Abstract
Background
Patients with cancer may be at high risk of adverse outcomes from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We analyzed a cohort of patients with cancer and coronavirus 2019 (COVID-19) reported to the COVID-19 and Cancer Consortium (CCC19) to identify prognostic clinical factors, including laboratory measurements and anticancer therapies.
Patients and methods
Patients with active or historical cancer and a laboratory-confirmed SARS-CoV-2 diagnosis recorded between 17 March and 18 November 2020 were included. The primary outcome was COVID-19 severity measured on an ordinal scale (uncomplicated, hospitalized, admitted to intensive care unit, mechanically ventilated, died within 30 days). Multivariable regression models included demographics, cancer status, anticancer therapy and timing, COVID-19-directed therapies, and laboratory measurements (among hospitalized patients).
Results
A total of 4966 patients were included (median age 66 years, 51% female, 50% non-Hispanic white); 2872 (58%) were hospitalized and 695 (14%) died; 61% had cancer that was present, diagnosed, or treated within the year prior to COVID-19 diagnosis. Older age, male sex, obesity, cardiovascular and pulmonary comorbidities, renal disease, diabetes mellitus, non-Hispanic black race, Hispanic ethnicity, worse Eastern Cooperative Oncology Group performance status, recent cytotoxic chemotherapy, and hematologic malignancy were associated with higher COVID-19 severity. Among hospitalized patients, low or high absolute lymphocyte count; high absolute neutrophil count; low platelet count; abnormal creatinine; troponin; lactate dehydrogenase; and C-reactive protein were associated with higher COVID-19 severity. Patients diagnosed early in the COVID-19 pandemic (January-April 2020) had worse outcomes than those diagnosed later. Specific anticancer therapies (e.g. R-CHOP, platinum combined with etoposide, and DNA methyltransferase inhibitors) were associated with high 30-day all-cause mortality.
Conclusions
Clinical factors (e.g. older age, hematological malignancy, recent chemotherapy) and laboratory measurements were associated with poor outcomes among patients with cancer and COVID-19. Although further studies are needed, caution may be required in utilizing particular anticancer therapies.
Clinical trial identifier
Key words: SARS-CoV2, neoplasm, cancer, anticancer therapy, laboratory measurements, outcomes
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has resulted in at least 1.5 million deaths worldwide.1 , 2 Patients with cancer may have increased risk for SARS-CoV-2 infection3, 4, 5 and worse outcomes.6, 7, 8, 9, 10, 11, 12, 13 Estimates of 30-day mortality associated with coronavirus 2019 (COVID-19) for patients with cancer range from 13% to 33%,6 , 7 compared with 0.5% to 2% in the general population.1 , 14
Patients with cancer comprise a heterogeneous population, and a better understanding of specific risk factors associated with poor outcomes may help guide clinical management. The COVID-19 and Cancer Consortium (CCC19) is an international consortium that collects data on patients with cancer and COVID-19.6 , 15 , 16 Studies from CCC19 and other cohorts have suggested that older age, male sex, smoking status, worse performance status (PS), presence of comorbidities, hematological malignancies, and active cancer are associated with more severe outcomes.6, 7, 8, 9 , 13
Prior studies were limited by modest statistical power. There is also conflicting data regarding the impact of timing and modality of recent anticancer therapy on COVID-19 severity.7 , 8 , 17 In addition, few studies have investigated the role of laboratory measurements as possible prognostic indicators, particularly among patients with cancer hospitalized with COVID-19.
Leveraging detailed information from almost 5000 patients with COVID-19 and cancer, we evaluated the hypothesis that specific demographic characteristics, clinical factors, and laboratory measurements would be associated with higher COVID-19 severity. We also explored the impact of specific anticancer therapies on COVID-19 severity and 30-day all-cause mortality.
Methods
Study design
CCC19 maintains a centralized multi-institution registry of patients with COVID-19 who have a current or past diagnosis of cancer. Details of the schema and data elements have been previously described.6 , 13 Study data are collected and managed using REDCap software hosted at Vanderbilt University Medical Center.18 , 19
Reports were accrued from 17 March to 18 November 2020 and included patients who had a laboratory-confirmed diagnosis of SARS-CoV-2 by PCR and/or serology. Patients with noninvasive cancers including nonmelanoma skin cancer, in situ carcinoma (except bladder carcinoma in situ), or precursor hematologic neoplasms (e.g. monoclonal gammopathy of undetermined significance) were excluded. Reports with low-quality data (quality score >4 using our previously defined metric20; Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.02.024) or incomplete outcome ascertainment, resulting in unknown status of the primary outcome, were also excluded.
This study was exempt from Institutional Review Board (IRB) review (VUMC IRB#200467) and was approved by IRBs at participating sites per respective institutional policy. This ongoing study is registered on ClinicalTrials.gov (NCT04354701).
Outcome definitions
The primary outcome was a five-level ordinal scale of COVID-19 severity based on a patient's most severe reported disease status: none of the following complications (hereafter, uncomplicated); admitted to the hospital, admitted to an intensive care unit (ICU), mechanically ventilated at any time after COVID-19 diagnosis; or died from any cause within 30 days of COVID-19 diagnosis. We performed a secondary analysis of 30-day all-cause mortality and a descriptive analysis of patterns of anticancer therapy received within 3 months of COVID-19 diagnosis.
Prognostic factors
Potential prognostic variables were identified a priori and included age; sex; race/ethnicity; country of patient residence (United States versus non-United States); month of COVID-19 diagnosis (January-April, May-August, September-November; year 2020); smoking status; obesity; cardiovascular and pulmonary comorbidities; renal disease; diabetes mellitus; Eastern Cooperative Oncology Group (ECOG) PS; type of malignancy (solid tumor, hematological neoplasm); cancer status at time of COVID-19 diagnosis; timing of the most recent anticancer therapy; modality of anticancer therapy received within 3 months of COVID-19 diagnosis; and anti-COVID-19 treatments. Cancer status was defined as remission or no evidence of disease versus active disease, with active further defined as responding to therapy, stable, or progressing. Timing of anticancer therapy was categorized as never treated, 0-4 weeks, 1-3 months, and >3 months prior to COVID-19 diagnosis. Anticancer modalities were defined as cytotoxic chemotherapy, immunotherapy, targeted therapy, endocrine therapy, locoregional therapy (radiation and/or surgery), and other (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.02.024). Anti-COVID-19 treatments included hydroxychloroquine, corticosteroids, remdesivir, and other (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.02.024).
Survey respondents were instructed to report the earliest measured laboratory measurements during the COVID-19 disease course. Laboratory measurements included absolute lymphocyte count (ALC), absolute neutrophil count (ANC), platelet count, creatinine, D-dimer, troponin, lactate dehydrogenase (LDH), and C-reactive protein (CRP). Hematological measurements (ALC, ANC, platelets) were recorded as high, normal, or low; nonhematological measurements were defined as normal or abnormal. Except for low ALC, which was centrally defined as ALC <1500/μl, ascertainment of upper and lower limits of normal was left to the discretion of survey respondents.
Statistical methods
All statistical methods were specified before database lock (18 November 2020) and the subsequent initiation of the analysis.
Standard descriptive statistics were used to summarize baseline prognostic factors overall and stratified by levels of the ordinal COVID-19 severity outcome. Adjusted odds ratios and 95% confidence intervals for COVID-19 severity and 30-day mortality were estimated from multivariable ordinal and binary logistic regression models, respectively.21 Exploratory analyses with smoothing splines were used to determine the association of age (as a continuous variable) with outcomes,22 which appeared nonlinear (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2021.02.024). A linear regression spline with a knot at 40 years, which allowed a different linear association less than and greater than 40 years, provided an adequate fit. All other covariates were categorical.
For analyses among all patients, we included all prespecified covariates in a single model, given a sufficient number of events (and corresponding degrees of freedom) to enable full multivariable models. In the primary analysis for COVID-19 severity, we did not adjust for anti-COVID-19 treatments due to suspected confounding by indication16; these were adjusted for in a sensitivity analysis. Results between minimally adjusted (age, sex, and race/ethnicity) and fully adjusted models, variance inflation factors, and clinical judgment were used to assess stability of the results. We considered interactions among specific comorbidities (cardiovascular, pulmonary, renal disease), specific anti-COVID-19 treatments (hydroxychloroquine, corticosteroids, other), specific anticancer therapies (cytotoxic chemotherapy, immunotherapy, targeted therapy), and between timing and modality of anticancer therapy.
