Abstract
Background:
Older adult delirium is often unrecognized in the emergency department (ED), yet the most compelling research questions to overcome knowledge-to-practice deficits remain undefined. The Geriatric Emergency care Applied Research (GEAR) Network was organized to identify and prioritize delirium clinical questions.
Methods:
GEAR identified and engaged 49 transdisciplinary stakeholders including emergency physicians, geriatricians, nurses, social workers, pharmacists, and patient advocates. Adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews, clinical questions were derived, medical librarian electronic searches were conducted, and applicable research evidence was synthesized for ED delirium detection, prevention, and management. The scoping review served as the foundation for a consensus conference to identify the highest priority research foci.
Results:
In the scoping review, 27 delirium detection “instruments” were described in 48 ED studies and used variable criterion standards with the result of delirium prevalence ranging from 6% to 38%. Clinician gestalt was the most common “instrument” evaluated with sensitivity ranging from 0% to 81% and specificity from 65% to 100%. For delirium management, 15 relevant studies were identified, including one randomized controlled trial. Some intervention studies targeted clinicians via education and others used clinical pathways. Three medications were evaluated to reduce or prevent ED delirium. No intervention consistently prevented or treated delirium. After reviewing the scoping review results, the GEAR stakeholders identified ED delirium prevention interventions not reliant on additional nurse or physician effort as the highest priority research.
Conclusions:
Transdisciplinary stakeholders prioritize ED delirium prevention studies that are not reliant on health care worker tasks instead of alternative research directions such as defining etiologic delirium phenotypes to target prevention or intervention strategies.
Delirium occurs in 8% to 10% of older adults in the emergency department (ED) and is the underlying reason for about 1.5 million ED visits annually in the United States.1–4 Delirium manifests clinically as sudden onset neuropsychiatric dysfunction with a waxing and waning course characterized by inattention, disorganized thinking, and changes in level of consciousness. Delirium is not attributable to an established neurocognitive disorder, but is a direct consequence of one or more medical conditions.5 Older adults are susceptible to developing delirium while in the ED.6,7 Delirium is often missed in the ED, because emergency staff are only 35% sensitive in detecting delirium.1,8–12 Even when recognized, delirium may lead to prolonged hospital stay, functional decline, accelerated cognitive decline, and postdischarge depression.13–16 When unrecognized in the ED, delirium is associated with increased 6-month mortality.8
In 2007, the Society for Academic Emergency Medicine (SAEM) Geriatric Task Force identified cognitive impairment (including delirium) as one of three conditions with substantial quality gaps for geriatric ED patients.17 In 2010, this SAEM Task Force identified four high-priority delirium research foci: 1) optimal methods of screening for and diagnosing delirium in the ED, 2) risk factors for delirium in the ED and interventions for prevention and moderation, 3) criteria for safe discharge for older ED patients with delirium, and 4) interventions to improve outcomes for older patients with delirium in the ED.18 In response, efforts to improve the timely recognition and management of older ED patient delirium have developed. These ED-focused responses include resident core competencies,19 multiorganizational operational guidelines,20 and quality indicators.21,22 Despite these efforts, the best practices for preventing, identifying, and managing delirium in the ED are still unknown.
The Geriatric Emergency care Applied Research (GEAR) Network is an interdisciplinary group of clinicians and researchers focused on the identification and execution of high-impact research questions for geriatric emergency medicine domains with substantial and clinically important knowledge gaps. GEAR has addressed questions in medication safety, elder abuse, falls, and care transitions using a framework of patient–intervention–control–outcome (PICO questions), scoping literature review, and building consensus on best practices and areas of future research.23 The primary objective was to prioritize high-yield older adult delirium research questions for the ED setting through an evidence-based consensus statement. A secondary objective was to report ED best practices for delirium prevention, recognition, and treatment based on a scoping review of published emergency medicine research.
METHODS
Study Design
Participating GEAR researchers were identified by their active membership in geriatric emergency medicine interest groups (within the SAEM, American Geriatrics Society, and Gerontological Society of America) and prior pertinent published research. GEAR researchers chose to participate in one of five subgroups as determined by the GEAR core team: cognitive impairment, medication safety, falls, geriatric abuse, and care transitions. The GEAR Cognitive Impairment (GEAR CI) subgroup included three geriatricians (UO, WWH, LAL); four emergency physicians (CRC, SMD, MS, UH); three research assistants (NH, EAL, DW); and one PhD researcher (KJK) with expertise in geriatrics, gerontology, and health services research.
The GEAR CI subgroup performed a scoping review that adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) reporting guidelines.24 The 14 GEAR CI members participated in monthly teleconferences to derive pertinent PICO questions, prioritize the PICO questions, derive a reproducible search strategy, independently filter the results of the electronic search, abstract key study data from the studies that met inclusion criteria, and synthesize the research findings into best practices based on currently available research.
PICO Questions
The GEAR CI subgroup derived and refined the following PICO questions:
PICO-1
Population: ≥65-year-old ED patients from any pre-ED setting;
Intervention: Risk stratification for delirium during ED episode of care using feasible and validated instrument;
Comparison: Clinician gestalt for delirium;
Outcome: Sensitivity, specificity, likelihood ratio, receiver operating characteristic area under the curve (ROC AUC), association of delirium identification with ED revisits and mortality, comparison of claims data delirium incidence with prospective ED study data.
PICO-2
Population: ≥65-year-old ED patients identified as high-risk for delirium during ED episode of care;
Intervention: Action to reduce the occurrence (i.e. prevent), duration, or severity of prevalent or incident delirium;
Comparison: Standard care;
Outcome: ED length of stay, hospital length of stay, ED returns, quality of life, mortality at 1 year.
PICO-3
Population: ≥65-year-old ED patients from any pre-ED setting;
Intervention: Risk stratification for dementia during ED episode of care using feasible and validated instrument;
Comparison: Clinician gestalt for dementia;
Outcome: Sensitivity, specificity, likelihood ratio, ROC AUC, association of dementia with ED revisit rates, comparison of claims data dementia incidence with prospective ED study data.
PICO-4
Population: ≥65-year-old ED patients identified as high risk for dementia during ED episode of care;
Intervention: Referral for definitive dementia diagnostic testing;
Comparison: Standard care;
Outcome: ED length of stay, ED returns, quality of life, institutionalization at 1 year.
The GEAR CI subgroup identified exemplar articles for each PICO question and surveyed 33 GEAR investigators from all five cores to prioritize the PICO questions via teleconference discussions and online voting. PICO questions 1 and 2 were selected as highest priority.
Search Strategy
Based on PICO questions 1 and 2 as well as exemplar articles that a search should identify, a medical librarian (MD) created electronic search strategies for OVID MEDLINE, Embase, CINAHL, and CENTRAL from inception through November 2019. Full details of the search terms are provided in Data Supplement S1, Appendix S1 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14166/full).
Study Selection and Data Abstraction
Two authors independently reviewed the titles and abstracts for the search results of each PICO question (PICO-1 = CRC, KJK; PICO-2 = CRC, UO). Inclusion criteria for PICO-1 were adults over age 65 with quantitative evaluation of one or more delirium identification methods in ED settings. Inclusion criteria for PICO-2 studies were one or more intervention(s) intended to improve the identification or outcomes of delirium in ED patients over age 65. No publication date or language exclusion criteria were applied. Unweighted Cohen’s kappa (κ) was used to quantify interrater agreement. An adjudicator (UH) was named a priori to resolve continued interrater discrepancies.
Two authors (PICO-1 = CRC, NH; PICO-2 = CRC, EAL) reviewed the selected articles and conference abstracts independently collecting key elements using a predesigned template. Information abstracted included the study setting, inclusion/exclusion criteria, study design, comparator reference standard or control group, and primary/secondary outcomes. The first authors of unpublished abstracts were contacted to determine whether that research has since been published. Manuscript and abstract authors were also contacted for additional study details if key elements could not be abstracted from the publication. Studies reporting none of the PICO outcomes were excluded. Data abstractors identified additional studies for potential inclusion by reviewing full-text articles’ references.
