Abstract
Background:
Chronic facial paralysis can lead to significant functional and psychosocial impairment. Treatment often involves free muscle flap-based facial reanimation surgery. Although surgical techniques have advanced considerably over the years, consensus has yet to be reached for postoperative outcome evaluation. To facilitate outcome comparison between the various techniques for free muscle-flap-based reanimation, a standardized, widely accepted functional outcomes assessment tool must be adopted.
Methods:
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we performed a systematic review of the PubMed, Cochrane, and Embase databases to identify the reported functional outcome measurement tools used in the free muscle flap-based reanimation literature.
Results:
The search yielded 219 articles, 43 of which met our inclusion and exclusion criteria. We noted an increase in publications reporting the utilization of objective measures over time, particularly software-based tools, as well as increased utilization of patient reported outcomes measures.
Conclusions:
Based on the trends identified in the literature, we suggest standardization of outcome measures following facial reanimation surgery with free muscle-flap using a combination of the Facial Assessment by Computer Evaluation (FACEgram) software and the Facial Clinimetric Evaluation (FaCE) Scale.
Introduction
Facial paralysis may arise from congenital syndromes, acquired conditions, trauma, or malignancy, and poses significant functional and psychosocial burdens on patients regardless of etiology. Functional impairments include difficulty with forming facial expressions, speaking, eating, and drinking.1 Facial disfigurement leads to increased psychological distress, including anxiety and depression, with a direct correlation between patients’ perception of their deficit and degree of distress experienced.2
Treatment strategy for facial paralysis often varies based on the chronicity of paralysis. Acute facial paralysis, lasting on the order of weeks, is typically treated with facial nerve decompression or facial nerve repair. Intermediate duration paralysis, lasting up to 2 years, is treated with nerve transfer procedures. Meanwhile, chronic facial paralysis, lasting longer than 2 years, typically requires regional or free muscle transfer. More recently, free functional muscle flap (FFMF) transfer has become the standard of care for chronic facial paralysis, with the gracilis being most commonly used.3,4 The use of other muscles, including serratus anterior and latissimus dorsi, has also been described.5
Although there have been significant surgical advancements in facial reanimation surgery, there still remains a lack of standardization for outcome monitoring.6 This presents a significant barrier to accurate comparison of outcomes following reanimation procedures. Historically, subjective, observer-based scales have been used because they are efficient and easy to administer. These tools aim to assess facial nerve function by evaluating outcomes, including facial symmetry and movement. However, many of these subjective measures were not initially developed for use in evaluating postsurgical reanimation outcomes, and they lack quantitative and objective data.6 In the past, objective instruments were not widely implemented because they were time-intensive and required specialized equipment. However, recent technological advances are mitigating some of these barriers to use, leading to increased utilization in recent years. An ideal standardized form of functional outcome tracking will be easy to administer, objective, and readily reproducible. The aim of our study was to review the reported functional outcome measurement tools used in the FFMF reanimation literature to inform the readers about future innovations in the field of facial reanimation surgery. Our findings may translate to other fields that draw on principles of FFMF reconstruction of the face, such as autologous facial reconstruction and facial transplantation.
Methods
Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Three electronic databases (PubMed, Embase, and Cochrane CENTRAL) were queried from inception through September 13, 2020 with the following search terms: “facial reanimation” or “facial paralysis surgery” or “reanimation surgery” or “Bell’s palsy” or “facial palsy” and “free muscle graft” or “free muscle transfer” or “free muscle flap” or “muscle transposition” or “muscle transfer” and “outcomes” or “objective measurements” or “functional outcomes” or “scoring system” or “scale” or “objective analysis” or “functional analysis” or “subjective analysis” or “subjective outcomes.”
Selection Criteria
All resulting articles were compiled, and duplicate titles were removed. Two independent reviewers (JJP, RRC) screened the remaining titles and abstracts. Disagreements regarding article identification and final selection for inclusion were resolved by a third reviewer (DB). All studies reporting outcomes for FFMF reanimation procedures were included. Although regional flaps are also used for reanimation, we focused our study on FFMF, as it is the gold standard for chronic facial paralysis.4 Studies that described facial reanimation via regional muscle transfer or nerve transfer and studies that reported outcomes of revision surgeries following facial reanimation were excluded, as were case reports, non-English articles, cadaveric studies, technical papers, and systematic reviews. If relevance could not be determined from the abstract alone, full text was retrieved and reviewed.
Data Collection
Included articles were examined and the following data points were recorded: author, year of publication, and functional outcome measurement tool used. Additional operative information on specific muscle and nerve used and coaptation technique were collected when available. If studies appeared to contain some but not all of the same patients, they were listed together in the data table but counted as individual studies when tallying total studies. We classified a tool as subjective if the evaluation relied on observer interpretation or patient input, and thus may vary based on observer or patient. We classified a tool as objective if it utilized a quantifiable measure that could be readily replicated and was not susceptible to interpretation.