Associations of laboratory measurements with outcomes were assessed among hospitalized patients due to current common clinical practice to avoid a laboratory blood draw for outpatients.23 Because of the reduced sample size, we adjusted for a smaller set of potential clinical confounders: age, sex, race/ethnicity, country of patient residence, month of COVID-19 diagnosis, type of malignancy, cancer status, and active anticancer therapy. No interactions were considered for this analysis.
Multiple imputation using additive regression, bootstrapping, and predictive mean matching was used to impute missing and unknown data for all variables included in the analysis,24 with the following exceptions: unknown ECOG PS and unknown cancer status were included as ‘unknown’ categories; and laboratory values were imputed only among hospitalized patients. Separate imputation models were developed for the full cohort (10 iterations; missingness rates were <5%) and the hospitalized cohort (20 iterations; missingness rates for laboratory values were >10%).
We conducted an exploratory analysis of specific anticancer drug exposures, which are collected in optional free-text fields. Two curators (JLW and XL) independently abstracted the fields for all patients with systemic anticancer therapy reported (cytotoxic chemotherapy, immunotherapy, endocrine therapy, and/or targeted therapy) within 3 months prior to COVID-19 diagnosis; disagreements were resolved by consensus. Specific drugs were grouped by similar mechanisms of action (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.02.024) based on consensus among authors. The results were visualized using UpSet plots.25
Analyses were performed in R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), including the Hmisc, rms, and UpSetR26 extension packages.
Results
A total of 6968 reports were evaluable in the REDCap database and 4966 were included in our analysis after exclusions (Supplementary Figure S2, available at https://doi.org/10.1016/j.annonc.2021.02.024). Among these patients, who had a median follow-up of 42 days [interquartile range (IQR) 22-90 days], 2872 (58%) were hospitalized during their COVID-19 course (Table 1 ). The median age of the entire cohort and hospitalized subgroup was 66 (IQR 56-76) and 70 (IQR 60-79) years, respectively. Approximately half of the patients were female and non-Hispanic white in each group, while non-Hispanic black patients represented 22% and 24%, respectively. Approximately 80% had solid tumors, 51% had cancer in remission, and 40% received anticancer therapy within 3 months of COVID-19 diagnosis. Altogether, 61% had cancer that was present, active, or treated within the past year. Additional baseline characteristics are summarized in Table 1.
Table 1.
Baseline prognostic factors among all patients and hospitalized patients
| All patients |
Hospitalized patients |
|
|---|---|---|
| (n = 4966) | (n = 2872) | |
| Median agea, years (IQR) | 66 (56-76) | 70 (60-79) |
| Sex | ||
| Female | 2527 (51) | 1323 (46) |
| Male | 2436 (49) | 1546 (54) |
| Missing/unknown | 3 (<1) | 3 (<1) |
| Race and ethnicityb | ||
| Non-Hispanic white | 2485 (50) | 1371 (48) |
| Non-Hispanic black | 1068 (22) | 697 (24) |
| Hispanic | 722 (15) | 390 (14) |
| Other | 578 (12) | 359 (12) |
| Missing/unknown | 113 (2) | 55 (2) |
| Smoking status | ||
| Never | 2615 (53) | 1356 (47) |
| Ever | 2161 (44) | 1386 (48) |
| Missing/unknown | 190 (4) | 130 (5) |
| Obesity status | ||
| Not obese | 3220 (65) | 1909 (66) |
| Obese | 1704 (34) | 944 (33) |
| Missing/unknown | 42 (1) | 19 (1) |
| Comorbiditiesb | ||
| Cardiovascular | 1582 (32) | 1175 (41) |
| Pulmonary | 1091 (22) | 762 (27) |
| Renal disease | 831 (17) | 644 (22) |
| Diabetes mellitus | 1385 (28) | 994 (35) |
| Missing/unknown | 56 (1) | 26 (1) |
| ECOG performance status | ||
| 0 | 1731 (35) | 725 (25) |
| 1 | 1296 (26) | 794 (28) |
| ≥2 | 806 (16) | 675 (24) |
| Unknown | 1121 (23) | 671 (23) |
| Missing | 12 (<1) | 7 (<1) |
| Type of malignancyb | ||
| Solid tumor | 4021 (81) | 2260 (79) |
| Hematological neoplasm | 1097 (22) | 717 (25) |
| Cancer status | ||
| Remission or no evidence of disease | 2546 (51) | 1366 (48) |
| Active and responding | 556 (11) | 293 (10) |
| Active and stable | 813 (16) | 467 (16) |
| Active and progressing | 613 (12) | 452 (16) |
| Unknown | 426 (9) | 283 (10) |
| Missing | 12 (<1) | 11 (<1) |
| Timing of anticancer therapy | ||
| Never treated | 413 (8) | 252 (9) |
| 0-4 weeks | 1609 (32) | 907 (32) |
| 1-3 months | 375 (8) | 231 (8) |
| >3 months | 2344 (47) | 1324 (46) |
| Missing/unknown | 225 (5) | 158 (6) |
| Modality of active anticancer therapyb,c | ||
| None | 2807 (57) | 1625 (57) |
| Cytotoxic chemotherapy | 802 (16) | 491 (17) |
| Immunotherapy | 248 (5) | 137 (5) |
| Targeted therapy | 693 (14) | 426 (15) |
| Endocrine therapy | 483 (10) | 229 (8) |
| Locoregional therapy | 422 (8) | 249 (9) |
| Other | 33 (1) | 18 (1) |
| Missing/unknown | 176 (4) | 110 (4) |
| Anti-COVID-19 treatmentb | ||
| None | 2816 (57) | 1048 (36) |
| Remdesivir | 438 (9) | 435 (15) |
| Hydroxychloroquine | 829 (17) | 796 (28) |
| Corticosteroids | 708 (14) | 634 (22) |
| Other | 1166 (23) | 1023 (36) |
| Missing/unknown | 259 (5) | 143 (5) |
| Country of patient residence | ||
| United States | 4739 (95) | 2714 (94) |
| Outside United States | 227 (5) | 158 (6) |
| Month of COVID-19 diagnosis | ||
| January-April | 1927 (39) | 1284 (45) |
| May-August | 2508 (51) | 1325 (46) |
| September-November | 433 (9) | 211 (7) |
| Missing/unknown | 98 (2) | 52 (2) |
| Absolute lymphocyte countd | ||
| Low | — | 1402 (49) |
| Normal | — | 891 (31) |
| High | — | 74 (3) |
| Missing/unknown | — | 505 (18) |
| Absolute neutrophil countd | ||
| Low | — | 217 (8) |
| Normal | — | 1739 (61) |
| High | — | 474 (17) |
| Missing/unknown | — | 442 (15) |
| Platelet countd | ||
| Low | — | 733 (26) |
| Normal | — | 1675 (58) |
| High | — | 119 (4) |
| Missing/unknown | — | 345 (12) |
| Creatinined | ||
| Normal | — | 1498 (52) |
| Abnormal | — | 1049 (37) |
| Missing/unknown | — | 325 (11) |
| D-dimerd | ||
| Normal | — | 236 (8) |
| Abnormal | — | 1321 (46) |
| Missing/unknown | — | 1315 (46) |
| Troponind | ||
| Normal | — | 983 (34) |
| Abnormal | — | 608 (21) |
| Missing/unknown | — | 1281 (45) |
| Lactate dehydrogenased | ||
| Normal | — | 358 (12) |
| Abnormal | — | 1128 (39) |
| Missing/unknown | — | 1386 (48) |
| C-reactive proteind | ||
| Normal | — | 137 (5) |
| Abnormal | — | 1434 (50) |
| Missing/unknown | — | 1301 (45) |
Data presented as n (%) unless otherwise indicated. The ‘Missing/unknown’ category indicates either missingness due to nonresponse for optional survey questions or a response of unknown; an unknown category was provided for all survey questions.