Synthesis of Best Practices and Building Consensus
Abstracted data were independently reviewed and summarized by three authors (UH, UO, SMD). We reported preliminary results to the CI subgroup, refined concepts and recommendations via e-mail and teleconference, and presented at an in-person consensus conference in October 2019 that included the entire GEAR network. The GEAR CI subgroup presented recommendations for prioritizing ED delirium research and GEAR Consensus Conference attendees voted on the final rank order of research priorities. Two non-emergency medicine delirium experts subsequently reviewed, edited, and endorsed a statement regarding the impact of the scoping review by the CI subgroup. Two patient, family, and caregiver representatives (LH, LN) reviewed this scoping review and PowerPoint presentations from the GEAR Consensus Conference and then provided their perspectives via e-mail and teleconference calls.
RESULTS
Evidence Synthesis
PICO-1: Delirium Risk Stratification.
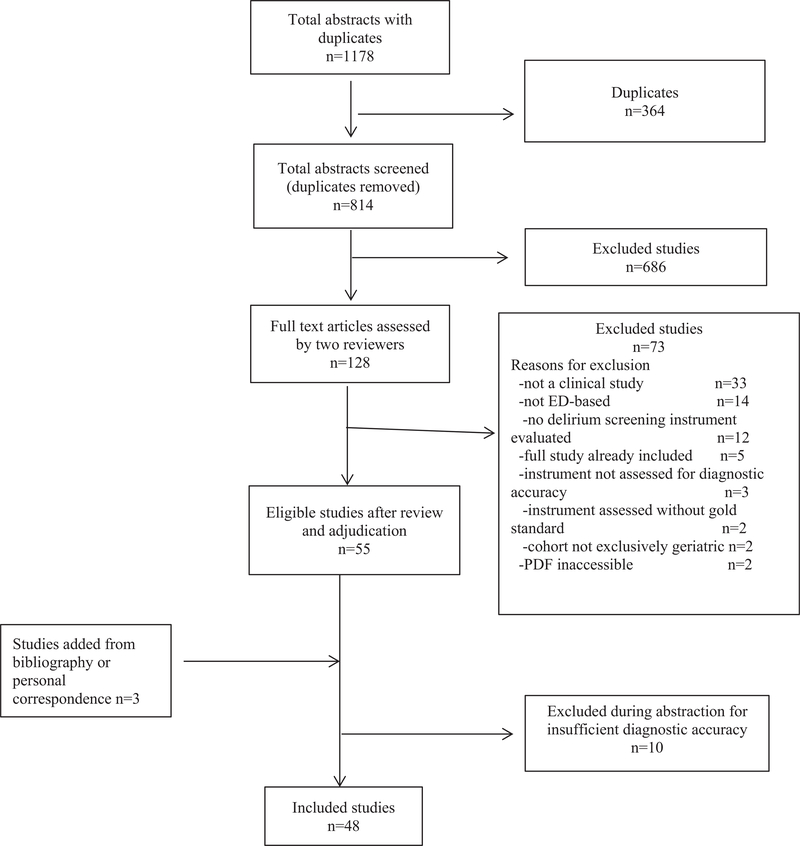
After duplicates were removed, the literature search identified 814 potential studies. One study was identified when one author served as an invited peer reviewer for a journal submission. The initial independent review demonstrated moderate agreement (κ = 0.50, 95% confidence interval [CI] = 0.41 to 0.59).25 After full-text review, adjudication, and abstraction, 48 articles were included in the scoping review (see Figure 1 for details of the inclusion and exclusion of these studies).24
Figure 1.
Summary of scoping review results for delirium screening for older adults in emergency settings.
Study Characteristics.
Of the 48 selected studies, eight were reported only as conference abstracts.26–33 These studies analyzed 27 different instruments’ accuracy to risk stratify for delirium. The most commonly evaluated stratification tool was clinician (nurse or physician) judgment (“gestalt”; studied in 12 papers).11,12,27,28,30,32,34–39 The next most commonly evaluated instruments were the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU; five),39–43 Confusion Assessment Method (CAM; four),27,29,44,45 Brief Confusion Assessment Method (bCAM; four),46–49 Richmond Agitation Sedation Scale (RASS; four),50–53 4AT (three),29,54,55 and the tablet “serious game” (three).56–58
Participant Characteristics.
The sum of study populations totaled 15,860 patients and two systematic reviews of 12 ED-based studies.45,59 One group published seven manuscripts reporting diagnostic accuracy of different delirium screening instruments on the same population, so these patients are only counted once.42,47,48,51,52,60,61 Patients were recruited from 1992 to 2016 and the manuscripts were published between 1995 and 2019. The majority of studies were prospective observational (35),11,12,26,27,30,32,34–36,38,40–43, 46–52,54–58,60–68 followed by chart review (three),37,39,69 before/after (two),31,70 case–control (one),71 randomized controlled trial (one),29 and a mixed prospective/retrospective study (one).53 Three studies failed to report their research design.28,33,44
Delirium Screening Instrument Characteristics.
Reported delirium prevalence in the ED ranged from 6% to 38%.30,70 Sensitivity of the instruments ranged from 0% for clinician documentation27 to 64% for 4AT.53 Specificity of the instruments reported ranged from 27% for the Bergman-Paris question67 to 100% for clinician gestalt.12 The most commonly used reference standards were the CAM (18);12,26–28,30,32,33,36,38,54, 56–58,62,64,66,67,70 Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria applied by neurologist, geriatrician, or psychiatrist (16);29,39,42,44,46–48,50,51, 53,55,60,61,65,68,69 and the CAM-ICU (three).43,52,63 Two original data studies did not report their reference standard31,37 (for a summary table of each included study’s population, inclusion/exclusion criteria, study design, reference standard, and diagnostic accuracy outcomes, see Data Supplement S2, Appendix S2). The components and original derivation or validation study for each delirium instrument are provided in Data Supplement S3, Appendix S3 (reproduced with permission from their original sources), while Data Supplement S4, Appendix S4, provides pragmatic instrument-level details regarding diagnostic accuracy, time to administer, and potential advantages or disadvantages for ED use.
Clinician gestalt was the most commonly studied risk assessment method for delirium. However, clinician gestalt varies significantly depending on how “gestalt” is measured and which physician’s gestalt is measured. Sensitivity is as low as 0%27,32 (when relying on a diagnosis recorded in the medical record) or as high as 81% when prospectively asking emergency physicians if they believe there is more than a 5% chance that their patient has delirium.30 Specificity ranges from 65% (when prospectively asking an emergency physician if they believe there is more than a 5% chance their patient has delirium) to 100% when delirium is documented in the medical record.12
Time on task for formal screening tools range from 20 seconds (Delirium Triage Screen)47 to 20 minutes (CAM).72 RASS (1 minute) and bCAM (2 minutes) demonstrated intermediate-range times on task. Interrater reliability was measured in 11 studies: CAM (κ = 0.7–0.91),70 bCAM (κ = 0.87–0.88),46–48 individual questions on the bCAM (κ = 0.25–0.50),48 CAM-ICU (κ = 0.92),42 mCAM-ED (κ = 0.79),35 “single question” (κ = 0.75),61 and RASS (weighted κ = 0.63).51 For the “serious game,” test–retest reliability was reported by Spearman’s rho and ranged 0.56 to 0.85.57
PICO-2: Delirium Interventions to Reduce the Occurrence (i.e., Prevent), Duration, or Severity of Delirium.
The literature search identified 761 potential studies (see Figure 2 for details of the inclusion and exclusion of these studies).24 The initial independent review of the raw search results demonstrated fair agreement (κ = 0.36, 95% CI = 0.22 to 0.50).25 After full-text review, adjudication, and abstraction, 15 “intervention” studies were included in the scoping review.
Figure 2.