Results
Description of Studies
Our initial database search yielded 219 articles based on our search parameters, and 43 were included in our study (Fig. 1). Thirteen studies reported only subjective functional outcome measures, 11 used only objective functional outcome measures, and 19 used a combination of objective and subjective outcome measures (Table 1). We identified an increase over time in utilization of objective outcome tools, including software-based tools. Additionally, while the use of subjective outcomes measures has declined overall in the last 10 years, the use of patient reported outcomes measures (PROMs) has become increasingly popular. Figure 2 summarizes the trends in utilization of subjective, objective, and combination tools from 1998 to 2010 versus 2011 to 2020.
Fig. 1.
PRISMA flow chart of studies included in the systematic review of the literature.
Table 1.
Included Studies in Reverse Chronological Order
| Author | Year | Category of Tool | Tool |
|---|---|---|---|
| Kim MJ, et al7 | 2020 | Combined | FACE software (FACEgram), 3D spatiotemporal analysis, Terzis grading scale |
| Kim MJ, et al8 | 2020 | Objective | FACE software (FACEgram) |
| Roy M, et al9 | 2019 | Objective | FACE software (FACEgram) |
| Mohanty AJ, et al10 | 2019 | Objective | FACE software (FACEgram) |
| Sakuma H, et al11 | 2019 | Subjective | Terzis grading scale |
| Oyer SL, et al12 | 2018 | Objective | FACE software (FACEgram) |
| van Veen MM, et al13 | 2018 | Combined | FACE software (FACEgram), FaCE QoL scale |
| Greene JJ, et al14 | 2018 | Combined | Emotrics, FaCE QoL scale |
| Amer TA, et al15 | 2018 | Objective | SMILE software |
| Faris C, et al16 | 2018 | Combined | FACE software (FACEgram), FaCE QoL scale, eFACE facial grading scale |
| Braig et al17 | 2017 | Objective | Oral commissure excursion measurement |
| Sforza C, et al18 | 2015 | Objective | 3D and 2D facial motion measurement |
| Okazaki M, et al19 | 2015 | Subjective | Harii scale |
| Snyder-Warwick AK, et al20 | 2015 | Objective | SMILE software |
| Cardenas-Mejia A, et al21 | 2015 | Combined | Terzis grading scale, EMG |
| Lindsay RW, et al22,23 | 2014*, 2014 | Combined | FACE software (FACEgram), FaCE QoL scale, Facial Grading Scale |
| Placheta et al24 | 2014 | Objective | Three-dimensional video analysis of facial movements and blink reflex |
| Hontanilla B, et al25 | 2013 | Objective | FACIAL CLIMA system |
| Takushima A, et al26 | 2013 | Subjective | Harii scale |
| Liu AT, et al27 | 2012 | Subjective | Toronto Facial Grading System, Facial Nerve Function Index |
| Vakharia KT, et al28 | 2012 | Combined | SMILE software, FaCE QoL scale |
| Harrison DH, et al29 | 2012 | Subjective | Clinical improvement scale, Hay score |
| Gousheh J, et al30 | 2011 | Objective | Oral commissure excursion measurement |
| Lin CH, et al31 | 2011 | Subjective | Terzis grading scale |
| Hadlock TA, et al32 | 2011 | Combined | FACE software (FACEgram), FaCE QoL scale |
| Krishnan KG, et al33 | 2010 | Combined | Commissural excursion indices, investigator determined smile reaction, patient self-evaluation of function |
| Terzis JK, et al34 | 2010 | Combined | Terzis grading scale, EMG |
| Terzis JK, et al35 | 2010 | Combined | Terzis and Bruno methodology of interpalpebral distance ratios measurement, Terzis and Bruno blink grading scale |
| Takushima A, et al36 | 2009 | Combined | FEMAS-1 (Facial Expression and Motion Analysis System-1) software, Harii scale |
| Terzis JK, et al37,38 | 2009, 2009 | Combined | Terzis grading scale, EMG |
| Terzis JK, et al39 | 2009 | Combined | Terzis grading scale, EMG |
| Manktelow RT, et al40 | 2006 | Combined | FaceMS, patient survey on the functional effects on eating, drinking, and speech |
| Sajjadian A, et al41 | 2006 | Combined | Facial Grading System, EMG |
| Kauhanen SC, et al42 | 2006 | Subjective | House-Brackmann grading scale |
| Ylä-Kotola TM, et al5,43,44 | 2004, 2005, 2008 | Subjective | House-Brackmann grading scale |
| Takushima A, et al45 | 2002 | Subjective | Harii scale |
| Schliephake H, et al46 | 2000 | Combined | Harii scale, SF-36 questionnaire, oral commissure excursion measurement, EMG |
| Wei W, et al47 | 1999 | Subjective | Clinical evaluation, patient questionnaire for appearance |
| Harii K, et al3 | 1998 | Subjective | Harii scale |
*This study does not include Facial Grading Scale.
Fig. 2.
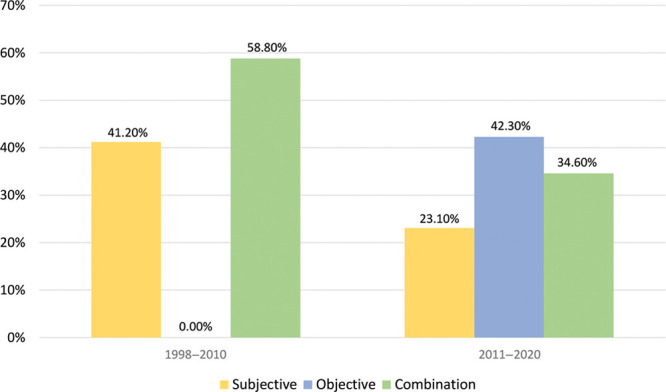
Trends in tool category between 1998–2010 and 2011–2020.
