Abstract
Background
Understanding malaria vector’s population dynamics and their spatial distribution is important to define when and where the largest infection risks occur and implement appropriate control strategies. In this study, the seasonal spatio-temporal dynamics of the malaria vector population and transmission intensity along intermittent rivers in a semi-arid area of central Ethiopia were investigated.
Methods
Mosquitoes were collected monthly from five clusters, 2 close to a river and 3 away from a river, using pyrethrum spray catches from November 2014 to July 2016. Mosquito abundance was analysed by the mixed Poisson regression model. The human blood index and sporozoite rate was compared between seasons by a logistic regression model.
Results
A total of 2784 adult female Anopheles gambiae sensu lato (s.l.) were collected during the data collection period. All tested mosquitoes (n = 696) were identified as Anopheles arabiensis by polymerase chain reaction. The average daily household count was significantly higher (P = 0.037) in the clusters close to the river at 5.35 (95% CI 2.41–11.85) compared to the clusters away from the river at 0.033 (95% CI 0.02–0.05). Comparing the effect of vicinity of the river by season, a significant effect of closeness to the river was found during the dry season (P = 0.027) and transition from dry to wet season (P = 0.032). Overall, An. arabiensis had higher bovine blood index (62.8%) as compared to human blood index (23.8%), ovine blood index (9.2%) and canine blood index (0.1%). The overall sporozoite rate was 3.9% and 0% for clusters close to and away from the river, respectively. The overall Plasmodium falciparum and Plasmodium vivax entomologic inoculation rates for An. arabiensis in clusters close to the river were 0.8 and 2.2 infective bites per person/year, respectively.
Conclusion
Mosquito abundance and malaria transmission intensity in clusters close to the river were higher which could be attributed to the riverine breeding sites. Thus, vector control interventions including targeted larval source management should be implemented to reduce the risk of malaria infection in the area.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-021-03697-z.
Keywords: Clusters, Ethiopia, Intermittent rivers, Malaria, Seasonal dynamics, Transmission intensity
Background
The global decline of malaria incidence due to scaling up of existing interventions has led to increased interest by many endemic countries to plan for malaria elimination. In such an elimination agenda, the understanding of the spatio-temporal heterogeneity in areas of unstable transmission is of primordial importance [1, 2]. Spatio-temporal heterogeneity leads to restricted areas, so-called malaria hotspots, where mosquito breeding and transmission is ongoing even in the dry season [3] and from where malaria transmission can spread to a wider area when conditions are more conducive [4]. The smallest spatial unit capable of supporting transmission is the household, where peri-domestic transmission occurs [5].
Malaria case distribution is characterized by spatio-temporal heterogeneity in unstable transmission areas [2, 6]. Thus, malaria distribution in low incidence settings appears patchy, and local transmission hotspots are a continuous source of infection [7, 8]. Also in malaria endemic regions micro-epidemiologic variation in malaria incidence and transmission exists and high-risk areas (hotspots) for malaria infection are constantly present [9, 10]. Hence, it is critical to define malaria spatial units at micro-epidemiological level, identify spatial heterogeneity of transmission and assess the effectiveness of malaria control programmes within these units. To this end, studies generated fine scale maps of malaria endemicity and identified spatial units that have higher transmission than their surroundings [11–13].
Though malaria transmission intensity is associated with rainfall apart from other factors, there is evidence that transmission persists late in the dry season. A study in Senegal supports the hypothesis that malaria transmission is maintained during the dry season in an area of low and seasonal transmission [14]. Malaria parasite transmission and clinical disease are characterized by important micro-geographic variations, even between adjacent villages, households or families [15]. Among other factors, the presence or absence of breeding sites contributes to heterogeneity of malaria transmission. A study in Bandiagara, Mali, confirms that the existence of marked spatial heterogeneity of malaria transmission was related to the occurrence of seasonal breeding sites [16].
Understanding the dynamics of malaria transmission and knowing the spatial vector distribution helps to define when and where the largest infection risks occur and supports the development of appropriate and targeted control strategies [17]. Moreover, it is important when malaria transmission rate is on decline, that the control measures are directed towards the transmission foci [18].
Patterns of malaria transmission along river basins was studied in Eastern Sudan in which infectivity and transmission rates increased with proximity to the river following peak rainfall and subsequent recession of rivers [19]. In Ethiopia, malaria transmission is seasonal, and is determined by altitude, rainfall and temperature in addition to other local factors [20]. Despite its seasonality, there are substantial small scale spatial variations in malaria transmission [21]. This is supported by a study that showed a high variation in malaria incidence and Anopheles mosquito abundance and distribution among villages [22].