Summary of scoping review results for delirium interventions for older adults in emergency settings.
Study Characteristics.
All included studies had interventions implemented in the ED from 1996 to 2015 and published between 2001 and 2018. Among the 15 studies, one was a randomized, placebo-controlled trial73 while five were retrospective observational and pre-/post- studies.74–78 Seven of the studies were prospective observational and pre-/post- studies,1,34,79–83 while another was a case–control study84 and another was a pre-/post- survey following a workshop85 (for a summary table of each included study’s population, inclusion/exclusion criteria, study design, intervention, and outcomes see Data Supplement S5, Appendix S5).
Participant Characteristics.
The sum of study populations totaled 9,507 individuals. However, significant between-study differences in subjects recruited exist. For example, some interventions focused on older adults admitted for acute medical illnesses,81 whereas others were solely hip fracture patients77,80,82–84 and others were critically ill patients admitted to the ICU from the ED.79 In other studies, the subjects were members of the health care team.85 For these studies focusing on health care team subjects, the numbers and characteristics of ED nurses and physicians who participated in workshops and grand rounds were not reported.
Intervention Characteristics and Outcomes
Study intervention fell into three different categories. The first consisted of clinician-targeted interventions, ranging from educational interventions for nurses and physicians81,82,85 to provision of reminders to providers.78 Second were care process interventions consisting of implementation of emergency care bundles,83 clinical pathways,80,84 screening processes,34 and provision of screening results to physicians.1 Other care process interventions included patient evaluations in the ED by geriatricians and interdisciplinary team rounds75,76 or early hospitalization or admission to the acute geriatric unit when delirium was recognized.74 Finally, the third category was pharmacologic interventions which included an adapted pain management pathway84 and use of antipsychotics (haloperidol and blonanserin).73,79
Primary outcomes included detection and incidence of delirium in seven studies,34,73,75–77,83,84 change in patient’s care plan measured by alteration in disposition or additional diagnostic evaluation in two studies,1,81 and repeat ED visits and hospital readmissions in one study.74 Primary outcomes were not defined in five of the studies.78–82 Secondary outcomes studied include severity and duration of delirium;73 secondary complications such as falls, fractures, aspiration pneumonias, and mortality;34 and health care utilization characteristics such as ED revisits, hospital readmissions and associated cost, length of stay, or placement in a long-term care facility.1,34,73,74 Other secondary outcomes studied included reduction in pain84 and referral for a geriatric assessment at home.85 Eight of the studies did not report any secondary outcomes.
Clinician Targeted Interventions
Among the interventions, there were six provider-targeted interventions. Three of these interventions focused on provider education through workshops, grand rounds, and small group meetings.81,82,85 Two of the educational interventions also included implementation of process changes.34,80 The other three provider interventions focused on provider reminders for delirium screening and provision of screening results to the physicians.1,78 Two before–after retrospective studies by the same investigators evaluated geriatrician care in the ED for Parkinson’s disease patients.75,76 Among the educational interventions was a 1-day workshop for clinicians that was associated with an improvement in the outcomes of increased delirium screening (p = 0.006) and referral for home care and geriatrics evaluation.85 Educational interventions were also significantly associated with reduced delirium duration from a median of 4 days to 1 day and reduced severity by 2.94 points using a modification of CAM,82 reduced use of benzodiazepines (p < 0.01) and antihistamines (p < 0.05), and reduced length of stay. Naughton et al.81 used small group meetings between the ED and an acute geriatrics unit with audit and feedback of nurses to reduce delirium and estimated that each case of delirium prevented saved a mean of 3.4 hospital days. An educational intervention paired with development and preferential admission to acute geriatric unit for patients with cognitive impairment or delirium was associated with a decrease in prevalent delirium on hospital day 4 from 41% to 19%.81 Among interventions focused on providing reminders to providers, no significant increase in proportion of patients diagnosed with delirium,1 change in patient care plans by emergency physicians,1 nor reduction in secondary complications of delirium postintervention were observed.34 Using an electronic medical record reminder for ED triage nurses to obtain RASS and bCAM with an automatic physician alert when delirium screening was positive, Delaney et al.78 demonstrated a reduction in 30-day ED return visits from 90 to 35 per month.
Care Process Interventions
These studies focused on establishing clinical pathways and implementation of emergency care bundles along with interdisciplinary team rounds and geriatrician ED visit.74–76,80,83 For example, the pathway described by Fleury et al.80 for ED patients with suspected proximal femur fractures included imaging protocols and requisite labs followed by a geriatric consult “to detect, treat, or prevent delirium.” The description of components of emergency care bundles by Thomson et al.75,76 are completely lacking and are only evaluated in individuals with Parkinson’s disease so may not be applicable to other populations. Emergency care bundles were associated with increased delirium detection rates from 22% to 29%, reductions in hospital length of stay from 16 to 11 days, and reduced mortality from 24% to 14% for patients with identified delirium.75,76,80 Other care processes evaluated included maintaining oxygen saturation, intravenous fluids, preventing hypotension, and temperature changes.83 A multifactorial protocol extending from the community prehospital through the ED to postoperative settings for patients with suspected hip fracture was associated with lower rates of postoperative delirium (33% to 21%) and more rapid ED-to-ward time (4.6 to 2.7 hours).83 In one observational study using the National Health Insurance Research Database examining 2,780 ED visits for patients with delirium between 2000 and 2008, decisions to admit patients with delirium at the initial ED visit reduced ED returns and subsequent hospitalizations within 28 days when compared with discharge home during the initial ED evaluation.74
Pharmacologic Interventions
Three medications were explored to reduce or prevent ED delirium: haloperidol,73 blonanserin,79 and regional anesthesia.84 Neither haloperidol nor opioid analgesics demonstrated significant reductions in delirium incidence or severity. On the other hand, an open-label, noncontrolled, nonrandomized trial of blonanserin reduced hyperactive delirium by 33% and mixed delirium by 35%.79 Patients who received scheduled prophylactic haloperidol had more severe and longer duration of delirium.73 LeBlanc et al.84 described no significant decrease in delirium for hip fracture patients receiving a nerve block compared with opioid therapy.
Consensus of Highest-yield ED Delirium Research Priorities
Based on the scoping review above and prior to the consensus conference the GEAR CI subgroup attained agreement on the following primary research objectives in order of highest to lowest priority with the objective to accelerate the identification and reduction of ED delirium:
Phenotyping: Define ED delirium phenotypes based on clinical features (presenting complaint, underlying cognitive frailty, and suspected delirium precipitant) and/or readily available delirium biomarkers.86–88 Then, by phenotype and severity89,90 stratify delirium identification strategy accuracy and intervention effectiveness.
Building for Implementation: Evaluate the pragmatic reproducibility, fidelity, adaptability, and sustainability91 of different ED delirium detection protocols ranging from nurse/physician education to trained volunteers, computer-based screening, and family report.
Phenotype-targeted Interventions: Rather than apply monomorphic intervention(s) to every delirium identified, base ED intervention studies on biologically plausible delirium precipitant informed by delirium phenotype research and ED-validated delirium identification strategies.
Transdisciplinary Expansion: Harmonize delirium identification and phenotype-guided intervention strategies across medical and surgical specialties to build the foundation for more transparent interdisciplinary comparative analyses and catalyze meaningful transdisciplinary research.92,93
Organized Knowledge Acquisition: Develop a Pediatric Emergency Care Applied Research Network (PECARN)-like multi-institutional data repository of ED delirium cases identified using the same methods permitting stratification by delirium phenotype.94 The data repository would include key prognostic variables (dementia,95 frailty96); pathophysiology-guided interventions; and both process-and patient-centered outcomes including the burden of delirium on caregivers.97
During the consensus conference, attendees added to, reworded, and reranked the proposed research priorities. The GEAR work group included 49 members. Those who were able to attend the consensus conference in person voted on research priorities using polleverywhere.com. Those who did not attend completed their rankings through a RedCap Survey link that was e-mailed. There was 100% voting participation by all 49 members of the task force. Table 1 represents the final GEAR research recommendations for ED delirium care.