Subjective Measures
Subjective functional outcome measures were most commonly reported. Thirty-two papers included a subjective measure, and it was the only outcome tool reported in 13 of those studies. Our findings also suggest a decrease in the prevalence of subjective tools used in recent years. Between 1998 and 2010, 41.2% (7/17) of studies utilized subjective measures as their sole form of functional outcome tracking, whereas only 23.1% (6/26) did so between 2011 and 2020. In total, all papers from 1998 to 2010 contained a form of subjective measurement, whereas only 57.7% (15/26) had the same from 2011 to 2020. Overall, 11 studies included PROMs, 63.6% (7/11) of which were published between 2011 and 2020. The most frequently employed measure was the Terzis grading scale (8 studies), followed by the Facial Clinimetric Evaluation (FaCE) and Harii grading scales (6 studies each) (Table 2).
Table 2.
Subjective Tools in Reverse Chronological Order
| Author | Year | Tool |
|---|---|---|
| Sakuma H, et al11 | 2019 | Terzis grading scale |
| Okazaki M, et al19 | 2015 | Harii scale |
| Takushima A, et al26 | 2013 | Harii Scale |
| Liu AT, et al27 | 2012 | Toronto Facial Grading System, Facial Nerve Function Index |
| Harrison DH, et al29 | 2012 | Clinical improvement scale, Hay score |
| Lin CH, et al31 | 2011 | Terzis grading scale |
| Kauhanen SC, et al42 | 2006 | House-Brackmann grading scale |
| Ylä-Kotola TM, et al5,43,44 | 2004, 2005, 2008 | House-Brackmann grading scale |
| Takushima A, et al45 | 2002 | Harii scale |
| Wei W, et al47 | 1999 | Clinical evaluation, patient questionnaire on appearance |
| Harii K, et al3 | 1998 | Harii scale |
Objective Measures
Overall, 30 papers included some form of objective functional outcome assessment, with 11 reporting it as their sole form of assessment. While we noted a decrease in the use of subjective measures, we found the use of objective tools has become more common, with 42.3% (11/26) of papers from 2011 to 2020 utilizing them as the sole instrument, up from 0% from 1998 to 2010. The number of publications reporting use of objective tools rose from 58.8% (10/17) from 1998 to 2010 to 76.9% (20/26) from 2011 to 2020. Facial Assessment by Computer Evaluation (FACE) software, also referred to as FACEgram, was the most common objective tool used (9 studies). Software-based tools accounted for 85% (17/20) of objective tools used from 2011 to 2020 (Table 3).
Table 3.
Objective Tools in Reverse Chronological Order
| Author | Year | Tool |
|---|---|---|
| Kim MJ, et al8 | 2020 | FACE software (FACEgram) |
| Roy M, et al9 | 2019 | FACE software (FACEgram) |
| Mohanty AJ, et al10 | 2019 | FACE software (FACEgram) |
| Oyer SL, et al12 | 2018 | FACE software (FACEgram) |
| Amer TA, et al15 | 2018 | SMILE software |
| Braig et al17 | 2017 | Oral commissure excursion measurement |
| Sforza C, et al18 | 2015 | 3D and 2D facial motion measurement |
| Snyder-Warwick AK, et al20 | 2015 | SMILE software |
| Placheta et al24 | 2014 | Three-dimensional video analysis of facial movements and blink reflex |
| Hontanilla B, et al25 | 2013 | FACIAL CLIMA system |
| Gousheh J, et al30 | 2011 | Oral commissure excursion measurement |
Combined Measures
The use of a subjective measure in combination with an objective measure was described in 19 papers. From 1998 to 2010, 58.8% (10/17) of articles used a combination of measures, whereas 34.6% (9/26) did so between 2011 and 2020. The most frequent combination was a software-based-tool used along with a PROM (Table 4).
Table 4.
