Abstract
Background
Chest computed tomography (CT) is a useful tool for the diagnosis of coronavirus disease-2019 (COVID-19), although its exact value for predicting critical illness remains unclear. This study evaluated the efficacy of chest CT to predict disease progression, pulmonary complications, and viral positivity duration.
Methods
A single-center cohort study was conducted by consecutively including hospitalized patients with confirmed COVID-19. The chest CT patterns were described and a total severity score was calculated. The predictive accuracy of the severity score was evaluated using the receiver operating characteristic analysis, while a Cox proportional hazards regression model was implemented to identify the radiological features that are linked to prolonged duration of viral positivity.
Results
Overall, 42 patients were included with 10 of them requiring intensive care unit admission. The most common lesions were ground glass opacities (92.9%), consolidation (66.7%), and crazy-paving patterns (61.9%). The total severity score significantly correlated with inflammatory and respiratory distress markers, as well as with admission CURB-65 and PSI/PORT scores. It was estimated to predict critical illness with a sensitivity and specificity of 75% and 70%, respectively. Time-to-event analysis indicated that patients without ground-glass opacities presented significantly shorter median viral positivity (16 vs. 27 days).
Conclusions
Chest CT severity score positively correlates with markers of COVID-19 severity and presents promising efficacy in predicting critical illness. It is suggested that ground-glass opacities are linked to prolonged viral positivity. Further studies should confirm the efficacy of the severity score and elucidate the long-term pulmonary effects of COVID-19.
Keywords: COVID-19, Computed tomography, Chest, Predict, Critical illness
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-2019, Coronavirus disease-2019; CT, computed tomography; ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; CURB, Confusion, Urea, Respiratory rate, Blood pressure; PSI/PORT, Pneumonia Severity Index/Pneumonia Outcome Research Trial; MEWS, Modified Early Warning Score; SOFA, Sequential Organ Failure Assessment; APACHE II, Acute Physiology and Chronic Health Evaluation II; GGO, ground glass opacity; ROC, receiver operating characteristics; AUC, area under the curve
1. Introduction
Coronavirus disease-2019 (COVID-19) represents an emerging, potentially life-threatening, respiratory syndrome caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a betacoronavirus fist recognized in Wuhan, China [1]. It is mainly transmitted by respiratory droplets with a mean incubation time of five days, while pre-symptomatic carriers are suggested to contribute significantly to the disease spread [2]. Viral cell entry is based on the binding of its spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor, which is mainly expressed by type II alveolar epithelial cells [3]. COVID-19 may range from asymptomatic disease to acute respiratory distress syndrome (ARDS), sepsis, and multiorgan failure, depending on patients’ age, comorbidities, and host immune response [4]. Moreover, the disease may be linked to a hypercoagulable state, which may lead to arterial and venous thrombotic complications and even diffuse intravascular coagulation [5]. Various treatment strategies have been proposed, with promising evidence supporting the potential efficacy of dexamethasone [6] in reducing COVID-19-related mortality and remdesivir [7] in shortening the time to recovery in hospitalized patients.
Effective risk stratification is essential to guide clinical decisions regarding patient triaging and allocation of healthcare resources. To this point, much research effort has been devoted to the development of prognostic scores aiming to identify COVID-19 patients at increased risk of complications early during the course of the disease [8]. In this context, various predictive models have been constructed including several demographical and laboratory parameters, such as age, sex, comorbidities, lymphopenia, elevated D-dimers, and inflammatory markers [9]. However, the existing models have been criticized for being at high risk of bias, and lacking external validation; hence, no single clinical score is currently recommended to be widely applied in the clinical setting [10].
Chest computed tomography (CT) represents a useful tool for the initial evaluation of patients with suspected SARS-CoV-2 infection, presenting high sensitivity for the diagnosis of the disease [11]. Diffuse ground-glass opacities have been suggested as the hallmark of COVID-19, while several atypical manifestations have been also reported [12]. Ground-glass opacities as a radiological feature is non-specific for COVID-19, though it is typical and highly suggestive for the disease, especially when noted bilaterally and in the peripheral, subpleural location. This probably explains the low sensitivity of chest radiography for the depiction of lung changes during the early stages of the disease. The extent, distribution, and morphology of ground-glass opacities may vary on chest CT; therefore, diffuse, confluent, or patchy areas of ground glass may be observed as well as round-shaped. Furthermore, ground-glass opacities admixed with consolidation, as well as ground glass opacities with superimposed interlobular septal thickening, resulting in a crazy-paving pattern, have been described as frequent but non-specific radiological findings of COVID-19 pneumonia [13,14].
Nonetheless, the exact efficacy of chest CT in the prediction of disease progression remains still under investigation. The aim of the present study is to describe the time course of lung lesions on chest CT among COVID-19 individuals, as well as to assess the accuracy of a chest CT severity score for the prediction of critical illness. In addition, the role of chest CT in the detection of pulmonary complications, as well as the association of imaging findings with the duration of viral positivity are also evaluated.
2. Materials and methods
2.1. Study design and participants
The present cohort study was approved by the institutional review board of our institution (“Sotiria” Chest Diseases General Hospital, University of Athens, March 2020). Informed consent was obtained by all patients or their next of kin. A total of 42 patients with confirmed COVID-19 infection were consecutively enrolled. The diagnosis was solely based on the detection of SARS-CoV-2 by real-time reverse transcription polymerase chain reaction (rRT-PCR) analysis of nasopharyngeal swabs. All patients had at least one chest CT scan upon admission; selected cases had also a follow-up scan based on clinical indication. CT scans were categorized in four groups based on their timing: group 1 (CT performed 0–9 days after symptom onset), group 2 (CT performed 10–19 days after symptom onset), group 3 (CT performed 20–29) days after symptom onset) and group 4 (CT performed ≥ 30 days after symptom onset).