Table 1.
Consensus Conference Ranking of ED Delirium Research Priorities
| Research Priority Rank—Descriptor |
| 1—Prevention focus including developing screening instrument/risk score that does not entail additional nurse/physician workload |
| 2—Testing delirium prevention strategies in ED once high-risk individuals identified |
| 3—Identifying high-risk patients for cognitive impairment (lumping delirium and dementia together) and then unique care bundles depending on dementia, delirium, or delirium superimposed on dementia |
| 4- Build a multi-institutional data repository and research infrastructure for delirium hypothesis testing similar to PECARN |
| 5- Structured implementation science evaluation of ED delirium detection and intervention strategy pragmatic reproducibility, fidelity, adaptability, and sustainability |
| 6—Identifying delirium phenotypes via pathophysiology, anticipated disease trajectory, anticipated response to interventions, and accuracy of different ED screening instruments |
| 7—Targeting delirium prevention or treatment interventions based on delirium phenotypes based on biologic plausibility of effect and pathophysiologic etiology of episode delirium |
DISCUSSION
This PRISMA-ScR adherent scoping review and GEAR consensus process provides a rigorous approach to defining the current state of ED delirium research, while outlining a high-yield path forward for multiple stakeholders. Delirium is prevalent, harmful, detectable, and potentially preventable in the ED, but will require a shift from tool-based research to pragmatic interventional trials.98–100 Over two dozen ED delirium screening instruments exist of varying complexity and levels of validation. Unfortunately, many delirium-screening studies focus on diagnostic accuracy without measuring or contemplating health care personnel acceptance, incorporation into practice, or sustainability of use in the busy ED.100 Although researchers from non-ED settings have evaluated psychometric properties such as tool development methods, reliability, validity, feasibility, and implementation, these delirium screening instrument properties are rarely evaluated in emergency medicine settings.101 The psychometric properties of delirium screening instruments from non-ED settings in non-English languages have also been studied.102,103
Emergency department delirium instrument diagnostic accuracy studies also rarely cite adherence to Standards for Reporting Diagnostic Accuracy (STARD) guidelines calling into question the rigor of the studies,104,105 notably the risk of common biases that skew observed sensitivity/specificity such as incorporation bias, partial verification bias, differential verification bias, imperfect criterion standard bias, or spectrum effect.106 The relevance of these diagnostic biases for clinicians and researchers is that the true sensitivity and specificity of delirium screening instruments is unknown. If researchers minimized or eliminated these biases, sensitivity and specificity may be higher or lower than reported. In addition, delineating accuracy (the ability to distinguish those with delirium from those without) is the lowest tier of diagnostic research. More impactful research is required to determine whether using any of the ED delirium instruments identifies a subset of delirium patients not previously recognized or whether that newly identified subset of delirium patients can be positively impacted in any measurable way by any intervention.107,108 Once a diagnostic screening instrument demonstrates accuracy, randomized controlled trials are helpful to evaluate patient-centric benefit or harm.108 Thus far, these types of efficacy trials for ED delirium-screening instruments do not exist. As an example of this diagnostic impact research, brain natriuretic peptide (BNP) is undeniably accurate to distinguish congestive heart failure from other causes of acute decompensated dyspnea, but multiple trials randomizing ED physicians to be aware of the BNP result or to not be aware of it demonstrate no consistent differences in length of stay, admission rates, mortality, or readmission rates.109
Gestalt is the most frequently evaluated approach to identify delirium and has the advantage of not adding burden to the clinician workload. However, significant interphysician variability exists so a role for an accurate and reliable delirium instrument exists. Among existing delirium screening instruments, CAM has been most extensively validated for accuracy in hospital settings with 94% sensitivity and 89% specificity,110 but far fewer ED studies exist.27,29,44,71 The time and equipment (photos, training) required to administer CAM in real-world ED settings is an inconvenient but often ignored reality between inpatient and ED environments that limits feasibility and acceptance of CAM. ED health care teams, which have limited time and are often interrupted while performing tasks, judge usability on brevity and accuracy.111 CAM requires more time and training to administer, so ED providers may sacrifice a degree of accuracy for feasibility.72 For example, bCAM requires 2 minutes to administer with a positive likelihood ratio of 10 to 20 that is quite accurate to rule-in delirium.46,47 The Delirium Triage Screen requires 20 seconds to administer with a negative likelihood ratio of 0.04, which is quite accurate to rule out delirium.47
Furthermore, ED delirium intervention studies depend on accurate ED delirium screening instruments, yet ED-specific validity of the tools to distinguish patients with delirium is rarely reported in intervention studies. Interventional trials to reduce the occurrence or duration of delirium during or immediately following an episode of ED care are limited in number and varied in interventional focus, populations evaluated, and outcomes assessed. The handful of ED delirium intervention studies explores a variety of unrelated interventions on heterogeneous patient populations with different outcomes assessed at variable time frames postintervention. Almost every interventional study is a nonrandomized trial design with significant risk of bias for any measured effects (or lack thereof). Clinician-targeted interventions are associated with increasing rates of delirium screening and referral to home care and geriatrics evaluation, but unclear impact on patient-oriented outcomes.
There is a paucity of evidence for effective ED-based prevention and treatment strategies for delirium. This lack of evidence stands in stark contrast to evidence on delirium prevention and management for ICU, ward, and postoperative settings.112–116 The ED is often the first point of entry to the hospital and presents an upstream opportunity to improve older adult delirium outcomes across the continuum of care. Identifying patients with delirium or those at risk for delirium is particularly important in the ED since delirium commonly occurs with acute illness or injury. Early identification of delirium (or risk for delirium) may catalyze more effective prevention and treatment protocols. The challenges in studying and adopting delirium screening and intervention practices in the ED setting pertain largely to time constraints. Pragmatically, less time is available to assess older adults in the ED, collateral information is often limited, and quicker decision making is expected. Optimizing the environment and resources used for prevention or treatment of delirium mandates adaptation to the ED setting.
The available single-center observational studies of changes in the processes of care for patients with possible delirium report reductions in delirium prevalence and duration and hospital length of stay.74–76,80,83 Unfortunately, these process intervention studies inadequately report the components of care process changes and evaluate heterogeneous outcomes including delirium detection rates, ED and hospital lengths of stay, mortality, postoperative delirium incidence, and incidence of hypoxia. Because of the observational study designs, variability of care process components implemented, study environments, and outcomes assessed, additional studies are required to delineate the specific care processes or combinations that positively or negatively influence delirium outcomes.
More ED-based delirium research is essential to guide clinicians on best practices for delirium detection in the ED setting, along with interventions to reduce incident delirium and to treat prevalent delirium. Compared with operative, ICU, and ward settings, the ED environment is uniquely challenging for the identification, treatment, and investigation of delirium, but extrapolating delirium research from dissimilar settings is imperfect and possibly harmful. Interventions to identify, prevent, or treat delirium need to consider aspects of feasibility, acceptability, and effectiveness when adopted in the ED setting, ideally utilizing implementation science and human factors engineering approaches.117 Because pharmacologic studies have demonstrated disappointing results on the prevention or treatment of delirium in the ED setting, future ED delirium studies might evaluate the pragmatic application of nonpharmacologic interventions currently demonstrating effectiveness in hospital settings.112 Potential intervention strategies include targeting delirium risk factors such as immobility, vision or hearing impairment, functional decline, dehydration, and sleep deprivation as well as pharmacologic measures of avoiding or reserving the use of antipsychotics or sedation only in patients with severe agitation posing risk to patient or staff safety.118
Existing ED delirium screening and intervention research conceptualizes delirium as a monomorphic entity without contemplating potentially unique patient phenotypes.119,120 Do medical, surgical, or traumatic emergencies lead to different pathophysiology of delirium? Does delirium in the cognitively frail individual differ in neurobiologic etiology from those without underlying cognitive deficit? Does the delirium precipitant (e.g., medications, infections, pain, or other physiologic precipitant) affect the diagnostic accuracy of ED delirium screening instruments or the observed effectiveness of delirium interventions? ED delirium interventions require extensive funding to facilitate research and perhaps more importantly time to fully understand the pathophysiology of delirium as these frail individuals enter the front door of the hospital. In addition, the timing of delirium identification and interventions might be associated with effectiveness, so ED researchers could explore collaborative approaches with long-term care facilities and/or ambulance services. For example, long-term care facilities could provide a video depicting an individual patient’s baseline mental status for emergency medical services or ED personnel to review when assessing for delirium. Much in the same way that early sepsis treatment has been effective even though similar interventions had not worked later in the hospital course for critically ill patients, delirium may be best treated at the earliest possible moment.121–123 Interventions that have been ineffective in the hospital may be more beneficial when delivered earlier in the course of delirium and deserve to be evaluated in the ED with a better understanding of delirium phenotypes and stratified analyses of those subtypes, to understand whether some subtypes may be more responsive to different interventions.
