Abstract
Aims:
Sustained heavy alcohol consumption is associated with a range of neurocognitive deficits. Yet, past research centers on a severe profile of alcohol use disorder (AUD), with persons recruited from in-patient settings. The current project aims to compare neurocognitive performance between individuals seeking AUD outpatient treatment with healthy comparisons while considering the association between performance, disorder severity, and sex.
Methods:
Enrollment included two matched groups (N = 125; 34 % female): 77 treatment-seeking individuals with AUD; 48 healthy comparison individuals with low drinking patterns. Neurocognitive performance on NIH Toolbox subtests measuring attention, inhibition, episodic memory, working memory, language, and processing speed were compared across groups. Within the AUD group, analyses examined the relationship between performance, disorder severity, recent alcohol consumption, and sex.
Results:
AUD group did not perform significantly lower than healthy comparisons on neurocognition subtests assessed. Within AUD group, females displayed significantly higher processing speeds than males (p = .007). Disorder severity and alcohol consumption were not significantly related to performance. However, a significant interaction between disorder severity and sex emerged (p = .010), with higher severity associated with poorer performance in males but not females, on a subtest measuring attention and inhibition.
Conclusions:
Effect of heavy alcohol use on neurocognitive performance was not detected in this outpatient AUD sample. Weaknesses in domains of attention and inhibition may be correlated with AUD severity among males, but not females. Further research on AUD severity and sex in understanding individual differences in neurocognition is warranted, particularly using novel tools for large scale phenotyping, such as the NIH Toolbox.
Keywords: Neurocognition, Severity, Alcohol use disorder, Alcohol, Sex, Treatment-seeking
1. Introduction
Alcohol-related problems represent a national health concern with estimates of up to 29 million adults having an alcohol use disorder (AUD) in a given year (Grant et al., 2017). One of the harms associated with AUD is neurocognitive impairment, which contributes to continued alcohol-seeking behavior and relapse (Koob and Volkow, 2010). Worsened neurocognitive functioning in patients with AUD is associated with poorer treatment outcomes, as neurocognitive processes allow individuals to engage in goal-directed actions, plan, and self-regulate, among other tasks (Bates et al., 2006). Moreover, neurocognitive impairments are related to functional and structural brain abnormalities and are suggestive of accelerated brain aging (Chanraud et al., 2007; Guggenmos et al., 2017; Pfefferbaum et al., 2013, 1997). These deficits are well-documented in the literature (Bernardin and Maheut-Bosser, 2014; Horner et al., 1999; Sullivan et al., 2000), particularly in domains of executive function, which includes measures of response inhibition (Stavro et al., 2013), working memory (Bernardin and Maheut-Bosser, 2014), and processing speed (Stavro et al., 2013). More specifically, scores in various dimensions of memory, including episodic memory (Le Berre et al., 2017; Pitel et al., 2007), verbal memory (Davies et al., 2005), and visual memory (Kopera et al., 2012) were lower in individuals with AUD than healthy comparisons. A recent meta-analysis identified that, relative to healthy controls, short-term abstinent individuals with AUD exhibited significantly lower functioning across all 12 measured neurocognitive domains (Stavro et al., 2013). This analysis also showed that attention had the largest effect size of any domain but has been the least researched domain (Kopera et al., 2012; Loeber et al., 2009; Stavro et al., 2013).
Although Stavro and colleagues’ (2013) meta-analysis provides evidence that neurocognitive impairment is a prevalent issue among individuals with AUD, the vast majority of these studies have centered on severe alcohol use disorder, with persons recruited from in-patient settings and compared to healthy individuals. However, deficits of heavy drinking adults recruited from outpatient treatment settings have been largely understudied (Davies et al., 2005; Horner et al., 1999). One study that enrolled healthy outpatients with AUD found evidence of neurocognitive impairment for the AUD group as compared to controls in areas of attention, visuospatial scanning, inhibition, and verbal but not non-verbal memory (Davies et al., 2005). Since research in less severe outpatient samples is sparse, it is thus unclear whether neurocognitive differences potentially precede the onset of AUD, or alternatively, when in the disease course of addiction these impairments arise, become detectable, and may start to impact recovery. Research investigating the relationship between severity of AUD and neurocognitive performance may contribute to this understanding as well. For example, among non-treatment seeking individuals with alcohol dependence, a composite measure of alcohol severity was correlated with higher delay discounting rates (i.e., greater impulsivity) and associated dysregulations in neural control regions (Lim et al., 2017). Further, indicators of alcohol use history including years of problem drinking (Duka et al., 2003), age of initiation (Nguyen-Louie et al., 2017), and recent and lifetime alcohol consumption (Horner et al., 1999; Sullivan et al., 2002; Woods et al., 2016) have been related to neurocognitive performance across the lifespan on measures such as visual attention, cognitive inhibition, and working memory. This literature provides some initial evidence that neurocognitive deficits are graded correlates of chronic alcohol use.
Moreover, accounting for and understanding the impact of other factors, such as age, education, and sex may improve our understanding of individual differences in neurocognitive impairments in addiction, success in treatment, and recovery (Le Berre et al., 2017). There is mixed evidence on whether heavy alcohol use differentially affects neurocognitive performance for females as compared with males. While several studies have evidenced neurocognitive deficits for females with problematic alcohol use (Acker, 1986; Glenn and Parsons, 1991; Sullivan et al., 2002), others suggest that they may be less sensitive than males to the toxic effects of alcohol and may thus display less neurocognitive impairment (Sparadseo et al., 1983; Yonker et al., 2005). However, these findings have varied widely across domains; for example, findings inconsistently report both the presence and absence of deficits for females in areas of memory (e.g., short-term memory, immediate recall, episodic memory; Oscar-Berman and Marinkovic, 2007), and other research suggests that for individuals with alcohol dependence, females perform lower than males on tests of psychomotor speed (Acker, 1986) but similarly on measures of abstraction and visuoperceptual functioning (Sparadseo et al., 1983). Yet, there remains a lack of females enrolled in studies on AUD to adequately capture these potentially important and nuanced differences (Stavro et al., 2013). Additionally, other findings suggest that age (Bates et al., 2006; Fein et al., 1990), education, cigarette use (Durazzo and Meyerhoff, 2007), and psychopathology (Gierski et al., 2013) have also been related to performance on tasks of neurocognition among those with AUD; accounting for these factors may help refine group differences in neurocognitive performance, as such psychiatric comorbidities are common in individuals with AUD (Castillo-Carniglia et al., 2019).
Another gap in the literature is the use of multiple neurocognitive batteries that complicate cross-test comparisons for neurocognitive domains (Stavro et al., 2013). This limitation may be addressed by the NIH Toolbox, a standardized cognitive battery that was developed through NIH’s Blueprint for Neuroscience Research initiative (Hodes et al., 2013). The NIH Toolbox may be a useful new instrument for researchers and clinicians in that it is computer-based, brief, and easy to administer (Weintraub et al., 2013b). Notably, while this battery broadly measures neurocognitive abilities, it is not intended to replace comprehensive neuropsychological evaluation. Additional investigation of this toolbox is warranted in substance-using populations, as its designers expressed the necessity of validation in various clinical populations (Weintraub et al., 2013b). To our knowledge, no published studies to date have used the NIH Toolbox to study neurocognition in AUD. This is an important gap as the NIH Toolbox seeks to facilitate neurocognitive testing for the purpose of large-scale phenotyping.