Combination Subjective and Objective Tools in Reverse Chronological Order
| Author | Year | Tool |
|---|---|---|
| Kim MJ, et al7 | 2020 | FACE software (FACEgram), 3D spatiotemporal analysis, Terzis grading scale |
| van Veen MM, et al13 | 2018 | FACE software (FACEgram), FaCE QoL scale |
| Greene JJ, et al14 | 2018 | Emotrics, FaCE QoL scale |
| Faris C, et al16 | 2018 | FACE software (FACEgram), FaCE QoL scale, eFACE facial grading scale |
| Cardenas-Mejia A, et al21 | 2015 | Terzis grading scale, EMG |
| Lindsay RW, et al22,23 | 2014*, 2014 | FACE software (FACEgram), FaCE QoL scale, Facial Grading Scale |
| Vakharia KT, et al28 | 2012 | SMILE software, FaCE QoL scale |
| Hadlock TA, et al32 | 2011 | FACE software (FACEgram), FaCE QoL scale |
| Krishnan KG, et al33 | 2010 | Commissural excursion indices, investigator determined smile reaction, patient self-evaluation of function |
| Terzis JK, et al34 | 2010 | Terzis grading scale, EMG |
| Terzis JK, et al35 | 2010 | Terzis and Bruno methodology of interpalpebral distance ratios measurement, Terzis and Bruno blink grading scale |
| Takushima A, et al36 | 2009 | FEMAS-1 (Facial Expression and Motion Analysis System-1) software, Harii scale |
| Terzis JK, et al37,38 | 2009, 2009 | Terzis grading scale, EMG |
| Terzis JK, et al39 | 2009 | Terzis grading scale, EMG |
| Manktelow RT, et al40 | 2006 | FaceMS, patient survey on the functional effects on eating, drinking, and speech |
| Sajjadian A, et al41 | 2006 | Facial Grading System, EMG |
| Schliephake H, et al46 | 2000 | Harii scale, SF-36 questionnaire, oral commissure excursion measurement, EMG |
*This study does not include Facial Grading Scale.
Discussion
Intact and functional facial musculature dictates our ability to perform essential communication and other vital tasks, such as verbalization, expression, and feeding.1 Loss of these functions, as seen in chronic facial paralysis, is associated with impaired function and poor quality of life (QoL).48 Surgical management of chronic facial paralysis, which aims to return function to the paralyzed musculature, has seen many advancements in recent years. However, outcome comparison between different procedures and techniques has been limited by the lack of a universally accepted functional outcome monitoring tool.4,6
To move toward a widely accepted, standardized form of outcome tracking, the instrument of choice must be easy to use, objective, and reproducible with strong intra- and interrater reliability. Moreover, such a tool could be beneficial in parallel fields, such as facial transplantation, which face similar challenges in evaluating patients’ subjective and objective recovery. In this study, we aimed to identify the historical trends in functional outcome tracking within the FFMF facial reanimation literature to establish a consensus for outcome tracking.
In our review, we found an increase in utilization of objective outcome tools, particularly software-based tools. While the overall use of subjective tools has declined in the last 10 years, the use of PROMs has become increasingly popular. Although we had initially believed PROMs, House-Brackmann, Terzis scale, and the Hari scale to all be examples of subjective tools, our findings suggest PROMs to be in a category of their own. More specifically, many of these other tools were initially designed to serve as objective tools but have since been found to be highly user-dependent. PROMs, on the other hand, are subjective in design and execution. Therefore, while the use of other tools has declined, the use of PROMs has increased along with other new, technologically advanced objective tools. Our investigation suggests that PROMs, in combination with an objective tool, offer surgeons a genuine pair of subjective and objective tools, which had previously been unavailable.
In analyzing these studies, there was no clear standardization in the timing of scale utilization postoperatively. The most frequently utilized measures have been FACEgram (a software-based objective tool) and FaCE scale (a PROM focusing on QoL). Although multiple outcome tracking tools were identified, we will focus our discussion on those most frequently used.
Objective Tools
FACEgram
The most frequently described functional outcome tool in this review, FACEgram, was developed at Massachusetts Eye and Ear Infirmary as a more comprehensive and accessible version of their SMILE software. FACEgram, unlike SMILE, does not require access to MATLAB.49 FACEgram is a validated tool that uses a number of important facial landmarks to analyze key facial movements as identified by facial reanimation specialists. These include oral commissure movement and palpebral fissure narrowing during eyelid closure, among others. FACEgram also evaluates symmetry during these movements.49 FACEgram utilizes standard photographs, distinguishing it from other objective tools that require a 3-dimensional camera or other advanced technology that may limit accessibility.49 Furthermore, FACEgram allows remote analysis of photographs, which is beneficial for the many patients who would otherwise travel long distances for appointments. A limitation of FACEgram, however, is that it cannot discern whether patients are able to effectively express emotions, as it exclusively detects facial movement. Additionally, FACEgram does not address the psychosocial effects of facial reanimation surgery, limiting its ability to offer a comprehensive assessment of patients’ functional outcome.
Patient Reported Outcome Measures
FaCE Scale
As facial paralysis may have a significant impact on patients’ interpersonal relationships, career success, and overall wellbeing, validated QoL measures are essential for a holistic approach to the evaluation of outcomes following facial reanimation surgery. The use of PROMs has been proposed to evaluate these aspects and are becoming increasingly important across multiple fields, even considered a requirement by some.50,51 These metrics offer insight into an essential aspect of patient recovery. In patients with hemifacial paralysis, it has been shown that psychological distress has a larger impact on social disability than functional limitation.52 This further highlights the essential role of PROMs. FaCE scale, developed in 2001, is a validated questionnaire for disease-specific patient reported QoL in facial palsy.53 It readily evaluates important secondary effects of facial paralysis, such as oral incompetence, difficulty communicating, eye irritation, excessive lacrimation, facial pain, and social stigmatization.53 FaCE scale has been widely used to report QoL following multiple treatments for both flaccid and non-flaccid facial paralysis.22 This allows for comparison among patients who have undergone FFMF as well as those who have undergone local muscle or nerve transfer. FaCE scale has also been validated in several languages, improving accessibility.54 If conducted pre and postoperatively, clinicians can use FaCE scale to evaluate patient-reported functional and psychosocial recovery. Scales which omit this critical metric are limited in their ability to offer a comprehensive assessment.