However, currently, there is only limited evidence that local hotspots fuel transmission and little is known about the seasonal variation and intensity of malaria transmission in clusters close to intermittent rivers in Ethiopia. Such information is required for planning and implementing effective vector control interventions. Therefore, this study aimed at investigating seasonal dynamics of malaria vectors and transmission intensity along intermittent rivers in a semi-arid area of central Ethiopia.
Methods
Study area
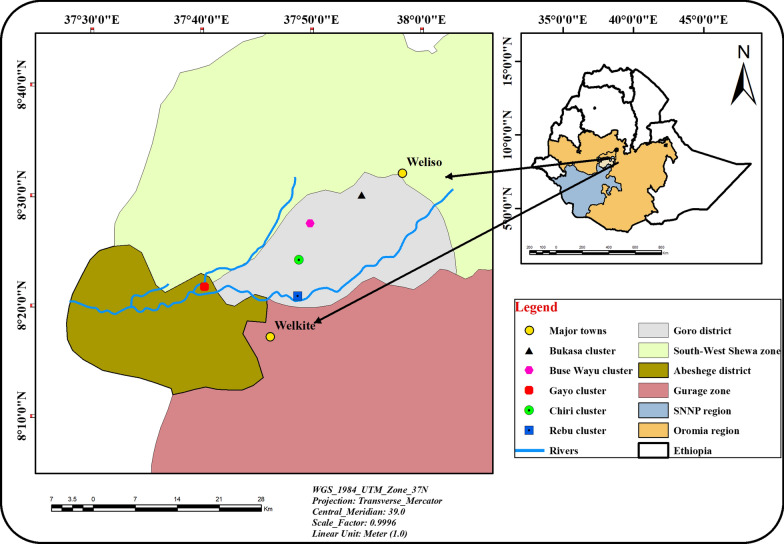
This study was conducted in two neighbouring kebeles, Rebu and Dire Lafto, which are located 144 km south west of Addis Ababa, the capital of Ethiopia (Fig. 1). Rebu kebele is located in the Goro district of the south west shewa Zone, Oromia Regional National State while Dire Lafto kebele is located in the Abeshege district, Southern Nations, Nationalities and Peoples Regional State, Ethiopia. Geographically located between latitudes 07o40′0′′N and 09o0′0′′N and longitudes 37o30′0′′E and 38o50′0′′E with mean altitude of 1748 m above sea level (Fig. 1).
Fig. 1.
Map of study area
Goro had a total population of 45,486, of which 22,912 were males and 22,574 were females according to the 2007 CSA report. From the total population, only 8.2% were urban dwellers. The majority (70.2%) of the inhabitants were Muslims, while 26.7% were Orthodox Christians and 2.2% were Protestants. Based on the 2007 Census conducted by the CSA, Abeshege Woreda had a total population of 61,424, of which 32,450 were males and 28,974 females; none of these were urban inhabitants. Half (50%) of the inhabitants were Orthodox Christians followed by Muslims (32.0%), Protestants (15.8%) and Catholics (1.3%) [23].
In the selected study sites, the major rainy season occurs from June to September, whereas the minor rainy season is from February to March. The sites are delineated by two intermittent streams, Rebu in the South East and Bala in the West, both of which have ample small water pools during the dry season.
Mosquito collection
Adult mosquito collection was carried out monthly from November 2014 to July 2016. At the study sites, 5 clusters were chosen, with one in the vicinity of the Rebu River and one in the vicinity of the Bala River, whereas the other three clusters were at least 1.5–2.7 km away from any of the two rivers. In each cluster, 12 households were selected, making a total of 60 households. The average altitude for clusters Rebu, Gayo, Buse-Wayu, Chirri and Bukasa is 1769, 1614, 1813, 1766 and 1940 m above mean sea level, respectively. Four seasons were considered and the surveys represented wet season (June, July, August and September), transmission from wet to dry season (October and November), dry season (December, January, February and March) and transmission from dry to wet (April and May).
Indoor resting mosquitoes were sampled using Pyrethrum Spray Catches (PSCs) from the selected sixty houses from 06:00 am to 7:30 am, following a standard protocol [24] using insecticide aerosol (BAYGON®, S. C. Johnson & Son) procured from a local supermarket. Consent was obtained from the head of each household to conduct the monthly PSC and the head was informed one day prior to the PSC. Food and water were removed from the house and white sheets spread on the floor and over the furniture in the house. Two field workers, one inside the house and one outside, sprayed around the eaves. The mosquito collectors in the house then sprayed the roof and walls. The house was closed for 15 min after which the white sheets were brought outside to have sufficient light to see the dead and dying mosquitoes. After 15 min, dead and immobilized mosquitoes were collected and sorted by sex and genus. Collected mosquitoes were transported to a field laboratory for morphological identification.