To advance our understanding of neurocognitive functioning in AUD, the current project aims to address important gaps in the literature by examining neurocognitive functioning in outpatient treatment-seeking individuals with AUD. These individuals, who have a potentially less severe profile than those previously studied, were compared to a community-based sample of healthy comparison individuals. All neurocognitive assessments were conducted through the novel NIH Toolbox. Given the literature above, we hypothesized that the AUD group, relative to the healthy comparison group, would have significantly lower scores on all neurocognition subtests administered, which measure attention, inhibitory control, processing speed, working memory, episodic memory, and language. A secondary aim was to explore the association between neurocognitive performance within the AUD group and an AUD severity factor, total recent consumption of alcoholic drinks, and sex, after accounting for important demographic factors and measures of nicotine and cannabis co-use.
2. Methods
2.1. Participants
After an initial phone screening interview, a total of 182 participants (118 for AUD group; 64 for healthy comparison group (HC group)) were screened in person to determine eligibility in the current study. Of the 182 individuals who came in for this in-person screening, 125 were deemed eligible based on criteria noted below (77 participants in the AUD group; 48 in the healthy comparison group).
2.2. Screening procedures
2.2.1. Recruitment and enrollment
Recruitment for the alcohol treatment-seeking group was a part of a larger, ongoing NIH-funded randomized clinical trial of ibudilast for AUD (NCT03594435). Screening data for this trial is used in the current analyses. A healthy comparison group was recruited to match the group with AUD on key demographics variables including sex, age, race, ethnicity, and education. Both groups were recruited from the metropolitan Los Angeles area using similar recruitment tools, including online advertisements and flyers placed in the community. Interested participants completed a phone screener and if determined potentially eligible for the appropriate study, were asked to come into the research lab for an in-person visit. In order to participate in the in-person visit, participants were required to have a blood alcohol concentration of 0.000 g/dl and a urine toxicology screen negative for all drugs tested, except cannabis. All participants provided written informed consent after receiving a full explanation of the study procedures and were compensated for their time. All study procedures were approved by the University of California, Los Angeles Institutional Review Board.
2.2.2. Healthy comparison group
Eligible healthy comparison participants met the following eligibility criteria: age 18–65; fluency in English; engage in low past 30-day alcohol drinking patterns including < 7 drinks per week for females, < 14 for males, and report no binge drinking in the past month (defined by more than 4 alcoholic drinks per occasion for males or more than 3 alcoholic drinks for females); score < 8 on the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993). Exclusion criteria for healthy comparison participants included: current psychiatric diagnoses defined by DSM-5 for major depressive episode, anxiety disorder (i.e., generalized anxiety disorder, panic disorder, agoraphobia, obsessive-compulsive disorder), posttraumatic stress disorder, eating disorder (i.e., anorexia nervosa, bulimia nervosa, binge eating disorder); lifetime diagnoses defined by the DSM-5 for alcohol or other substance use disorder (SUD), manic episode, or psychotic symptoms; history of any major medical or neurological condition that may affect neurocognitive functioning, including traumatic brain injury, dementia, seizures, or serious or repeated concussions; history of cognitive impairment or learning/ developmental disability; past month active suicidal ideation or attempt; or currently taking any psychotropic medications (e.g., psychostimulants, benzodiazepines, or antidepressants).
2.2.3. Group with alcohol use disorder
For the current study, eligibility for the group with AUD is based generally on criteria required to enroll in a larger pharmacotherapy trial for individuals seeking treatment for alcohol use. For the current analyses, eligibility criteria included: meet current DSM-5 criteria for AUD; and report interest in treatment for alcohol use, as assessed using a single-item question: “Do you have a desire to reduce or quit drinking?” Exclusion criteria for participants with AUD included: lifetime DSM-5 diagnosis for manic episode or endorsement of lifetime psychotic symptoms; DSM-5 diagnosis for a non-alcohol SUD (except for cannabis: only severe cannabis use disorder was exclusionary); clinically significant alcohol withdrawal symptoms (score > 9 on the Clinical Institute Withdrawal Assessment for Alcohol, Revised (CIWA-Ar; Sullivan et al., 1989)); pregnancy, nursing, or planning to become pregnant during the larger trial, or decision to not use birth control (if female); past month active suicidal ideation or attempt; or currently taking any psychotropic medications with the exception of a stable antidepressant regimen.
2.3. Measures
2.3.1. Clinical interviews
Participants completed clinical interviews with trained graduate students or staff members, including the Timeline Follow-Back (TLFB) Interview (Sobell and Sobell, 1992) to assess past month self-reported quantity and frequency of alcohol, cigarette, and cannabis use; selected modules from the Structured Clinical Interview for DSM-5 (First et al., 2016) to assess inclusion and exclusion criteria regarding psychiatric diagnoses and symptoms; Columbia Suicide Severity Rating Scale (C-SSRS (Posner et al., 2011)), if indicated, to assess reports of suicidal ideation and attempts; CIWA-Ar (group with AUD only; Sullivan et al., 1989) to identify clinically significant alcohol withdrawal symptoms.
2.3.2. Neurocognitive testing via NIH Toolbox
2.3.2.1. Validation.
In order to examine neurocognitive functioning, participants across the two groups completed a portion of the NIH Toolbox Cognition battery (Weintraub et al., 2013a), which was administered by trained graduate students in clinical psychology or bachelor’s level research coordinators. The Cognition battery measured domains of attention, inhibitory control, episodic memory, working memory, language, and processing speed. Through previous validation and standardization procedures, the NIH toolbox showed good discriminant (ranging from r = .05 to r = .30) and convergent (ranging from r = .48 to r = .93) validity when tested against “gold standards” in the field of cognitive assessment (Weintraub et al., 2013b), high test-retest reliability (ranging from r = .72 to r = .96), robust age-related performance results and was normed in a diverse population to match the U.S. demographics (Beaumont et al., 2013).
2.3.2.2. Scoring.
The Cognition battery is a brief (45–60 min) and convenient multidimensional assessment tool; raw scores are electronically normed to provide three performance scores (Age-Corrected Standard Scores, Uncorrected Standard Scores, and Fully Corrected Standard Scores; see NIH Scoring and Interpretation Guide for more details). For the current analyses, Fully Corrected Standard Scores were used: raw scores are normed based on a nationally representative sample, while adjusting for demographic variables including age, sex, educational attainment, and race/ ethnicity (Weintraub et al., 2013b). These scores are based on T-score metric, with a mean of 50 and standard deviation of 10 with higher scores indicating better performance.