Information regarding patients’ comorbidities (hypertension, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, asthma, cancer, or immunodeficiency), symptoms, and clinical and laboratory characteristics were collected. Two pneumonia severity scores (Confusion, Urea, Respiratory rate, Blood pressure, age ≥ 65 years-CURB-65 [15] and Pneumonia Severity Index/Pneumonia Outcome Research Trial-PSI/PORT [16]) were calculated at admission aiming to predict the risk of complications, while the maximum scores of Modified Early Warning Score (MEWS) [17], Sequential Organ Failure Assessment (SOFA) [18], and Acute Physiology and Chronic Health Evaluation II (APACHE II) [19] were estimated in order to identify critical illness. Duration of SARS-CoV-2 positivity was measured from the first day of symptoms (or the day of molecular diagnosis when the first day of symptoms was not available) to the day of the first negative nasopharyngeal swab. All data were collected by two researchers independently, while any potential discrepancies were resolved by their consensus or discussion with all authors.
2.2. CT image acquisition technique
Chest CT scans were performed using a 16-Slice CT Scanner (GE Medical Systems, Optima CT540) with 2.5-mm section thickness, 1.25-mm reconstruction slice thickness, and 120 KVp tube voltage. Non-contrast chest CT scan was obtained with the patient in supine position and at end-inspiration when possible, while a dedicated CT Pulmonary Angiography protocol with bolus tracking was acquired in cases of suspected pulmonary embolism.
2.3. Image interpretation
Two senior chest radiologists (14 and 15 years of experience) evaluated separately all CT images, without access to the clinical or laboratory characteristics of patients. The presence of the following radiological lesions and patterns was retrospectively assessed [20]: ground-glass opacity (GGO), consolidation, GGO with consolidation, round GGO, crazy-paving, organizing pneumonia pattern, interlobular septal thickening, parenchymal bands, halo sign, reverse halo sign, air-bronchogram, traction bronchiectasis, cavitation, nodules, subsegmental vessel enlargement, pleural effusion, and lymphadenopathy. In addition, the location (unilateral/bilateral), distribution (peripheral/peribronchovascular), zonal predominance (upper/mid/lower), and the lung background (normal/emphysema/fibrosis) were evaluated. Potential complications, especially pulmonary embolism, radiological signs of bacterial superinfection, mainly consolidation in a specific lobe or lung segment, mucoid impactions, centrilobular nodules with tree-in-bud and cavitation, as well as predictive signs of pulmonary fibrosis including a combination of traction bronchiectasis, parenchymal bands, and atelectasis were also taken into account.
A chest CT severity score was calculated by assessing the degree of lobe involvement for each of the five lung lobes separately as follows: 0% (no involvement), 1%–25% (minimal involvement), 26%–50% (mild involvement), 51%–75% (moderate involvement), and 76%–100% (severe involvement). Corresponding scores for each degree of lobe involvement were classified from lobe score 0 (no involvement) to lobe score 4 (severe involvement). The overall lung “total severity score” was calculated by adding the five lobe scores reaching a range of possible scores from 0 to 20 [21]. Follow-up CT scans were judged to show progression, resolution, or no change of pulmonary lesions. Any disagreements between the two radiologists were resolved through their consensus.
2.4. Data analysis
Statistical analysis was performed in R-3.6.3. The threshold of a two-sided p-value < 0.05 was chosen to define statistical significance. Inter-rater agreement was judged by estimating the Cohen's kappa (κ) coefficient [22]. Patients were categorized depending on whether they were admitted to the intensive care unit (ICU), while CT scans were classified based on their timing since symptom onset. The normality of continuous variables was tested by the Shapiro-Wilk test [23]. Normally distributed data were expressed as mean and standard deviation and were compared using the Student's t-test. Otherwise, the median and interquartile range were reported and comparisons were conducted with the Mann-Whitney U-test. The Fischer's exact test was used for the analysis of binary variables, while comparisons of three or more groups were performed with the one-way analysis of variance method or the Kruskal-Wallis test, as appropriate [24]. The potential correlations of the chest CT severity score with inflammatory (white blood cells, neutrophil-to-lymphocyte ratio, C-reactive protein, procalcitonin, ferritin and fibrinogen) and respiratory distress markers (PaO2/FiO2 ratio and arterial-alveolar gradient), as well as with clinical scores (initial CURB-65 and PSI/PORT, worst MEWS, SOFA, and APACHE II) were tested using the Pearson or the non-parametric Spearman correlation coefficients, depending on the normality of the data [25]. Multiplicity correction was performed via false discovery rate estimation following the Benjamini-Hochberg method.
The accuracy of the chest CT severity score in the prediction ICU admission was evaluated by constructing the receiver operating characteristics (ROC) curve and calculating the area under the curve (AUC). Only the initial CT scans were taken into consideration for the diagnostic accuracy analysis in order to ensure the predictive nature of the CT score measurements. The optimal threshold was identified by calculating the Youden index [26], and the corresponding sensitivity and specificity were reported. Moreover, ROC analysis was planned to be implemented, aiming to assess the efficacy of maximum C-reactive protein and procalcitonin in predicting bacterial superinfection, as detected by chest CT imaging. A time-to-event analysis was also conducted by applying a Cox proportional hazards regression model, aiming to identify which radiological features are associated with prolonged SARS-CoV-2 positivity.