Mosquito processing
The mosquitoes were first classified into species using standard morphological keys [25] and then into different abdominal (gonotrophic) status, i.e. unfed, fed, half-gravid and gravid [26] at the field laboratory using a dissecting microscope. Then, each mosquito was kept in a labelled 1.5 ml Eppendorf tube containing silica gel desiccant and cotton wool. Samples were stored at − 20 °C at the Tropical and Infectious Diseases Research Center Laboratory, Jimma University until shipped to Imperial College London, VigiLab, UK for further processing. At the VigiLab, the mosquito samples were identified at species level using Polymerase Chain Reaction (PCR) following the protocols developed by Scott et al. for Anopheles gambiae sensu lato (s.l.) [27]. The presence of Plasmodium sporozoites and the source of the blood meal was assessed using PCR [28, 29].
Statistical analysis
Mosquito abundance was analysed by a mixed Poisson regression model including household as random effect and season, distance to the river (close or away) and their interaction as categorical fixed effects. Human blood index (HBI) was calculated as the proportion of Anopheles samples with human blood (with possibly other species sources) out of the total number of Anopheles blood samples tested [30–32]. Bovine, goat and dog blood indices were calculated in the same way. The human blood index and sporozoite rate were compared between seasons using logistic regression model with household as random effect, and were restricted to mosquito catches from clusters close to the river as mosquito catches were too low for the three clusters away from the river.
The monthly entomological inoculation rate (EIR) of Anopheles mosquitoes was determined for each season as follows [33]:
Results
Seasonal density
A total of 2784 adult female An. gambiae s.l. were collected during the study period (Additional file 1). Of the 696 An. gambiae s.l. specimens analysed by PCR, all belonged to Anopheles arabiensis. The majority (99.1%) of the mosquitoes were collected from the clusters close to Rebu and Bala rivers. Table 1 shows the average daily household counts of An. arabiensis in the clusters close to and away from the river. The average daily household count was significantly higher (P = 0.037) in the clusters close to the river 5.35 (95% CI 2.41–11.85) compared to the clusters away from the river 0.033 (95% CI 0.02–0.05).
Table 1.
The average daily household count (95% CI) of Anopheles arabiensis as a function of season and distance from the river
| Season | Distance from the river | |
|---|---|---|
| Close to the river | Away from the river | |
| Wet | 2.42 (0.83–7.08) | 0.06 (0.02–0.12) |
| Transition wet–dry | 14.56 (4.76–44.55) | 0.04 (0.01–0.09) |
| Dry | 4.53 (1.96–10.48) | 0.01 (0.004–0.04) |
| Transition dry–wet | 5.13 (2.15–12.21) | 0.04 (0.02–0.10) |
| Total | 5.35 (2.41–11.85) | 0.033 (0.02–0.05) |
Comparing the effect of vicinity of the river by season, a significant effect of closeness to the river was found during the dry season (P = 0.027) and transition from dry to wet season (P = 0.032), but not in the wet season (P = 0.084) and transition from wet to dry season (P = 0.090).
Mosquito physiological state
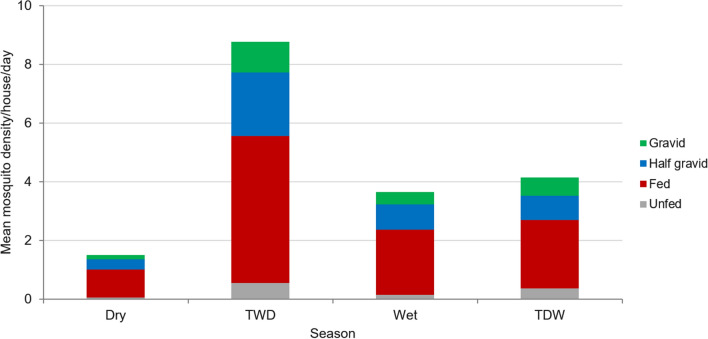
The majority (58.9%) of the collected An. arabiensis were blood fed followed by half-gravid (23.5%), gravid (11.9%) and unfed (5.7%). The proportion of blood fed An. arabiensis was higher in all seasons (Fig. 2).
Fig. 2.
Physiological state of Anopheles arabiensis
Blood meal indices
Overall, An. arabiensis had higher bovine blood index (62.8%) as compared to human blood index (23.8%), ovine blood index (9.2%) and canine blood index (0.1%). The HBI of An. arabiensis during the wet, TWD, dry and TDW season was 23.3%, 14.5%, 26.6% and 28.2%, respectively (Table 2). There was no overall significant difference between the four seasons in terms of the HBI (P = 0.231).
Table 2.