Subjective Tools
House-Brackmann Scale
The House-Brackmann grading scale (HBGS), developed in 1983, is the most frequently used tool to assess degree of facial weakness.55 Although it has been used to evaluate outcomes following facial reanimation surgery, it was initially created to grade facial nerve disability.6 The HBGS has documented pitfalls, including that it does not fully represent facial function, lacks sensitivity to subtle differences in severity of weakness, and has documented high inter-observer variability.56 Some limitations were addressed in 2009 with the updated HBGS, known as Facial Nerve Grading Scale 2.0, but the scale is still tailored to patients with acute facial paralysis, limiting its generalizability for use in chronic cases.
Terzis Scale
Created in 1997 to evaluate surgical outcomes following FFMF reanimation procedures, the Terzis Facial Grading System evaluates facial symmetry at rest and quality of smile.57 Shortcomings of Terzis Facial Grading System include that it does not evaluate recovery of essential facial functions, nor does it assess psychosocial recovery. Moreover, it is time-intensive and the scores fail to track improvement over time, limiting its utility.
Harii Scale
The Harii Scale was developed in 1998 to evaluate outcomes following facial reanimation surgery.3 Unlike other subjective tools, the Harii scale includes an objective measure, electromyography (EMG), as a component of the overall evaluation.3 However, the overall score depends primarily on the subjective component. Moreover, the scale fails to provide important information on psychosocial recovery and certain functional outcomes, making it a poor choice as a generalizable scale.
Newer Outcome Tracking Tools
Numerous newer tools have been reported in the literature, although they were less frequently seen in our review. These include Emotrics and clinician-graded electronic facial paralysis assessment (eFACE). Emotrics is a facial measurement software that utilizes machine learning technology for facial landmark identification and evaluation of facial movement.58 Another potentially significant tool, eFACE, overcomes a limitation of other clinician-based tools, in that it independently analyzes static, dynamic, and synkinetic facial features.59 As newer technologies are developed and their clinical utility demonstrated, they should be considered as additions to a universal outcome tracking method.
Shared Outcome Measure
Comparing non-standardized outcomes poses a significant challenge to further progress the field. Having a universal method for tracking functional outcomes would facilitate a data-driven comparison of surgical techniques, not only for FFMF reanimation, but for all facial paralysis interventions. These comparisons would ultimately be important for guiding appropriate selection of technique and managing patient expectations.
We propose a combined outcome tracking measure that employs FACEgram together with FaCE scale. FACEgram has proved to be user-friendly, objective, efficient, accurate and reproducible, and does not rely on special equipment or lighting.49 Additionally, as the utility of PROMs is well documented in both surgical and non-surgical fields, the inclusion of a PROM would provide further insight into patient-perceived QoL. To that effect, we propose the inclusion of FaCE scale in tracking postoperative outcomes, as it is a PROM specific for facial paralysis.53
Potential Applications
In consideration of the potential impact of this work, we believe that novel, related reconstructive areas such as facial transplantation may be informed by our findings, given the degree of overlap in technique with facial reanimation. Facial transplantation and FFMF reanimation both employ free-tissue transfer and rely on motor reinnervation for facial function. Functional outcome tracking in facial transplantation has been largely based on clinical evaluation by the transplant team, with adjunct tools occasionally employed.60 At our institution, we utilize clinical evaluation by a multidisciplinary team of surgeons and therapists, along with a patient questionnaire for self-reported functional outcomes. We have also employed tools such as optical tracking software (Vicon 460; Vicon Motion Systems, 2001, Denver, Colo.) and the Sunnybrook facial grading system. Like reanimation, no consensus exists on the ideal method of tracking and reporting outcomes after facial transplantation, which limits advancement within this growing field. Although not currently utilized in facial transplantation, software-based facial analysis tools such as FACEgram are an intriguing option with the potential for significant impact within the field. Additionally, as stated earlier, PROMs are an integral aspect of assessing recovery and should be included as part of a holistic evaluation after facial transplantation.
Limitations and Future Directions
Our study is limited by the potential exclusion of relevant non-English papers or any other relevant papers that were not identified by our search strategy. Furthermore, the heterogeneity of the included studies made it difficult to draw other specific conclusions due to differences in the way studies were conducted. However, we feel this investigation enabled us to report valuable data on the functional outcome measures utilized for FFMF reanimation. As developing a universally accepted scale for all reanimation procedures has proved challenging, we offer suggestions on how to move closer to this goal. We believe initial steps may include standardizing outcome tracking tools based on individual procedures. For chronic facial paralysis treated with FFMF, we suggest investigating the use of FACEgram in combination with FaCE scale as the standardized outcome tracking method. Standardization would allow for scientifically useful comparison of the different FFMF reanimation procedures and consequently improve outcomes for patients. To facilitate accurate comparison of outcomes, a consensus on the postoperative timing of evaluations using these tools must be agreed upon.