3. Results
3.1. Clinical characteristics
A total of 42 patients were included and 10 of them were admitted to the ICU, requiring invasive mechanical ventilation. The clinical and laboratory characteristics of the participants are summarized in Table 1 . Specifically, 29 patients (69%) were males, while the mean age of the study population was 56.64 years (SD: 14.12, range: 33 to 92). The most common comorbidity was hypertension (28.6%), while the most prevalent symptoms were fever (97.6%) and cough (66.7%), followed by fatigue (61.9%) and shortness of breath (59.5%). Patients who were subsequently admitted to the ICU presented at hospital admission significantly lower PaO2/FiO2 ratio (p-value < 0.001) and higher arterial-alveolar gradient (p-value: 0.002), as well as lower lymphocyte count (p-value < 0.001) and higher serum C-reactive protein (p-value: 0.024), procalcitonin (p-value: 0.011), ferritin (p-value: 0.025), aspartate aminotransferase (p-value: 0.035), and fibrinogen (p-value: 0.029). In addition, critically-ill ICU patients had significantly higher initial CURB-65 (p-value < 0.001) and PSI/PORT (p-value: 0.005) scores, as well as significantly higher maximum values of MEWS (p-value < 0.001), SOFA (p-value: 0.001), and APACHE II (p-value < 0.001) scores.
Table 1.
Clinical and laboratory characteristics of the study population.
| Clinical characteristics | All patients (N = 42) | Admission to ICU |
p-value | |
|---|---|---|---|---|
| Yes (N = 10) | No (N = 32) | |||
| Age (years) | 56.64±14.12 | 61.30±8.51 | 55.19±15.28 | 0.120 |
| Male gender | 29 (69.0%) | 9 (90%) | 20 (62.5%) | 0.134 |
| Obesity | 12 (28.6%) | 4 (40%) | 8 (25%) | 0.433 |
| Smoking history | 11 (26.2%) | 4 (40%) | 7 (21.9%) | 0.418 |
| Comorbidities | ||||
| Hypertension | 12 (28.6%) | 6 (60%) | 6 (18.8%) | 0.019 |
| Diabetes mellitus | 2 (4.8%) | 2 (20%) | 0 (0%) | 0.052 |
| Heart failure | 1 (2.4%) | 0 (0%) | 1 (3.1%) | 1 |
| Chronic kidney disease | 4 (9.5%) | 1 (10%) | 3 (9.4%) | 1 |
| Chronic obstructive pulmonary disease | 1 (2.4%) | 0 (0%) | 1 (3.1%) | 1 |
| Asthma | 4 (9.5%) | 1 (10%) | 3 (9.4%) | 1 |
| Cancer | 2 (4.8%) | 0 (0%) | 2 (6.3%) | 1 |
| Immunodeficiency | 2 (4.8%) | 1 (10%) | 1 (3.1%) | 0.424 |
| Symptoms | ||||
| Fever | 41 (97.6%) | 10 (100%) | 31 (96.9%) | 1 |
| Cough | 28 (66.7%) | 5 (50%) | 23 (71.9%) | 0.241 |
| Fatigue | 26 (61.9%) | 4 (40%) | 22 (68.8%) | 0.130 |
| Shortness of breath | 25 (59.5%) | 9 (90%) | 16 (50%) | 0.031 |
| Diarrhea | 13 (30.9%) | 0 (0%) | 13 (40.6%) | 0.018 |
| Vomiting | 4 (9.5%) | 2 (20%) | 2 (6.3%) | 0.245 |
| Loss of smell | 4 (9.5%) | 0 (0%) | 4 (12.5%) | 0.556 |
| Vital signs – Arterial blood gases at admission | ||||
| Mean arterial pressure (mmHg) | 90 [80.83–95.83] | 93.33 [83.33–99.17] | 89.17 [80–93.33] | 0.458 |
| Heart rate (beats/minute) | 81.88±14.55 | 89.11±15.89 | 79.84±13.73 | 0.139 |
| PaO2/FiO2 ratio (mmHg) | 340.9 [288.7–376.5] | 220 [190.6–271.4] | 361.9 [328.6–388.1] | <0.001 |
| Arterial-alveolar gradient (mmHg) | 37.93 [30.93–68.93] | 130.85 [60.09–160.45] | 36.73 [27.84–42.03] | 0.002 |
| Lactate (mmol/L) | 1.14±0.32 | 1.23±0.30 | 1.12±0.32 | 0.350 |
| Laboratory tests at admission | ||||
| White blood cells (/μL) | 6572±2518 | 6807±3586 | 6499±2152 | 0.801 |
| Neutrophils (/μL) | 4457±1997 | 5258±2954 | 4206±1571 | 0.304 |
| Lymphocytes (/μL) | 1335 [922–1875] | 1214 [741–1560] | 1500 [950–1912] | <0.001 |
| Neutrophil-to-lymphocyte ratio | 3.04 [1.88–4.72] | 4.15 [3.04–4.55] | 3.01 [1.63–3.96] | 0.090 |