Number of blood samples (%) of An. arabiensis according to host species, season and vicinity to the river
| Season | Close to the river | Away from the river | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Bovine | Goat | Dog | H + B | H + G | B + G | Unknown | Human | Bovine | H + B | Unknown | |
| Wet | 16 (14.8) | 34 (31.5) | 3 (2.8) | 1 (0.9) | 7 (6.5) | 1 (0.9) | 0 | 46 (42.6) | 2 (25.0) | 2 (25.0) | 1 (12.5) | 3 (37.5) |
| TWD | 4 (2.6) | 66 (43.4) | 0 | 0 | 18 (11.9) | 0 | 54 (35.5) | 10 (6.6) | 0 | 0 | 0 | 0 |
| Dry | 53 (14.4) | 192 (52.0) | 0 | 0 | 45 (12.2) | 0 | 9 (2.4) | 70 (19.0) | 0 | 0 | 0 | 0 |
| TDW | 13 (12.0) | 22 (20.4) | 1 (0.9) | 0 | 17 (15.8) | 0 | 1 (0.9) | 54 (50.0) | 1 (50.0) | 0 | 0 | 1 (50.0) |
| Total | 86 (11.7) | 314 (42.6) | 4 (0.6) | 1 (0.1) | 87 (11.8) | 1 (0.1) | 64 (8.7) | 180 (24.4) | 3 (30.0) | 2 (20.0) | 1 (10.0) | 4 (40.0) |
TWD transition from wet to dry, TDW transition from dry to wet, H + B human and bovine, H + G human and goat; number in parentheses indicates percentage, Unknown unidentified blood meal sources
Sporozoite and entomological inoculation rates
A total of 747 An. arabiensis samples were tested for Plasmodium circumsporozoite proteins (CSPs) using PCR (Table 3). Of these, 29 specimens were positive, 8 for Plasmodium falciparum and 21 for Plasmodium vivax. The overall Plasmodium sporozoite rate was 3.9% with 1.1% and 2.8% for P. falciparum and P. vivax, respectively. None of the mosquito specimens collected from clusters away from the river were positive for CSP (Table 3).
Table 3.
Sporozoite rates of An. arabiensis in clusters close to and away from the river according to season
| Season | Close to the river | Away from the river | ||||
|---|---|---|---|---|---|---|
| No tested | Pf + ve (%) | Pv + ve (%) | No tested | Pf + ve (%) | Pv + ve (%) | |
| Wet | 108 | 2 (1.85) | 4 (3.70) | 8 | 0 | 0 |
| TWD | 152 | 2 (1.32) | 2 (1.32) | 0 | 0 | 0 |
| Dry | 369 | 4 (1.08) | 12 (3.25) | 0 | 0 | 0 |
| TDW | 108 | 0 (0.00) | 3 (2.78) | 2 | 0 | 0 |
| Total | 737 | 8 (1.09) | 21(2.85) | 10 | 0 | 0 |
TWD transition from wet to dry, TDW transition from dry to wet, Pf + ve positive for P. falciparum circumsporozoite protein (CSP), Pv + ve positive for P. vixax CSP, % percentage
The entomological inoculation rate was determined only for the clusters close to the river due to low mosquito count for clusters away from the river. The overall P. falciparum and P. vivax EIR for An. arabiensis was 0.18 and 0.07 infective bites per person/month, respectively. Peak P. falciparum EIR was observed during the TWD season with EIR of 0.13 infective bites per person/month whereas peak P. vivax EIR was observed during the TDW and dry season with EIR of 0.18 infective bites per person/month in those seasons.
Discussion
Understanding the seasonal dynamics of malaria-vector populations and malaria transmission intensity in semi-arid areas, where breeding sites are limited to rivers during dry season, is essential to design effective strategies for malaria control and elimination. Moreover, accurate information on dynamics of malaria transmission and its spatial distribution helps to define when and where the largest infection risks occur, and facilitates the development of appropriate and targeted control strategies [17]. In the present study, key entomological parameters such as species composition, seasonal distribution, physiological status, blood meal sources, sporozoite rates and EIR were assessed in a semi-arid area to determine seasonal dynamics of mosquitoes and transmission intensity in clusters close and away from intermittent rivers. The EIR was used as a surrogate for malaria transmission intensity, as no data on the incidence of malaria cases were available. Thus, understanding the heterogeneity in malaria vector density and transmission intensity along rivers enables spatially targeted mosquito vector control.
In this study, all specimens of An. gambiae s.l. identified by the PCR were An. arabiensis. In line with the study, the presence of a single malaria vector species in a locality has also been observed elsewhere [19, 34, 35]. Anopheles arabiensis is certainly the dominant species in south west Ethiopia [36], whether using the PSC, the CDC light trap, exit traps or the artificial pit shelter. In this study, only PSC was used as a method of mosquito collection, which might have been the reason why we only observed An. arabiensis. Other mosquito species, such as Anopheles coustani and Anopheles pharoensis might be present in the study region, but as they are exophilic they are rarely captured by indoor collection methods like PSC. Anopheles arabiensis was the predominant anopheline species in lowland areas of Kenya [37], south-central Ethiopia [38] and south-west Ethiopia [39, 40]. This might be due to the fact that An. arabiensis adopts better to hot and arid climatic conditions [41].
