Abstract
Objective:
To investigate the natural history of coagulation factor perturbation after injury, and identify longitudinal differences in clotting factor repletion by RBC:FFP transfusion ratio.
Summary Background Data:
Hemostatic transfusion ratios of red blood cell (RBC) to fresh frozen plasma (FFP) approaching 1:1 are associated with a survival advantage in traumatic hemorrhage, even in patients with normal coagulation studies.
Methods:
Plasma was prospectively collected from 336 trauma patients during their ICU stay for up to 72h from 2/05 – 10/11. Standard coagulation studies as well as pro- and anticoagulant clotting factors were measured. RBC:FFP transfusion ratios were calculated at 6h after arrival, and dichotomized into ‘low ratio’ (RBC:FFP ≤1.5:1) and ‘high ratio’ (RBC:FFP >1.5:1) groups.
Results:
Factor level measurements from 193 non-transfused patients provide an early natural history of clotting factor level changes after injury. In comparison, 143 transfused patients had more severe injury, prolonged PT and PTT, and lower levels of both pro- and anticoagulants up to 24h. PTT was prolonged up to 12h and only returned to admission baseline at 48h in ‘high ratio’ patients, versus correction by 6h in ‘low ratio’ patients. Better repletion of Factors V, VIII, and IX was seen longitudinally, and both unadjusted and injury-adjusted survival was significantly improved in ‘low ratio’ versus ‘high ratio’ groups.
Conclusions:
Resuscitation with a ‘low ratio’ of RBC:FFP leads to earlier correction of coagulopathy, and earlier and prolonged repletion of some but not all procoagulant factors. This prospective evidence suggests hemostatic resuscitation as an interim standard of care for transfusion in critically injured patients pending the results of ongoing randomized study.
MINIABSTRACT
In traumatic hemorrhage, ‘low ratio’ transfusion with ratios of RBC:FFP approaching 1:1 leads to more effective repletion of specific coagulation factor deficits and improved injury-adjusted survival. This data provides prospectively-collected evidence suggesting that low ratio transfusion may have both coagulation dependent and independent mechanisms accounting for improved survival after trauma.
INTRODUCTION
Trauma remains a major cause of morbidity and mortality in both civilian and military populations, and the majority of preventable traumatic deaths occur as a result of hemorrhage. 1–3 While transfusion as a bridge to surgical hemostasis is an undisputed mainstay of therapy for hemorrhagic shock, the optimal practice of transfusion has changed in the last 10 years in response to the recognition of coagulopathy as a critical predictor of poor outcomes. Specifically, 25–38% of traumatically injured patients are found to have abnormal coagulation on arrival to the hospital, and patients with this acute traumatic coagulopathy (ATC) go on to be four times more likely to die.2, 4–6 Thus, a thorough understanding of optimal transfusion strategies to both treat the end-organ hypoperfusion of hemorrhagic shock as well as to correct (or prevent) coagulopathy are critical to the acute care of trauma patients. To this end, the practice of ‘hemostatic resuscitation’ – the empiric transfusion of plasma at a fixed ratio with red blood cells – has evolved over many years as a potential therapeutic strategy to address both goals.7–11
Multiple large retrospective studies have demonstrated that empiric transfusion of red blood cell (RBC) and fresh frozen plasma (FFP) units in RBC:FFP ratios approaching 1:1 are associated with a survival advantage in hemorrhagic shock after traumatic injury in both civilian12, 13 and military settings.14–16 Alongside clinical enthusiasm for this novel approach, however, exists significant concern regarding the survival bias inherent in such retrospective studies. 17, 18 One of the major barriers to interpreting the observed survival advantage associated with hemostatic resuscitation is the lack of a well-understood biological mechanism, making dynamic monitoring of efficacy difficult and meaningful clinical endpoints unclear. Two competing hypotheses exist: one primary hypothesis suggests that plasma-based resuscitation leads to better repletion of coagulation factors by earlier and higher-volume FFP transfusion. While this is clinically intuitive, there are currently no data examining the relationship of plasma-based resuscitation and coagulation factor repletion. Alternatively, others have suggested that earlier and increased plasma transfusion may modulate the inflammomodulatory cascade triggered by severe injury,19, 20 thus treating an ‘endotheliopathy’ of trauma. Evidence to support this hypothesis exists in the fact that the survival benefit to plasma-based resuscitation strategies is evident even in patients with normal-range coagulation studies on admission.21
To address these competing hypotheses, the first aim of this study was to investigate differences in longitudinal clotting factor levels in a critically-injured cohort of trauma patients who were not transfused, in order to establish an early natural history of coagulation after injury. The second aim was to examine longitudinal changes in coagulation profiles in transfused patients in relation to RBC:FFP transfusion ratios, assessed by dichotomizing patients into ‘low ratio’ (RBC:FFP ≤1.5:1) and ‘high ratio’ (RBC:FFP >1.5:1) groups. Overall, these data provide a novel and comprehensive assessment of changes in the clotting factor milieu after critical injury by describing an early natural history to coagulation abnormalities, as well as identifying the differential effects of transfusion strategies on the correction of these abnormalities over time.
METHODS
Patient Sample, Study Design, Clinical Data:
We performed a prospective cohort study of patients requiring highest-level trauma activation and subsequent intensive care unit (ICU) admission, beginning on arrival to the Emergency Department of San Francisco General Hospital from February 2005 to October 2011 as part of a longitudinal study examining perturbations in coagulation and inflammation after injury. Highest-level trauma activation was triggered by either pre-specified physiologic (at least one pre-hospital or hospital systolic blood pressure <90, heart rate >110, or Glasgow Coma Score ≤8) or anatomic criteria (penetrating torso trauma or evidence of high-energy blunt trauma), or the clinical discretion of pre-hospital providers, ED triage nurses, or attending physicians. ICU admission was at the discretion of the attending trauma surgeon; patients who died in the operating room or emergency department prior to ICU admission were also included. During the study period, 3,775 patients met criteria for highest-level trauma activation, with 2,066 subsequently admitted to the ICU and thus eligible for study enrollment. Of these, 289 patients were prospectively excluded for age less than 18 years old, incarceration, pregnancy, transfer from another hospital, or administration of >2 liters of crystalloid prior to initial blood draw. Patients were retrospectively excluded if they were found to be on warfarin or possessed a preexisting bleeding diathesis at the time of injury. Sample and data collection was performed for these 1,777 patients under a waiver of consent approved by the University of California Institutional Review Board. Consent was subsequently obtained from patients or appropriate surrogates in 535 cases; of these, 336 patients had serial clotting factor measurements performed and complete transfusion data available, constituting the final patient population for analysis.
As an observational study, the decision to transfuse and the specific array of blood products transfused were entirely at the discretion of the attending trauma surgeon. An institutional massive transfusion protocol exists, whose activation prompts delivery of ‘packs’ of 4U RBC / 4U FFP and prompts consideration of a pooled donor platelet ‘6-pack’ for platelets <100,000/mL and 2U pooled cryoprecipitate for fibrinogen <100mg/dL; protocol activation is at the discretion of the attending trauma surgeon or anesthesiologist. A minimum of 4U thawed AB plasma and 6U of type O blood are available in the blood bank at all times for immediate release. Adjunct hemostatic agents such as recombinant Factor VIIa (NovoSeven RT; Novo Nordisk Inc.; Plainsboro, NJ) and prothrombin complex concentrate (Bebulin VH; Baxter; Westlake Village, CA) were administered at the discretion of the attending trauma surgeon or anesthesiologist; no enrolled patient received tranexamic acid or other antifibrinolytic agent during the study period.