Conclusions
Accurate comparison of outcomes between the various techniques for FFMF reanimation is essential, and a universal functional outcome assessment method is needed. Although no standardized tool currently exists to evaluate outcomes following facial reanimation procedures, there has been a trend toward more objective measures, particularly software-based tools, and toward specific subjective measures, namely PROMs. Standardizing evaluative tools and reaching consensus on their use will inform future innovations in the field of facial reanimation surgery, as well as other methods of autologous facial reconstruction and transplantation.
Footnotes
Published online 18 March 2021.
Disclosure: The authors have no financial interest in relation to content of this article.
References
- 1.Shindo M. Management of facial nerve paralysis. Otolaryngol Clin North Am. 1999; 32:945–964. [DOI] [PubMed] [Google Scholar]
- 2.Fu L, Bundy C, Sadiq SA. Psychological distress in people with disfigurement from facial palsy. Eye (Lond). 2011; 25:1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harii K, Asato H, Yoshimura K, et al. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: a new alternative. Plast Reconstr Surg. 1998; 102:941–951. [DOI] [PubMed] [Google Scholar]
- 4.Bos R, Reddy SG, Mommaerts MY. Lengthening temporalis myoplasty versus free muscle transfer with the gracilis flap for long-standing facial paralysis: a systematic review of outcomes. J Craniomaxillofac Surg. 2016; 44:940–951. [DOI] [PubMed] [Google Scholar]
- 5.Ylä-Kotola TM, Kauhanen MS, Asko-Seljavaara SL. Facial reanimation by transplantation of a microneurovascular muscle: long-term follow-up. Scand J Plast Reconstr Surg Hand Surg. 2004; 38:272–276. [DOI] [PubMed] [Google Scholar]
- 6.Niziol R, Henry FP, Leckenby JI, et al. Is there an ideal outcome scoring system for facial reanimation surgery? A review of current methods and suggestions for future publications. J Plast Reconstr Aesthet Surg. 2015; 68:447–456. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Kim HB, Jeong WS, et al. Comparative study of 2 different innervation techniques in facial reanimation: cross-face nerve graft-innervated versus double-innervated free gracilis muscle transfer. Ann Plast Surg. 2020; 84:188–195. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Oh TS. A nasolabial fold reset technique for enhancing midface lifts in facial reanimation: three-dimensional volumetric analysis. J Craniomaxillofac Surg. 2020; 48:162–169. [DOI] [PubMed] [Google Scholar]
- 9.Roy M, Klar E, Ho ES, et al. Segmental gracilis muscle transplantation for midfacial animation in Möbius syndrome: a 29-year experience. Plast Reconstr Surg. 2019; 143:581e–591e. [DOI] [PubMed] [Google Scholar]
- 10.Mohanty AJ, Hembd A, Thrikutam N, et al. Reuse of the masseteric nerve for dynamic reanimation in facial palsy patients with previously failed one-stage dynamic smile reanimation. Plast Reconstr Surg. 2019; 143:567–571. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma H, Tanaka I, Yazawa M, et al. Multivector functioning muscle transfer using superficial subslips of the serratus anterior muscle for longstanding facial paralysis. J Plast Reconstr Aesthet Surg. 2019; 72:964–972. [DOI] [PubMed] [Google Scholar]
- 12.Oyer SL, Nellis J, Ishii LE, et al. Comparison of objective outcomes in dynamic lower facial reanimation with temporalis tendon and gracilis free muscle transfer. JAMA Otolaryngol Head Neck Surg. 2018; 144:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Veen MM, Dijkstra PU, le Coultre S, et al. Gracilis transplantation and temporalis transposition in longstanding facial palsy in adults: patient-reported and aesthetic outcomes. J Craniomaxillofac Surg. 2018; 46:2144–2149. [DOI] [PubMed] [Google Scholar]
- 14.Greene JJ, Tavares J, Mohan S, et al. Long-term outcomes of free gracilis muscle transfer for smile reanimation in children. J Pediatr. 2018; 202:279–284.e2. [DOI] [PubMed] [Google Scholar]
- 15.Amer TA, El Kholy MS. The split hypoglossal nerve versus the cross-face nerve graft to supply the free functional muscle transfer for facial reanimation: a comparative study. J Plast Reconstr Aesthet Surg. 2018; 71:750–757. [DOI] [PubMed] [Google Scholar]
- 16.Faris C, Heiser A, Hadlock T, et al. Free gracilis muscle transfer for smile reanimation after treatment for advanced parotid malignancy. Head Neck. 2018; 40:561–568. [DOI] [PubMed] [Google Scholar]
- 17.Braig D, Bannasch H, Stark GB, et al. Analysis of the ideal muscle weight of gracilis muscle transplants for facial reanimation surgery with regard to the donor nerve and outcome. J Plast Reconstr Aesthet Surg. 2017; 70:459–468. [DOI] [PubMed] [Google Scholar]