Previous studies documented higher Anopheles mosquito abundance in houses near the river than houses far from the river [42–44]. Similarly, in this study, the mean density of the An. arabiensis was significantly higher in clusters close to the river compared to clusters away from the river. This could be explained by the availability of breeding sites in riverbeds [19, 45–48].
A significant effect of proximity to the river was found during the dry season and transition from dry to wet season, whereas no statistically significant effect of closeness to the river was found in the wet and transition from wet to dry seasons. This might be due to the fact that riverbeds were the only breeding sites in the study region during dry and transition from dry to wet seasons whereas there were ample breeding sites in all clusters during the wet and transmission from wet to dry seasons. The findings of this study match with the results of the previous studies [35, 49] which recorded variation in vector densities over seasons. The results also showed that the bovine blood index (BBI) of An. arabiensis was higher compared to the human blood index (HBI) and this is in line with previous reports [50–52]. The unidentified blood meal sources could be of donkey, horse, sheep and chicken for which we did not test. The zoophilic behaviour of An. arabiensis observed in the present study is consistent with the results of other studies [39, 53–57].
The overall sporozoite rate was 3.9% and 0% for clusters close to and away from the river, respectively. This is comparable with earlier reports in Africa where sporozoite rates in An. arabiensis ranged from 2 to 7% [58, 59]. In southern and south-central Ethiopia, much lower sporozoite rate of An. arabiensis for P. falciparum was documented [38, 39, 60]. The variation in sporozoite rate could be related to the different feeding behaviour of An. arabiansis. Sporozoite rates are typically lower when the vector is more zoophilic [61] which is consistent with the findings of this study. Sporozoites were only found in clusters close to the river. Similarly, a clustered distribution of Plasmodium CSPs-positive An. arabiensis in a sub-village near the shore of a lake was observed in Ethiopia [20, 39]. This study is in line with a study conducted in Eastern Sudan where CSP was higher in villages along Rahad river basin [19].
The overall P. falciparum and P. vivax EIR of An. arabiensis in clusters close to the rivers in Goro and Abeshege districts were 0.8 and 2.19 infective bites per person/year, respectively. This indicates that the entomological inoculation rates in the study area were in agreement with previous reports [42, 62]. The results are also consistent with the findings in Tanzania [4] where malaria transmission was strongly associated with proximity to the river. The EIR in the present study is substantially higher than the one found in southern Ethiopia where P. falciparum and P. vivax EIR of An. arabiensis was 0.1 infective bites per person/year for houses close to a breeding site [52]. Plasmodium CSP positive An. arabiensis were abundant in clusters close to river, similar to the findings of previous studies [20, 39, 42]. High P. falciparum EIR was recorded during transition from wet to dry season whereas high P. vivax EIR was recorded during transition from dry to wet season. This indicates that malaria transmission persists immediately after the wet season and late in the dry season [14].
Conclusions
This study showed that riverbeds contribute substantially to malaria transmission during the dry and transition from dry to wet seasons suggesting that riverine breeding sites are the main driving transmission hotspots in the area. Infectivity and transmission intensity were recorded in clusters close to the river when the rivers became intermittent and large number of pockets of breeding sites were created. The results presented here support that vector control strategies to eradicate mosquitoes should target the larval stage at the time when the mosquito population is at its lowest and confined to few remaining hotspots in the semi-arid areas of central Ethiopia. Moreover, identifying and assessing mosquito breeding sites using Geographic Information System (GIS) is useful with river locations as a tool for more effective larval source management in the highlands of Ethiopia. Since clustering of malaria transmission intensity was observed, targeted vector control interventions should be implemented to reduce the overall transmission and sustain malaria control to move towards elimination.
Supplementary Information
Acknowledgements
We acknowledge the financial support from the Institutional University Cooperation IUC-JU program to LD, in the framework of the Flemish Interuniversity Council (VLIR-UOS).
Abbreviations
- EIR
Entomological inoculation rate
- TWD
Transmission from wet to dry
- TDW
Transmission from dry to wet
- Pf + ve
Positive for P. falciparum circumsporozoite protein (CSP)
- Pv + ve
Positive for P. vixax circumsporozoite protein
- %
Percentage
- H + B
Human and bovine
- H + G
Human and goat
- PSC
Pyrethrum space spray catches
- CSP
Circumsporozoite protein
Authors’ contributions
TH and GKC developed the research idea. KE performed the mosquito field studies. TH took care of all laboratory analyses. LD analysed and interpreted the data and supported the field work. KE, TH, DY, GKC and LD wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the Institutional University Cooperation IUC-JU project in the framework of the Flemish Interuniversity Council (VLIR-UOS).