Our sample collection methodology has been described in detail previously.5, 22 Briefly, serial 10 mL samples of blood were drawn in citrated vacuum tubes upon arrival to the ED, and then serially at 6, 12, 24, 48, and 72 hours after admission to the ICU. Samples were immediately centrifuged, and plasma extracted and stored at –80°C; all analysis was performed by researchers blinded to all patient data. Levels of fibrinogen, the procoagulant Factors II, V, VII, VIII, IX, and X, the endogenous anticoagulants antithrombin III (AT III), Protein C, activated Protein C (aPC), and plasminogen activator inhibitor-1 (PAI-1) were assayed. Fibrinogen, Factors II, V, VII, VIII, IX, and X, antithrombin III, and Protein C were measured with a Stago Compact Coagulation Analyzer (Diagnostica Stago Inc.; Parsippany, NJ) in accordance with manufacturer instructions. Activated Protein C measurements were performed on samples collected in citrated tubes containing 10mM benzamidine using an enzyme capture assay described in detail elsewhere.5 PAI-1 measurements were performed with an enzyme-linked immunosorbent assay (eBiosciences; San Diego, CA).
Statistical Analysis:
All data are presented as mean ± standard deviation, median (inter-quartile range [IQR]), or percentage. The study population was subdivided based on two criteria: transfusion status and RBC:FFP ratio. First, we dichotomized the cohort into those who were transfused within 24h and those who received no blood products within 24h of admission. In the transfused patient group, RBC:FFP ratios were calculated as the number of whole RBC units divided by FFP units received in the first 6h. The 6h time point was the earliest time point with available data after arrival; this was selected in order to capture patients requiring acute transfusion in response to hemorrhagic shock. Transfused patients were dichotomized into ‘low ratio’ and ‘high ratio’ groups using the median RBC:FFP ratio in the group rounded to the nearest ½ unit (1.5 RBC:1 FFP) as a cut-point to ensure relative equality of group size. Univariate comparisons were made using Student’s t-test for normally distributed data, Wilcoxon rank-sum testing for skewed data, and Fisher’s exact test for proportions. Paired continuous factor level measurements were compared using the paired t-test for normally distributed data and the sign-rank test for skewed data. Kaplan-Meier time-to-event analysis and log-rank testing were used to assess differences in in-hospital mortality between groups. Cox proportional hazards regression was used to adjust for baseline demographic, injury, and physiology characteristics. An alpha of 0.05 was considered significant. All data analysis was performed by the authors using Stata version 12 (StataCorp; College Station, TX).
RESULTS
We prospectively enrolled, collected serial blood samples from, and assayed coagulation factors in 336 patients. This population represents a critically injured cohort of trauma patients: mean age was 42.1 ± 19.6y, penetrating injury occurred in 30.6%, mean ISS was 27.0 ± 15.9, mean base deficit was −6.9 ± 6.4, and in-hospital mortality was 31.8% (Table 1a). 40 (11.9%) patients were coagulopathic (INR ≥1.5) and 131 (40.0%) patients were in shock (base deficit ≤−6) on arrival. Other cohort demographic information is detailed in Table 1a.
TABLE 1a.
Summary statistics for all patients
| Age | 42.1 ± 19.6 |
| Penetrating injury | 30.6% |
| ISS | 27.0 ± 15.9 |
| AIS-head | 4 (0 – 5) |
| GCS | 8 (3 – 14) |
| pH | 7.27 ± 0.15 |
| Base deficit | −6.9 ± 6.4 |
| Prehospital IVF | 100 (0 – 500) |
| Temperature (°C) | 35.6 ± 0.9 |
| RBC units / 24h | 0 (0 – 7) |
| FFP units / 24h | 0 (0 – 5) |
| Platelet units / 24h | 0 (0 – 0) |
| Factor VIIa given | 5.1% |
| Total hospital days | 8 (3 – 24) |
| ICU days | 4 (2 – 12) |
| Ventilator-free days / 28d | 15 (0 – 26) |
| Ventilator-associated pneumonia | 27.4% |
| Acute lung injury | 29.9% |
| Multiorgan failure | 16.2% |
| Mortality | 31.8% |
ISS = injury severity score; AIS = abbreviated injury score; GCS = Glasgow coma score; IVF = intravenous fluid; RBC = red blood cells; FFP = fresh frozen plasma; ICU = intensive care unit. N = 336.
To begin to investigate resuscitation practices in these patients, we dichotomized the cohort into those who were transfused within 24h (143 patients) and those who received no blood products within 24h of admission (193 patients); demographics of these cohorts are given in Table 1b. As expected, the 143 transfused patients had more severe injury, a higher percentage of penetrating injury, higher ISS, lower pH, more severe base deficit, fewer ventilator-free days, a higher incidence of acute lung injury, multi-organ failure, and higher overall mortality (all p < 0.001, Table 1b). 64 patients (44.8% of transfused patients, 19.0% of the overall cohort) received ‘massive’ transfusions of ≥10 units RBC/24h. 17 transfused patients (11.9%) received recombinant Factor VIIa. The median RBC:FFP ratio in transfused patients at 6h was 1.25:1; therefore, we selected a clinically accessible ratio of 1.5:1 in order to subdivide the transfused cohort into ‘low ratio’ (RBC:FFP ratio ≤1.5:1) and ‘high ratio’ (RBC:FFP >1.5:1) based on the number of whole units of RBC and FFP transfused by 6h of admission. Of the 143 transfused patients, 91 patients received a ‘low ratio’ with a median RBC:FFP ratio of 1:1 (IQR 0.8–1.2:1). The other 52 transfused patients received a ‘high ratio’ of RBC:FFP with a median of 2.2:1 (IQR 2.0–3.0:1; Table 1c). As expected, the 91 patients transfused with a ‘low ratio’ received fewer RBC units per 24 hours, with a median of 7 (4–14) units versus 10 (7–22) units in the ‘high ratio’ group (p=0.001; Table 1c). The ‘low ratio’ group was comparable in age, mechanism of injury, and ISS to the ‘high ratio’ group; however, they had higher AIS-head score (median 5 vs. 1, p < 0.05) and lower GCS (median 6 vs. 12, p < 0.05). In addition, those transfused with a ‘high ratio’ had a significantly more severe base deficit (mean −11.3 vs. −7.8, p=0.004), lower temperature (mean 35.0 vs. 35.8 degrees Celsius, p=0.004), and a trend towards higher mortality rate (55.8% vs. 42.9%, p=0.165). Patients transfused with a ‘low ratio’ had trends towards longer hospital stay (median 11 vs. 6.5 days, p=0.135), and higher rates of ventilator associated pneumonia (40.0% vs. 23.4%, p=0.074) and multi-organ failure (25.6% vs. 13.5%, p=0.134), as well as significantly longer ICU stay (median 5 vs. 3 days, p=0.028; Table 1c).
TABLE 1b.