Through the GEAR consensus conference process, two new research priorities were added in addition to the five presented by the GEAR CI subgroup (Table 1). These two priorities (1—prevention focused research, including developing a screening instrument or risk score that does not entail additional nurse or physician workload and 2—testing delirium prevention strategies in the ED once high-risk individuals are identified) were subsequently selected as the top research priorities by the GEAR Consensus Conference participants after some debate. GEAR members who were not involved in the scoping review, but who were the first to hear its results, added these two priorities. These recommendations are the most basic of the seven research priorities ultimately identified. Other research priorities representing broader tiers of inquisition included: 3—providing unique care bundles, 4—building a multi-institutional data repository and research infrastructure, 5—a structured implementation science evaluation of delirium, 6—identifying delirium phenotypes, and 7—targeting delirium prevention or treatment based on delirium phenotypes. Although GEAR Consensus Conference attendees did not provide rationale for their votes, their votes seem to reflect the paucity of data for ED-based delirium risk-stratification and interventions, thereby favoring a back-to-basics approach.
After the scoping review and consensus conference we obtained further stakeholder input.93 Two experts in delirium (a geriatrician and an intensivist) endorsed a statement on the scoping review and original research priorities in Data Supplement S6, Appendix S6. In summary, they note that GEAR has revealed significant delirium knowledge gaps between management recommendations and objective evidence of benefit for delirium patients and their families. There is not a single controlled ED study on which to base actionable protocols of delirium prevention or delirium reduction interventions. GEAR’s priorities to refocus ED delirium detection research on motor and etiology-subtypes aligns with emerging delirium research priorities outside of emergency medicine. The collaborative research laboratory envisioned by GEAR is highly appealing beyond emergency medicine and provides the accelerant needed for improved delirium outcomes. It is notable that these experts highlight the more advanced and complex research priorities, which were high priority for the GEAR CI subgroup, but ultimately deprioritized in the GEAR Consensus Conference.
Patient, family, and caregiver stakeholders provided the summary of the scoping review and consensus conference in Data Supplement S7, Appendix S7. This summary notes that screening for delirium or risk of developing delirium is important to minimize the longer-term negative effects of delirium; however, screening is difficult because of delirium’s complexity. Several screening tools are available, but few have been tested in the unique environment of the ED. Also, timing of the screening is important and it appears from the reviewed research that early screening is preferable to prevent downstream complications. Important gaps in current knowledge that are priorities for future research projects include studying the effects of delirium on patient health outcomes and improving the pragmatic implementation of delirium screening in the ED. Future treatment or prevention trials should focus on patient-centered outcomes in quantifying potential benefits and harms of delirium interventions aligned with individualized risk factors and goals of care.
LIMITATIONS
This scoping review and evidence-based consensus statement has several limitations. PICO-1’s focus on risk stratification might be interpreted to mean research to evaluate the risk of developing delirium (prognostic, incident delirium) rather than identifying delirium at a point in time (diagnostic, prevalent, or incident delirium). The GEAR CI subgroup’s intended focus was the latter diagnostic accuracy priority. PICO-2’s intervention also broadly targeted primary delirium prevention with secondary prevention and prevalent delirium amelioration strategies. Each of these delirium interventions may require quite different strategies and lumping them together may be unhelpful. In addition, delirium can accelerate cognitive decline, but the well-recognized morbidity was not an outcome of either PICO-1 or PICO-2. Dementia is another subtype of cognitive impairment commonly encountered and often unrecognized in ED settings, yet GEAR stakeholders excluded PICO-3 and PICO-4, which pertained to dementia. The National Institute of Aging recently awarded investigators to develop and pilot test strategies to improve the process and outcomes of care for ED dementia patients and their caregivers, so these PICO questions will soon be explored.
We also report no quality assessment because this scoping review was not designed as a systematic review. Nonetheless, our scoping review demonstrates an overall paucity of evidence regardless of the quality, particularly in the area of delirium interventions. Therefore, current research cannot adequately inform effective ED-based delirium interventions. Significant effort was made to identify all studies in accordance with PRISMA-ScR guidelines;24 however, it is possible that our search strategy missed relevant studies or that new studies have emerged since the scoping review was performed (November 2019). Finally, a diverse group of stakeholders, including clinicians of varying specialties, researchers, patients, and their advocates, attended the consensus conference. However, this group does not encompass all possible stakeholders. A significant divergence of opinion between the GEAR CI subgroup and the remainder of GEAR’s stakeholders occurred in that research priorities presented by the GEAR CI subgroup and endorsed by delirium experts in intensive care and geriatrics were ultimately rejected by the rest of GEAR. Finally, all participants in the scoping review and consensus conference are from North America. Additional perspectives are important to develop more globally relevant ED delirium research priorities.
CONCLUSIONS
The results of this scoping review demonstrate over two dozen ED delirium-screening instruments of varying complexity and degrees of validation. However, ED-based interventional studies to prevent or treat delirium are almost nonexistent. Top research priorities for cognitive impairment in the ED continue to focus on the basics of identification, prevention, and treatment of delirium in the ED including: 1) development of a screening instrument or risk score that does not entail additional clinician workload and 2) testing delirium prevention strategies in the ED once high-risk individuals are identified.
Supplementary Material
Data Supplement S2. Delirium screening study synopsis.
Data Supplement S3. Appendix of ED delirium screening instruments.
Data Supplement S4. Administration time, accuracy, and pragmatic features of delirium screening instruments.
Data Supplement S5. ED delirium intervention study synopsis.
Data Supplement S6. Transdisciplinary commentary of GEAR cognitive impairment.
Data Supplement S7. Patient/Family caregiver perspective on GEAR CI recommendations.
Data Supplement S1. Search strategy details.
ACKNOWLEDGEMENT
The authors acknowledge the contributions of Dr. Malaz Boustani and Dr. Wes Ely for reviewing early versions of the manuscript and providing the expert commentary provided in Appendix S6.
GEAR is supported by the National Institute on Aging grant R21AG058926 (to UH), the John A Hartford Foundation, and the Gary and Mary West Health Institute. Dr. Shah is funded by National Institute on Aging (NIA) (K24AG054560–01).
Footnotes
The authors have no relevant financial information or potential conflicts to disclose.
Reprints will not be available.