| Platelets (/μL) | 197.29±64.85 | 174.97±60.94 | 204.27±65.37 | 0.211 |
| Platelet-to-lymphocyte ratio | 216 [160.5–394.5] | 202.69 [175.3–249.07] | 260.3 [159.8–449.6] | 0.163 |
| Hemoglobin (g/dL) | 13.96±1.26 | 14.39±1.48 | 13.82±1.18 | 0.288 |
| C-reactive protein (mg/dL) | 6.4 [1.34–10.90] | 12.52 [6.72–15.95] | 3.34 [1.15–10.24] | 0.024 |
| Procalcitonin (ng/mL) | 0.09 [0.04–0.13] | 0.13 [0.10–0.20] | 0.04 [0.03–0.11] | 0.011 |
| Ferritin (ng/mL) | 496.2 [245–949.25] | 989 [682.2–2259.5] | 434.8 [188–833] | 0.025 |
| Lactate dehydrogenase (U/L) | 265 [219–365] | 359 [262.2–384.1] | 258 [206.5–348] | 0.071 |
| Urea (mg/dL) | 28.5 [23.25–37.5] | 35 [30.5–50] | 28 [22.75–33] | 0.065 |
| Creatinine (mg/dL) | 0.9 [0.8–1.0] | 1.0 [0.9–1.08] | 0.9 [0.8–1.0] | 0.062 |
| Aspartate aminotransferase (U/L) | 33 [24.5–43.75] | 47 [31–68] | 30.5 [22.75–43] | 0.035 |
| Alanine aminotransferase (U/L) | 33.5 [21–54] | 46.5 [24.25–62.75] | 33 [21–45.5] | 0.330 |
| Total bilirubin (mg/dL) | 0.6 [0.48–0.8] | 0.6 [0.53–0.68] | 0.6 [0.43–0.8] | 0.875 |
| Fibrinogen (mg/dL) | 563.9±169.8 | 673.7±156.7 | 525.9±159.7 | 0.029 |
| D-dimers (μg/mL) | 0.56 [0.38–1.31] | 0.74 [0.64–1.20] | 0.49 [0.32–1.61] | 0.249 |
| Treatment | ||||
| Oseltamivir | 16 (38.1%) | 5 (50%) | 11 (34.4%) | 0.465 |
| Hydroxychloroquine | 31 (73.8%) | 10 (100%) | 21 (65.6%) | 0.041 |
| Azithromycin | 32 (76.2%) | 10 (100%) | 22 (68.8%) | 0.084 |
| Ceftriaxone | 17 (40.5%) | 7 (70%) | 10 (31.3%) | 0.062 |
| Clinical scores | ||||
| Initial CURB-65 | 1 [0–1] | 2 [1,2] | 0 [0–1] | <0.001 |
| Initial PSI/PORT | 68 [52–81] | 74.5 [71.5–111] | 58 [47–78] | 0.005 |
| Maximum MEWS | 1 [0–2] | 3 [[2], [3], [4]] | 1 [0–2] | <0.001 |
| Maximum SOFA | 2 [[1], [2], [3]] | 3 [3–3] | 2 [1,2] | 0.001 |
| Maximum APACHE II | 6.5 [3.75–9] | 11 [[9], [10], [11]] | 5 [2–7.5] | <0.001 |
Bold text indicates statistical significance (p-value <0.05). Continuous data are expressed as mean ± standard deviation or median [interquartile range].
3.2. Radiological findings
Inter-rater agreement was assessed to be strong, as the estimated values of Cohen's kappa were found to be > 0.8 in all outcomes (Suppl. Table 1). The radiological features of patients are presented in Table 2 . Evaluation of pulmonary background revealed normal lung in 88.1% of cases, while emphysema was detected in three patients (7.1%). In the majority of patients, lesions were present bilaterally (92.9%), affecting predominantly the lower lobes in 61.9% of cases. Peripheral and peribronchovascular distribution were detected in 30.9% and 4.8% of patients, respectively, while both patterns were present in 24 individuals (57.1%) (Fig. 1 ). The most common types of lesions were GGO (92.9%), consolidation (66.7%), crazy-paving (61.9%), interlobular septal thickening (59.5%), parenchymal bands (54.8%), GGO with consolidation (50%), organizing pneumonia pattern (47.6%), and traction bronchiectasis (45.2%). Less common findings were round GGO (26.2%), pleural effusion (21.4%), lymphadenopathy (23.8%), air-bronchogram (9.5%), reverse halo sign (7.1%), halo sign (2.4%), and nodules (2.4%). Patients admitted to the ICU presented significantly higher rates of both peripheral and peribronchovascular distribution of lesions (p-value: 0.015), as well as a higher risk of observing sings predictive of pulmonary fibrosis (p-value: 0.01). Pulmonary embolism was observed in three cases after admission to the ICU (p-value: 0.01).
Table 2.