Summary statistics for patients by transfusion within 6h of arrival
| Non-transfused (n = 193) | Transfused (n = 143) | p-value | |
|---|---|---|---|
| Age | 43.4 ± 19.6 | 40.2 ± 19.5 | 0.135 |
| Penetrating injury | 18.8% | 46.5% | <0.001* |
| ISS | 21.8 ± 13.7 | 34.1 ± 16.0 | <0.001* |
| AIS-head | 4 (0 – 5) | 4 (0 – 5) | 0.976 |
| GCS | 7 (4 – 14) | 8.5 (3 – 15) | 0.615 |
| pH | 7.31 ± 0.14 | 7.22 ± 0.15 | <0.001* |
| Base deficit | −4.9 ± 5.5 | −9.1 ± 6.7 | <0.001* |
| Prehospital IVF | 200 (0 – 500) | 100 (0 – 300) | 0.141 |
| Temperature (°C) | 35.6 ± 0.9 | 35.5 ± 0.9 | 0.372 |
| RBC units / 24h | 0 | 9 (5 – 17) | - |
| FFP units / 24h | 0 | 6 (4 – 12) | - |
| Platelet units / 24h | 0 | 1 (0 – 2) | - |
| Factor VIIa given | 0.0% | 11.9% | <0.001* |
| Total hospital days | 8 (3 – 20) | 9 (2 – 30) | 0.573 |
| ICU days | 4 (2 – 9) | 4 (2 – 19) | 0.487 |
| Ventilator-free days / 28d | 24 (0 – 26) | 0 (0 – 22) | <0.001* |
| Ventilator-associated pneumonia | 22.9% | 33.3% | 0.073 |
| Acute lung injury | 19.7% | 44.4% | <0.001* |
| Multiorgan failure | 12.5% | 21.1% | <0.001* |
| Mortality | 20.2% | 47.6% | <0.001* |
ISS = injury severity score; AIS = abbreviated injury score; GCS = Glasgow coma score; IVF = intravenous fluid; RBC = red blood cells; FFP = fresh frozen plasma; ICU = intensive care unit. N = 336.
p <0.05.
TABLE 1c.
Summary statistics for patients by RBC:FFP transfusion ratio within 6h of arrival
| Low ratio RBC:FFP <=1.5:1 (n = 91) | High ratio RBC:FFP >1.5:1 (n = 52) | p-value | |
|---|---|---|---|
| RBC:FFP ratio | 1 (0.8 – 1.2) | 2.2 (2.0 – 3.0) | - |
| Age | 38.7 ± 18.3 | 42.8 ± 21.5 | 0.253 |
| Penetrating injury | 42.2% | 53.8% | 0.222 |
| ISS | 32.7 ± 16.2 | 36.6 ± 15.5 | 0.164 |
| AIS-head | 5 (0 – 5) | 1 (0 – 5) | 0.009* |
| GCS | 6 (3 – 15) | 12 (4 – 15) | 0.029* |
| pH | 7.23 ± 0.15 | 7.19 ± 0.14 | 0.145 |
| Base deficit | −7.8 ± 6.4 | −11.3 ± 6.7 | 0.004* |
| Prehospital IVF | 150 (0 – 300) | 0 (0 – 325) | 0.196 |
| Temperature (°C) | 35.8 ± 0.8 | 35.0 ± 0.9 | 0.004* |
| RBC units / 24h | 7 (4 – 14) | 10 (7 – 22) | 0.001* |
| FFP units / 24h | 7 (4– 12) | 4.5 (3 – 11) | 0.160 |
| Platelet units / 24h | 0 (0 – 2) | 1 (0 – 2) | 0.525 |
| Factor VIIa given | 11.0% | 13.5% | 0.789 |
| Total hospital days | 11 (2 – 32) | 6.5 (1.5 – 24) | 0.135 |
| ICU days | 5 (2 – 22) | 3 (1 – 12) | 0.028* |
| Ventilator-free days / 28d | 0 (0 – 24) | 0 (0 – 20.5) | 0.551 |
| Ventilator-associated pneumonia | 40.0% | 23.4% | 0.074 |
| Acute lung injury | 46.0% | 41.7% | 0.834 |
| Multiorgan failure | 25.6% | 13.5% | 0.134 |
| Mortality | 42.9% | 55.8% | 0.165 |
ISS = injury severity score; AIS = abbreviated injury score; GCS = Glasgow coma score; IVF = intravenous fluid; RBC = red blood cells; FFP = fresh frozen plasma; ICU = intensive care unit. N = 143.
p <0.05.
After separately evaluating the demographics of the study population based on transfusion and resuscitation ratios, we then sought to describe specific differences in admission and longitudinal coagulation factor levels. The 193 non-transfused patients represent a cohort of injured patients in which serial coagulation factor measurements reflect an unperturbed course, thus describing the natural history of factor levels after injury. Differences between admission and later factor levels at all subsequent time points up to 48 hours were statistically significant for PT, PTT, Factor II, Factor V, Factor VIII, Factor X, and activated Protein C, as described in detail in Table 2a. Overall, compared to admission baseline, PT and PTT became progressively prolonged, and factor levels of both endogenous procoagulants and anticoagulants became progressively depleted in the non-transfused cohort; Factors V and IX were exceptions, in that they progressively increased over time (Table 2a). In the cohort of 143 transfused patients, differences between admission and later factor levels at all subsequent time points up to 48 hours were statistically significant for PTT, Factor VII, Factor VIII, Factor IX, Protein C, and activated Protein C, as described in Table 2b. Overall, compared to admission baseline, PTT became progressively prolonged for 48h after admission and only returned to baseline at 72h. Similarly to the non-transfused cohort, Factor VIII levels remained lower than admission baseline for 72h, Factor IX levels rose from admission baseline to 72h, and activated Protein C levels dropped significantly by 6h and remained low through 72h. In transfused patients, Protein C levels remained depleted relative to admission baseline for up to 72h, compared to the repletion by 24h seen in the non-transfused cohort. Factor VII levels showed a varied pattern, with early repletion within 12h, followed by depletion relative to admission levels from 24–48h, and subsequent return to admission levels by 72h. Given the critical differences in the transfused versus non-transfused patient population, we also compared baseline factor levels between the transfused and non-transfused cohorts: PT and PTT were prolonged, Factors II, V, VII, IX, and X as well as ATIII and Protein C were lower, and activated Protein C and tPA were elevated in the transfused cohort on admission (all p < 0.05, Table 2a and 2b).
TABLE 2a.