2.3.2.3. Neurocognitive domains.
Participants in the current study completed five of the seven available NIH Toolbox cognition battery subtests (List Sorting Working Memory Test (LSWM); Pattern Comparison Processing Speed Test (PCPS); Picture Sequence Memory Test (PSM); Flanker Inhibitory Control and Attention Test (FICA); Oral Reading Recognition Test (ORR)). LSWM test measures working memory (processing and storage of information); food and animal items were presented visually along with simultaneous audio recording stating item name and participants were then asked to repeat item names back in size order. PCPS test measures processing speed; participants were asked to respond as quickly as possible to indicate whether two simple pictures were the same or different. PSM test measures episodic memory; participants were presented with a sequence of events (visually and via audio recording) and then attempted to place scrambled pictures into the correct temporal order. FICA test measures attention and inhibitory control domain of executive functioning; participants were asked to focus on a middle arrow stimulus while inhibiting attention to other arrows during both congruent (all arrows pointing the same direction) and incongruent (middle arrow pointing a different direction) trials. ORR test measures language; words were presented visually, and participants were asked to pronounce and read words accurately.
2.3.3. Individual Difference Measures
Self-report questionnaires were completed to collect information on demographics, mood, health, and substance use patterns, including the following: AUDIT (Saunders et al., 1993) to measure alcohol-related problems; Alcohol Dependence Scale (ADS; Skinner and Allen, 1982) to assess alcohol dependence severity; Beck Anxiety Inventory (BAI; Beck et al., 1988) to capture anxiety symptomatology; Beck Depression Inventory-II (BDI-II; Beck et al., 1996) to capture mood symptomatology; and Penn Alcohol Craving Scale (PACS) to measure past-week craving for alcohol (Flannery et al., 1999).
2.4. Data analysis
Chi-square, Fisher’s exact, and independent samples T-tests were performed to test potential differences in demographic and individual difference variables between the AUD and healthy comparison group. To test the study hypothesis that participants with AUD would display deficits of neurocognitive functioning as compared to healthy individuals with low drinking patterns, a series of general linear model analyses were conducted using SAS Statistical Software version 9.4. Specifically, general linear model analyses were conducted with group (AUD vs. HC), sex (male vs. female) and their interaction (group × sex) as dichotomous factors and years of education and mood symptomatology (BDI-II and BAI total scores) as a continuous covariates, with the dependent measures being the five NIH Toolbox standardized subtest scores (tested separately): attention/inhibitory control, episodic memory, language, processing speed, and working memory. In the interest of parsimony, final models are presented with non-significant covariates removed, including sex and group x sex interaction term; results between full and final models are consistent.
For the AUD group only, a series of mixed general linear model analyses were conducted to explore the association between neurocognitive performance and a number of severity-related factors. To reduce the number of variables to be examined in primary analyses, consistent with previous studies (Lim et al., 2017; Moallem et al., 2013), a principal components analysis (PCA) with varimax rotation was conducted to create an AUD Severity Factor on the AUD group data including: (a) DSM-5 AUD symptom count; (b) PACS total score; (c) ADS total score; (d) AUDIT total score; (e) BDI-II total score; (f) BAI total score; and (h) CIWA-Ar total score. One model for each of the five neurocognitive subtest scores (outcome variable) were run, which included the AUD Severity Factor score as a continuous predictor, sex (male vs. female) as a dichotomous predictor, and their interaction (AUD Severity Factor × sex) with the following variables added as covariates: education, age, nicotine and cannabis use status (user or non-user, as determined by past 30-day TLFB reports). Significant interactions were probed with post-hoc tests of simple effects. Additionally, given previous research identifying quantity of alcohol consumption as a relevant measure implicated in neurocognitive performance (Horner et al., 1999; Sullivan et al., 2002; Woods et al., 2016), we conducted an exploratory analysis; one model for each of the neurocognitive subtest scores were run in which total number of recent alcoholic drinks served as the focal predictor and the following variables served as covariates: sex, education, age, and nicotine and cannabis use status. In the interest of parsimony, final models are presented with non-significant covariates removed; sex and sex x AUD severity interaction terms were retained. Results between full and final models are consistent. Chi-square and independent samples T-tests were performed to ensure that males and females within the group with AUD did not significantly differ on any demographic or individual difference variables. Independent samples T-tests were conducted to compare neurocognitive performance for those in the AUD group who tested positive for THC vs. negative for THC on the urine toxicology screen.
3. Results
3.1. Participant characteristics
The current sample (N = 125; AUD group n = 77; HC group n = 48) was found to have the following characteristics: 34 % female, average age of 46 years, 15 years of education, 53 % identifying as Caucasian, 32 % identifying as African American, and 21 % identifying as Latinx. The two groups were adequately matched on age, race, ethnicity, and financial status (p’s > .350; see Table 1). Despite efforts to match the two groups, the group with AUD and healthy comparison group differed significantly on the following: level of education (p < .001), employment status (p = .028), and sex (p = .034). As expected, the group with AUD had significantly higher past 30-day reports of substance use and mood symptomatology (see Table 1; p’s < .001). On average, the AUD group drank alcohol 21 of the past 30 days and had 5.2 alcoholic drinks per day with 30 % and 36 % reporting cigarettenicotine and cannabis use in the last 30 days, respectively.
Table 1.
Participant characteristics by group.
| Characteristic | Group with AUD Mean (SD) N = 77 | Healthy Comparisons GroupMean (SD) N = 48 | Sign. (p-value) |
|---|---|---|---|
| Age | 44.1 (11.0) | 45.8 (13.4) | .452 |
| Range (20–65) | |||
| Sex (% Female) | 27.3 % | 45.8 % | .034* |
| Education (Years) | 13.9 (2.4) | 15.6 (2.2) | < .001* |
| Range (7–20) | |||
| Ethnicity (%) | .420 | ||
| Caucasian | 52.0 % | 54.2 % | |
| African American | 32.5 % | 31.3 % | |
| Asian | 2.6 % | 6.3 % | |
| Pacific Islander | 0.0 % | 2.1 % | |
| Native American | 2.6 % | 0.0 % | |
| Multi-ethnic | 10.4 % | 6.3 % | |
| % Latinx | 22.1 % | 18.8 % | .656 |
| Employment Status | .028* | ||
| Full Time | 35.1 % | 47.9 % | |
| Part Time | 15.6 % | 29.2 % | |
| Retired or Disability | 14.3 % | 6.3 % | |
| Unemployed | 35.1 % | 16.7 % | |
| Financial Status | .376 | ||
| Not enough money to pay bills | 15.6 % | 6.3 % | |
| Enough money to pay bills but cut back | 36.4 % | 41.7 % | |
| Enough money to pay bills but no extras | 18.2 % | 25.0 % | |
| Enough money for extras | 29.9 % | 27.1 % | |
| Alcohol (Past 30 Days) | |||
| Drinking Days | 21.2 (8.3) | 1.9 (4.4) | < .001* |
| Range (0–30) | |||
| Drinks per Day | 5.2 (3.6) | .09 (0.2) | < .001* |
| Range (0–16.9) | |||
| AUDIT Total | 21.5 (7.2) | 1.1 (1.4) | < .001* |
| Range (0–37) | |||
| % Cannabis Users (TLFB Past 30) | 36.4 % | 2.1 % | < .001* |
| % THC Positive | 28.6 % | 4.2 % | < .001* |
| % Nicotine Users (TLFB Past 30) | 29.9 % | 4.2 % | < .001* |
| Beck Depression Inventory-II Total Score | 13.1 (10.0) | 5.9 (7.9) | < .001* |
| Range (0–40) | |||
| Beck Anxiety Inventory Total Score | 10.1 (9.3) | 3.1 (4.2) | < .001* |
| Range (0–46) | |||
| % Meeting Criteria for Current MDEa | 9.1 % | 0.0 % | .043* |
| % Meeting Criteria for Current CUDa | 14.3 % | 0.0 % | .008* |
| Penn Alcohol Craving Scale Total Scoreb | 14.2 (7.2) | – | – |
| Range (0–30) | |||
| Alcohol Dependence Scale Total Scoreb | 16.9 (7.8) | – | – |
| Range (1–41) | |||
| DSM-5 SCID AUD Symptom Countb | 6.4 (2.4) | – | – |
| Range (2–11) | |||
| % Mild AUD | 10.4 % | – | – |
| % Moderate AUD | 29.9 % | —— | |
| % Severe AUD | 59.7 % |
Note: AUDIT = Alcohol Use Disorders Identification Test; TLFB = Timeline Follow-back; MDE = Major Depressive Episode; CUD = Cannabis Use Disorder; AUD = Alcohol Use Disorder;
diagnostic criteria according to DSM-5;
substance use information collected for group with AUD group only.