- 18.Sforza C, Frigerio A, Mapelli A, et al. Double-powered free gracilis muscle transfer for smile reanimation: a longitudinal optoelectronic study. J Plast Reconstr Aesthet Surg. 2015; 68:930–939. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki M, Mutsumi O, Kentaro T, et al. One-stage dual latissimus dorsi muscle flap transfer with a pair of vascular anastomoses and double nerve suturing for long-standing facial paralysis. J Plast Reconstr Aesthet Surg. 2015; 68:e113–e119. [DOI] [PubMed] [Google Scholar]
- 20.Snyder-Warwick AK, Fattah AY, Zive L, et al. The degree of facial movement following microvascular muscle transfer in pediatric facial reanimation depends on donor motor nerve axonal density. Plast Reconstr Surg. 2015; 135:370e–381e. [DOI] [PubMed] [Google Scholar]
- 21.Cardenas-Mejia A, Covarrubias-Ramirez JV, Bello-Margolis A, et al. Double innervated free functional muscle transfer for facial reanimation. J Plast Surg Hand Surg. 2015; 49:183–188. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay RW, Bhama P, Weinberg J, et al. The success of free gracilis muscle transfer to restore smile in patients with nonflaccid facial paralysis. Ann Plast Surg. 2014; 73:177–182. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay RW, Bhama P, Hadlock TA. Quality-of-life improvement after free gracilis muscle transfer for smile restoration in patients with facial paralysis. JAMA Facial Plast Surg. 2014; 16:419–424. [DOI] [PubMed] [Google Scholar]
- 24.Placheta E, Tzou CJ, Hold A, et al. Facial synkinesia before and after surgical reanimation of the paralyzed face. Plast Reconstr Surg. 2014; 133:842e–851e. [DOI] [PubMed] [Google Scholar]
- 25.Hontanilla B, Marre D, Cabello Á. Facial reanimation with gracilis muscle transfer neurotized to cross-facial nerve graft versus masseteric nerve: a comparative study using the Facial Clima evaluating system. Plast Reconstr Surg. 2013; 131:1241–1252. [DOI] [PubMed] [Google Scholar]
- 26.Takushima A, Harii K, Asato H, et al. Fifteen-year survey of one-stage latissimus dorsi muscle transfer for treatment of longstanding facial paralysis. J Plast Reconstr Aesthet Surg. 2013; 66:29–36. [DOI] [PubMed] [Google Scholar]
- 27.Liu AT, Lin Q, Jiang H, et al. Facial reanimation by one-stage microneurovascular free abductor hallucis muscle transplantation: personal experience and long-term outcomes. Plast Reconstr Surg. 2012; 130:325–335. [DOI] [PubMed] [Google Scholar]
- 28.Vakharia KT, Henstrom D, Plotkin SR, et al. Facial reanimation of patients with neurofibromatosis type 2. Neurosurgery. 2012; 70(2 suppl Operative):237–243. [DOI] [PubMed] [Google Scholar]
- 29.Harrison DH, Grobbelaar AO. Pectoralis minor muscle transfer for unilateral facial palsy reanimation: an experience of 35 years and 637 cases. J Plast Reconstr Aesthet Surg. 2012; 65:845–850. [DOI] [PubMed] [Google Scholar]
- 30.Gousheh J, Arasteh E. Treatment of facial paralysis: dynamic reanimation of spontaneous facial expression-apropos of 655 patients. Plast Reconstr Surg. 2011; 128:693e–703e. [DOI] [PubMed] [Google Scholar]
- 31.Lin CH, Wallace C, Liao CT. Functioning free gracilis myocutaneous flap transfer provides a reliable single-stage facial reconstruction and reanimation following tumor ablation. Plast Reconstr Surg. 2011; 128:687–696. [DOI] [PubMed] [Google Scholar]
- 32.Hadlock TA, Malo JS, Cheney ML, et al. Free gracilis transfer for smile in children: the Massachusetts Eye and Ear Infirmary Experience in excursion and quality-of-life changes. Arch Facial Plast Surg. 2011; 13:190–194. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan KG, Schackert G, Seifert V. Outcomes of microneurovascular facial reanimation using masseteric innervation in patients with long-standing facial palsy resulting from cured brainstem lesions. Neurosurgery. 2010; 67:663–74; discussion 674. [DOI] [PubMed] [Google Scholar]
- 34.Terzis JK, Konofaos P. Novel use of C7 spinal nerve for Moebius. Plast Reconstr Surg. 2010; 126:106–117. [DOI] [PubMed] [Google Scholar]
- 35.Terzis JK, Karypidis D. The outcomes of dynamic procedures for blink restoration in pediatric facial paralysis. Plast Reconstr Surg. 2010; 125:629–644. [DOI] [PubMed] [Google Scholar]
- 36.Takushima A, Harii K, Okazaki M, et al. Availability of latissimus dorsi minigraft in smile reconstruction for incomplete facial paralysis: quantitative assessment based on the optical flow method. Plast Reconstr Surg. 2009; 123:1198–1208. [DOI] [PubMed] [Google Scholar]
