People with addiction differ from casual drug users by their uncontrollable drug intake despite the adverse consequences associated with drug use. The compulsive drug-seeking and drug-taking behaviors that define addiction are also observed in rodent models that were first established almost 2 decades ago. Studies have shown that there is a percentage of individuals in human and rodent populations that are susceptible to drug use while others are resilient (1,2). Susceptibility to developing any substance use disorder is influenced by genetic and epigenetic variation along with psychosocial factors. To date, despite the identification of genes involved in the process of addiction, there is a lack of knowledge about the molecular mechanisms that drive individual susceptibly or resiliency to the development of substance use disorders.
Decades of work on this subject have laboriously characterized the cellular and molecular changes induced by drugs in discrete regions of the brain. Much attention has been placed on the striatum, which serves as a key reward processing center in the mesolimbic dopamine system. All drugs of abuse engage dopamine pathways, which are critical for signaling incentive salience as well as reward learning and prediction (3). Therefore, vulnerability to addiction might involve members of the dopamine system that could alter dopamine neurotransmission in essential reward areas such as the nucleus accumbens (NAcc). In the current issue of Biological Psychiatry, Lee et al. (4) identify one dopamine receptor whose expression differs between mice that are susceptible and those that are resilient to cocaine intravenous self-administration (IVSA). Indeed, their study suggests that dopamine D2 receptor (D2R)–mediated regulation of NAcc cholinergic interneurons (ChINs) is crucial in establishing vulnerability to cocaine use (4).
These data are supported by human imaging studies that have found a substantial reduction of D2R availability in the striatum, including the NAcc (5). The novelty in this study also resides in the specific effect of IVSA on D2R expression in the ChINs. D2R, together with the D1R, is highly expressed and localized in the major cell type and sole output neurons of the striatum, the medium spiny neurons (MSNs). However, Lee et al. (4) focus on D2R expression in ChINs, which make up only 1% to 2% of striatal neurons. Despite their low abundance, ChINs form dense arborizations that allow for numerous connections to be made with MSNs, afferent fibers, and other striatal interneurons; ChIN activity profoundly modulates striatal output through acetylcholine (ACh) release to these targets. The activity of ChINs is under negative and positive dopaminergic regulation by D2R and D5R, respectively (6). Thus, the induction of dopamine signaling by drugs of abuse invariably modulates ChIN activity, which in turn affects striatal output.
Lee et al. (4) used an IVSA protocol to identify mice that demonstrated either high or low motivation to IVSA based on their breakpoint in a progressive ratio schedule of reinforcement. IVSA together with progressive ratio schedules are powerful models that are useful for measuring the reinforcing efficacy of drugs of abuse. In doing so, the authors succeeded in identifying 2 groups of mice: a high-motivation group (cocaine susceptible) and a low-motivation group (cocaine resilient). Interestingly, cocaine-susceptible mice showed increased drug consumption despite receiving a shock at the time of self-administration, as previously observed (1,2). Cocaine-susceptible mice also showed increased drug-seeking behavior during an extinction protocol compared with cocaine-resilient mice. These results convincingly support previous reports and show that susceptibility and resiliency are observed in mice using this IVSA protocol.
ChINs are also called tonically active neurons because of their constant activity, which is regulated by an astounding number of neuromodulators and neurotransmitters (6). Some of these inputs induce pausing of ChIN’s tonic activity, which is believed to be critical for encoding the salience value of external stimuli. In particular, loss of D2R signaling in these interneurons impairs their pausing (7). Lee et al. (4) used single-unit recording to measure the activity of NAcc ChINs in cocaine-susceptible and cocaine-resilient mice. They observed that cocaine increased ChIN activity in both drug-naïve and resilient mice while, on the contrary, susceptible mice showed diminished ChIN activity.
Cocaine intake leads to alterations in gene expression in the NAcc, although the transcriptomic changes in the ChINs have remained elusive because of their low abundance. Lee et al. (4) performed RNA sequencing on isolated ChINs from the NAcc of mice after a progressive ratio schedule. This led to the identification of 2909 differentially expressed genes when comparing cocaine-susceptible and cocaine-resilient mice. Pathway analyses indicated that genes involved in dopaminergic signaling were among the most differentially expressed between the susceptible and resilient groups. Interestingly, Drd2, which encodes D2R, was found to be upregulated in cocaine-susceptible mice compared with cocaine-resilient mice.