COVID-19, coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
For patients younger than 18 years (n = 9), age was truncated to 18 years; for patients older than 89 years (n = 161), age was truncated to 90 years. Truncation was done in concordance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and to reduce the risk of re-identifiability.
Percentages could sum to >100% because categories are not mutually exclusive.
Within 3 months of COVID-19 diagnosis.
Laboratory data were systemically not collected for nonhospitalized patients.
Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2021.02.024, provides unadjusted rates of hospitalization and 30-day mortality. Of note, the 30-day mortality rate (95% CI) for patients diagnosed with COVID-19 during January-April, May-August, and September-November was 21% (20%-23%), 10% (9%-11%), and 7% (5%-10%), respectively.
COVID-19 severity
Of the 4966 patients, 2072 had an uncomplicated disease course (Table 2 ). For the 2894 patients with complications, 1675 were admitted to the hospital but did not require ICU care or mechanical ventilation and did not die. An additional 232 were admitted to the ICU without mechanical ventilation, 292 required mechanical ventilation, and 695 died within 30 days. Patients who died were older (median age 75 versus 61-69 years for other outcomes). Males had worse COVID-19 severity compared with females, as indicated by greater proportions of males among those who received mechanical ventilation or died. Table 2 and Supplementary Table S5, available at https://doi.org/10.1016/j.annonc.2021.02.024, provide summaries stratified by the ordinal outcome for the entire cohort and the hospitalized subgroup, respectively.
Table 2.
Baseline prognostic factors stratified by levels of COVID-19 severitya among all patients
| Prognostic factor | No complications |
Admitted to hospital |
Admitted to ICU |
Received mechanical ventilation |
Died within 30 days |
|---|---|---|---|---|---|
| (n = 2072, 42%) | (n = 1675, 34%) | (n = 232, 5%) | (n = 292, 6%) | (n = 695, 14%) | |
| Median ageb, years (IQR) | 61 (50-70) | 69 (59-78) | 66.5 (58-76) | 66 (57-72.25) | 75 (66-83) |
| Sex | |||||
| Female | 1193 (58) | 832 (50) | 109 (47) | 111 (38) | 282 (41) |
| Male | 879 (42) | 841 (50) | 123 (53) | 180 (62) | 413 (59) |
| Missing/unknown | 0 (0) | 2 (<1) | 0 (0) | 1 (<1) | 0 (0) |
| Race and ethnicityc | |||||
| Non-Hispanic white | 1100 (53) | 802 (48) | 116 (50) | 125 (43) | 342 (49) |
| Non-Hispanic black | 369 (18) | 389 (23) | 51 (22) | 76 (26) | 183 (26) |
| Hispanic | 328 (16) | 239 (14) | 27 (12) | 46 (16) | 82 (12) |
| Other | 217 (10) | 211 (13) | 36 (16) | 38 (13) | 76 (11) |
| Missing/unknown | 58 (3) | 34 (2) | 2 (1) | 7 (2) | 12 (2) |
| Smoking status | |||||
| Never | 1248 (60) | 842 (50) | 105 (45) | 154 (53) | 266 (38) |
| Ever | 764 (37) | 768 (46) | 113 (49) | 126 (43) | 390 (56) |
| Missing/unknown | 60 (3) | 65 (4) | 14 (6) | 12 (4) | 39 (6) |
| Obesity status | |||||
| Not obese | 1293 (62) | 1125 (67) | 148 (64) | 165 (57) | 489 (70) |
| Obese | 756 (36) | 538 (32) | 82 (35) | 125 (43) | 203 (29) |
| Missing/unknown | 23 (1) | 12 (1) | 2 (1) | 2 (1) | 3 (<1) |
| Comorbiditiesc | |||||
| Cardiovascular | 393 (19) | 629 (38) | 96 (41) | 110 (38) | 354 (51) |
| Pulmonary | 323 (16) | 414 (25) | 65 (28) | 67 (23) | 222 (32) |
| Renal disease | 179 (9) | 331 (20) | 49 (21) | 63 (22) | 209 (30) |
| Diabetes mellitus | 385 (19) | 540 (32) | 82 (35) | 113 (39) | 265 (38) |
| Missing/unknown | 30 (1) | 15 (1) | 2 (1) | 4 (1) | 5 (1) |
| ECOG performance status | |||||
| 0 | 1004 (48) | 476 (28) | 65 (28) | 96 (33) | 90 (13) |
| 1 | 499 (24) | 490 (29) | 62 (27) | 79 (27) | 166 (24) |
| ≥2 | 115 (6) | 328 (20) | 50 (22) | 35 (12) | 278 (40) |
| Unknown | 449 (22) | 378 (23) | 54 (23) | 80 (27) | 160 (23) |
| Missing | 5 (<1) | 3 (<1) | 1 (<1) | 2 (1) | 1 (<1) |
| Type of malignancyc | |||||
| Solid tumor | 1744 (84) | 1361 (81) | 167 (72) | 213 (73) | 536 (77) |
| Hematological neoplasm | 373 (18) | 368 (22) | 74 (32) | 91 (31) | 191 (27) |
| Cancer status | |||||
| Remission | 1173 (57) | 831 (50) | 125 (54) | 148 (51) | 269 (39) |
| Active and responding | 262 (13) | 194 (12) | 17 (7) | 27 (9) | 56 (8) |
| Active and stable | 344 (17) | 275 (16) | 38 (16) | 48 (16) | 108 (16) |
| Active and progressing | 153 (7) | 243 (15) | 23 (10) | 32 (11) | 162 (23) |
| Unknown | 139 (7) | 129 (8) | 29 (12) | 34 (12) | 95 (14) |
| Missing | 1 (<1) | 3 (<1) | 0 (0) | 3 (1) | 5 (1) |
| Timing of anticancer therapy | |||||
| Never treated | 159 (8) | 144 (9) | 21 (9) | 26 (9) | 63 (9) |
| 0-4 weeks | 697 (34) | 530 (32) | 66 (28) | 96 (33) | 220 (32) |
| 1-3 months | 139 (7) | 130 (8) | 14 (6) | 15 (5) | 77 (11) |
| >3 months | 1012 (49) | 793 (47) | 113 (49) | 137 (47) | 289 (42) |
| Missing/unknown | 65 (3) | 78 (5) | 18 (8) | 18 (6) | 46 (7) |
| Modality of active anticancer therapyc,d | |||||
| None | 1171 (57) | 953 (57) | 142 (61) | 167 (57) | 374 (54) |
| Cytotoxic chemotherapy | 305 (15) | 293 (17) | 29 (12) | 31 (11) | 144 (21) |
| Immunotherapy | 108 (5) | 75 (4) | 15 (6) | 11 (4) | 39 (6) |
| Targeted therapy | 264 (13) | 243 (15) | 34 (15) | 48 (16) | 104 (15) |
| Endocrine therapy | 252 (12) | 149 (9) | 11 (5) | 24 (8) | 47 (7) |
| Locoregional therapy | 173 (8) | 140 (8) | 20 (9) | 24 (8) | 65 (9) |
| Other | 15 (1) | 9 (1) | 0 (0) | 2 (1) | 7 (1) |
| Missing/unknown | 65 (3) | 63 (4) | 10 (4) | 14 (5) | 24 (3) |
| Anti-COVID-19 treatmentc | |||||
| None | 1752 (85) | 744 (44) | 54 (23) | 44 (15) | 222 (32) |
| Remdesivir | <5 (<1) | 210 (13) | 72 (31) | 69 (24) | 84 (12) |
| Hydroxychloroquine | 32 (2) | 380 (23) | 57 (25) | 122 (42) | 238 (34) |
| Corticosteroids | 73 (4) | 281 (17) | 92 (40) | 104 (36) | 158 (23) |
| Other | 142 (7) | 465 (28) | 100 (43) | 175 (60) | 284 (41) |
| Missing/unknown | 112 (5) | 84 (5) | 11 (5) | 14 (5) | 38 (5) |
| Country of patient residence | |||||
| United States | 2004 (97) | 1573 (94) | 221 (95) | 282 (97) | 659 (95) |
| Outside United States | 68 (3) | 102 (6) | 11 (5) | 10 (3) | 36 (5) |
| Month of COVID-19 diagnosis | |||||
| January-April | 627 (30) | 651 (39) | 75 (32) | 163 (56) | 411 (59) |
| May-August | 1177 (57) | 842 (50) | 129 (56) | 115 (39) | 245 (35) |
| September-November | 222 (11) | 148 (9) | 26 (11) | 6 (2) | 31 (4) |
| Missing/unknown | 46 (2) | 34 (2) | 2 (1) | 8 (3) | 8 (1) |
Data presented as n (%) unless otherwise indicated. The ‘Missing/unknown’ category indicates either missingness due to nonresponse for optional survey questions or a response of unknown; an unknown category was provided for all survey questions.