Denotes significance at the p < .05 level for corresponding significance test (Chi-Square, Fisher’s Exact, or independent samples T).
3.2. NIH Ttoolbox cognition scores
Education emerged as a significant covariate for three subtests measuring working memory (p = .022), episodic memory (p = .001), and language (p = .020) with higher education associated with higher neurocognitive subtest performance across groups. BAI score was a significant covariate for the subtest measuring attention and inhibitory control (p = .002) with higher anxiety symptomatology related to lower subtest performance. Neither sex (p’s > .05) nor the sex × group interaction term (p’s > .05) were significantly related to performance on any of the five subtests. Overall, final general linear models (non-significant covariates removed), revealed no significant differences in NIH Toolbox subtest standard scores (Fully Corrected norms) between healthy comparison group vs. AUD group (see Table 2; p’s > .05), except for the subtest measuring working memory (p = .025). On average, the group with AUD unexpectedly had significantly higher working memory performance than the healthy comparison group.
Table 2.
NIH toolbox cognition subtest scores by group.
| NIH Toolbox Subtest | Group with Alcohol Use Disorder Mean Score (SD) [%tile] | Healthy Comparison Group Mean Score (SD) [%tile] | ||||
|---|---|---|---|---|---|---|
| Males(n = 56) | Females(n = 21) | Combined(N = 77) | Males(n = 26) | Females(n = 22) | Combined(N = 48) | |
| List Sorting Working Memory | 49.93 (8.54) | 50.24 (10.30) | 50.01 (8.98)* [50 %] |
48.42 (11.22) | 45.86 (9.24) | 47.25 (10.33) [39 %] |
| Pattern Comparison Processing Speed | 43.14 (12.61) | 52.23 (13.03) | 45.62 (13.28) [33 %] |
46.85 (12.56) | 46.91 (14.41) | 46.88 (13.29) [38 %] |
| Picture Sequence Memory | 51.14 (9.11) | 48.67 (11.41) | 50.47 (9.77) [52 %] |
50.15 (10.0) | 49.55 (12.33) | 49.88 (10.99) [50 %] |
| Flanker Inhibitory Control and Attention | 38.64 (8.84) | 40.52 (7.88) | 39.16 (8.58) [14 %] |
39.08 (5.93) | 41.55 (8.54) | 40.21 (7.27) [16 %] |
| Oral Reading Recognition | 58.13 (10.87) | 58.19 (13.11) | 58.14 (11.44)a [79 %] |
54.46 (6.66) | 58.36 (8.66) | 56.25 (7.81) [73 %] |
Note: Table presents mean Fully-Corrected norms derived from the NIH Toolbox not adjusted for model covariates;
N = 76 (n = 55 Males) for Oral Reading Recognition Test for the AUD group.
Denotes a significant difference between groups (p = .025) after covarying for education.
3.3. Principal component analysis for AUD severity
The principal components analysis of DSM-5 AUD symptom count, PACS, AUDIT, CIWA-Ar, ADS, BDI-II, and BAI, yielded one factor with all variables loading > .40 on the AUD Severity factor, except CIWA which loaded < .40 on this factor. Thus, CIWA was removed and the PCA analysis was rerun with the 6 remaining variables, which yielded one factor with all variables loading > .40 that explained 60 % of the variance (Eigenvalue = 3.574; see Table 3). This supports the use of the AUD severity factor score in subsequent analyses.
Table 3.
Principal components analysis factor loadings for alcohol use disorder severity.
| Variable | AUD Severity Factor |
|---|---|
| PACS Total Score | .67 |
| DSM-5 SCID AUD Symptom Count | .82 |
| AUDIT Total Score | .85 |
| ADS Total Score | .88 |
| Beck Depression Inventory -II Total Score | .58 |
| Beck Anxiety Inventory Total Score | .79 |
Note: AUD = Alcohol Use Disorder; PACS = Penn Alcohol Craving Scale; AUDIT = Alcohol Use Disorders Identification Test; ADS = Alcohol Dependence Scale.
3.4. Neurocognitive functioning in AUD
3.4.1. AUD Severity Factor
Of all the covariates (age, education, cannabis use, and cigarette use) added into the five models, only education was significantly related to neurocognitive performance; this was true for only one subtest measuring language abilities (F = 6.99, p = .010) after adjusting for other covariates, with higher education level associated with higher performance. Overall, two of the five general linear models (i.e., final models with non-significant covariates removed) produced significant full model equations: Flanker Inhibitory Control and Attention subtest, F(3, 73) = 5.35, p = 0.002, R2 = 0.18; Pattern Comparison Processing Speed subtest, F(3, 73) = 2.91, p = 0.040, R2 = 0.11 (see Table 4).
Table 4.
Modeling of processing speed and inhibitory control/ attention performance by alcohol use disorder severity and sex.
| Flanker Inhibitory Control and Attention Performance Model N = 77 |
Picture Comparison Processing Speed Performance Model N = 77 |
|||||||
|---|---|---|---|---|---|---|---|---|
| F-value | p-value | R2 | Degrees of Freedom (Model, Error) |
F-value | p-value | R2 | Degrees of Freedom (Model, Error) |
|
| Full Model | 5.35** | .002 | 0.180 | 3, 73 | 2.91* | .040 | 0.107 | 3, 73 |
| b Estimate | Standard Error | t-value | p-value | b Estimate | Standard Error | t-value | p-value | |
| Effect | ||||||||
| Intercept | 40.47** | 1.73 | 23.39 | < .0001 | 52.19** | 2.80 | 18.66 | < .0001 |
| AUD Severity Factor | 1.66 | 1.85 | 0.90 | .372 | 1.63 | 2.99 | 0.54 | .588 |
| Sex (Male vs. Female) | −1.88 | 2.03 | −0.92 | .359 | −9.06** | 3.28 | −2.76 | .007 |
| Interaction | −5.63** | 2.13 | −2.65 | .010 | −3.07 | 3.43 | −0.89 | .374 |
| Male | −3.96** | 1.04 | −3.79 | < .001 | – | – | – | – |
| Female | 1.66 | 1.85 | 0.90 | .372 | – | – | – | – |
Note: AUD = Alcohol Use Disorder; beta estimates (b) are unstandardized; interaction term includes Sex and AUD Severity Factor.