Chest CT findings of the included patients.
| Radiological characteristics | All patients (N = 42) | Admission to ICU |
p-value | |
|---|---|---|---|---|
| Yes (N = 10) | No (N = 32) | |||
| Background | ||||
| Normal lung | 37 (88.1%) | 8 (80%) | 29 (90.6%) | 0.341 |
| Emphysema | 3 (7.1%) | 1 (10%) | 2 | |
| Fibrosis | 1 (2.4%) | 1 (10%) | 0 | |
| Emphysema + fibrosis | 1 (2.4%) | 0 (0%) | 1 | |
| Location | ||||
| Unilateral | 0 (%) | 0 (%) | 0 (%) | 1 |
| Bilateral | 39 (92.9%) | 10 (100%) | 29 (90.6%) | |
| No. of affected lobes | 5 [4,5] | 5 [5–5] | 5 [[3], [4], [5]] | 0.080 |
| Distribution | ||||
| Peripheral | 13 (30.9%) | 0 (0%) | 13 (40.6%) | 0.015 |
| Peribronchovascular | 2 (4.8%) | 1 (10%) | 1 (3.1%) | |
| Both | 24 (57.1%) | 9 (90%) | 15 (46.9%) | |
| Zonal predominance | ||||
| No predominance | 7 (16.7%) | 2 (20%) | 5 (15.6%) | 1 |
| Upper | 7 (16.7%) | 2 (20%) | 5 (15.6%) | |
| Mid | 2 (4.8%) | 0 (0%) | 2 (6.3%) | |
| Lower | 26 (61.9%) | 6 (60%) | 20 (62.5%) | |
| Pattern of lesions | ||||
| Ground glass opacity | 39 (92.9%) | 10 (100%) | 29 (90.6%) | 1 |
| Consolidation | 28 (66.7%) | 7 (70%) | 21 (65.6%) | 1 |
| Crazy paving | 26 (61.9%) | 8 (10%) | 18 (56.3%) | 0.270 |
| Interlobular septal thickening | 25 (59.5%) | 8 (80%) | 17 (53.1%) | 0.162 |
| Parenchymal bands | 23 (54.8%) | 7 (70%) | 16 (50%) | 0.305 |
| Ground glass opacity with consolidation | 21 (50%) | 7 (70%) | 14 (43.8%) | 0.277 |
| Organizing pneumonia pattern | 20 (47.6%) | 3 (30%) | 17 (53.1%) | 0.284 |
| Traction bronchiectasis | 19 (45.2%) | 7 (70%) | 12 (37.5%) | 0.144 |
| Round ground glass opacity | 11 (26.2%) | 1 (10%) | 10 (23.8%) | 0.245 |
| Pleural effusion | 9 (21.4%) | 2 (20%) | 7 (21.9%) | 1 |
| Lymphadenopathy | 10 (23.8%) | 2 (20%) | 8 (25%) | 0.181 |
| Air-bronchogram | 4 (9.5%) | 2 (20%) | 2 (6.3%) | 0.236 |
| Reverse halo sign | 3 (7.1%) | 0 (0%) | 3 (9.4%) | 1 |
| Halo sign | 1 (2.4%) | 0 (0%) | 1 (3.1%) | 1 |
| Nodules | 1 (2.4%) | 0 (0%) | 1 (3.1%) | 1 |
| Cavitation | 0 (0%) | 0 (0%) | 0 (%) | N/A |
| Subsegmental vessel enlargement | 0 (0%) | 0 (0%) | 0 (%) | N/A |
| Complications | ||||
| Radiological superinfection | 5 (11.9%) | 2 (20%) | 3 (9.4%) | 0.577 |
| Pulmonary embolism | 3 (7.1%) | 3 (30%) | 0 (0%) | 0.010 |
| Signs predictive of fibrosis | 22 (52.4%) | 9 (90%) | 13 (40.6%) | 0.010 |
| Severity score | 11.75±4.75 | 12.60±4.25 | 7.38±4.23 | 0.004 |
Bold text indicates statistical significance (p-value <0.05).Continuous data are expressed as mean ± standard deviation or median [interquartile range].
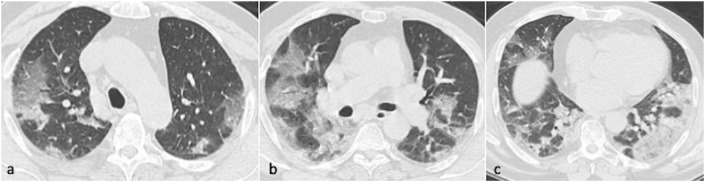
Fig. 1.
Axial CT chest images in a 57-year-old male patient in the upper, mid and lower zones showing extensive, bilateral predominantly ground glass opacities in peripheral and peribronchovascular distribution. Estimated total CT severity score 18.
3.3. Time course
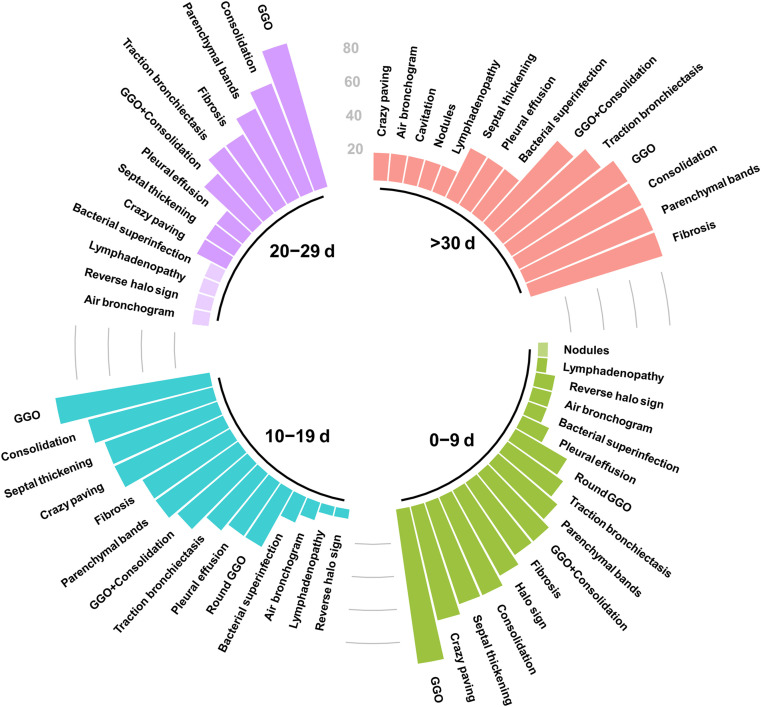
Totally, 14 follow-up CT scans were performed, with seven of them showing progression, two regression, and five no significant change of pulmonary lesions. The time course of lung changes is depicted in Fig. 2 . GGO was the most prevalent type of lesion during all phases of the infection. On the other hand, late stages of the disease were linked to significantly lower rates of crazy-paving (p-value: 0.002), interlobular septal thickening (p-value: 0.023), and round GGO (p-value: 0.015). It should be noted that the proportion of patients showing radiological signs predictive of pulmonary fibrosis was highest (83.3%) in group 4 (≥30 days from symptom onset) (Suppl. Table 2).