Longitudinal factor levels in patients receiving no blood products within 72h of arrival
| 0h | 6h | 12h | 24h | 48h | 72h | |
|---|---|---|---|---|---|---|
| PT (sec) | 13.8 (13.1 – 15.4)* | 14.7 (13.8 – 15.6)# | 14.4 (13.7 – 15.7)# | 15.6 (14.2 – 16.8)# | 15.2 (14.0 – 16.2)# | 14.6 (14.2 – 16.5)# |
| PTT (sec) | 29.8 (26.7 – 33.8)* | 30.9 (28.8 – 34.9)# | 33.1 (29.3 – 36.5)# | 38.6 (33.7 – 42.8)# | 42.0 (37.1 – 49.5)# | 44.6 (39.9 – 46.9)# |
| Fibrinogen (ng/mL) | 234 (151 – 289) | 260 (233 – 306) | 251 (211 – 303) | 233 (208 – 259) | 506 (350 – 582) | 720 (622 – 818) |
| Factor II (%) | 81.4 ± 19.1* | 74.7 ± 18.2# | 75.9 ± 18.0# | 73.1 ± 17.6# | 69.5 ± 19.1# | 83.2 ± 18.7 |
| Factor V (%) | 56.5 ± 27.9* | 56.7 ± 24.0# | 56.6 ± 21.2# | 57.2 ± 22.1# | 68.0 ± 22.3# | 81.3 ± 29.3# |
| Factor VII (%) | 95.4 ± 33.9* | 95.9 ± 28.9 | 94.0 ± 30.6 | 67.6 ± 26.9# | 61.3 ± 33.0# | 81.1 ± 29.4 |
| Factor VIII (%) | 218.8 ± 171.8 | 136.6 ± 106.5# | 128.5 ± 76.3# | 137.6 ± 127.7# | 139.4 ± 53.2# | 177.0 ± 63.4# |
| Factor IX (%) | 129.2 ± 40.9* | 130.1 ± 45.9 | 138.1 ± 46.8# | 140.8 ± 43.5# | 172.2 ± 58.4# | 226.2 ± 93.1# |
| Factor X (%) | 86.3 ± 22.9* | 78.7 ± 23.1# | 79.8 ± 22.0# | 74.5 ± 18.6# | 66.3 ± 17.1# | 76.2 ± 20.6 |
| AT III (%) | 89.8 ± 21.8* | 87.3 ± 31.4 | 84.8 ± 22.2# | 83.7 ± 21.0# | 83.1 ± 23.4 | 84.1 ± 28.3 |
| Protein C (%) | 96.4 ± 28.8* | 92.1 ± 23.3# | 89.5 ± 27.0# | 81.5 ± 24.7# | 81.9 ± 23.0 | 87.0 ± 25.0 |
| aPC (ng/mL) | 3.1 (1.5 – 9.5)* | 0.9 (0.2 – 2.1)# | 1.0 (0.2 – 1.7)# | 1.2 (0.2 – 1.7)# | 1.0 (0.1 – 1.8)# | 0.6 (0.3 – 1.4)# |
| D-dimer (mcg/mL) | 1.7 (0.5 – 4.0)* | 1.6 (0.7 – 3.6)# | 2.3 (0.9 – 4.1)# | 2.4 (1.0 – 4.0) | 1.7 (1.0 – 4.2) | 1.9 (1.3 – 3.1) |
| tPA (ng/mL) | 15.2 (8.4 – 27.3)* | 12.1 (6.9 – 19.0)# | 6.0 (4.6 – 14.2)# | 6.7 (5.7 – 9.4)# | 6 | 6.7 |
| PAI-1 (ng/mL) | 38.0 (9.6 – 60.7) | 73.4 (44.1 – 91.4) | 38.8 (19.3 – 55.2) | 33.2 (17.8 – 71.0) | 11.7 (8.1 – 15.0) | 17.4 |
PT = prothrombin time; PTT = activated partial thromboplastin time; AT III = antithrombin III; aPC = activated Protein C; tPA = tissue plasminogen activator; PAI-1 = plasminogen activator inhibitor-1. N=193.
p <0.05 for comparison between transfused and non-transfused patients on arrival only.
p <0.05 for comparison between admission factor level and factor level at the indicated time.
TABLE 2b.
Longitudinal factor levels in patients receiving blood products within 72h
| 0h | 6h | 12h | 24h | 48h | 72h | |
|---|---|---|---|---|---|---|
| PT (sec) | 15.7 (14.2 – 19.6)* | 15.6 (14.4 – 17.8) | 15.7 (14.7 – 17.1) | 16.7 (15.5 – 18.2) | 16.6 (14.9 – 18.8) | 15.5 (14.3 – 17.3) |
| PTT (sec) | 30.8 (26.9 – 38.3)* | 32.8 (28.9 – 38.0)# | 34.3 (31.8 – 39.6)# | 38.6 (34.5 – 43.6)# | 40.8 (36.6 – 47.4)# | 39.0 (33.8 – 43.8)# |
| Fibrinogen (ng/mL) | 160 (125 – 221) | 153 (124 – 226) | 196 (152 – 237) | 288 (189 – 340)# | 398 (281 – 566)# | 499 (294 – 716)# |
| Factor II (%) | 66.5 ± 21.2* | 62.2 ± 16.6# | 63.8 ± 16.0# | 64.0 ± 16.0# | 65.6 ± 17.5 | 70.0 ± 15.4 |
| Factor V (%) | 41.5 ± 26.1* | 41.4 ± 19.6# | 43.3 ± 18.7 | 46.5 ± 21.0 | 54.6 ± 27.5# | 74.2 ± 39.2# |
| Factor VII (%) | 75.1 ± 34.9* | 99.3 ± 61.8# | 94.5 ± 60.2# | 52.7 ± 28.8# | 58.6 ± 29.4# | 76.2 ± 30.3 |
| Factor VIII (%) | 219.6 ± 144.8 | 128.6 ± 94.5# | 115.6 ± 73.7# | 119.1 ± 71.8# | 150.7 ± 124.2# | 179.5 ± 88.9# |
| Factor IX (%) | 103.6 ± 40.2* | 114.9 ± 38.8# | 115.6 ± 33.0# | 119.6 ± 35.0# | 155.7 ± 46.0# | 185.2 ± 50.4# |
| Factor X (%) | 66.7 ± 24.4* | 68.3 ± 26.3 | 66.2 ± 21.3# | 61.4 ± 15.7# | 64.1 ± 15.6# | 70.7 ± 17.6# |
| AT III (%) | 78.1 ± 24.6* | 72.1 ± 19.4# | 76.3 ± 17.9 | 73.9 ± 18.3# | 67.3 ± 19.6# | 74.3 ± 23.0 |
| Protein C (%) | 80.6 ± 28.0* | 69.9 ± 20.6# | 71.7 ± 18.7# | 66.8 ± 19.6# | 67.0 ± 22.6# | 73.7 ± 28.0 |
| aPC (ng/mL) | 12.1 (3.5 – 37.8)* | 1.9 (0.7 – 3.5)# | 1.2 (0.5 – 2.4)# | 1.0 (0.2 – 1.7)# | 1.3 (0.5 – 2.3)# | 1.2 (0.6 – 2.4)# |
| D-dimer (mcg/mL) | 6.8 (2.9 – 9.8)* | 6.5 (3.3 – 9.4) | 6.6 (3.8 – 9.9) | 5.2 (2.9 – 9.4)# | 3.3 (1.9 – 6.1)# | 3.6 (2.7 – 5.6)# |
| tPA (ng/mL) | 27.9 (13.0 – 44.0)* | 11.6 (9.9 – 18.9)# | 11.8 (8.7 – 15.1)# | 9.0 (5.9 – 12.9)# | 4.6 (4.3 – 5.3) | 4.5 (3.5 – 5.4) |
| PAI-1 (ng/mL) | 25.3 (12.1 – 33.4) | 130.2 (79.8 – 149.8)# | 135.1 (84.8 – 237.4)# | 70.4 (46.8 – 120.4)# | 26.0 (14.0 – 33.2) | 19.2 (13.8 – 24.6) |
PT = prothrombin time; PTT = activated partial thromboplastin time; AT III = antithrombin III; aPC = activated Protein C; tPA = tissue plasminogen activator; PAI-1 = plasminogen activator inhibitor-1. N=143.
p <0.05 for comparison between transfused and non-transfused patients on arrival only.
p <0.05 for comparison between admission factor level and factor level at the indicated time.