Availability of data and materials
Data are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from Jimma University institute of health Institutional Review Boards (IRB), Ethiopia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alemu K, Worku A, Berhane Y. Malaria infection has spatial, temporal, and spatiotemporal heterogeneity in unstable malaria transmission areas in northwest Ethiopia. PLoS ONE. 2013;8:e79966. doi: 10.1371/journal.pone.0079966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson JC, Pinchoff J, Muleba M, Lupiya J, Chilusu H, Mwelwa I, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: implications for vector control. Parasit Vectors. 2016;9:510. doi: 10.1186/s13071-016-1786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousema T, Stevenson J, Baidjoe A, Stresman G, Griffin JT, Kleinschmidt I, et al. The impact of hotspot-targeted interventions on malaria transmission: study protocol for a cluster-randomized controlled trial. Trials. 2013;14:1–2. doi: 10.1186/1745-6215-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oesterholt MJ, Bousema JT, Mwerinde OK, Harris C, Lushino P, Masokoto A, et al. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J. 2006;5:98. doi: 10.1186/1475-2875-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stresman G, Bousema T, Cook J. Malaria hotspots: is there epidemiological evidence for fine-scale spatial targeting of interventions? Trends Parasitol. 2019;35:822–834. doi: 10.1016/j.pt.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Knudson A, González-Casabianca F, Feged-Rivadeneira A, Pedreros MF, Aponte S, Olaya A, et al. Spatio-temporal dynamics of Plasmodium falciparum transmission within a spatial unit on the Colombian Pacific Coast. Sci Rep. 2020;10:3756. doi: 10.1038/s41598-020-60676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basurko C, Hanf M, Han-Sze R, Rogier S, Héritier P, Grenier C, et al. Influence of climate and river level on the incidence of malaria in Cacao. French Guiana Malar J. 2011;10:26. doi: 10.1186/1475-2875-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin DM. Hotspots of malaria in a low-transmission setting in Southern Zambia. Doctoral dissertation, Johns Hopkins University; 2019.
- 9.Badu K. Evaluation of immuno-epidemiological markers for assessing malaria transmission intensity in the hypo-endemic highlands of Kenya and the accuracy of malaria diagnosis in the Holo-endemic forest zone of Ghana. Doctoral dissertation, Kwame Nkrumah University of Science and Technology; 2013.
- 10.Bautista CT, Chan AS, Ryan JR, Calampa C, Roper MH, Hightower AW, Magill AJ. Epidemiology and spatial analysis of malaria in the Northern Peruvian Amazon. Am J Trop Med Hyg. 2006;75:1216–1222. doi: 10.4269/ajtmh.2006.75.1216. [DOI] [PubMed] [Google Scholar]
- 11.Bejon P, Williams TN, Nyundo C, Hay SI, Benz D, Gething PW, et al. A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. Elife. 2014;3:e02130. doi: 10.7554/eLife.02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okami S, Kohtake N. Spatiotemporal modeling for fine-scale maps of regional malaria endemicity and its implications for transitional complexities in a routine surveillance network in western Cambodia. Public Health Front. 2017;26:262. doi: 10.3389/fpubh.2017.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagna AB, Gaayeb L, Sarr JB, Senghor S, Poinsignon A, Boutouaba-Combe S, et al. Plasmodium falciparum infection during dry season: IgG responses to Anopheles gambiae salivary gSG6-P1 peptide as sensitive biomarker for malaria risk in Northern Senegal. Malar J. 2013;12:301. doi: 10.1186/1475-2875-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooker S, Clarke S, Njagi JK, Polack S, Mugo B, Estambale B, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 16.Coulibaly D, Rebaudet S, Travassos M, Tolo Y, Laurens M, Kone AK, et al. Spatio-temporal analysis of malaria within a transmission season in Bandiagara. Mali Malar J. 2013;12:82. doi: 10.1186/1475-2875-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noor AM, Mutheu JJ, Tatem AJ, Hay SI, Snow RW. Insecticide-treated net coverage in Africa: mapping progress in 2000–07. Lancet. 2009;373:58–67. doi: 10.1016/S0140-6736(08)61596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himeidan YE, Elzaki MM, Kweka EJ, Ibrahim M, Elhassan IM. Pattern of malaria transmission along the Rahad River basin. Eastern Sudan Parasit Vectors. 2011;4:109. doi: 10.1186/1756-3305-4-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loha E, Lindtjørn B. Predictors of Plasmodium falciparum malaria incidence in Chano Mille, South Ethiopia: a longitudinal study. Am J Trop Med Hyg. 2012;87:450–459. doi: 10.4269/ajtmh.2012.12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeshiwondim AK, Gopal S, Hailemariam AT, Dengela DO, Patel HP. Spatial analysis of malaria incidence at the village level in areas with unstable transmission in Ethiopia. Int J Health Geogr. 2009;8:5. doi: 10.1186/1476-072X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gari T, Kenea O, Loha E, Deressa W, Hailu A, Balkew M, et al. Malaria incidence and entomological findings in an area targeted for a cluster-randomized controlled trial to prevent malaria in Ethiopia: results from a pilot study. Malar J. 2016;15:145. doi: 10.1186/s12936-016-1199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CSA. Population and housing census of Ethiopia: administrative report. Addis Ababa: Central Statistical Agency; 2012.