COVID-19, coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group; ICU, intensive care unit; IQR, interquartile range.
Five-level ordinal scale based on a patient's most severe reported disease status. For example, patients who were admitted to the intensive care unit without mechanical ventilation and did not die within 30 days of COVID-19 diagnosis are classified as ‘admitted to intensive care unit’, whereas patients who were admitted to the intensive care unit with mechanical ventilation and did not die within 30 days of COVID-19 diagnosis are classified as ‘received mechanical ventilation’.
For patients younger than 18 years, age was truncated to 18 years; for patients older than 89 years, age was truncated to 90 years.
Percentages could sum to >100% because categories are not mutually exclusive.
Within 3 months of COVID-19 diagnosis.
Multivariable analysis revealed higher COVID-19 severity among patients older than 40 years, males, and non-Hispanic black and Hispanic patients compared with non-Hispanic white patients (Table 3 ). In addition, obesity, cardiovascular and pulmonary comorbidities, renal disease, diabetes mellitus, worse ECOG PS, and hematological malignancy were associated with higher COVID-19 severity. Active and progressing cancer, recent active cytotoxic chemotherapy, and COVID-19-directed treatments were also associated with higher severity. Notably, noncytotoxic systemic anticancer therapies including immunotherapy, targeted therapy, and endocrine therapy were not associated with higher COVID-19 severity. Of the 483 patients receiving endocrine therapy, 214 (44%) were in remission, which was a higher proportion than for those receiving cytotoxic chemotherapy (11%), targeted therapy (15%), or immunotherapy (6%).
Table 3.
Adjusted associations of baseline prognostic factors with COVID-19 severity (primary) and 30-day all-cause mortality (secondary) among all patients
| COVID-19 severity |
30-day mortality |
|
|---|---|---|
| ORa (95% CI) | ORb (95% CI) | |
| Age, per decadec | ||
| Age <40 years | 0.91 (0.72-1.15) | 0.58 (0.35-0.97) |
| Age >40 years | 1.38 (1.31-1.45) | 1.75 (1.59-1.93) |
| Sex, male versus female | 1.47 (1.31-1.65) | 1.46 (1.20-1.77) |
| Race and ethnicity, versus non-Hispanic white | ||
| Non-Hispanic black | 1.46 (1.27-1.68) | 1.38 (1.09-1.75) |
| Hispanic | 1.38 (1.16-1.64) | 1.31 (0.96-1.80) |
| Other | 1.27 (1.05-1.53) | 0.97 (0.70-1.36) |
| Smoking status, ever versus never | 1.10 (0.98-1.24) | 1.20 (0.98-1.46) |
| Obesity status, obese versus not obese | 1.14 (1.01-1.29) | 1.09 (0.88-1.35) |
| Cardiovascular comorbidities, yes versus no | 1.46 (1.29-1.67) | 1.17 (0.95-1.43) |
| Pulmonary comorbidities, yes versus no | 1.52 (1.33-1.74) | 1.34 (1.09-1.66) |
| Renal disease, yes versus no | 1.38 (1.19-1.60) | 1.31 (1.05-1.63) |
| Diabetes mellitus, yes versus no | 1.53 (1.35-1.73) | 1.23 (1.00-1.50) |
| ECOG performance status, versus 0 | ||
| 1 | 1.42 (1.22-1.64) | 1.53 (1.14-2.05) |
| ≥2 | 3.44 (2.88-4.10) | 4.48 (3.34-6.00) |
| Unknown | 1.75 (1.50-2.04) | 2.04 (1.51-2.76) |
| Type of malignancy, versus solid tumor | ||
| Hematological neoplasm | 1.70 (1.46-1.99) | 1.44 (1.10-1.87) |
| Multipled | 1.21 (1.01-1.44) | 1.30 (1.00-1.70) |
| Cancer status, versus remission or no evidence of disease | ||
| Active and responding | 0.84 (0.67-1.04) | 0.79 (0.52-1.18) |
| Active and stable | 0.97 (0.81-1.16) | 1.06 (0.77-1.44) |
| Active and progressing | 2.19 (1.80-2.67) | 2.88 (2.13-3.90) |
| Unknown | 1.93 (1.55-2.41) | 2.19 (1.56-3.07) |
| Timing of anticancer therapy, versus >3 months | ||
| Never treated | 1.05 (0.83-1.32) | 1.10 (0.75-1.62) |
| 0-4 weeks | 1.04 (0.79-1.36) | 1.10 (0.70-1.72) |
| 1-3 months | 1.03 (0.75-1.41) | 1.39 (0.84-2.29) |
| Modality of active anticancer therapye | ||
| Cytotoxic chemotherapy, yes versus no | 1.28 (1.04-1.58) | 1.61 (1.15-2.24) |
| Immunotherapy, yes versus no | 0.86 (0.64-1.16) | 0.91 (0.56-1.47) |
| Targeted therapy, yes versus no | 1.09 (0.87-1.36) | 0.90 (0.63-1.31) |
| Endocrine therapy, yes versus no | 0.79 (0.61-1.03) | 0.68 (0.43-1.09) |
| Locoregional therapy, yes versus no | 1.18 (0.93-1.50) | 0.96 (0.65-1.42) |
| Other, yes versus no | 0.97 (0.47-2.00) | 1.31 (0.44-3.94) |
| Anti-COVID-19 treatmentf | ||
| Remdesivir, yes versus no | — | 1.55 (1.10-2.18) |
| HCQ alone, yes versus no | — | 1.64 (1.16-2.32) |
| Corticosteroids alone, yes versus no | — | 1.86 (1.35-2.56) |
| Other alone, yes versus no | — | 1.64 (1.23-2.17) |
| HCQ + corticosteroids, yes versus no | — | 1.91 (1.21-3.01)g |
| HCQ + other, yes versus no | — | 2.98 (2.24-3.97)g |
| Country of residence, United States versus outside United States | 1.07 (0.81-1.41) | 0.85 (0.54-1.35) |
| Month of COVID-19 diagnosis, versus January-April | ||
| May-August | 0.50 (0.45-0.57) | 0.43 (0.35-0.54) |
| September-November | 0.42 (0.34-0.52) | 0.26 (0.16-0.41) |
Models for COVID-19 severity and 30-day all-cause mortality include all variables listed, except where noted. There were no indications of model instability, except for timing of anticancer therapy (variance inflation factor 5.4); however, multicollinearity is not unexpected because timing and modality are both defined by receipt of anticancer therapy.
CI, confidence interval; COVID-19, coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group; HCQ, hydroxychloroquine; OR, odds ratio.
Odds ratios >1 indicate higher COVID-19 severity.
Odds ratios >1 indicate higher odds of 30-day all-cause mortality.
Obtained from a linear regression spline with a knot at age 40 years, such that odds ratios for ‘Age <40 years’ correspond to the per-decade difference in age for ages <40 years and odds ratios for ‘Age >40 years’ correspond to the per-decade difference in age for ages >40 years.
Includes two or more solid tumors or hematological neoplasms.
Within 3 months of COVID-19 diagnosis.
The model for COVID-19 severity did include anti-COVID-19 treatments due to suspected confounding by indication.
Interaction P = 0.19 (2 degrees of freedom).
More recent diagnosis of COVID-19 compared with diagnosis earlier in the pandemic (between January and April) was associated with lower COVID-19 severity. Significant interactions were observed among anti-COVID-19 treatments (Supplementary Table S6, available at https://doi.org/10.1016/j.annonc.2021.02.024). However, there were no meaningful interactions among medical comorbidities, anticancer therapies, or between timing of anticancer therapy and modality of anticancer therapies (Supplementary Table S7, available at https://doi.org/10.1016/j.annonc.2021.02.024).