The authors also noted a correlation between cocaine susceptibility, ChIN firing rate, and Drd2 expression (4). Indeed, cocaine-susceptible mice had reduced ChIN firing and higher Drd2 expression compared with cocaine-resilient mice, which showed increased ChIN firing and lower Drd2 expression. Together, these results support a critical role for D2R signaling in the control of ChIN activity and cocaine effects in IVSA.
To determine if D2R signaling in ChINs is both necessary and sufficient for the control of IVSA, Lee et al. (4) used overexpression and knockdown strategies targeting D2R in NAcc ChINs. They showed that D2R overexpression reduces the firing rate upon activation using a D2R-specific ligand, while D2R knockdown prevents this firing rate reduction. In addition, D2R overexpression in NAcc ChINs or the chemogenetic silencing of these neurons shows increased cocaine susceptibility, while D2R knockdown causes increased cocaine resiliency. Their chemogenetic results are at odds with previously reported data in which optogenetic silencing of ChIN disrupted cocaine-induced conditioned place preference (8). However, it is important to note that conditioned place preference and IVSA have different time frames and the experimental conditions between chemogenetics and optogenetics differ, which likely explains these diverging results. In line with data from Lee et al. (4), studies performed on ChIN-specific D2R knockout mice showed that the selective ablation of D2R in ChINs disrupts cocaine-induced conditioned place preference (9).
Repeated administration of cocaine increases dendritic spine density and long-term potentiation in MSNs (10). Interestingly, mice that overexpress D2R in NAcc ChINs show increased dendritic spiny density in D1R-MSNs compared with control mice in the absence of cocaine exposure (4). The NAcc is home to many glutamatergic inputs from the ventral tegmental area, cortex, thalamus, basolateral amygdala (BLA), and the ventral hippocampus that might drive striatal long-term potentiation and are important for reinforcement learning, pyschostimulation, and context value processing. Lee et al. (4) found that overexpression of D2R in NAcc ChINs strengthened D1-MSN excitatory postsynaptic currents induced by optogenetic stimulation of the BLA–NAcc pathway, but not of the ventral hippocampus–NAcc pathway. The authors also found that D2R knockdown in NAcc ChINs increases the D1/D2 excitatory postsynaptic current amplitude ratio in the ventral hippocampus–NAcc pathway and attenuates the D1/D2 excitatory postsynaptic current amplitude ratio in the BLA–NAcc pathway after 5 days of noncontingent cocaine treatment. It remains unknown which type of ACh receptors and what other inputs might drive these effects in MSNs. In addition to direct D1R-MSN modulation, ACh could also be affecting afferents from the cortex, thalamus, and ventral tegmental area, which may collectively alter the release of GABA (gamma-aminobutyric acid), glutamate, and dopamine. Muscarinic M4 receptors, which are enriched in the striatum, might be a candidate because they are expressed at varying levels in each of these regions and because blocking this receptor subtype in mice lacking D2R in ChINs (9) is sufficient to restore deficits in the psychomotor and rewarding effects of cocaine.