- 24.WHO . Manual on practical entomology in malaria. Geneva: World Health Organization; 1995. [Google Scholar]
- 25.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. S Afr Inst Med Res. 1987;55:1–43. [Google Scholar]
- 26.WHO . Malaria entomology and vector control-guide for participants training module on malaria control. Geneva: World Health Organization; 2013. [Google Scholar]
- 27.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 28.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. doi: 10.4269/ajtmh.2007.76.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinheirob VE, Thaithongc S, Browna KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 30.Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- 31.Pappa V, Reddy M, Overgaard HJ, Abaga S, Caccone A. Estimation of the human blood index in malaria mosquito vectors in Equatorial Guinea after indoor antivector interventions. Am J Trop Med. 2011;84:298–301. doi: 10.4269/ajtmh.2011.10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmerman RH, Galardo AK, Lounibos LP, Aruda M, Wirtz R. Blood meal hosts of Anopheles species (Diptera: Culicidae) in a malaria-endemic area of the Brazilian Amazon. J Med Entmol. 2006;43:947–956. doi: 10.1093/jmedent/43.5.947. [DOI] [PubMed] [Google Scholar]
- 33.WHO . Malaria entomology and vector control. Geneva: World Health Organization; 2003. [Google Scholar]
- 34.Al-Eryani SM, Kelly-Hope L, Harbach RE, Briscoe AG, Barnish G, Azazy A, et al. Entomological aspects and the role of human behaviour in malaria transmission in a highland region of the Republic of Yemen. Malar J. 2016;15:130. doi: 10.1186/s12936-016-1179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirebvu E, Chimbari MJ. Characterization of an indoor-resting population of Anopheles arabiensis (Diptera: Culicidae) and the implications on malaria transmission in Tubu village in Okavango subdistrict. Botswana J Med Entomol. 2016;53:569–576. doi: 10.1093/jme/tjw024. [DOI] [PubMed] [Google Scholar]
- 36.Getachew D, Gebre-Michael T, Balkew M, Tekie H. Species composition, blood meal hosts and Plasmodium infection rates of Anopheles mosquitoes in Ghibe River Basin, southwestern Ethiopia. Parasit Vectors. 2019;12:257. doi: 10.1186/s13071-019-3499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16:443. doi: 10.1186/s12936-017-2098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Animut A, Balkew M, Gebre-Michael T, Lindtjørn B. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malar J. 2013;12:76. doi: 10.1186/1475-2875-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Entomologic inoculation rates of Anopheles arabiensis in Southwestern Ethiopia. Am J Trop Med Hyg. 2013;89:466–473. doi: 10.4269/ajtmh.12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taye A, Hadis M, Adugna N, Tilahun D, Wirtz RA. Biting behavior and Plasmodium infection rates of Anopheles arabiensis from Sille. Ethiopia Acta Trop. 2006;97:50–54. doi: 10.1016/j.actatropica.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Kirby MJ, Lindsay SW. Responses of adult mosquitoes of two sibling species, Anopheles arabiensis and An. gambiae s.s (Diptera: Culicidae), to high temperatures. Bull Entomol Res. 2004;94:441–448. doi: 10.1079/BER2004316. [DOI] [PubMed] [Google Scholar]
- 42.Esayas E, Woyessa A, Massebo F. Malaria infection clustered into small residential areas in lowlands of southern Ethiopia. Parasit Epidemiol Control. 2020;10:e00149. doi: 10.1016/j.parepi.2020.e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getachew D, Balkew M, Tekie H. Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J. 2020;19:65. doi: 10.1186/s12936-020-3145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCann RS, Messina JP, MacFarlane DW, Bayoh MN, Gimnig JE, Giorgi E, et al. Explaining variation in adult Anopheles indoor resting abundance: the relative effects of larval habitat proximity and insecticide-treated bed net use. Malar J. 2017;16:288. doi: 10.1186/s12936-017-1938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruz RM, Gil LH, de Almeida e Silva A, da Silva Araújo M, Katsuragawa TH. Mosquito abundance and behavior in the influence area of the hydroelectric complex on the Madeira River, Western Amazon, Brazil. Trans R Soc Trop Med Hyg. 2009;103:1174–1176. doi: 10.1016/j.trstmh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Dida GO, Anyona DN, Abuom PO, Akoko D, Adoka SO, Matano AS, Owuor PO, Ouma C. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect Dis Poverty. 2018;7:2. doi: 10.1186/s40249-017-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gil LH, Alves FP, Zieler H, Salcedo JM, Durlacher RR, Cunha RP, et al. Seasonal malaria transmission and variation of anopheline density in two distinct endemic areas in Brazilian Amazonia. J Med Entomol. 2003;40:636–641. doi: 10.1603/0022-2585-40.5.636. [DOI] [PubMed] [Google Scholar]