Next, we analyzed comprehensive factor levels in those transfused with a ‘low ratio’ versus ‘high ratio’ of RBC:FFP units within the first 6h of admission, in order to identify differences in clotting factor levels over time associated with the two different transfusion strategies. On arrival and prior to transfusion, patients ultimately receiving ‘low ratio’ transfusion had significantly higher levels of baseline Factor II, and lower baseline levels of Factor VIII compared to the ‘high ratio’ cohort (Table 3a and 3b). In the group of 91 patients transfused with a ‘low ratio’, differences in admission and later factor levels at all subsequent time points up to 24 hours were statistically significant for Factor VII, Factor VIII, Factor IX, activated Protein C, and PAI-1 (Table 3a). Within the group of 52 patients transfused with a ‘high ratio’, differences in admission and later factor levels at all subsequent time points up to 24 hours were statistically significant for PTT, Factor VIII, ATIII, Protein C, activated Protein C, tPA, and PAI-1 (Table 3b). Evaluating point-by-point differences in factor levels between the two transfusion strategy cohorts, significant differences were seen at several time points. While admission PT and PTT for both ratio groups were similar, the PTT was comparatively prolonged in the group transfused with a ‘high ratio’ compared to those transfused with a ‘low ratio’ at 6h and 12h after admission; furthermore, the prolonged PTT took 72h to return to admission baseline in the ‘high ratio’ group, compared to correction within 24h in the ‘low ratio’ group. Similarly, Protein C levels remained depleted relative to admission baseline at all time points in the ‘high ratio’ group, while Protein C levels were repleted to baseline by 72h in the ‘low ratio’ group. Early absolute clotting factor deficits in levels of Factor V (at 6h), Factor IX (at 6 and 12h), and Factor X (at 6h) were better-corrected by ‘low’ versus ‘high’ ratio transfusion (Table 3a and 3b).
TABLE 3a.
Longitudinal factor levels in patients transfused with ‘low ratio’ (RBC:FFP <=1.5:1) at 6h
| 0h | 6h | 12h | 24h | 48h | 72h | |
|---|---|---|---|---|---|---|
| PT (sec) | 16.7 (14.5 – 21.2) | 15.3 (14.2 – 17.6) | 15.6 (14.6 – 16.9)# | 16.9 (15.5 – 19.1) | 16.2 (14.9 – 18.8) | 15.5 (14.1 – 17.8)# |
| PTT (sec) | 32.5 (27.5 – 42.2) | 33.2 (28.6 – 36.1)* | 33.7 (30.8 – 38.7)* | 38.2 (34.8 – 43.1)# | 39.2 (36.4 – 47.4) | 38.5 (32.9 – 43.8) |
| Fibrinogen (ng/mL) | 157 (106 – 200) | 142 (111 – 205) | 180 (133 – 234) | 288 (162 – 362)# | 325 (264 – 514)# | 494 (395 – 559)# |
| Factor II (%) | 66.2 ± 21.3* | 61.6 ± 17.1 | 64.4 ± 17.5 | 64.0 ± 18.5 | 66.8 ± 20.2 | 72.2 ± 14.4# |
| Factor V (%) | 38.7 ± 24.7 | 43.2 ± 20.7* | 43.7 ± 19.3 | 45.3 ± 23.4 | 58.8 ± 29.0# | 79.6 ± 44.2# |
| Factor VII (%) | 72.3 ± 31.7 | 107.0 ± 77.6# | 100.3 ± 69.7# | 48.2 ± 22.9# | 61.8 ± 34.6 | 79.6 ± 29.8 |
| Factor VIII (%) | 188.1 ± 128.5* | 115.3 ± 80.3# | 105.2 ± 62.2# | 110.6 ± 51.0# | 179.3 ± 147.7 | 192.9 ± 94.6 |
| Factor IX (%) | 101.2 ± 40.6 | 116.3 ± 39.4*# | 114.0 ± 35.4*# | 114.6 ± 36.7# | 151.6 ± 45.6# | 184.4 ± 46.2# |
| Factor X (%) | 64.1 ± 23.9 | 72.6 ± 33.4* | 65.0 ± 19.9 | 60.7 ± 17.9 | 66.2 ± 16.8 | 72.6 ± 15.7 |
| AT III (%) | 75.5 ± 24.9 | 67.6 ± 18.9 | 73.8 ± 17.6 | 69.8 ± 16.7 | 66.8 ± 21.0 | 72.4 ± 22.6 |
| Protein C (%) | 77.4 ± 29.0 | 66.8 ± 17.0 | 72.0 ± 18.5 | 64.5 ± 22.4# | 65.9 ± 21.6# | 73.4 ± 30.4 |
| aPC (ng/mL) | 15.3 (5.4 – 49.5) | 2.8 (0.7 – 4.7)# | 1.2 (0.6 – 2.5)# | 1.0 (0.5 – 1.9)# | 0.5 (0.3 – 1.7)# | 0.8 (0.6 – 2.4)# |
| D-dimer (mcg/mL) | 6.7 (2.4 – 9.8) | 6.4 (3.2 – 9.4) | 6.8 (3.8 – 10.6) | 7.6 (3.9 – 11.4) | 3.3 (2.1 – 5.0) | 3.7 (3.1 – 6.1) |
| tPA (ng/mL) | 31.0 (9.0 – 46.7) | 15.3 (11.2 – 22.6) | 13.5 (11.4 – 18.0)# | 9.8 (6.1 – 15.3)# | 4.3 | - |
| PAI-1 (ng/mL) | 25.3 (11.8 – 30.2) | 146.9 (138.7 – 372.4)# | 142.4 (134.2 – 278.9)# | 86.1 (57.7 – 124.6)# | 25.5 (8.3 – 33.5) | - |
PT = prothrombin time; PTT = activated partial thromboplastin time; AT III = antithrombin III; aPC = activated Protein C; tPA = tissue plasminogen activator; PAI-1 = plasminogen activator inhibitor-1. N=91.
p <0.05 for comparison between ‘low’ and ‘high ratio’ patients at each time point.
p <0.05 for comparison between admission factor level and factor level at the indicated time.
Table 3b.