Denotes significance at the p < .05 level.
Denotes significance at the p < .01 level.
Specifically, results from the Flanker model revealed a significant sex X AUD Severity Factor interaction (F = 7.01, p = .010; see Fig. 1). Post-hoc simple effects tests found a significant effect of AUD Severity Factor score on this subtest performance for males (t(73)=−3.79, p < .001) but not females (t(73) = 0.90, p = .372), such that among males, higher AUD severity was associated with lower attention and inhibitory control performance. While the sex × Severity Factor interaction was not significant for any other subtest models (p’s > .05), a marginally significant interaction was found for the working memory subtest (LSWM; F = 3.69, p = .059). Post-hoc simple effects tests similarly found a significant effect of AUD Severity Factor score on this LSWM subtest performance for males (t(73)=−2.27, p = .026) but not females (t(73) = 0.92, p = .359), such that among males, higher AUD severity was associated with lower working memory performance. The AUD Severity Factor score not significantly related to neurocognitive performance on any of the five subtests across sex (p’s > .05).
Fig. 1.
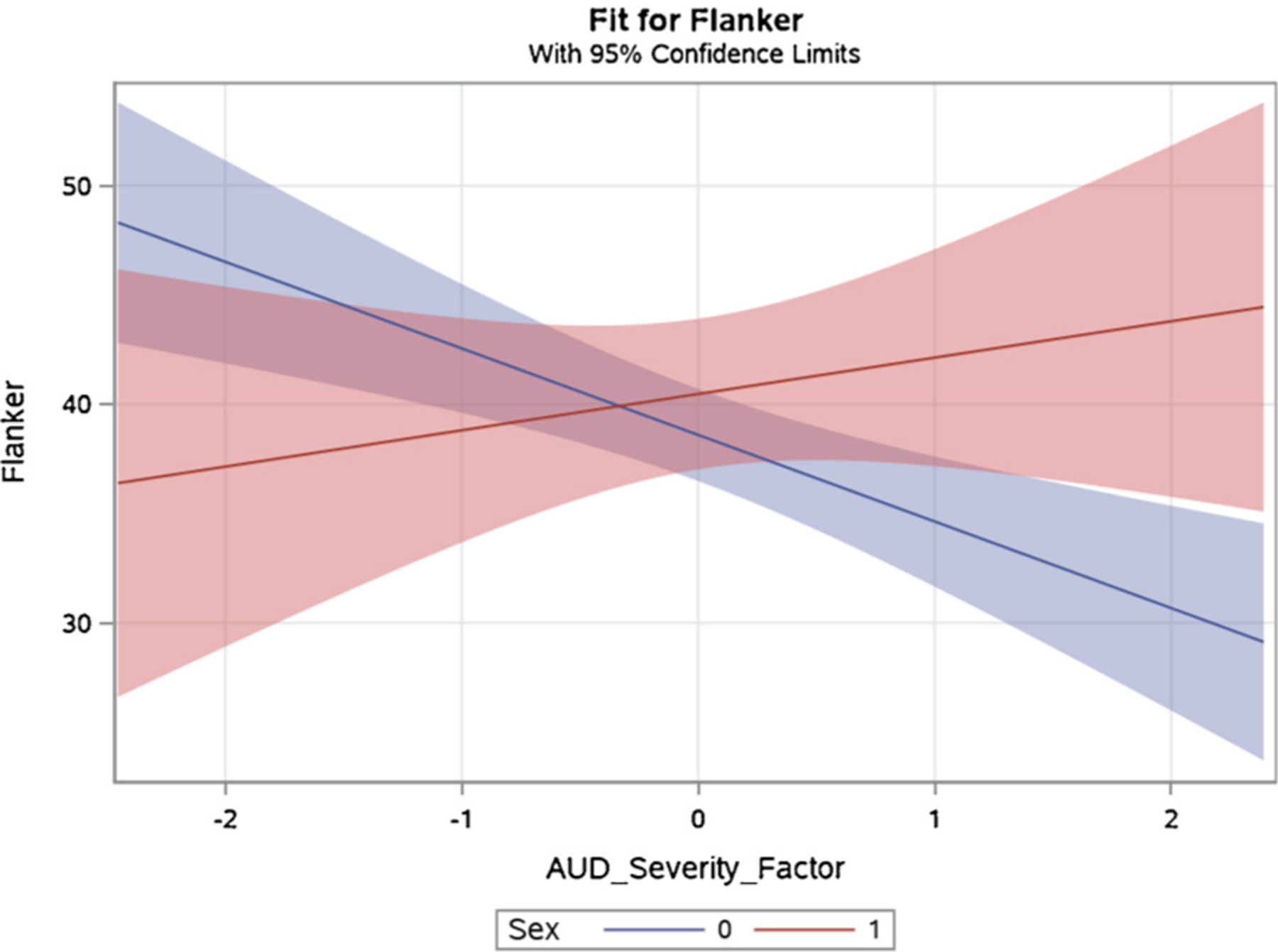
This graphic represents a significant interaction between AUD Severity Factor score and sex (male = 0 [blue], female = 1 [red]) on Flanker Inhibitory Control and Attention subtest performance (average model-adjusted standard Tt-score). Males but not females with greater AUD severity had lower levels of attention and inhibitory control.
Results from the processing speed model show a significant effect of sex on processing speed performance (F = 7.64, p = .007; see Fig. 2), such that females exhibited higher scores on average (adjusted mean = 52.19, SE = 2.80) compared with males (adjusted mean = 43.13, SE = 1.71). There was not a significant effect of sex on performance for any other subtest (p’s > .05). Chi-square and independent samples T-test confirmed no significant differences between males and females on demographic or individual difference variables listed in Table 1 (p’s > .05). Exploratory comparison analyses (independent samples T-tests) suggested that THC status (positive vs. negative result on toxicology screen) was not significantly associated with neurocognitive performance on any of the subtests (p-range: .20–.98).
Fig. 2.