- 37.Terzis JK, Olivares FS. Long-term outcomes of free-muscle transfer for smile restoration in adults. Plast Reconstr Surg. 2009; 123:877–888. [DOI] [PubMed] [Google Scholar]
- 38.Terzis JK, Tzafetta K. “Babysitter” procedure with concomitant muscle transfer in facial paralysis. Plast Reconstr Surg. 2009; 124:1142–1156. [DOI] [PubMed] [Google Scholar]
- 39.Terzis JK, Olivares FS. Long-term outcomes of free muscle transfer for smile restoration in children. Plast Reconstr Surg. 2009; 123:543–555. [DOI] [PubMed] [Google Scholar]
- 40.Manktelow RT, Tomat LR, Zuker RM, et al. Smile reconstruction in adults with free muscle transfer innervated by the masseter motor nerve: effectiveness and cerebral adaptation. Plast Reconstr Surg. 2006; 118:885–899. [DOI] [PubMed] [Google Scholar]
- 41.Sajjadian A, Song AY, Khorsandi CA, et al. One-stage reanimation of the paralyzed face using the rectus abdominis neurovascular free flap. Plast Reconstr Surg. 2006; 117:1553–1559. [DOI] [PubMed] [Google Scholar]
- 42.Kauhanen SC, Ylä-Kotola TM, Leivo IV, et al. Long-term adaptation of human microneurovascular muscle flaps to the paralyzed face: an immunohistochemical study. Microsurgery. 2006; 26:557–565. [DOI] [PubMed] [Google Scholar]
- 43.Ylä-Kotola TM, Kauhanen MS, Koskinen SK, et al. Magnetic resonance imaging of microneurovascular free muscle flaps in facial reanimation. Br J Plast Surg. 2005; 58:22–27. [DOI] [PubMed] [Google Scholar]
- 44.Ylä-Kotola TM, Kauhanen MS, Asko-Seljavaara SL, et al. P75 nerve growth factor receptor is expressed in regenerating human nerve grafts. J Surg Res. 2008; 146:254–261. [DOI] [PubMed] [Google Scholar]
- 45.Takushima A, Harii K, Asato H, et al. Neurovascular free-muscle transfer to treat facial paralysis associated with hemifacial microsomia. Plast Reconstr Surg. 2002; 109:1219–1227. [DOI] [PubMed] [Google Scholar]
- 46.Schliephake H, Schmelzeisen R, Tröger M. Revascularized muscle transfer for facial reanimation after long-standing facial paralysis. Int J Oral Maxillofac Surg. 2000; 29:243–249. [PubMed] [Google Scholar]
- 47.Wei W, Zuoliang Q, Xiaoxi L, et al. Free split and segmental latissimus dorsi muscle transfer in one stage for facial reanimation. Plast Reconstr Surg. 1999; 103:473–480. [DOI] [PubMed] [Google Scholar]
- 48.Coulson SE, O’dwyer NJ, Adams RD, et al. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004; 25:1014–1019. [DOI] [PubMed] [Google Scholar]
- 49.Hadlock TA, Urban LS. Toward a universal, automated facial measurement tool in facial reanimation. Arch Facial Plast Surg. 2012; 14:277–282. [DOI] [PubMed] [Google Scholar]
- 50.Wong KW, Forrest CR, Goodacre TE, et al. Measuring outcomes in craniofacial and pediatric plastic surgery. Clin Plast Surg. 2013; 40:305–312. [DOI] [PubMed] [Google Scholar]
- 51.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009; 124:345–353. [DOI] [PubMed] [Google Scholar]
- 52.VanSwearingen JM, Cohn JF, Turnbull J, et al. Psychological distress: linking impairment with disability in facial neuromotor disorders. Otolaryngol Head Neck Surg. 1998; 118:790–796. [DOI] [PubMed] [Google Scholar]
- 53.Kahn JB, Gliklich RE, Boyev KP, et al. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001; 111:387–398. [DOI] [PubMed] [Google Scholar]
- 54.Barry P, Mancini J, Alshukry A, et al. Validation of French versions of the Facial Disability Index and the Facial Clinimetric Evaluation Scale, specific quality of life scales for peripheral facial palsy patients. Clin Otolaryngol. 2019; 44:313–322. [DOI] [PubMed] [Google Scholar]
- 55.House JW. Facial nerve grading systems. Laryngoscope. 1983; 93:1056–1069. [DOI] [PubMed] [Google Scholar]
- 56.Reitzen SD, Babb JS, Lalwani AK. Significance and reliability of the House-Brackmann grading system for regional facial nerve function. Otolaryngol Head Neck Surg. 2009; 140:154–158. [DOI] [PubMed] [Google Scholar]
- 57.Terzis JK, Noah ME. Analysis of 100 cases of free-muscle transplantation for facial paralysis. Plast Reconstr Surg. 1997; 99:1905–1921. [DOI] [PubMed] [Google Scholar]
- 58.Guarin DL, Dusseldorp J, Hadlock TA, et al. A machine learning approach for automated facial measurements in facial palsy. JAMA Facial Plast Surg. 2018; 20:335–337. [DOI] [PubMed] [Google Scholar]
- 59.Banks CA, Bhama PK, Park J, et al. Clinician-graded electronic facial paralysis assessment: the eFACE. Plast Reconstr Surg. 2015; 136:223e–230e. [DOI] [PubMed] [Google Scholar]
- 60.Kantar RS, Wake N, Alfonso AR, et al. Magnetic resonance imaging volumetry of facial muscles in a face transplant recipient. Plast Reconstr Surg Glob Open. 2019; 7:e2515. [DOI] [PMC free article] [PubMed] [Google Scholar]