- 48.Staedke SG, Nottingham EW, Cox J, Kamya MR, Rosenthal PJ, Dorsey G. Proximity to mosquito breeding sites as a risk factor for clinical malaria episodes in an urban cohort of Ugandan children. Am J Trop Med Hyg. 2003;69:244–246. doi: 10.4269/ajtmh.2003.69.244. [DOI] [PubMed] [Google Scholar]
- 49.Van Der Hoek W, Konradsen F, Amerasinghe PH, Perera D, Piyaratne MK, Amerasinghe FP. Towards a risk map of malaria for Sri Lanka: the importance of house location relative to vector breeding sites. Int J Epidemiol. 2003;32:280–285. doi: 10.1093/ije/dyg055. [DOI] [PubMed] [Google Scholar]
- 50.Emami SN, Ranford-Cartwright LC, Ferguson HM. The transmission potential of malaria-infected mosquitoes (An. gambiae-Keele, An. arabiensis-Ifakara) is altered by the vertebrate blood type they consume during parasite development. Sci Rep. 2017;7:40520. doi: 10.1038/srep40520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayagaya VS, Nkwengulila G, Lyimo IN, Kihonda J, Mtambala H, Ngonyani H, et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17. doi: 10.1186/s12936-014-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Blood meal origins and insecticide succesptablity of Anopheles arabiansis from Chano in South-West Ethiopia. Parasit Vectors. 2013;6:44. doi: 10.1186/1756-3305-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duchemin JB, Tsy JM, Rabarison P, Roux J, Coluzzi M, Costantini C. Zoophily of Anopheles arabiensis and An. gambiae in Madagascar demonstrated by odour-baited entry traps. Med Vet Entomol. 2001;15:50–57. doi: 10.1046/j.1365-2915.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 54.Habtewold T, Walker AR, Curtis CF, Osir EO, Thapa N. The feeding behaviour and Plasmodium infection of Anopheles mosquitoes in southern Ethiopia in relation to use of insecticide-treated livestock for malaria control. Trans R Soc Trop Med Hyg. 2001;95:584–586. doi: 10.1016/S0035-9203(01)90086-0. [DOI] [PubMed] [Google Scholar]
- 55.Mahande A, Mosha F, Mahande J, Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Zoophagic behaviour of anopheline mosquitoes in southwest Ethiopia: opportunity for malaria vector control. Parasit Vectors. 2015;8:645. doi: 10.1186/s13071-015-1264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 58.Abdalla H, Matambo TS, Koekemoer LL, Mnzava AP, Hunt RH, Coetzee M. Insecticide susceptibility and vector status of natural populations of Anopheles arabiensis from Sudan. Trans R Soc Trop Med Hyg. 2008;102:263–271. doi: 10.1016/j.trstmh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Temu EA, Minjas JN, Coetzee M, Hunt RH, Shiff CJ. The role of four anopheline species (Diptera: Culicidae) in malaria transmission in coastal Tanzania. Trans R Soc Trop Med Hyg. 1998;92:152–158. doi: 10.1016/S0035-9203(98)90724-6. [DOI] [PubMed] [Google Scholar]
- 60.Abraham M, Massebo F, Lindtjørn B. High entomological inoculation rate of malaria vectors in area of high coverage of interventions in southwest Ethiopia: implication for residual malaria transmission. Parasit Epidemiol Control. 2017;2:61–69. doi: 10.1016/j.parepi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor KA, Koros JK, Nduati J, Copeland RS, Collins FH, Brandling-Bennett AD. Plasmodium falciparum infection rates in Anopheles gambiae, An. arabiensis, and An. funestus in western Kenya. Am J Trop Med Hyg. 1990;43:124–129. doi: 10.4269/ajtmh.1990.43.124. [DOI] [PubMed] [Google Scholar]
- 62.Msugh-Ter MM, Gbilekaa VC, Nyiutaha IG. Sporozoite infection rates of female Anopheline mosquitoes in Makurdi, an endemic area for malaria in central Nigeria. Int J Entomol Res. 2014;2:103–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon request.