Longitudinal factor levels in patients with 6h RBC:FFP ‘high ratio’ at 6h
| 0h | 6h | 12h | 24h | 48h | 72h | |
|---|---|---|---|---|---|---|
| PT (sec) | 16.2 (14.6 – 20.6) | 16.2 (15.1 – 18.3) | 15.9 (15.0 – 17.0) | 17.0 (15.8 – 17.7) | 17.6 (15.6 – 18.8) | 15.6 (15.2 – 17.3) |
| PTT (sec) | 32.5 (27.6 – 41.8) | 36.6 (32.5 – 41.7)*# | 35.9 (32.8 – 40.4)*# | 39.8 (36.0 – 46.4)# | 47.0 (38.6 – 49.5)# | 41.5 (39.4 – 45.8) |
| Fibrinogen (ng/mL) | 139 (125 – 205) | 124 (101 – 153) | 183 (157 – 230) | 258 (213 – 291)# | 547 (530 – 566)# | 668 (566 – 780)# |
| Factor II (%) | 57.7 ± 18.3* | 54.1 ± 14.9 | 59.4 ± 12.1 | 60.6 ± 12.4 | 61.8 ± 14.2 | 70.2 ± 15.4 |
| Factor V (%) | 38.0 ± 26.8 | 33.3 ± 16.2*# | 39.7 ± 16.7# | 43.2 ± 17.6 | 47.7 ± 25.3 | 61.0 ± 23.0 |
| Factor VII (%) | 73.9 ± 46.3 | 86.3 ± 51.8 | 101.1 ± 59.4 | 61.1 ± 36.3# | 52.9 ± 19.3# | 78.7 ± 32.7 |
| Factor VIII (%) | 266.6 ± 172.0* | 106.4 ± 77.4# | 106.2 ± 78.5# | 112.5 ± 43.7# | 114.1 ± 53.4# | 152.6 ± 78.1# |
| Factor IX (%) | 96.5 ± 44.2 | 86.5 ± 29.7* | 100.7 ± 18.5* | 107.1 ± 25.1 | 142.9 ± 36.1# | 179.0 ± 53.1# |
| Factor X (%) | 61.0 ± 23.1 | 54.7 ± 16.9* | 62.3 ± 20.9 | 58.2 ± 10.4# | 57.9 ± 12.9# | 72.7 ± 13.0 |
| AT III (%) | 73.8 ± 23.4 | 67.2 ± 19.7# | 74.7 ± 16.7# | 71.9 ± 20.0# | 61.2 ± 18.9# | 66.8 ± 24.1# |
| Protein C (%) | 79.5 ± 27.1 | 63.9 ± 17.8# | 69.7 ± 12.9# | 64.4 ± 15.1# | 57.7 ± 11.9# | 61.3 ± 18.6# |
| aPC (ng/mL) | 13.7 (3.5 – 51.2) | 1.4 (0.6 – 2.7)# | 1.0 (0.4 – 2.3)# | 0.5 (0.0 – 1.4)# | 1.2 (1.1 – 1.4)# | 1.2 (0.5 – 13.4) |
| D-dimer (mcg/mL) | 7.5 (3.8 – 12.0) | 6.5 (2.6 – 23.0) | 7.4 (4.2 – 14.6) | 7.4 (3.8 – 10.6)# | 4.7 (2.8 – 6.7) | 4.4 (3.2 – 6.7) |
| tPA (ng/mL) | 25.0 (14.7 – 38.3) | 12.5 (8.0 – 15.6)# | 10.2 (8.4 – 14.3)# | 6.8 (5.8 – 11.1)# | 5.0 (4.6 – 5.3) | 4.5 (3.5 – 5.4) |
| PAI-1 (ng/mL) | 25.7 (12.4 – 38.4) | 125.1 (49.5 – 132.6)# | 141.6 (84.1 – 251.4)# | 64.6 (43.8 – 143.4)# | 27.1 (19.1 – 33.2) | 19.2 (13.8 – 24.6) |
PT = prothrombin time; PTT = activated partial thromboplastin time; AT III = antithrombin III; aPC = activated Protein C; tPA = tissue plasminogen activator; PAI-1 = plasminogen activator inhibitor-1. N=52.
p <0.05 for comparison between ‘low’ and ‘high ratio’ patients at each time point.
p <0.05 for comparison between admission factor level and factor level at the indicated time.
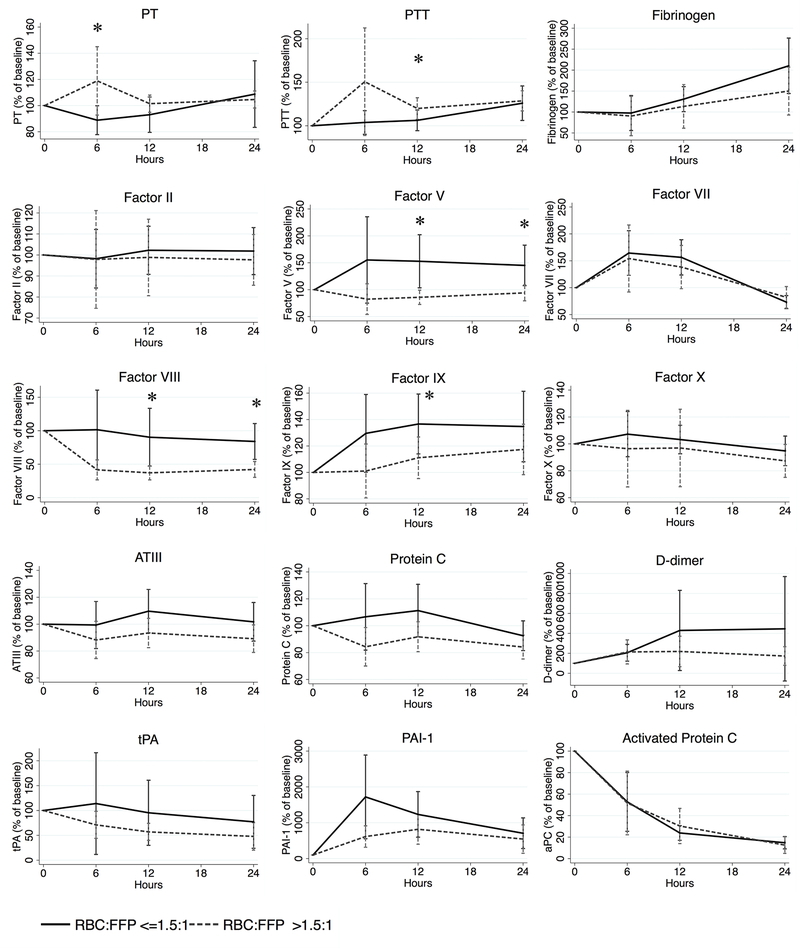
In order to better understand differences between transfusion practices given the differences in baseline population characteristics (Table 1c) and clotting factor levels on admission (Tables 3a and 3b), we then assessed longitudinal clotting factor levels as a percentage of their arrival baseline (Figure 1). Overall, patients transfused with a ‘low ratio’ had significantly less prolonged PT (at 6h) and PTT (at 12h) compared to those transfused at a ‘high ratio’. In terms of specific factor repletion, Factors V and VIII were significantly better repleted at both 12h and 24h, as well as Factor IX at 12h. No significant differences were seen compared to admission baseline for fibrinogen, procoagulant Factors II, VII, or X, anticoagulants ATIII, Protein C, or activated Protein C, or fibrinolysis-associated molecules D-dimer, tPA, or PAI-1.
Figure 1.
Longitudinal percentage change in coagulation studies by ‘high’ versus ‘low’ RBC:FFP resuscitation ratio compared to admission baseline
RBC = red blood cells; FFP = fresh frozen plasma; PT = prothrombin time; PTT = activated partial thromboplastin time; AT III = antithrombin III; aPC = activated Protein C; tPA = tissue plasminogen activator; PAI-1 = plasminogen activator inhibitor-1. N=143. *p <0.05 for comparison between ‘low’ and ‘high ratio’ patients at each time point.