Fig. 2.
Frequency of lung changes at chest CT depending on the time of scan since symptom onset. Chest CT scans were categorized based on whether they were performed 0–9, 10–19, 20–29 or ≥30 days from symptoms onset.
3.4. Chest CT severity score
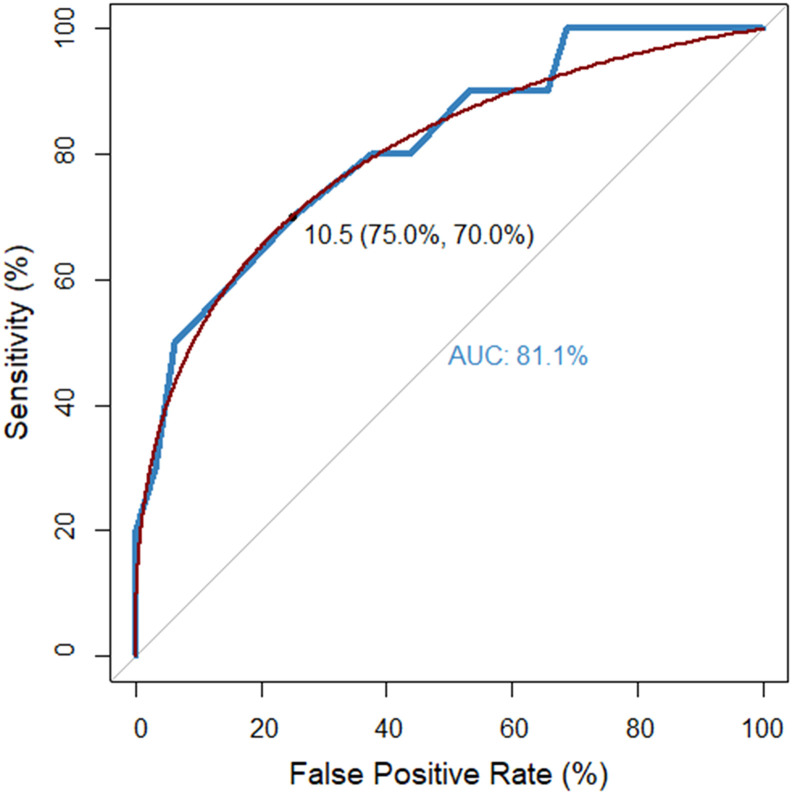
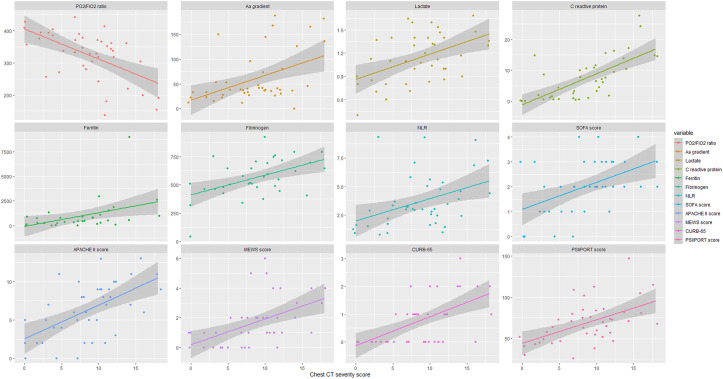
The mean chest CT severity score was estimated to be 11.75 (SD: 4.75, range: 0–18). Patients developing critical illness presented significantly higher chest CT severity scores (12.60 ± 4.25 vs. 7.38 ± 4.23, p-value: 0.004). The AUC of the chest CT severity score for the prediction of ICU admission was calculated to be 81.1%; hence, the marker was estimated to provide a sensitivity of 75% and specificity of 70% at the threshold of 10.5 (Fig. 3 ). Moreover, significant correlation was observed between chest CT severity score and PaO2/FiO2 ratio (r: 0.555, p-value < 0.001), arterial-alveolar gradient (r: 0.458, p-value: 0.004), blood lactate (r: 0.433, p-value: 0.007), serum C-reactive protein (r: 0.694, p-value < 0.001), ferritin (r: 0.487, p-value: 0.007), fibrinogen (r: 0.474, p-value: 0.006), neutrophil-to-lymphocyte ratio (r: 0.411, p-value: 0.008), initial CURB-65 (r: 0.581, p-value < 0.001), and PSI/PORT scores (r: 0.512, p-value < 0.001), as well as with worst MEWS (r: 0.560, p-value < 0.001), SOFA (r: 0.470, p-value: 0.004), and APACHE II (r: 0.576, p-value <0.001) scores (Fig. 4 ). No significant correlation of chest CT severity score with white blood cell count (r: 0.105, p-value: 0.507) and procalcitonin (r: 0.204, p-value: 0.234) was estimated. A correlation matrix is depicted in Suppl. Fig. 1.