References
- 1.Hustey FM, Meldon SW, Smith MD, Lex CK. The effect of mental status screening on the care of elderly emergency department patients. Ann Emerg Med 2003;41:678–84. [DOI] [PubMed] [Google Scholar]
- 2.Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med 2009;16:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCaig LF, Burt CW. National hospital ambulatory medical care survey: 2002 emergency department summary. Adv Data 2004;18:1–34. [PubMed] [Google Scholar]
- 4.De J, Wand AP. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist 2015;55:1079–99. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993;119:474–81. [DOI] [PubMed] [Google Scholar]
- 7.Bo M, Bonetto M, Bottignole G, et al. Length of stay in the emergency department and occurrence of delirium in older medical patients. J Am Geriatr Soc 2016;64:1114–9. [DOI] [PubMed] [Google Scholar]
- 8.Kakuma R, Du Fort GG, Arsenault L, et al. Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc 2003;51:443–50. [DOI] [PubMed] [Google Scholar]
- 9.Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med 2002;39:248–53. [DOI] [PubMed] [Google Scholar]
- 10.Naughton BJ, Moran MB, Kadah H, Heman-Ackah Y, Longano J. Delirium and other cognitive impairment in older adults in an emergency department. Ann Emerg Med 1995;25:751–5. [DOI] [PubMed] [Google Scholar]
- 11.Lewis LM, Miller DK, Morley JE, Nork MJ, Lasater LC. Unrecognized delirium in ED geriatric patients. Am J Emerg Med 1995;13:142–5. [DOI] [PubMed] [Google Scholar]
- 12.Elie M, Rousseau F, Cole M, Primeau F, McCusker J, Bellavance F. Prevalence and detection of delirium in elderly emergency department patients. CMAJ 2000;163:977–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Cole MG, McCusker J, Bellavance F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ 2002;167:753–9. [PMC free article] [PubMed] [Google Scholar]
- 14.McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc 2003;51:1539–46. [DOI] [PubMed] [Google Scholar]
- 15.Langan C, Sarode DP, Russ TC, Shenkin SD, Carson A, Maclullich AMJ. Psychiatric symptomatology after delirium: a systematic review. Psychogeriatrics 2017;17: 327–35. [DOI] [PubMed] [Google Scholar]
- 16.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009; 72:1570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrell KM, Hustey FM, Hwang U, Gerson LW, Wenger NS, Miller DK. Quality indicators for geriatric emergency care. Acad Emerg Med 2009;16:441–9. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter CR, Shah MN, Hustey FM, Heard K, Gerson LW, Miller DK. High yield research opportunities in geriatric emergency medicine: prehospital care, delirium, adverse drug events, and falls. J Gerontol A Biol Sci Med Sci 2011;66:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan TM, Losman ED, Carpenter CR, et al. Development of geriatric competencies for emergency medicine residents using an expert consensus process. Acad Emerg Med 2010;17:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg M, Carpenter CR, Bromley M, et al. Geriatric emergency department guidelines. Ann Emerg Med 2014;63:e7–25. [DOI] [PubMed] [Google Scholar]
- 21.Schnitker LM, Martin-Khan M, Burkett E, Beattie ER, Jones RN, Gray LC. Process quality indicators targeting cognitive impairment to support quality of care for older people with cognitive impairment in emergency departments. Acad Emerg Med 2015;22:285–98. [DOI] [PubMed] [Google Scholar]
- 22.Schnitker LM, Martin-Khan M, Burkett E, et al. Structural quality indicators to support quality of care for older people with cognitive impairment in emergency departments. Acad Emerg Med 2015;22:273–84. [DOI] [PubMed] [Google Scholar]
- 23.The Geriatric Emergency Care Applied Research (GEAR) Network. The Geriatric Emergency Department Collaborative. 2020. Available at: https://gedcollaborative.com/research/gear/. Accessed Jun 24, 2020.
- 24.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33: 159–74. [PubMed] [Google Scholar]
- 26.Bédard C, Voyer P, Eagles D, et al. Validation of the Ottawa 3DY in community seniors in the ED. CJEM 2017;19:S47. [Google Scholar]
- 27.Ellis R, Loo GT, Rivera L, Richardson LD, Sze J, Hwang U. Detection of delirium in older emergency department patients: standardizing screening using 3D-confusion assessment method. Acad Emerg Med 2017;24:S276. [Google Scholar]
- 28.Emond M, Nadeau A, Boucher V, et al. Underreport of incident delirium in elderly patients treated in the emergency department. CJEM 2018;20:S44.28920564 [Google Scholar]
- 29.Fox C, Shenkin S, Goodacre S, et al. Utility of the 4AT rapid assessment instrument in assessment of delirium and cognitive impairment in acute care. Alzheimer’s Dementia 2018;14:P1559–60. [Google Scholar]
- 30.Kennedy M, Enander RA, Wolfe RE, Marcantonio ER, Shapiro NI. How accurate are emergency department physicians and nurses at assessing delirium among elderly emergency department patients? Acad Emerg Med 2011;18:S96. [Google Scholar]
- 31.Miller C, Yelinevich I, Momeni M, Musa I, Stoneley S. Improving recognition of delirium in the emergency department: a quality improvement project. Age Ageing 2018;47:ii25. [Google Scholar]
- 32.Rangel Selvera OA, Gonzalez Esparza SA, Baztan Cortes JJ. Prevalence of delirium in patients older than 65 years who come to emergency with medical pathology. Eur Geriatr Med 2011;2:S64–5. [Google Scholar]
- 33.Yeon H, Saczynski J. Comparison of delirium detection rates in dementia and non-dementia elderly population by family caregivers using family confusion assessment methods (FAM-CAM) in the emergency department-an observational study. Value Health 2017;20:A237–8. [Google Scholar]
- 34.Arendts G, Love J, Nagree Y, Bruce D, Hare M, Dey I. Rates of delirium diagnosis do not improve with emergency risk screening: results of the emergency department delirium initiative trial. J Am Geriatr Soc 2017;65:1810–5. [DOI] [PubMed] [Google Scholar]
- 35.Grossmann FF, Hasemann W, Graber A, Bingisser R, Kressig RW, Nickel CH. Screening, detection and management of delirium in the emergency department - a pilot study on the feasibility of a new algorithm for use in older emergency department patients: the modified Confusion Assessment Method for the Emergency Department (mCAM-ED). Scand J Trauma Resusc Emerg Med 2014;22:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hare M, Wynaden D, McGowan S, Speed G. Assessing cognition in elderly patients presenting to the emergency department. Int Emerg Nurs 2008;16:73–9. [DOI] [PubMed] [Google Scholar]
- 37.Press Y, Margulin T, Grinshpun Y, et al. The diagnosis of delirium among elderly patients presenting to the emergency department of an acute hospital. Arch Gerontol Geriatr 2009;48:201–4. [DOI] [PubMed] [Google Scholar]
- 38.Singler K, Thiem U, Christ M, et al. Aspects and assessment of delirium in old age. First data from a German interdisciplinary emergency department. Z Gerontol Geriatr 2014;47:680–5. [DOI] [PubMed] [Google Scholar]
- 39.Van de Meeberg EK, Festen S, Kwant M, Georg RR, Izaks GJ, Ter Maaten JC. Improved detection of delirium, implementation and validation of the CAM-ICU in elderly emergency department patients. Eur J Emerg Med 2017;24:411–6. [DOI] [PubMed] [Google Scholar]
- 40.Han JH, Hayhurst CJ, Chandrasekhar R, et al. Delirium’s arousal subtypes and their relationship with 6-month functional status and cognition. Psychosomatics 2019;60:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han JH, Shintani A, Eden S, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med 2010;56:244–52.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han JH, Wilson A, Graves AJ, et al. Validation of the confusion assessment method for the intensive care unit in older emergency department patients. Acad Emerg Med 2014;21:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisch A, Miller T, Haag A, Martin-Gill C, Guyette FX, Suffoletto BP. Diagnostic accuracy of a rapid checklist to identify delirium in older patients transported by EMS. Prehosp Emerg Care 2013;17:230–4. [DOI] [PubMed] [Google Scholar]
- 44.Fabbri RM, Moreira MA, Garrido R, Almeida OP. Validity and reliability of the Portuguese version of the Confusion Assessment Method (CAM) for the detection of delirium in the elderly. Arq Neuropsiquiatr 2001;59:175–9. [DOI] [PubMed] [Google Scholar]