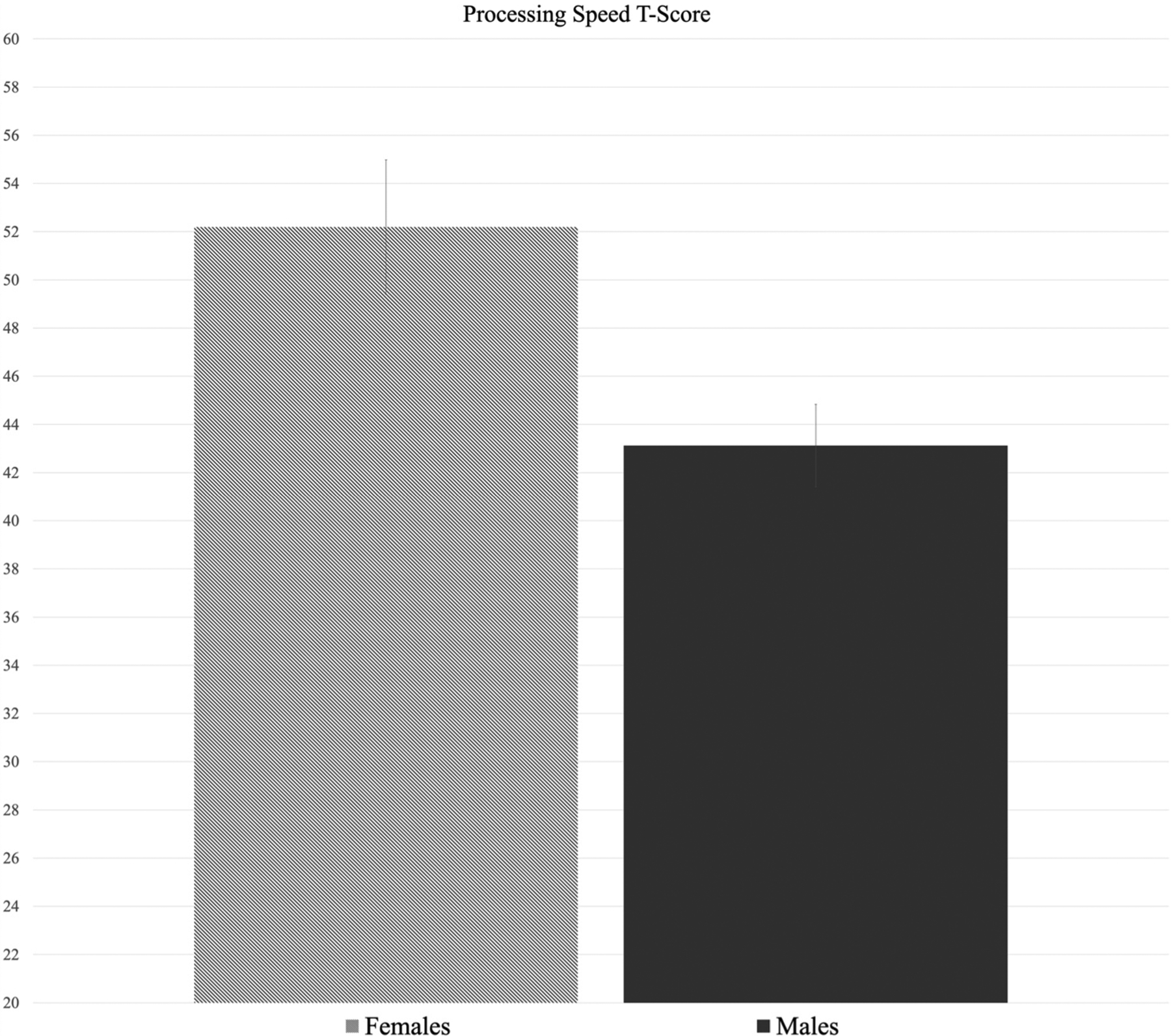
Average model-adjusted standard Tt-score by sex on the Picture Comparison Processing Speed subtest. This represent a significant effect of sex on processing speed performance (p = .007), in which females (adjusted mean = 52.19) scored higher than males (adjusted mean = 43.13).
3.4.2. Total recent alcohol consumption
Education and sex emerged as the only significant covariates with higher education being significantly related to both higher language performance (p = .009) and attention/inhibitory control performance (p = .046); sex was significantly associated with processing speed performance (p = .006). For final models with non-significant covariates removed, only one model produced a significant full model equation: Pattern Comparison Processing Speed subtest, F(2, 74) = 4.49, p = .014, R2 = 0.11. However, this was driven by significant performance differences by sex (p = .004; females performing higher than males) and not total recent alcohol consumption (p = .280). Total recent alcohol consumption was not significantly related to neurocognitive performance on any of the other four subtests models (p’s > .05) after accounting for covariates.
4. Discussion
The current study is the first to utilize the NIH Toolbox to assess neurocognitive function in individuals with AUD as compared with healthy individuals with low alcohol drinking patterns. This is important as the NIH Toolbox seeks to facilitate neurocognitive testing for large-scale phenotyping of psychiatric populations and healthy individuals. In contrast to much of the previous literature, our AUD sample was comprised of individuals presenting to an outpatient alcohol treatment trial as opposed to those enrolled in an in-patient care setting (Horner et al., 1999; Stavro et al., 2013). We sought to test our hypothesis that the group with AUD would perform significantly lower than the healthy comparison group across all neurocognitive domains assessed, as heavy, sustained alcohol consumption is putatively related to neurocognitive deficits (Stavro et al., 2013). Surprisingly, results overall showed no detectable group differences in neurocognitive performance in domains assessed, including episodic memory, language, processing speed, and attention and inhibitory control. Explanations for this null finding could be that: (a) the NIH Toolbox is not as sensitive as other comprehensive and well-validated neuropsychological batteries;, (b) effect sizes may be smaller than the medium effect we are powered to detect;, or (c) the healthy comparison group may be “less healthy” than previously published studies, which may be a function of our efforts to recruit demographically and socioeconomically matched controls using similar community-based recruitment methods that might better account for pre-existing differences (Dean et al., 2013; Moritz et al., 2018). A combination of such factors may also account for the null findings. Moreover, across the two groups, sex was not significantly related to neurocognitive performance on any subtests tested. This is consistent with varied literature showing that while male and female differences in neurocognitive abilities for healthy adult individuals have been identified (e.g., spatial visualization, verbal fluency, processing speed), these effects are modest (Grissom and Reyes, 2019; Liu et al., 2013; Siedlecki et al., 2019).
Several intriguing patterns of neurocognitive performance emerged across domains assessed and these patterns were similar for both groups. Both groups performed in the average range (24th–75th percentile) on tests of working memory, episodic memory, and processing speed. Although the group with AUD performed significantly better on a subtest of working memory, the average score fell at the 50th percentile. On a measure of attention and inhibitory control, both groups performed approximately 1 SD below the mean (~15th percentile), suggesting this may be an area of relative weakness for both the AUD and comparison group. In contrast, on a measure of language ability, which may most closely approximate overall/ premorbid IQ or crystallized intelligence, both groups performed around the 75th percentile. We can conclude that although the performance varied across domains, groups performed similarly, which suggests that they were well matched. In sum, with the NIH Toolbox, we did not detect neurocognitive deficits in the AUD group when comparing performance to healthy individuals recruited from the community.