Many characteristics associated with higher COVID-19 severity, including cytotoxic chemotherapy, were also associated with 30-day mortality (Table 3). Factors such as Hispanic ethnicity and cardiovascular comorbidities had a weaker association that was no longer statistically significant. COVID-19-directed treatments had a substantial attenuation of the association in the 30-day mortality analysis, although all retained statistical significance.
Laboratory measurements among hospitalized patients
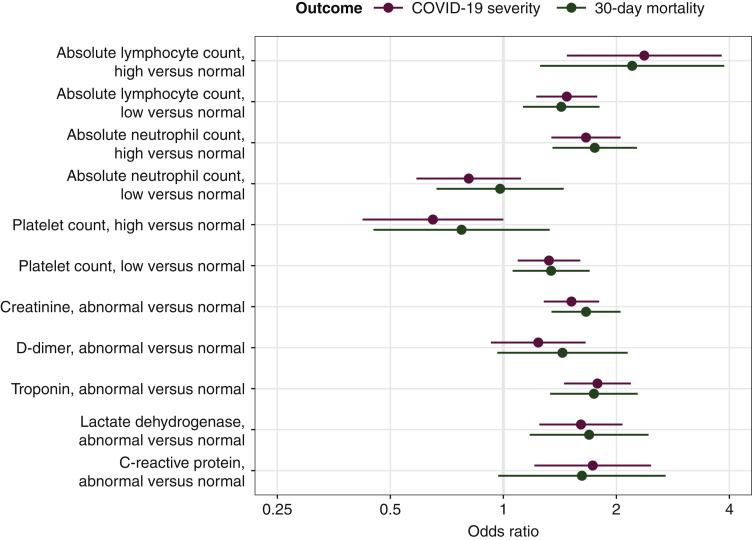
Laboratory measurements collected during SARS-CoV-2 diagnosis were analyzed among the hospitalized subgroup of 2872 patients (Figure 1 , Supplementary Table S8, available at https://doi.org/10.1016/j.annonc.2021.02.024). High ALC; low ALC; high ANC; and low platelets; as well as abnormal levels of creatinine; troponin; or LDH; were each associated with higher COVID-19 severity and 30-day mortality. Abnormal CRP was associated with higher COVID-19 severity.
Figure 1.
Adjusted odds ratios and 95% confidence intervals for laboratory measurements obtained from multivariable models for COVID-19 severity and 30-day all-cause mortality among hospitalized patients.
Odds ratios >1 indicate higher COVID-19 severity or higher odds of 30-day all-cause mortality. Adjusted for age, sex, race/ethnicity, country of patient residence, month of COVID-19 diagnosis, type of malignancy, cancer status, and active anticancer therapy. COVID-19, coronavirus disease 2019.
Anticancer therapies
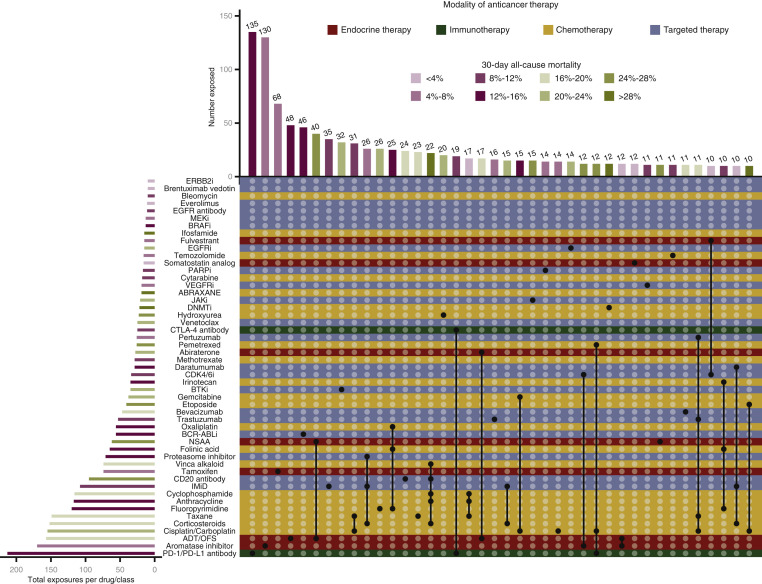
Of the 1803 patients receiving systemic anticancer therapy within 3 months of COVID-19 diagnosis, 1626 (90%) had extractable free-text drug exposure with 125 distinct drugs/classes reported. Most exposures (n = 856, 53%) were to a single drug or class; 357 (22%) patients received at least three drugs in combination. Drug/class exposures noted in at least 10 patients are shown in Figure 2 . The three treatment regimens with the lowest and highest observed 30-day and overall all-cause mortality are described in Table 4 . Platinum-etoposide, R-CHOP-like, and DNA methyltransferase inhibitor regimens were associated with the highest observed 30-day and overall all-cause mortality.
Figure 2.
Visualization of the most prevalent cancer therapies and associated 30-day all-cause mortality.
Individual anticancer drug exposures and their combinations are shown in an UpSet plot, which is an alternative to the Venn diagram for the visualization of high-dimensional data. Each row represents the individual anticancer therapies recorded as being given within 3 months of COVID-19 diagnosis that were present in ≥10 cases; rows are colored by treatment modality. Each column represents the intersection of one or more drugs given in combination (i.e. as a regimen) in ≥10 cases. A column with a single dark circle represents a monotherapy regimen; columns with multiple dark circles connected by dark lines represent multiagent regimens. Bars are colored by mortality for the patients receiving the drug or the combination, with darker hues representing higher mortality. This information is also shown in tabular format in Supplementary Table 9, available at https://doi.org/10.1016/j.annonc.2021.02.024.
ADT, androgen-deprivation therapy; BCR-ABLi, BCR-ABL tyrosine kinase inhibitor; BRAFi, serine/threonine-protein kinase B-Raf inhibitor; BTKi, Bruton tyrosine kinase inhibitor; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor; COVID-19, coronavirus disease 2019; DNMTi, DNA methyltransferase inhibitor; EGFRi, epidermal growth factor receptor tyrosine kinase inhibitor; ERBB2i, epidermal growth factor receptor 2 tyrosine kinase inhibitor; IMiD, immunomodulator; JAKi, Janus kinase inhibitor; MEKi, mitogen-activated protein kinase kinase inhibitor; NSAA, nonsteroid antiandrogen; OFS, ovarian function suppression; PARPi, poly (ADP-ribose) polymerase inhibitor; VEGFRi, vascular endothelial growth factor receptor inhibitor.
Table 4.
Characteristics for exposures associated with the lowesta and highest observed mortality among patients treated with systemic anticancer therapy within 3 months of COVID-19 diagnosis
| Lowest observed mortality |
Highest observed mortality |
|||||
|---|---|---|---|---|---|---|
| AC-T-likeb |
Dara-IMiD-Dex |
OFS + AI |
Platinum + Etoposide |
R-CHOP-likec |
DNMTi |
|
| (n = 17) | (n = 10) | (n = 12) | (n = 10) | (n = 22) | (n = 12) | |
| All-cause mortality | ||||||
| 30-day mortality | 0 (0) | 0 (0) | 0 (0) | 3 (30) | 8 (36) | 6 (50) |
| Any mortality | 0 (0) | 1 (10) | 0 (0) | 4 (40) | 10 (45) | 6 (50) |
| Most common primary cancer | Breast 17 (100) |
MM 10 (100) |
Breast 11 (92) |
SCLC 5 (50) |
DLBCL 17 (77) |
MDS 7 (58) |
| Median (IQR) age, years | 55 (49-62) | 69 (64-80.5) | 43.5 (41-46.5) | 66.5 (60-74.5) | 67.5 (45-79) | 67.5 (59-87) |
| ECOG PS 0-1 | 16 (94) | 5 (50) | 11 (92) | 6 (60) | 18 (82) | 7 (58) |
| Curative treatment intent | 17 (100) | 0 (0)d | 10 (83) | 2 (20) | 18 (82) | 1 (8) |
Data presented as n (%) unless otherwise indicated.
AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor; COVID-19, coronavirus disease 2019; Dara, daratumumab; DLBCL, diffuse large B-cell lymphoma; DNMTi, DNA methyltransferase inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; IMiD, immunomodulatory imide drugs; IQR, interquartile range; MDS, myelodysplastic syndrome; MM, multiple myeloma; OFS, ovarian function suppression; PS, performance status; SCLC, small-cell lung cancer.
Not shown: somatostatin analogs, and CDK4/6i + fulvestrant.
Combination of anthracycline, cyclophosphamide, and taxane.
Combination of CD20 antibody, cyclophosphamide, anthracycline, vinca alkaloid, and corticosteroid.
All treatment for multiple myeloma except allogeneic stem cell transplant was considered palliative by definition.
Discussion
COVID-19 poses a substantial risk to patients with cancer. It is essential to understand factors associated with high risk of adverse outcomes to inform clinical decision making. In this study, we used a novel ordinal outcome of COVID-19 severity and a cohort of almost 5000 patients with cancer to identify demographic factors (age, male sex, race/ethnicity), clinical factors (comorbidities, ECOG PS, hematological malignancy, active and progressing cancer, recent cytotoxic chemotherapy), and laboratory measurements (high or low ALC; high ANC; low platelets; abnormal creatinine, troponin, or LDH) associated with higher COVID-19 severity. While these data can certainly inform providers regarding prognostic factors and risk stratification, and also significantly broaden our understanding in this important topic, our findings are hypothesis generating and might not directly modify daily clinical practice.
Our findings confirm those from an earlier study from CCC19 and other studies.6, 7, 8, 9, 10 , 17 In particular, older age and male sex have been identified as negative prognostic factors among patients with or without cancer, although our study is the first, to our knowledge, to demonstrate a nonlinear relationship between age and risk.6, 7, 8, 9, 10 , 17 , 27
We also noted higher COVID-19 severity for patients of non-Hispanic, non-white race/ethnicity and higher 30-day mortality for non-Hispanic black patients. These differences may suggest disparities in health care access, delivery, and research,28 especially in the context of our prior finding and a recent systematic review suggesting that non-Hispanic black patients were less likely to receive novel anti-COVID-19 treatments.16 , 29 Future research from CCC19 is planned to investigate these disparities further.
Among patients with cancer, hematological malignancies,6 , 8 , 11 active cancer,6 , 9, 10, 11 , 17 and worse ECOG PS6 , 10 , 17 have been consistently associated with worse outcomes, which was also noted here. While prior studies observed a negative association between number of comorbidities and COVID-19 outcomes,6 , 7 , 9 few have investigated specific comorbidities as we included in our analysis.
In previous studies among patients without cancer, low ALC, low platelets, and abnormal CRP and creatinine were identified among laboratory values associated with severe COVID-19.30 , 31 Data among patients with cancer are limited, although prior studies suggested that abnormal CRP, LDH, and low ALC were associated with worse COVID-19 outcomes.10 , 17 Our study included a broader range of routinely collected laboratory measurements and identified new parameters associated with higher COVID-19 severity. However, we did not collect laboratory values now recognized to be associated with COVID-19 severity (e.g. ferritin32 and procalcitonin33); future efforts will include automated extraction of these and longitudinal values directly from electronic health records.
Receipt of cytotoxic chemotherapy was associated with higher COVID-19 severity and 30-day mortality. However, there is substantial variability of anticancer regimens, such that no one regimen containing cytotoxics was received by >31 patients (Figure 2). Some regimens may be subject to unmeasured confounding, for example, extent of lung involvement in patients with lung cancer receiving platinum doublets. It was very concerning to note the high mortality among those receiving R-CHOP, especially because most received it with curative intent. While grade 5 toxicities with R-CHOP may occur,34 a mortality rate >40% is very high. Although the exact etiology remains unclear, this regimen is broadly immunosuppressive. In addition to B-cell lymphodepleting effects, rituximab is known to alter the T-cell compartment, which may contribute to cytokine storm.13 , 35 On the contrary, the finding of relatively lower mortality among patients with multiple myeloma receiving daratumumab + IMiD + corticosteroid seems paradoxical given the high risk of infection in this patient population. Interestingly, inhibition of the CD38 pathway may reduce the inflammatory response.36 This relatively favorable prognosis is supported by several studies.37, 38, 39
Notably, immunotherapy alone was not associated with higher COVID-19 severity. This is in contrast to an earlier report in lung cancer,40 which was subsequently disproven after adjustment for smoking status from the same group.41 This finding is encouraging as immuno-therapeutics (specifically, immune checkpoint inhibitors) are the most prescribed regimen in our cohort and >40% of patients with advanced cancer may be eligible for immunotherapy.42
Similarly, endocrine therapy was not associated with higher COVID-19 severity, after adjustment for cancer status. There is a hypothetical possibility that antiandrogens could downregulate TMPRSS2 in the lung, limiting SARS-CoV-2 infection.13 , 43 Further investigation is needed.
The pandemic has substantially changed oncology practice in many deleterious ways, which may worsen cancer-related outcomes.13 , 44 Since the beginning, clinicians have attempted to balance the risks and benefits of cancer therapy by developing consensus-based algorithms to assist decision making45, 46, 47, 48; our data could further guide the optimization and refinement of those algorithms. Our finding of lower COVID-19 severity later in the pandemic may also suggest an overall improvement in COVID-19 care. Alternative explanations for this finding include that certain areas may have been overwhelmed earlier in the pandemic and that patients prone to severe disease and death, particularly those in skilled nursing facilities, may have been infected early. Notably, only 9% of included patients were diagnosed with COVID-19 during September-November, so that the observed improvement in outcomes should not be extrapolated to the surge in November-December 2020. Ultimately, an individualized risk–benefit discussion is critical when choosing systemic treatment, balancing carefully risks of cancer progression, associated risk of anticancer therapy, and COVID-19 severity.
While the majority of prior studies have suggested poor outcomes for patients with cancer and COVID-19, a recent study using a case-matched study design found patients with cancer and COVID-19 had similar outcomes to those without cancer, when matched by age, sex, and comorbidities.49 However, this study was limited to hospitalized patients in Manhattan, whereas our cohort includes any patient with cancer and COVID-19 and is diverse in multi-institutional representation.
Notable strengths of our study include detailed and granular information directly collected by health care professionals on a large and geographically diverse patient population with comprehensive follow-up. The novel ordinal scale of COVID-19 severity extends our previous research beyond 30-day mortality to capture other relevant complications of COVID-19 disease, and is consistent with newly recommended analytical approaches.50 The analysis of anticancer therapy elucidated specific regimens associated with increased mortality, which warrants detailed exploration.
Our study has several limitations, including those inherent to a retrospective, observational cohort study. Despite a robust data quality assurance system, survey-based data collection (voluntary, uncompensated) across multiple sites may result in selection biases, reporting errors, missing, and unknown data; the potential impact of these is mitigated by exclusion of low-quality reports and multiple imputation. Our results, particularly those for COVID-19 treatments, may be subject to confounding by indication and severity.16 Baseline laboratory measurements prior to COVID-19 diagnosis, which have been suggested to be associated with COVID-19 outcomes,51 were not collected due to the time-intensive nature of manually recording laboratories; automated data pulls from electronic health records may address this limitation in the future. Fixed dates are not captured due to the deidentified nature of the protocol; therefore time intervals are approximated at varying levels of granularity. We did not pursue subset analysis within individual cancer types, which is an area of future research.
In conclusion, we confirmed high COVID-19 severity and mortality among patients with cancer, in particular for those of older age, male sex, non-Hispanic non-white race/ethnicity, worse ECOG PS, hematologic malignancy, and select laboratory measurements. Certain chemotherapy regimens were associated with high all-cause mortality. These findings can inform novel translational research, clinical trial designs, and clinical decision making for patients with cancer and COVID-19. Future planned work from CCC19 includes further investigation into health care disparities, outcomes for specific cancer subtypes, and impact of particular anticancer therapies.
Acknowledgements
We thank Jake R. Conway for his helpful modifications to the UpSetR package.