- 45.Schnitker L, Martin-Khan M, Beattie E, Gray L. What is the evidence to guide best practice for the management of older people with cognitive impairment presenting to emergency departments? A systematic review. Adv Emerg Nurs 2013;35:154–69. [DOI] [PubMed] [Google Scholar]
- 46.Baten V, Busch HJ, Busche C, et al. Validation of the brief confusion assessment method for screening delirium in elderly medical patients in a German emergency department. Acad Emerg Med 2018;25:1251–62. [DOI] [PubMed] [Google Scholar]
- 47.Han JH, Wilson A, Vasilevskis EE, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med 2013;62:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han JH, Wilson A, Graves AJ, Shintani A, Schnelle JF, Ely EW. A quick and easy delirium assessment for nonphysician research personnel. Am J Emerg Med 2016;34:1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han JH, Vasilevskis EE, Chandrasekhar R, et al. Delirium in the Emergency Department and Its Extension into Hospitalization (DELINEATE) study: effect on 6-month function and cognition. J Am Geriatr Soc 2017;65:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossmann FF, Hasemann W, Kressig RW, Bingisser R, Nickel CH. Performance of the modified Richmond Agitation Sedation Scale in identifying delirium in older ED patients. Am J Emerg Med 2017;35:1324–6. [DOI] [PubMed] [Google Scholar]
- 51.Han JH, Vasilevskis EE, Schnelle JF, et al. The diagnostic performance of the Richmond agitation sedation scale for detecting delirium in older emergency department patients. Acad Emerg Med 2015;22:878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han JH, Brummel NE, Chandrasekhar R, et al. Exploring delirium’s heterogeneity: association between arousal subtypes at initial presentation and 6-month mortality in older emergency department patients. Am J Geriatr Psychiatry 2017;25:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morandi A, Han JH, Meagher D, et al. Detecting delirium superimposed on dementia: evaluation of the diagnostic performance of the Richmond agitation and sedation scale. J Am Med Dir Assoc 2016;17:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gagné AJ, Voyer P, Boucher V, et al. Performance of the French version of the 4AT for screening the elderly for delirium in the emergency department. CJEM 2018;80:903–10. [DOI] [PubMed] [Google Scholar]
- 55.O’Sullivan D, Brady N, Manning E, et al. Validation of the 6-Item cognitive impairment test and the 4AT test for combined delirium and dementia screening in older emergency department attendees. Age Ageing 2018;47:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tong T, Chignell M, Tierney MC, Lee J. A serious game for clinical assessment of cognitive status: validation study. JMIR Serious Games 2016;4:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong T, Chignell M, Tierney MC, Lee JS. Test-retest reliability of a serious game for delirium screening in the emergency department. Front Aging Neurosci 2016;8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JS, Tong T, Tierney MC, Kiss A, Chignell M. Predictive ability of a serious game to identify emergency patients with unrecognized delirium. J Am Geriatr Soc 2019;67:2370–5. [DOI] [PubMed] [Google Scholar]
- 59.LaMantia MA, Messina FC, Hobgood CD, Miller DK. Screening for delirium in the emergency department: a systematic review. Ann Emerg Med 2014;63:e2. [DOI] [PubMed] [Google Scholar]
- 60.Han JH, Schnelle JF, Ely EW. The relationship between a chief complaint of “altered mental status” and delirium in older emergency department patients. Acad Emerg Med 2014;21:937–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han JH, Wilson A, Schnelle JF, Dittus RS, Ely EW. An evaluation of single question delirium screening tools in older emergency department patients. Am J Emerg Med 2018;36:1249–52. [DOI] [PubMed] [Google Scholar]
- 62.Cirbus J, MacLullich AMJ, Noel C, Ely EW, Chandrasekhar R, Han JH. Delirium etiology subtypes and their effect on six-month function and cognition in older emergency department patients. Int Psychogeriatr 2019;31:267–76. [DOI] [PubMed] [Google Scholar]
- 63.Dyer AH, Briggs R, Nabeel S, O’Neill D, Kennelly SP. The Abbreviated Mental Test 4 for cognitive screening of older adults presenting to the emergency department. Eur J Emerg Med 2017;24:417–22. [DOI] [PubMed] [Google Scholar]
- 64.Giroux M, Sirois M-J, Boucher V, et al. Frailty assessment to help predict patients at risk of delirium when consulting the emergency department. J Emerg Med 2018;55:157–64. [DOI] [PubMed] [Google Scholar]
- 65.Hasemann W, Grossmann FF, Stadler R, et al. Screening and detection of delirium in older ED patients: performance of the modified Confusion Assessment Method for the Emergency Department (mCAM-ED). A two-step tool. Intern Emerg Med 2018;13:915–22. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy M, Enander RA, Tadiri SP, Wolfe RE, Shapiro NI, Marcantonio ER. Delirium risk prediction, healthcare use and mortality of elderly adults in the emergency department. J Am Geriatr Soc 2014;62:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laguë A, Voyer P, Ouellet MC, et al. Using the Bergman-Paris question to screen seniors in the emergency department. CJEM 2018;20:753–61. [DOI] [PubMed] [Google Scholar]
- 68.Marra A, Jackson JC, Ely EW, et al. Focusing on inattention: the diagnostic accuracy of brief measures of inattention for detecting delirium. J Hosp Med 2018;13: 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q 2014;85:211–24. [DOI] [PubMed] [Google Scholar]
- 70.Hullick C, Conway J, Higgins I, et al. An assistant workforce to improve screening rates and quality of care for older patients in the emergency department: findings of a pre- post, mixed methods study. BMC Geriatr 2018; 18:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monette J, Galbaud du Fort G, Fung SH, et al. Evaluation of the Confusion Assessment Method (CAM) as a screening tool for delirium in the emergency room. Gen Hosp Psychiatry 2001;23:20–5. [DOI] [PubMed] [Google Scholar]
- 72.Mariz J, Costa-Castanho T, Teixeira J, Sousa N, Correia-Santos N. Delirium diagnostic and screening instruments in the emergency department: an up-to-date systematic review. Geriatrics 2016;1:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schrijver EJ, de Vries OJ, van de Ven PM, et al. Haloperidol versus placebo for delirium prevention in acutely hospitalised older at risk patients: a multi-centre double-blind randomised controlled clinical trial. Age Ageing 2018;47:48–55. [DOI] [PubMed] [Google Scholar]
- 74.Ma IC, Chen KC, Chen WT, et al. Increased readmission risk and healthcare cost for delirium patients without immediate hospitalization in the emergency department. Clin Psychopharmacol Neurosci 2018;16:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomson A, Irving R, Miller C, Partington L, Cotton P. Impact of an in-reach programme & emergency care bundle on delirium in patients with idiopathic Parkinson’s disease. Eur Geriatr Med 2017;8:S154. [Google Scholar]
- 76.Thomson A, Irving R, Miller C, Partington L, Cotton P. Benefits of an in-reach programme & emergency care bundle for patients with idiopathic Parkinson’s disease. Eur Geriatr Med 2017;8:S145. [Google Scholar]
- 77.Thompson C, Brienza VJ, Sandre A, Caine S, Borgundvaag B, McLeod S. Risk factors associated with acute inhospital delirium for patients diagnosed with a hip fracture in the emergency department. CJEM 2018; 80:911–9. [DOI] [PubMed] [Google Scholar]
- 78.Delaney M, Pepin J, Somes J. Emergency department delirium screening improves care and reduces revisits for the older adult patient. J Emerg Nurs 2015;41:521–4. [DOI] [PubMed] [Google Scholar]
- 79.Kato K, Akama F, Yamada K, et al. Blonanserin for the treatment of delirium patients at an emergency medical care center: an open-label study. Asian J Psychiatr 2013;6:182–3. [DOI] [PubMed] [Google Scholar]
- 80.Fleury N, Chevalley F, Rubli E, Coti P, Farron A, Jolles BM. Efficiency of the lausanne clinical pathway for proximal femoral fractures. Front Surg 2015;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc 2005;53:18–23. [DOI] [PubMed] [Google Scholar]
- 82.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc 2001;49:523–32. [DOI] [PubMed] [Google Scholar]