Based on the hypothesized role of clinical severity of alcohol use disorder and quantity of alcohol consumption on neurocognitive function, we undertook analyses within the AUD sample only. Additionally, due to the mixed literature on whether sustained alcohol use differentially affects neurocognitive abilities among males and females, we tested the relationship between sex and neurocognitive performance. While the composite AUD Severity Factor score was not significantly associated with neurocognitive performance on any subtests, sex did serve to differentiate performance within the clinical sample in a number of ways. First, on the Flanker task, severity of AUD was significantly related to performance among males but not females. Higher AUD severity level was associated with lower attention and inhibitory control performance among males, suggesting that one’s degree of problematic alcohol use is associated with graded weaknesses in these areas specifically for males but not females. The same association between AUD severity and lower performance among male participants emerged for a working memory subtest as well, although the interaction between AUD severity and sex was only marginally significant. Relatedly, the group with AUD performed lowest on the Flanker subtest (albeit not differently than controls) and together this could potentially suggest that these areas of neurocognition may be affected earlier in the disease course than others. This is in line with previous research showing that moderate alcohol use may impact inhibitory control as early as young adulthood (Lopez-Caneda et al., 2014; Wetherill et al., 2013). Additionally, sex served to differentiate performance on a measure of processing speed within the AUD group, such that females exhibited significantly faster processing speeds than males and this was not a function of AUD severity, nor was this effect found for healthy comparison group. Contextually, previous research on neurocognitive functioning in healthy adults has demonstrated a slight but consistent advantage in processing speed for females, as compared with males (Siedlecki et al., 2019).
Overall, we interpret these findings in light of the literature, which present a rather mixed picture whereby some studies find that females are more vulnerable to the neurocognitive effects of alcohol use (Acker, 1986; Glenn and Parsons, 1991), while others suggest that females do not exhibit the same neurocognitive impairments as males (Oscar-Berman and Marinkovic, 2007; Sparadseo et al., 1983). In contrast to the current study, previous research on sex differences in the domain of inhibition suggests that in females only, indicators of heavy drinking were associated with response inhibition deficits on the stop signal task (Harper et al., 2018; Nederkoorn et al., 2009). However, our findings from an outpatient sample may suggest that males may beare more affected by the negative impacts of AUD severity than females in the areas of attention and inhibitory control. These findings serve to inform the literature on sex-dependent effects of alcohol use on neurocognitive function.
From a clinical viewpoint, a noteworthy dimension affecting neurocognitive performance is mood symptomatology namely, anxiety and depression. It is notable that high levels of depression and anxiety can impair neurocognitive performance and that the AUD severity construct encompassed measures of depression and anxiety symptoms, which fit well with the AUD Severity Factor from the principal component analysis. Including mood indicators in the AUD Severity Factor is a novel addition (Lim et al., 2017; Moallem et al., 2013). However, this addition is consistent with previous research indicating a positive relationship between AUD severity and mood symptomatology, such that individuals with more severe AUD exhibit higher levels of negative affectivity (Cano et al., 2017; Pavkovic et al., 2018). Importantly, males and females did not significantly differ in their levels of depression and anxiety. Overall, this suggests that levels of depression and anxiety were well-accounted for by AUD severity, and together were significantly associated with attention/ inhibitory control and working memory scores for males through the AUD Severity Factor composite score construct.
Another way in which this study contributes to the field of AUD and neurocognitive function is by employing the NIH toolbox. If proven useful in future research with AUD samples, the NIH Toolbox could be used as a standard neurocognitive assessment, allow for easier comparison across studies, and to better understand the effects of neurocognitive impairment on treatment outcomes over time. This is critical given that only 1 in 5 individuals with AUD seek treatment and neurocognitive assessment is rarely part of routine care (Copersino et al., 2009); outcomes for those who do seek treatment vary widely (Ray et al., 2018). Additionally, the field of addiction neuroscience is moving towards a personalized medicine approach, whereby understanding individual differences in neurocognition may impact how clinicians’ approach treatment in the future. This is in line with the Addictions Neuroclinical Assessment (ANA) framework, which suggests that executive function, along with incentive salience and negative emotionality, are important dimensions to assess (Kwako et al., 2017) to improve clinical translational research.
The present study should be interpreted in light of its strengths and weaknesses. Strengths include a well-characterized community samples with AUD and healthy comparison individuals that were recruited by the same methods. Overall, the two groups were well-matched on demographic and socioeconomic variables and models comparing the two groups included relevant covariates. Additionally, the NIH Toolbox is a valid, highly relevant, and a brief measure of neurocognitive function that accounts for demographic factors in scoring neurocognitive test results. Individuals with AUD were drawn from an outpatient clinical treatment trial and display a range of AUD severity allowing for examination of functioning across this range. Further, a component factor of AUD severity was derived to limit the number of individual comparisons and potential of making type-I errors. Study limitations include the moderate sample size which was not powered to detect small effect sizes, whichand may be particularly relevant when detecting sex-differences, especially given that only 27 % of the clinical sample were female. The NIH Toolbox is not widely used and fully evaluated in the context of psychiatric disorders, including substance-using populations. Moreover, while the NIH Toolbox broadly measures neurocognitive abilities, it is less comprehensive and likely less sensitive than in-depth and comprehensive neuropsychological assessments and comprises only one subtest per domain. As such, this battery may be less capable of detecting nuanced differences between substance-using and healthy comparison samples. Finally, given the lack of testing to account for pre-morbid differences, we cannot readily conclude that differences in neurocognitive performance are a consequence of alcohol use as opposed to pre-existing individual differences.
In conclusion, this study examined neurocognitive functioning in outpatient treatment seekers with AUD versus healthy community comparisons and found no evidence of neurocognitive impairment for the AUD sample, which was less severe than those previous studied. Analyses examining the relationship between clinical disorder severity and neurocognitive performance, restricted to the AUD sample only, indicated intriguing sex-dependent effects whereby males but not females with higher levels of AUD severity had lower levels of attention/inhibitory control and working memory performance. Females in the clinical sample had significantly faster processing speeds than males but this was not related to severity of AUD. These results contribute to the emerging literature on alcohol and sex differences (Nolen-Hoeksema and Hilt, 2006) and suggest that males and females may be differentially affected by AUD severity and highlight the need to investigate this further in understanding neurocognitive deficits. This study also serves as an initial evaluation of the NIH toolbox as an instrument that can potentially make neurocognitive assessments more accessible in a host of psychiatric and clinical contexts.
Role of funding source
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R01 AA026190 & K24 AA025704 to LAR], the National Institute on Drug Abuse [UCLA training Grant 5T32 DA024635], and the Tobacco Related Disease Research Program [Grant T29 DT0371].
Footnotes
Declaration of Competing Interest
No conflict declared.