Funding
REDCap is developed and supported by Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH). This study was partly supported by grants from the American Cancer Society and Hope Foundation for Cancer Research (No. MRSG-16-152-01-CCE to DPS); the National Cancer Institute [grant numbers P30 CA068485 to BF, CH, C-YH, BIR, JLW, SM, and YS; U01 CA231840 to JLW; T32CA009515 to ARK; T32CA236621 and P30 CA046592 to CRF; P30 CA177558 to EBD, 2UG1CA189859-06 and 2P30 CA 013330-47 to BH; 5P30CA056036-20 to BB; P30 CA013330 to AKV; P30CA013696 to JEH and GKS; P30 CA196521 to DBD and MDG; P30CA054174 to DPS, MS, and RAM; 1P30CA240139-01 to GdLL; P30CA015704 to GHL and PG; P30CA023100 to RRM; P30CA046934 to DWB and MZA]; National Center for Advancing Translational Sciences of the National Institutes of Health [grant numbers 2UL1TR001425-05A1 to TMW-D; P30 CA168524 to EW-B; 2UG1CA190140-06 to MAT; P30 CA016058 to DGS; UG1 CA189974 to GHL; UL1TR001873 to JEH]; DCV is supported by the Fonds de la récherche en santé du Québec Clinician-Scientist scholar's program (no grant number). MZA is supported by the Merck Investigator Studies Program (no grant number). SG acknowledges an award from North American Thrombosis Forum (NATF; no grant number). LZ is supported by the Spanish Society of Medical Oncology SEOM grant. VSK is supported by a grant from the Prostate Cancer Foundation (no grant number).
Disclosure
JDA reports research funding to the institution from Tesaro, outside the submitted work. ZB reports nonfinancial support from Bristol Myers Squibb and grants from Genentech/imCORE, outside the submitted work. BB reports research funding to the institution from Boehringer Ingelheim, Bicycle Therapeutics, Syros Pharmaceuticals, and Ikena Oncology, all outside the submitted work. TB-S reports research funding to the institution from Agios, Arys, Boston Biomedical, Bayer, Amgen, Merck, Celgene, Lilly, Ipsen, Clovis, Seattle Genetics, Array Biopharma, Genentech, Novartis, Mirati, Merus, AbGenomics, Incyte, Pfizer, BMS; consulting (to institution) for Ipsen, Array Biopharma, Pfizer, Seattle Genetics, Bayer, Genentech, Incyte, and Merck; consulting (to self) for AbbVie, Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo, Natera, Treos Bio, Celularity, Exact Science, Sobi, BeiGene, Xilis, Astra Zeneca, and Foundation Medicine; serving on Independent Data Monitoring Committee/Data and Safety Monitoring Board (to self) for AstraZeneca, Exelixis, Lilly, PanCAN, and 1Globe; positions on Scientific Advisory Board for Imugene, Immuneering, and Sun Biopharma; and inventions/patents (WO/2018/183488 and WO/2019/055687), all outside the submitted work. SB reports being on advisory boards for Bristol Meyers Squibb and Seattle Genetics. MAB reports personal fees from Exelixis, Bristol-Myers Squibb, Bayer, Eisai, Pfizer, AstraZeneca, Janssen, Genomic Health, Nektar, and Sanofi; grants from Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peloton Therapeutics, and Pfizer, outside the submitted work. NB reports honoraria from Novartis, Pfizer, Roche, and Lilly, outside the submitted work. DWB reports research funding to the institution from Exelixis, Ayala, Merck, and Elevar, all outside the submitted work. DDC declares consulting or advisory role with Exelixis, outside the submitted work. TKC reports institutional and personal research support from Alexion, Analysis Group, AstraZeneca, Aveo, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Calithera, Cerulean, Corvus, Eisai, Exelixis, F. Hoffmann-La Roche, Foundation Medicine Inc., Genentech, GlaxoSmithKline, Ipsen, Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Roche, Roche Products Limited, Sanofi/Aventis, Takeda, Tracon; consulting/honoraria or advisory role with Alexion, Analysis Group, AstraZeneca, Aveo, Bayer, Bristol Myers-Squibb/ER Squibb and sons LLC, Cerulean, Corvus, Eisai, EMD Serono, Exelixis, Foundation Medicine Inc., Genentech, GlaxoSmithKline, Heron Therapeutics, Infinity Pharma, Ipsen, Jansen Oncology, IQVIA, Lilly, Merck, NCCN, Novartis, Peloton, Pfizer, Pionyr, Prometheus Labs, Roche, Sanofi/Aventis, Surface Oncology, Tempest, Up-to-Date; CME-related events (e.g. OncLive, PVI, MJH Life Sciences); stock ownership in Pionyr, Tempest; patents filed, royalties, or other intellectual properties related to biomarkers of immune checkpoint blockers; fees for travel, accommodations, expenses, medical writing in relation to consulting, advisory roles, or honoraria; and no speaker's bureau; also supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE and Program, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at DFCI. DBD reports consulting for Ipsen, Boehringer Ingelheim; ASCO Young Investigator Award from Conquer Cancer Foundation, outside the submitted work. AE reports grant support from AstraZeneca, outside the submitted work. DF reports research funding to the institution from Viracor-Eurofins and Astellas, all outside the submitted work. LAF reports clinical trial funding to the institution from BMS, EMD Serono, Pfizer, Merck KGaA, Array, Kartos, Merck, and Incyte, ECOG-ACRIN study funding from Array; and personal fees from Elsevier and Via Oncology, outside the submitted work. DBF reports honoraria from Castle Biosciences. SMG reports Honoraria from AstraZeneca, Merck, Genentech/Roche; consulting or advisory role with Genentech/Roche, AstraZeneca, Bristol-Myers Squibb, Takeda, Xcovery, Boehringer Ingelheim, Novocure, Daiichi Sankyo, Novartis, Jazz Pharmaceuticals, Blueprint Medicines, Eli Lilly, Pfizer, Janssen Oncology; research funding (to self) from Merck, AstraZeneca; research funding (to institution) from Genentech/Roche, Merck, Blueprint Medicines, ARIAD/Takeda, Astellas Pharma, Lycera, Daiichi Sankyo, IMAB, Nektar, AstraZeneca, Pfizer, Amgen; travel, accommodations, expenses from Genentech/Roche, Merck; and other relationship from AstraZeneca, all outside the submitted work. MDG reports personal fees from Genentech, Pfizer, Astra Zeneca, Merck, Bristol Myers Squib, Dragonfly, Dracen, Seattle Genetics, and Astellas, outside the submitted work. PG reports consulting fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Dyania Health, Driver, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, Genzyme, GlaxoSmithKline, Heron Therapeutics, Immunomedics, Infinity Pharmaceuticals, Janssen, Merck, Mirati Therapeutics, Pfizer, Seattle Genetics, QED Therapeutics; research funding to institution from Merck, Mirati Therapeutics, Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Debiopharm, Bristol-Myers Squibb, QED Therapeutics, GlaxoSmithKline, and Kure It Cancer Research, all outside the submitted work. SG reports research funding to the institution from AstraZeneca and consulting/advisory role with Puma Biotechnology. SG reports consultancy fees from BMS, Merck, AstraZeneca, Seattle Genetics, Pfizer; and speaker fees from Seattle Genetics and Janssen, all outside the submitted work. TRH reports consulting or advisory role with Curium, ScioScientific, TERUMO, Lexicon, Ipsen, Advanced Accelerator; research funding from Ipsen, ArQule, Agios, Thermo Fisher Scientific, Basilea. BH reports research funding to the institution from Amgen, AbbVie, BI, Mirati, Merck, Eli-Lilly, AstraZeneca, BMS, Novartis, GSK, Pfizer, Advaxis, and Guardant Health; consulting/advisory role with Merck, BMS, Genentech, AstraZeneca, Amgen, Novartis, TPT, VI, Guardant Health; and honoraria from PER and OncLive, all outside the submitted work. JEH reports research funding from Regeneron and Dendreon; and travel, accommodations, and expenses from Genzyme. CH reports funding from the Henry Ford Cancer Institute supporting the current work; research funding to institution from Merck, Exelixis, Bayer, AstraZeneca, Genentech, Dendreon and Bausch; personal fees from