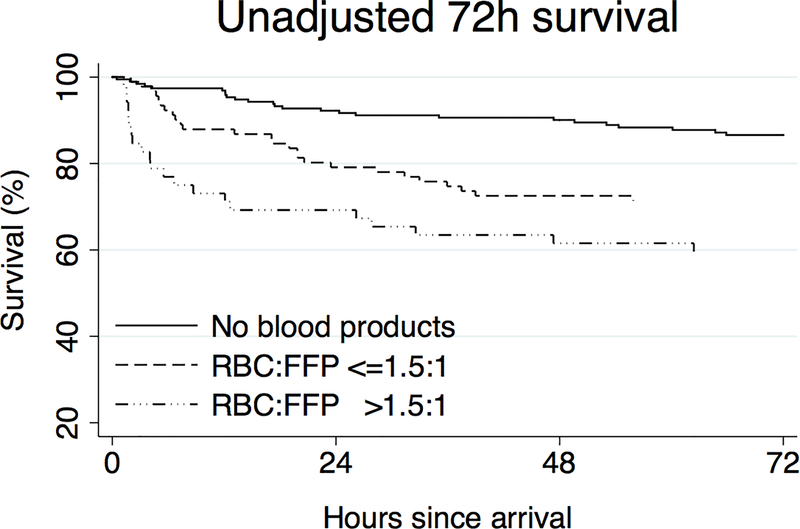
In order to determine the overall association with survival, we performed Kaplan-Meier survival analysis comparing non-transfused patients to those transfused with a ‘low’ versus ‘high’ ratio of RBC:FFP, identifying an association between lower RBC:FFP transfusion ratios and improved survival to discharge (Figure 2, log-rank p < 0.001). Given the significant baseline differences in injury severity in these populations detailed in Table 1b and 1c, we created a Cox proportional hazards model for in-hospital mortality in order to adjust for age, injury severity score, arrival GCS, arrival base deficit, arrival INR, penetrating versus blunt mechanism, and categorical transfusion status (non-transfused, low ratio, or high ratio); the study population had 107 in-hospital deaths, and Harrell’s C for the model was 0.829 (Table 4). Compared to the referent category of non-transfused patients, those transfused with a ‘low’ ratio had a non-significant hazard ratio for mortality of 1.661 (p=0.079, 95% confidence interval (CI) 0.943 – 2.925), while those transfused with a ‘high’ ratio had 3.402-fold significantly higher mortality (p=0.001, 95% CI 1.693–6.833). In terms of differences between transfusion strategies, those transfused with a ‘high’ ratio had a significant 2.048-fold higher mortality than those transfused with a ‘low’ ratio, even when adjusted for differences in age, injury characteristics, and admission physiology (p=0.027, 95% CI 1.087 – 3.858; Table 4).
Figure 2.
Unadjusted Kaplan-Meier survival curves by RBC:FFP ratio.
RBC = red blood cells; FFP = fresh frozen plasma. N=336. Log-rank p <0.001 between groups.
Table 4.
Cox proportional hazards model for in-hospital mortality.
| Variable | HR | P-value | 95% Confidence Interval |
|---|---|---|---|
| Age | 1.038 | <0.001 | (1.024 – 1.052) |
| ISS | 1.023 | 0.002 | (1.008 – 1.038) |
| Arrival GCS | 0.803 | <0.001 | (0.749 – 0.861) |
| Arrival base deficit | 1.029 | 0.154 | (0.989 – 1.069) |
| Arrival INR | 0.958 | 0.820 | (0.659 – 1.392) |
| Penetrating injury | 2.362 | 0.011 | (1.213 – 4.599) |
| Non-transfused | - | - | - |
| Low ratio | 1.661 | 0.079 | (0.943 – 2.925) |
| High ratio | 3.402 | 0.001 | (1.693 – 6.833) |
ISS = injury severity score; GCS = Glasgow coma score; INR = international normalized ratio. N=336. In-hospital mortality outcome occurred in 107 cases. Harrell’s C = 0.829.
DISCUSSION
Here we present the first ‘natural history’ study of longitudinal clotting factor levels after severe trauma. By prospective collection of both longitudinal biochemical and outcomes data beginning early after injury, we are here able to provide data on the course and serial effects of treatment regimes on coagulation after injury by presenting longitudinal profiles as affected by ‘low ratio’ and ‘high ratio’ hemostatic resuscitation, identifying significant differences in repletion of specific clotting factors based on transfusion strategy. In particular, hemostatic resuscitation ratio-based transfusion is associated with earlier correction of both PT and PTT, as well as earlier repletion of Factors V, VIII, IX, and X after severe injury, both in terms of absolute levels and when compared to admission baseline. We further identify a survival advantage to the ‘low ratio’ transfusion strategies, both in unadjusted analysis and when adjusted for patient demographics, injury characteristics, and admission physiology. Taken together, we believe this survival benefit appears most likely due to a mixed picture of both more optimal coagulopathy reversal as well as modulation of the inflammatory response to trauma.
The last ten years have seen a paradigm change in the care of the severely injured and bleeding patient. This change is a consequence of the identification of ATC as a clinical entity, and of hemostatic resuscitation as its effective (and empiric) treatment. Prior to the clinical recognition of ATC, classic trauma resuscitation literature identified iatrogenic and resuscitation-associated causes as responsible for bleeding after trauma: hypothermia, metabolic acidosis, and dilutional coagulopathy were identified as primary drivers, and were generally thought to be secondary to resuscitative efforts.9 In response to this pioneering literature, trauma surgeons began to incorporate rewarming efforts, early correction of acidosis, and limitation of crystalloid as prime tenants of resuscitation strategy. Once these major causes of coagulopathy were avoided or appropriately treated, however, a distinct coagulopathy present in patients immediately after injury and prior to any resuscitative interventions still remained. Brohi et al. and Macleod et al. concurrently elucidated this in 2003, and defined ATC separately from the iatrogenic causes previously identified in coagulopathic bleeding after trauma.2, 4 Our group and others subsequently codified the clinical and biochemical nature of this entity, reporting that ATC occurs only in the presence of both severe injury and shock, and is mediated by activation of the Protein C system.5, 22, 23 Concurrently, military and civilian studies on transfusion strategy suggested a survival benefits to earlier and more frequent use of plasma during massive transfusion. Beginning with Borgman’s data from military casualties, multiple studies clearly demonstrated that approaching balanced ratio transfusion of RBC: FFP led to improved survival.12–16, 21 As this considerable retrospective data is compelling and prospective randomized studies in this arena are in early stages of progress, plasma-based resuscitation conduct has been overwhelmingly accepted by the trauma community despite the fact that the biochemical mechanisms underlying the survival benefit of plasma remain unclear.24
Whether this apparent survival benefit stems from earlier and more durable reversal of coagulopathy or is the result of as-yet undescribed inflammomodulatory effects remains unclear. Early suggestions that there is more to balanced resuscitation than the repletion of depleted clotting factors comes from clinical and basic science data. Clinically, Brown et al. reported that trauma patients derive benefit from plasma-based resuscitation independent of the presence of coagulopathy.21 Groundbreaking preclinical basic science data also supports the inflammomodulatory hypothesis. Pati et al. described a cell culture model showing that plasma decreased endothelial cell permeability compared to crystalloid.20 Kozar et al. showed that in vivo endothelial glycocalyx degradation after hemorrhagic shock was partially restored by plasma, but not lactated Ringer’s in a rat model.19 These results suggest that an ‘endotheliopathy’ induced by hemorrhagic shock may be treated by plasma administration via an inflammomodulatory mechanism, above and beyond the simple correction of clotting factor depletion. Given the known mortality benefit of balanced resuscitation confirmed here, further work identifying the nature of this endotheliopathy as well as the specific inflammomodulatory effects of plasma is required for a complete understanding of the biochemical mechanisms involved.