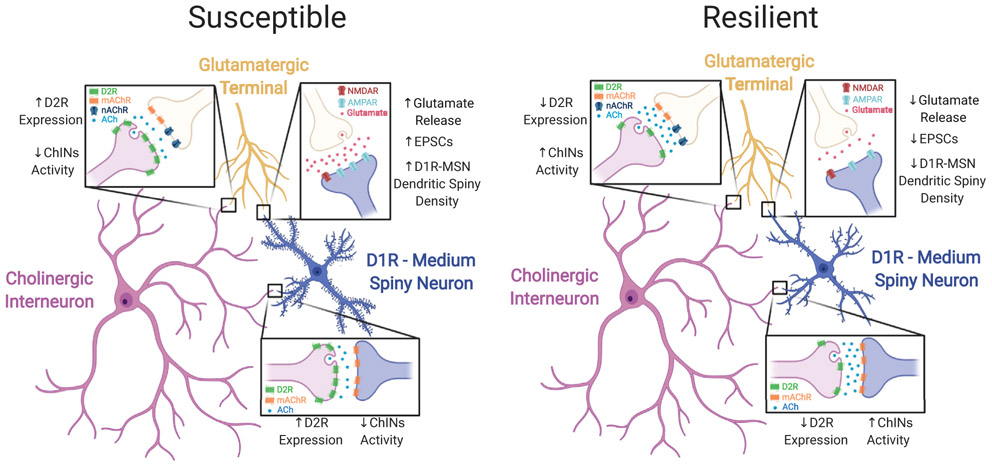
In conclusion, the study by Lee et al. (4) provides new evidence for a causal relationship between the dopaminergic control of NAcc ChINs and cocaine addiction susceptibility (Figure 1). Transcriptomic analyses support a critical role for gene expression in NAcc ChINs that differentiate cocaine-susceptible and cocaine-resilient mice. Strikingly, the expression of D2R in the ChINs and therefore the control of ACh signaling to MSNs and possibly other striatal convergent fibers appears necessary and sufficient to control cocaine susceptibility. Future studies aimed at understanding the precise mechanisms by which D2R expression is regulated in ChINs of susceptible animals will be critical to unraveling the molecular steps involved in the transition from casual drug use to abuse. Still, a burning question remains: is this mechanism and involvement of D2R in ChINs a property of other substances of abuse? It is tempting to speculate that a common feature of drugs is to alter ACh tone and disrupt the striatal dopamine/ACh balance. Thus, the results of this study may provide new insights for therapeutic strategies for the treatment and prevention of substance use disorders.
Figure 1.
Proposed model of cocaine self-administration susceptibility. Mice that are susceptible to cocaine intravenous self-administration have increased dopamine D2 receptor (D2R) expression in cholinergic interneurons (ChIN) of the nucleus accumbens compared with mice that are cocaine resilient. Elevated D2R expression in cocaine-susceptible mice lowers acetylcholine (ACh) levels, while decreased D2R expression may result in higher ACh levels in cocaine-resilient mice. The D2R-mediated control of ACh signaling may affect the activity of dopamine neurons, D1R-expressing medium spiny neurons (D1R-MSNs), and glutamatergic inputs from areas including the cortex, basolateral amygdala, and ventral hippocampus. Altered levels of ACh signaling could affect the synaptic strength of D1R-MSNs by directly inhibiting these neurons or modulating the activity of their glutamatergic inputs through the activation of muscarinic ACh receptors (mAChRs) and nicotinic ACh receptors (nAChRs). (Figure created using BioRender.com.) AMPAR, AMPA receptor; EPSC, excitatory postsynaptic current; NMDAR, NMDA receptor.
Acknowledgments and Disclosures
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) (to EB) and by a University of California, Irvine School of Medicine Dean’s Fellowship, the Dr. Lorna Carlin Scholar Award, a University of California, Irvine Graduate Dean’s Dissertation Fellowship, and National Institute on Drug Abuse T32 Predoctoral Training Grant in Substance Use and Use Disorders (Grant No. DA050558-01) (to RGL).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Deroche-Gamonet V, Belin D, Piazza PV (2004): Evidence for addiction-like behavior in the rat. Science 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- 2.Vanderschuren LJMJ, Everitt BJ (2004): Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019. [DOI] [PubMed] [Google Scholar]
- 3.Berridge KC, Robinson TE (2003): Parsing reward. Trends Neurosci 26:507–513. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Ribeiro EA, Kim J, Ko B, Kronman H, Jeong YH, et al. (2020): Dopaminergic regulation of nucleus accumbens cholinergic interneurons demarcates susceptibility to cocaine addiction. Biol Psychiatry 88:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Fowler JS, Wang G-J, Swanson JM (2004): Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol Psychiatry 9:557–569. [DOI] [PubMed] [Google Scholar]
- 6.Lim SAO, Kang UJ, McGehee DS (2014): Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharkwal G, Brami-Cherrier K, Lizardi-Ortiz JE, Nelson AB, Ramos M, Del Barrio D, et al. (2016): Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic interneurons. Neuron 91:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witten IB, Lin S-C, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. (2010): Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330:1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis RG, Serra M, Radl D, Gori M, Tran C, Michalak SE, et al. (2020): Dopaminergic control of striatal cholinergic interneurons underlies cocaine-induced psychostimulation. Cell Rep 31:107527. [DOI] [PubMed] [Google Scholar]
- 10.Lee K-W, Kim Y, Kim AM, Helmin K, Naim AC, Greengard P (2006): Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A 103:3399–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]