References
- Acker C, 1986. Neuropsychological deficits in alcoholics: the relative contributions of gender and drinking history. Br. J. Addict 81, 395–403. [DOI] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF, 2006. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychol. Addict. Behav 20, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont JL, Havlik R, Cook KF, Hays RD, Wallner-Allen K, Korper SP, Lai JS, Nord C, Zill N, Choi S, Yost KJ, Ustsinovich V, Brouwers P, Hoffman HJ, Gershon R, 2013. Norming plans for the NIH Toolbox. Neurology 80, S87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA, 1988. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol 56, 893–897. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bernardin F, Maheut-Bosser A, Paille F, 2014. Cognitive impairments in alcohol-dependent subjects. Front. Psychiatry 5, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano MA, de Dios MA, Correa-Fernandez V, Childress S, Abrams JL, Roncancio AM, 2017. Depressive symptom domains and alcohol use severity among Hispanic emerging adults: examining moderating effects of gender. Addict. Behav 72, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carniglia A, Keyes KM, Hasin DS, Cerda M, 2019. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry 6, 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL, 2007. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32, 429–438. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Fals-Stewart W, Fitzmaurice G, Schretlen DJ, Sokoloff J, Weiss RD, 2009. Rapid cognitive screening of patients with substance use disorders. Exp. Clin. Psychopharmacol 17, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A, 2005. Is there cognitive impairment in clinically’ healthy’ abstinent alcohol dependence? Alcohol Alcohol. 40, 498–503. [DOI] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED, 2013. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38, 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN, 2003. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol. Clin. Exp. Res 27, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, 2007. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci 12, 4079–4100. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L, 1990. Cognitive impairments in abstinent alcoholics. West. J. Med 152, 531–537. [PMC free article] [PubMed] [Google Scholar]
- First MB, Willams JBW, Karg RS, Spitzer RL, 2016. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM, 1999. Psychometric properties of the PennAlcohol Craving Scale. Alcohol. Clin. Exp. Res 23, 1289–1295. [PubMed] [Google Scholar]
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C, Bera-Potelle C, Cohen R, Kahn JP, Limosin F, 2013. Executive functions in adult offspring of alcohol-dependent probands: toward a cognitive endophenotype? Alcohol. Clin. Exp. Res 37 (Suppl 1), E356–E363. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA, 1991. Impaired efficiency in female alcoholics’ neuropsychological performance. J. Clin. Exp. Neuropsychol 13, 895–908. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74, 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom NM, Reyes TM, 2019. Let’s call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology 44 (1), 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenmos M, Schmack K, Sekutowicz M, Garbusow M, Sebold M, Sommer C, Smolka MN, Wittchen HU, Zimmermann US, Heinz A, Sterzer P, 2017. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl. Psychiatry 7, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Malone SM, Iacono WG, 2018. Impact of alcohol use of EEG dynamics of response inhibition: a cotwin control analysis. Addict. Biol 23, 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, Insel TR, Landis SC, The NIH Blueprint for Neuroscience Research, 2013. The NIH Toolbox: setting a standard for biomedical research. Neurology 80 S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MD, Waid LR, Johnson DE, Latham PK, Anton RF, 1999. The relationship of cognitive functioning to amount of recent and lifetime alcohol consumption in outpatient alcoholics. Addict. Behav 24, 449–453. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, Szelenberger W, 2012. Cognitive functions in abstinent alcohol-dependent patients. Alcohol 46, 665–671. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Grodin EN, Litten RZ, Koob GF, Goldman D, 2017. Addictions Neuroclinical Assessment: a reverse translational approach. Neuropharmacology 122, 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, Sullivan EV, 2017. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol. Clin. Exp. Res 41, 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AC, Cservenka A, Ray LA, 2017. Effects of alcohol dependence severity on neural correlates of delay discounting. Alcohol Alcohol. 52, 506–515. [DOI] [PubMed] [Google Scholar]
- Liu G, Hu PP, Fan J, Wang K, 2013. Gender differences associated with orienting attentional networks in healthy subjects. Chin. Med. J. (Engl.) 126 (12), 2308–2312. [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K, 2009. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 44, 372–381. [DOI] [PubMed] [Google Scholar]
- Lopez-Caneda E, Rodriguez Holguin S, Cadaveira F, Corral M, Doallo S, 2014. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol. 49, 173–181. [DOI] [PubMed] [Google Scholar]
- Moallem NR, Courtney KE, Bacio GA, Ray LA, 2013. Modeling alcohol use disorder severity: an integrative structural equation modeling approach. Front. Psychiatry 4, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz S, Irshaid S, Ludtke T, Schafer I, Hauschildt M, Lipp M, 2018. Neurocognitive functioning in alcohol use disorder: cognitive test results do not tell the whole story. Eur. Addict. Res 24, 217–225. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Baltus M, Guerrieri R, Wiers RW, 2009. Heavy drinking is associated with deficient response inhibition in women but not in men. Pharmacol. Biochem. Behav 93, 331–336. [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, Tapert SF, 2017. Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcohol. Clin. Exp. Res 41, 2082–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L, 2006. Possible contributors to the gender differences in alcohol use and problems. J. Gen. Psychol 133, 357–374. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K, 2007. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev 17, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavkovic B, Zaric M, Markovic M, Klacar M, Huljic A, Caricic A, 2018. Double screening for dual disorder, alcoholism and depression. Psychiatry Res. 270, 483–489. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO, 1997. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol. Clin. Exp. Res 21, 521–529. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV, 2013. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage 65, 176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, Desgranges B, Eustache F, 2007. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol. Clin. Exp. Res 31, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Grodin EPD, Hartwell E, Green R, Venegas A, Lim A, Gillis A, Miotto K, 2018. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am. J. Drug Alcohol Abuse 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M, 1993. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Siedlecki KL, Falzarano F, Salthouse TA, 2019. Examining gender differences in neurocognitive functioning across adulthood. J. Int. Neuropsychol. Soc 25 (10), 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA, 1982. Alcohol dependence syndrome: measurement and validation. J. Abnorm. Psychol 91, 199–209. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-back: a Technique for Assessing Self-reported Alcohol Consumption. Springer, pp. 41–72. [Google Scholar]
- Sparadseo FR, Zwick W, Butters N, 1983. Cognitive functioning of alcoholic females: an exploratory study. Drug Alcohol Depend. 12, 143–150. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S, 2013. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict. Biol 18, 203–213. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar). Br. J. Addict 84, 1353–1357. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A, 2000. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol. Clin. Exp. Res 24, 611–621. [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A, 2002. A profile of neuropscyhological deficits in alcoholic women. Neuropsychology 16, 74–83. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Bauer PJ, Zelazo PD, Wallner-Allen K, Dikmen SS, Heaton RK, Tulsky DS, Slotkin J, Blitz DL, Carlozzi NE, Havlik RJ, Beaumont JL, Mungas D, Manly JJ, Borosh BG, Nowinski CJ, Gershon RC, 2013a. I. NIH Toolbox cognition battery (CB): introduction and pediatric data. Monogr. Soc. Res. Child Dev 78, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC, 2013b. Cognition assessment using the NIH Toolbox. Neurology 80, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF, 2013. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology 230, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Porges EC, Bryant VE, Seider T, Gongvatana A, Kahler CW, de la Monte S, Monti PM, Cohen RA, 2016. Current heavy alcohol consumption is associated with greater cognitive impairment in older adults. Alcohol. Clin. Exp. Res 40, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker JE, Nilsson LG, Herlitz A, Anthenelli RM, 2005. Sex differences in spatial visualization and episodic memory as a function of alcohol consumption. Alcohol Alcohol. 40, 201–217. [DOI] [PubMed] [Google Scholar]