As an attempt to elucidate the biochemical framework of this clinical dilemma, in this study we describe the natural history of coagulation factor perturbation after injury, both with and without transfusion. Those patients requiring transfusion had prolonged PT and PTT, lower levels of procoagulants, and higher levels of endogenous anticoagulants at each time point up to 24h. Notably, transfusion with a ‘low ratio’ of RBC: FFP was associated with earlier correction of PT and PTT as well as repletion of Factor V, Factor VIII, and Factor IX deficits compared to those transfused with higher ratios, suggesting that ‘low ratio’ transfusion corrects specific critical clotting factor deficits more efficiently than higher RBC:FFP ratios. Interestingly, not all factors are significantly corrected in the ‘low ratio’ group compared to the ‘high ratio’ group. This suggests that reversal of ATC may be critically contingent on repletion of deficits in Factor V, VIII, IX, and X, identifying specific factors of interest to target therapeutically and follow clinically. Confirming the clinical significance of the differences in clotting factor repletion observed in ‘low’ versus ‘high’ ratio transfusion, we also identified a statistically significant survival benefit to ‘low ratio’ transfusion in Kaplan-Meier survival analysis. Of critical importance, this was robust even when adjusted for elements of patient demographics, injury characteristics, and admission physiology that potentially confound this finding.
As with other single-center prospective studies examining the relationship between clotting factor studies and outcomes, several limitations are important to interpretation of this data. The clinical utility of targeting and/or monitoring individual factors suggested here is contingent upon specific definitions of critical levels of depletion for each of these factors, which remain uncharacterized. Rizoli et al. have suggested that depletion to a 30% level represents a critical threshold that should guide the appropriateness of plasma therapy;25 however, the data presented here suggest that not all coagulation factors are critical mediators of ATC, and that each factor may have its own independent level of critical depletion. Further study from a coagulopathy reversal point of view will require viscoelastic assays such as thromboelastography, as opposed to static PT and PTT measures as used here, to specifically map out these critical definitions using a functionally meaningful (and clinically applicable) endpoint; in parallel, identification of the specific importance of improved Factor V, VIII, IX, and X repletion by ‘low ratio’ resuscitation also suggests specific biochemical targets of interest in unraveling the basic science behind potential inflammomodulatory mechanisms of plasma-based therapy. Importantly, this study is also not powered to identify potential unintended consequences of empiric plasma administration risks; as is well-documented elsewhere, the risks of inappropriate plasma administration are ominous, particularly in patients who undergo early plasma-based resuscitation but ultimately do not require massive transfusion.26 Most importantly, the recognition of potential survival bias is critical in the interpretation of the data presented here.18 Specifically, a conventional crystalloid and RBC-based approach to trauma resuscitation (placing many patients who die early after admission in a ‘high’ RBC:FFP ratio category, and reducing the apparent mortality rate in patients who simply survive long enough to receive substantial plasma) biases towards the finding a survival benefit in the ‘low ratio’ group, while exclusion of patients who die before the 6h time point used to calculate ratios here (thereby excluding patients who may stand the greatest chance to benefit from early plasma therapy) subtly biases against a ‘low ratio’ benefit; the ultimate balance of these potential biases cannot be derived from these data, mandating cautious interpretation of the survival benefit to ‘low ratio’ resuscitation seen here.27 Only ‘gold standard’ randomized clinical trials will eliminate this bias; however, given the logistic, clinical, and ethical challenges that face these ongoing studies, we suggest that the data presented here provide an evidence base to justify hemostatic resuscitation as a defensible interim standard of care for critically-injured trauma patients requiring transfusion until the results of randomized controlled trials are available to more definitively guide therapy.
In conclusion, we here detail the natural history of coagulation factors in both non-transfused and transfused patients after severe traumatic injury. We further demonstrate that targeting ratios of RBC:FFP <=1.5:1 leads to earlier correction of PT and PTT, and earlier and prolonged repletion of specific clotting factor deficits compared to higher ratio transfusion strategies. Given both the unadjusted and injury-adjusted survival benefit seen in plasma based resuscitation despite the correction of only a subset of factors, we hypothesize that hemostatic resuscitation may additionally prevent and/or treat the endotheliopathy of trauma via direct inflammomodulatory effects. Further studies must be done to continue to prospectively and definitively evaluate specific clotting factor deficits to trigger both plasma-based and clotting factor-targeted therapy, as well as to identify the specific biochemical mechanisms of both endotheliopathy and the inflammomodulatory cascade after trauma, and its specific responses to plasma-based therapy.
Acknowledgments
Grants: Supported by NIH GM-085689 (MJC), and NIH T32 GM-08258-20 (MEK), and NIH T32 GM-008258-25 (LZK).
Footnotes
Conflicts of interest: None declared
REFERENCES
- 1.Eastridge BJ, Hardin M, Cantrell J, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma 2011;71:S4–8. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma 2003;55:39–44. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira PG, Inaba K, Salim A, et al. Preventable morbidity at a mature trauma center. Arch Surg 2009;144:536–41; discussion 541–2. [DOI] [PubMed] [Google Scholar]
- 4.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma 2003;54:1127–30. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg 2012;255:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma 2008;64:1459–63; discussion 1463–5. [DOI] [PubMed] [Google Scholar]
- 7.Biffl WL, Smith WR, Moore EE, et al. Evolution of a multidisciplinary clinical pathway for the management of unstable patients with pelvic fractures. Ann Surg 2001;233:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma 2007;62:112–9. [DOI] [PubMed] [Google Scholar]
- 9.Kashuk JL, Moore EE, Millikan JS, et al. Major abdominal vascular trauma--a unified approach. J Trauma 1982;22:672–9. [DOI] [PubMed] [Google Scholar]
- 10.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg 1983;197:532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RF, Dulchavsky SA, Soullier G, et al. Problems with 20 or more blood transfusions in 24 hours. Am Surg 1987;53:410–7. [PubMed] [Google Scholar]
- 12.Shaz BH, Dente CJ, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion 2010;50:493–500. [DOI] [PubMed] [Google Scholar]
- 13.Zink KA, Sambasivan CN, Holcomb JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 2009;197:565–70; discussion 570. [DOI] [PubMed] [Google Scholar]
- 14.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 2007;63:805–13. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg 2008;248:447–58. [DOI] [PubMed] [Google Scholar]
- 16.Spinella PC, Perkins JG, Grathwohl KW, et al. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma 2008;64:S69–77; discussion S77–8. [DOI] [PubMed] [Google Scholar]
- 17.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma 2008;65:261–70; discussion 270–1. [DOI] [PubMed] [Google Scholar]
- 18.Snyder CW, Weinberg JA, McGwin G Jr., et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma 2009;66:358–62; discussion 362–4. [DOI] [PubMed] [Google Scholar]
- 19.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg 2011;112:1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pati S, Matijevic N, Doursout MF, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma 2010;69 Suppl 1:S55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown LM, Aro SO, Cohen MJ, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma 2011;71:S358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg 2007;245:812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 2007;13:680–5. [DOI] [PubMed] [Google Scholar]
- 24.Kautza BC, Cohen MJ, Cuschieri J, et al. Changes in massive transfusion over time: an early shift in the right direction? J Trauma Acute Care Surg 2012;72:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizoli SB, Scarpelini S, Callum J, et al. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma 2011;71(5 Suppl 1):S427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg 2010;210:957–65. [DOI] [PubMed] [Google Scholar]
- 27.Ho AM, Dion PW, Yeung JH, et al. Prevalence of survivor bias in observational studies on fresh frozen plasma:erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology 2012;116:716–28. [DOI] [PubMed] [Google Scholar]