Fig. 3.
Receiver operating characteristics curve of chest CT severity score for the prediction of admission to the intensive care unit. The threshold of 10.5 provided sensitivity and specificity of 75% and 70%, respectively. AUC: area under the curve.
Fig. 4.
Correlation of chest CT severity score with clinical scores, inflammatory and respiratory distress markers. The horizontal x-axis depicts the chest CT severity score and the vertical y-axis shows the values of clinical markers.
3.5. Bacterial superinfection
Bacterial superinfection was suspected by chest CT in nine cases (5 initial and 4 follow-up scans). Of them, the infection was microbiologically confirmed in five patients, with Acinetobacter baumannii being isolated in sputum in four cases and Klebsiella pneumoniae in one case. The diagnostic accuracy of maximum C-reactive protein and procalcitonin for the detection of radiological superinfection was estimated to be moderate (AUC: 74.1% and 70.3%, respectively). Specifically, C-reactive protein provided a sensitivity of 88.9% and specificity of 62.5% at the optimal threshold of 21.5 mg/L, while both the sensitivity and specificity of procalcitonin were calculated to be 75% at the cut-off of 0.2 ng/mL (Suppl. Fig. 2).
3.6. Duration of viral positivity
The median duration of SARS-CoV-2 positivity for the entire cohort was 25 days (95% confidence intervals-CI: 23 to 32) from the 1st day of symptoms. Univariate Cox proportional hazard regression analysis indicated that the only radiological feature associated with prolonged viral positivity was the presence of ground-glass opacities. No significant association with positivity duration was estimated for age, sex, consolidation, ground-glass opacities with consolidation, parenchymal bands, crazy paving, air bronchogram, round ground glass opacity, septal thickening, organizing pneumonia pattern, pleural effusion, or bacterial superinfection pattern (Suppl. Table 3). Therefore, as it is evident from the Kaplan-Meier curve, cases without ground-glass opacities on the admission chest CT presented significantly shorter median viral positivity (16 vs. 27 days, hazard ratio: 0.08, 95% CI: 0.02 to 0.35, p-value < 0.001) (Suppl. Fig. 3).
4. Discussion
The present study evaluated the imaging findings of patients with laboratory-confirmed SARS-CoV-2 infection in order to assess the role of chest CT in the prediction of disease progression and critical illness. To achieve this, the chest CT severity score was estimated and diagnostic accuracy analysis was performed, indicating promising efficacy of the score in the detection of patients prone to develop severe disease. This finding is in accordance with previous studies in the field [27,28], supporting the significant association of initial chest CT severity score with both short and long-term prognosis. Importantly, the chest CT score was suggested to positively correlate with admission inflammation markers (C-reactive protein, ferritin, neutrophil/lymphocyte ratio, fibrinogen), as well as with established clinical scores of pneumonia severity (CURB-65, PSI/PORT) and critical illness (MEWS, SOFA, APACHE II). In this context, it has been proposed that the extent of pulmonary lesions may reflect the degree of systemic inflammatory response, while preliminary data have indicated the potential beneficial effects of glucocorticoid therapy, leading to regression of lung infiltrates [29].
The majority of cases presented bilateral lung involvement, affecting mainly the lower lobes. Ground glass opacities and consolidations were the most prevalent lesions, especially during the initial phases of the disease. A crazy-paving pattern was commonly noticed during the first 20 days from symptom onset, while nodules, cavities, pleural effusion, and lymphadenopathy were rarely observed. Interestingly, the incidence of parenchymal bands and traction bronchiectasis was high in scans performed after 30 days from disease onset and thus indicative signs of pulmonary fibrosis were detected in the majority of patients during the late phase of the infection. This observation is in accordance with previous reports [30] and has been confirmed by recent autopsy findings, supporting that lung specimens obtained by patients who died after a long disease duration (i.e. > 30 days) presented pronounced histologic fibrotic remodeling [31]. However, whether COVID-19 may lead to long-term fibrosis and loss of pulmonary function remains to be determined by further longitudinal studies.
Sequential SARS-CoV-2 testing in nasopharyngeal swabs indicated that the duration of viral positivity was significantly shorter in patients without ground-glass opacities at admission. Moreover, the presence of ground-glass opacities was the only imaging feature associated with prolonged viral positivity. Previous studies have suggested that prolonged viral shedding may be linked to disease severity [32], as well as to higher levels of CD8+ T-lymphocytes [33], although the exact factors associated with the duration of SARS-CoV-2 positivity remain currently unclear. In this context, the combination of clinical and radiological features may enable the identification of patients at risk of longer viral presence in order to guide decisions about self-isolation and discharge of hospitalized patients. Nonetheless, whether increased duration of SARS-CoV-2 positivity translates to prolonged infectivity and transmission risk remains to be elucidated.