- 83.Bjorkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg D. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiol Scand 2010;54:678–88. [DOI] [PubMed] [Google Scholar]
- 84.LeBlanc P, Boucher V, Émond M, Courtemanche J, Ménassa M, Lee JS. Delirium prevention in the emergency department using regional anesthesia with ultrasound guidance in the elderly population with hip fracture: a pilot study. CJEM 2016;18:S103–4. [Google Scholar]
- 85.Brymer C, Cavanagh P, Denomy E, Wells K, Cook C. The effect of a geriatric education program on emergency nurses. J Emerg Nurs 2001;27:27–32. [DOI] [PubMed] [Google Scholar]
- 86.Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 2009;50:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fong TG, Vasunilashorn SM, Libermann T, Marcanatonio ER, Inouye SK. Delirium and Alzheimer disease: a proposed model for shared pathophysiology. Int J Geriatr Psychiatry 2019;34:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall RJ, Watne LO, Cunningham E, et al. CSF biomarkers in delirium: a systematic review. Int J Geriatr Psychiatry 2018;33:1479–500. [DOI] [PubMed] [Google Scholar]
- 89.Schulman-Green D, Schmitt EM, Fong TG, et al. Use of an expert panel to identify domains and indicators of delirium severity. Qual Life Res 2019;28:2565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones RN, Cizginer S, Pavlech L, et al. Assessment of instruments for measurement of delirium severity: a systematic review. JAMA Intern Med 2019;179:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neta G, Glasgow RE, Carpenter CR, et al. A framework for enhancing the value of research for dissemination and implementation. Am J Public Health 2015;105: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gross AL, Tommet D, D’Aquila M, et al. Harmonization of delirium severity instruments: a comparison of the DRS-R-98, MDAS, and CAM-S using item response theory. BMC Med Res Methodol 2018;18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carpenter CR, McFarland F, Avidan M, et al. Impact of cognitive impairment across specialties: summary of a report from the U13 conference series. J Am Geriatr Soc 2019;67:2011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pediatric Emergency Care Applied Research Network. The Pediatric Emergency Care Applied Research Network (PECARN): rationale, development, and first steps. Pdiatr Emerg Care 2003;19:185–93. [DOI] [PubMed] [Google Scholar]
- 95.Carpenter CR, Banerjee J, Keyes D, et al. Accuracy of dementia screening instruments in emergency medicine: a diagnostic meta-analysis. Acad Emerg Med 2019;26:226–45. [DOI] [PubMed] [Google Scholar]
- 96.Walston J, Robinson TN, Zieman S, et al. Integrating frailty research into the medical specialties-report from a U13 conference. J Am Geriatr Soc 2017;65:2134–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Racine AM, D’Aquila M, Schmitt EM, et al. Delirium burden in patients and family caregivers: development and testing of new instruments. Gerontologist 2019;59: e393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jackson TA, Wilson D, Richardson S, Lord JM. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int J Geriatr Psychiatry 2016;31:392–9. [DOI] [PubMed] [Google Scholar]
- 99.Shenvi C, Kennedy M, Austin CA, Wilson MP, Gerardi M, Schneider S. Managing delirium and agitation in the older emergency department patient: the ADEPT tool. Ann Emerg Med 2020;75:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kennedy M, Hwang U, Han JH. Delirium in the emergency department: moving from tool-based research to system-wide change. J Am Geriatr Soc 2020;68:956–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gélinas C, Bérubé M, Chevrier A, et al. Delirium assessment tools for use in critically Ill adults: a psychometric analysis and systematic Review. Crit Care Nurse 2018;38:38–49. [DOI] [PubMed] [Google Scholar]
- 102.Poikajärvi S, Salanterä S, Katajisto J, Junttila K. Validation of Finnish Neecham Confusion Scale and Nursing Delirium Screening Scale using Confusion Assessment Method algorithm as a comparison scale. BMC Nurs 2017;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gaspardo P, Peressoni L, Comisso I, Mistraletti G, Ely EW, Morandi A. Delirium among critically ill adults: evaluation of the psychometric properties of the Italian ‘Confusion Assessment Method for the intensive care unit. Intensive Crit Care Nurs 2014;30:283–91. [DOI] [PubMed] [Google Scholar]
- 104.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carpenter CR, Meisel ZF. Overcoming the tower of babel in medical science by finding the “EQUATOR”: research reporting guidelines. Acad Emerg Med 2017;24:1030–3. [DOI] [PubMed] [Google Scholar]
- 106.Kohn MA, Carpenter CR, Newman TB. Understanding the direction of bias in studies of diagnostic test accuracy. Acad Emerg Med 2013;20:1194–206. [DOI] [PubMed] [Google Scholar]
- 107.Carpenter CR. Understanding bias in diagnostic research. In: Pines JM, Carpenter CR, Raja AS, Schuur JD, editors. Evidence-Based Emergency Care: Diagnostic Testing and Clinical Decision Rules. Oxford, UK: Wiley-Blackwell, 2013:54–64. [Google Scholar]
- 108.El Dib R, Tikkinen KA, Akl EA, et al. Systematic survey of randomized trials evaluating the impact of alternative diagnostic strategies on patient-important outcomes. J Clin Epidemiol 2017;84:61–9. [DOI] [PubMed] [Google Scholar]
- 109.Carpenter CR, Keim SM, Worster A, Rosen P. Brain natriuretic peptide in the evaluation of emergency department dyspnea: is there a role? J Emerg Med 2012;42:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc 2008;56:823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chisholm CD, Weaver CS, Whenmouth L, Giles B. A task analysis of emergency physician activities in academic and community settings. Ann Emerg Med 2011;58:117–22. [DOI] [PubMed] [Google Scholar]
- 112.NIDUS - Network for Investigation of Delirium: Unifying Scientists. Available at: https://deliriumnetwork.org/. Accessed Jun 24, 2020.
- 113.Herling SF, Greve IE, Vasilevskis EE, et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database Syst Rev 2018;11:CD009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burry L, Hutton B, Williamson DR, et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev 2019;9: CD011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woodhouse R, Burton JK, Rana N, Pang YL, Lister JE, Siddiqu N. Interventions for preventing delirium in older people in institutional long-term care. Cochrane Database Syst Rev 2019;4:CD009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y, Ma J, Jin Y, et al. Benzodiazepines for treatment of patients with delirium excluding those who are cared for in an intensive care unit. Cochrane Database Syst Rev 2020;2:CD012670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carpenter CR, Pinnock H. Starry aims to overcome knowledge translation inertia: the standards for reporting implementation studies (StaRI) guidelines. Acad Emerg Med 2017;24:1027–9. [DOI] [PubMed] [Google Scholar]
- 118.Oh ES, Fong TG, Tt H, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017;318:1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018;33:1428–57. [DOI] [PubMed] [Google Scholar]
- 120.Oldham MA, Flaherty JH, Maldonado JR. Refining delirium: a transtheoretical model of delirium disorder with preliminary neurophysiologic subtypes. Am J Geriatr Psychiatry 2018;26:913–24. [DOI] [PubMed] [Google Scholar]
- 121.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med 1994;330:1717–22. [DOI] [PubMed] [Google Scholar]
- 122.Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically Ill patients. SvO2 Collaborative Group. N Engl J Med 1995;333:1025–32. [DOI] [PubMed] [Google Scholar]
- 123.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345:1368–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S2. Delirium screening study synopsis.
Data Supplement S3. Appendix of ED delirium screening instruments.
Data Supplement S4. Administration time, accuracy, and pragmatic features of delirium screening instruments.
Data Supplement S5. ED delirium intervention study synopsis.
Data Supplement S6. Transdisciplinary commentary of GEAR cognitive impairment.
Data Supplement S7. Patient/Family caregiver perspective on GEAR CI recommendations.
Data Supplement S1. Search strategy details.