanneganti T-D. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020:1–13. 10.1038/s41422-020-0295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;e1004462:10. 10.1371/journal.ppat.1004462 [DOI] [PMC free article] [PubMed] [Google Scholar]