The present study has several strengths. Data were registered in a comprehensive database, with radiologists being blinded to clinical and laboratory outcomes. Inter-rater agreement was high, supporting the robustness of radiological evaluations. Patient recruitment was consecutive and the examined variables and end-points were pre-specified; hence, the risk of selection bias was limited. To our knowledge, it is the first time that the relationship of chest CT features with the duration of viral shedding are assessed, suggesting that imaging may provide useful information about infectivity and contribute to the optimization of isolation strategies. Previous studies have demonstrated that prolonged viral shedding may be also associated with immunosuppression and elevated interleukin-6 levels [34], as well as with high viral load expressed as RNA copies. Conversely, detecting serum neutralizing antibody titer ≥1:20 has been linked to non-infectiousness [35]. On the other hand, the interpretation of outcomes is mainly limited by the available sample size; the study was a single-center one and thus generalizability of the results to populations of other countries cannot be ensured. In addition, only hospitalized patients were examined; hence, data from asymptomatic patients were not included in the analysis. It should be also noted that follow-up CT scans were performed by clinical indication and thus radiological information was not uniformly available during the late phase of the disease.
Several aspects remain to be clarified in order to shed light on the exact role of chest CT in the prediction of critical illness among COVID-19 patients. Specifically, the predictive accuracy of the chest CT severity score should be validated by further studies using predefined thresholds, based on the present outcomes. Moreover, the observed temporal changes of lung lesions should be confirmed by prospective cohorts performing CT scans at pre-specified time-points during the course of the disease. The long-term effects of COVID-19 on pulmonary parenchyma should be also assessed by examining whether the incipient fibrotic changes seen during the acute phase may lead to permanent interstitial lung disease. In addition, the influence of viral load on the radiological appearance and severity of lung lesions may be further evaluated by methods allowing absolute viral quantification. Finally, it is important to combine chest CT findings with clinical and laboratory data aiming to construct multivariate predictive models, achieving optimal discrimination of patients at high risk of disease progression.
5. Conclusions
The present study suggests that chest CT severity score constitutes a useful tool for the initial evaluation of COVID-19 patients as it positively correlates with markers of disease severity and presents promising efficacy in the prediction of critical illness and ICU admission. The temporal changes of pulmonary lesions during the course of the disease were described, suggesting that the presence of ground glass opacities is the most prevalent radiological feature among hospitalized patients, predisposing for prolonged viral positivity. Parenchymal bands and traction bronchiectasis are increasingly observed during the late phases of the infection, although whether COVID-19 may lead to long-term pulmonary fibrosis remains to be elucidated.
Funding
This study was carried out as part of our routine work.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resinv.2021.02.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/J.CLIM.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30:367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/S11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Recovery Collaborative Group H., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of covid-19 — final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) J Am Med Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 9.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynants L., Calster B Van, Collins G.S., Riley R.D., Heinze G., Schuit E. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369 doi: 10.1136/BMJ.M1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu B., Xing Y., Peng J., Zheng Z., Tang W., Sun Y. Chest CT for detecting COVID-19: a systematic review and meta-analysis of diagnostic accuracy. Eur Radiol. 2020;1 doi: 10.1007/s00330-020-06934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y., Wang L., Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. 2020;93(1):241–249. doi: 10.1002/jmv.26218. jmv.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Revel M.P., Parkar A.P., Prosch H., Silva M., Sverzellati N., Gleeson F. COVID-19 patients and the radiology department – advice from the European society of radiology (ESR) and the European society of thoracic imaging (ESTI) Eur Radiol. 2020;30:4903–4909. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson S., Kay F.U., Abbara S., Bhalla S., Chung J.H., Chung M. Radiological society of north America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine M.J., Auble T.E., Yealy D.M., Hanusa B.H., Weissfeld L.A., Singer D.E. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 17.Subbe C.P., Kruger M., Rutherford P., Gemmel L. Validation of a modified early warning score in medical admissions. QJM. 2001;94:521–526. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 18.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Knaus W., Draper E., Wagner D., Zimmerman J. Apache II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 20.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 21.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-NCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 23.Mishra P., Pandey C.M., Singh U., Gupta A., Sahu C., Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lantz B. The impact of sample non-normality on ANOVA and alternative methods. Br J Math Stat Psychol. 2013;66:224–244. doi: 10.1111/j.2044-8317.2012.02047.x. [DOI] [PubMed] [Google Scholar]
- 25.Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 26.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Ruch Y., Kaeuffer C., Ohana M., Labani A., Fabacher T., Bilbault P. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect. 2020;26(10):1417.E5–1417.E8. doi: 10.1016/j.cmi.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;1 doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Meng G., Li W., Shi B., Dong H., Su Z. Relationship of chest CT score with clinical characteristics of 108 patients hospitalized with COVID-19 in Wuhan, China. Respir Res. 2020;21:180. doi: 10.1186/s12931-020-01440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 31.Grillo F., Barisione E., Ball L., Mastracci L., Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis. 2021;21(4) doi: 10.1016/S1473-3099(20)30582-X. e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widders A., Broom A., Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Heal. 2020;25:210–215. doi: 10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin A., He Z.B., Zhang S., Zhang J.G., Zhang X., Yan W.H. Early risk factors for the duration of SARS-CoV-2 viral positivity in COVID-19 patients. Clin Infect Dis. 2020;71(16):2061–2065. doi: 10.1093/cid/ciaa490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vena A., Taramasso L., Di Biagio A., Mikulska M., Dentone C., De Maria A. Prevalence and clinical significance of persistent viral shedding in hospitalized adult patients with SARS-CoV-2 infection: a prospective observational study. Infect Dis Ther. 2021;10:387–398. doi: 10.1007/s40121-020-00381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Kampen Jja, van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12 doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.