Sanofi/Genzyme, Dendreon, Exelixis, Bristol Myers Squibb, Astellas, Medivation, Bayer, and Janssen Scientific, all outside the submitted work; and stock ownership by an immediate family member in Johnson and Johnson. DBJ reports advisory board participation for Array Biopharma, BMS, Catalyst Biopharma, Iovance, Jansen, Merck, Novartis, and OncoSec, and receives research funding from BMS and Incyte, all outside the submitted work. AK reports support to his institution from TESARO, Halozyme, Geistlich Pharma, Astellas Pharma, and Rafael Pharmaceuticals; and honoraria from OncLive, outside the submitted work. ARK (or an immediate family member) has currently or during the past 2 years owned stock or held an ownership interest in Merck, Sanofi, and BMS. VSK reports personal fees from Pfizer, Janssen, Dendreon, AstraZeneca, Seattle Genetics, and Clovis; grants (for institution) from Nektar, Novartis/Endocyte, Janssen, Clovis, and Prostate Cancer Foundation, all outside the submitted work. NMK reports personal fees from G1 Therapeutics, Invitae, Beyond Spring, Spectrum, BMS, Janssen, and Total Health, all outside the submitted work. PEL reports consulting/advisory role with Pfizer, Merck, Teva, BI, and Astra Zeneca, all outside the submitted work. AL-B reports personal fees from PSI CRO, Bayer, Blueprint, Astra-Zeneca, Medidata, Taiho, QED, Cardinal Health, BrightInsight, The Lynx Group, Boston Biomedical, Amgen, Bayer, Guardant, Natera, Eisai, Ipsen, and Merck; and stock options from Massive Bio, outside the submitted work. GdLL reports honoraria from Boehringer Ingelheim; consulting or advisory role for Pfizer and AstraZeneca; research funding from AstraZeneca; funding to his institution from Merck Sharp & Dohme, EMD Serono, AstraZeneca, Blueprint Medicines, Tesaro, Bavarian Nordic, NOVARTIS, G1 Therapeutics, Adaptimmune, BMS, GSK, AbbVie, Rgenix, Pfizer, Roche, Genentech, Lilly, and Janssen; travel, accommodations, and expenses from Boehringer Ingelheim, Pfizer, E.R. Squibb Sons, LLC, Janssen. GHL reports grants from AMGEN (institution); personal fees from G1 Therapeutics, TEVA, Samsung Bioepis, Beyond Spring, and Merck, outside the submitted work. RRM reports research funding from Bayer, Pfizer, Tempus; serves on Advisory Board for AstraZeneca, Bayer, Bristol Myers Squibb, Calithera, Exelixis, Janssen, Merck, Novartis, Pfizer, Sanofi, Tempus; is a consultant for Dendreon, Vividion; and serves on the molecular tumor board at Caris. RAM grants from Incyte, CTI, AbbVie, and Celgene; personal fees from Novartis, Genentech, Sierra Oncology, La Jolla, and Samus, outside the submitted work. VM has currently or during the past 2 years employment and stock or other ownership interest with Johnson & Johnson, all outside the submitted work. GN reports research funding to the institution from Novartis, all outside the submitted work. JN reports personal fees from AstraZeneca, Clovis Oncology; all outside the submitted work. CAP (or an immediate family member) has currently or during the past 2 years owned stock or held an ownership interest in Pfizer, Epizyme, Inovio, OPKO Health Inc, Roche. JMP reports grant from Dana-Farber/Harvard Cancer Center Breast SPORE Program, outside the submitted work. PP reports receiving payment for speakers' bureau from Novartis, Daichi Sankyo, Genentech, Seattle Genetics, and Pfizer, all outside the submitted work. NAP reports personal fees from Eli Lilly, Merck, BMS, Genentech, AstraZeneca, Inivata, and Regeneron, outside the submitted work. SP reports personal fees from AbbVie, Amgen, AstraZeneca, Bayer, Biocartis, Boehringer-Ingelheim, Bristol-Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F. Hoffmann-La Roche, Foundation Medicine, Illumina, Janssen, Merck Sharp and Dohme, Merck Serono, Merrimack, Novartis, Pharma Mar, Pfizer, Regeneron, Sanofi, Seattle Genetics and Takeda, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, Merck Sharp and Dohme, Novartis, Pfizer, and Takeda; nonfinancial support from Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Meyers Squibb, Clovis, F. Hoffmann-La Roche, Illumina, Merck Sharp and Dohme, Merck Serono, Novartis, Pfizer, and Sanofi; and personal fees from BioInvent (all fees to institution), outside the submitted work. DYR reports consulting/advisory role with and coverage of travel/accommodation expenses by Castle Biosciences, all outside the submitted work. BIR reports grants, personal fees, and nonfinancial support from Merck; grants and personal fees from BMS, Pfizer, Aveo, and Genentech; grants from Astra Zeneca; personal fees from Synthorx, 3D Medicines, Aravive, Surface Oncology, and Arrowhead Therapeutics; and other from PTC Therapeutics, outside the submitted work. RPR reports research grants to her institution from BMS and Janssen and has worked as a consultant/advisor and received honoraria from BMS and Janssen, all of which are outside the scope of submitted work. ALS reports travel support provided by Pfizer and Astellas. GKS reports personal fees from Apexigen, Array, Epizyme, GenCirq, Daiichi Sankyo, Fortress, Iovance Biotherapeutics, Bayer Pharmaceuticals, Pfizer Oncology, Array Advisory Board, Oncogenuity, Puretech, PTC Therapeutics, Ellipses Pharma, Concarlo; advisory board for Bionaut; grants from Astex; stock ownership in Pfizer, all outside the submitted work. SS reports stock and other ownership interests in Grand Rounds, Janssen, and Natera. YS reports honoraria from Boehringer Ingelheim, AstraZeneca, Novartis, and Eisai; consulting or advisory role with Pfizer, AstraZeneca, Novartis, Roche, Genentech, and Janssen, all outside the submitted work. MAT reports travel support from Syapse, Royalties from UpToDate, Connect MDS/AML Registry in Celgene (now owned by BMS), Myeloma Registry in Takeda; stock ownership in Doximity; personal fees from VIA Oncology (now owned by Elsevier ClinicalPath), Adaptive Advisory Board, and GSK; he is the local PI for Clinical Trials in AbbVie, BMS, CRAB CTC, Denovo, Research Network, Eli Lilly, LynxBio, Strata Oncology, and TG Therapeutics, all outside the submitted work. AKV reports research funding to the institution from BMS, MedPacto, Prelude, iOnctura, and Janssen; honoraria from Acceleron and Novartis; consulting/advisory role with Stelexis and Janssen; stock or other ownership in Stelexis; and an immediate family member with employment/leadership with CereXis, all outside the submitted work. DCV reports honoraria and speakers' bureau fees from CSL Behring, Merck Canada, Novartis Canada, Takeda, and UCB Biosciences GmbH, and travel accommodations from CSL Behring, and Avir Pharma, all outside the submitted work. He is supported by the Fonds de la recherche en santé du Québec (FRQS) Clinician-Scientist Junior 2 program. JLW reports grants from the National Cancer Institute during the conduct of the study; personal fees from Westat and IBM Watson Health; and other from HemOnc.org LLC, outside the submitted work. TMW-D reports stock and other ownership interests in High Enroll; honoraria from Physicians' Education Resource; consulting or advisory roles with Shattuck Labs, Rakuten Medical, Exicure; research funding from Merck, AstraZeneca/MedImmune, Bristol-Myers Squibb, GlaxoSmithKline, Caris Life Sciences, GlaxoSmithKline; travel, accommodations, expenses from Merck, Bristol-Myers Squibb, Bexion, AstraZeneca/MedImmune, Caris Life Sciences, Lilly, and Tesaro, all outside the submitted work. EW-B reports work in a consultant/advisor role for Astellas and BMS; funding support from Pfizer Global Medical Grants; other for Exelixis; and an immediate family member with stock ownership in Immunomedics and Nektar, all outside the submitted work. TZ reports research funding (to Duke) from Pfizer, Janssen, Acerta, AbbVie, Novartis, Merrimack, OmniSeq, PGDx, Merck, Mirati, Astellas, and Regeneron; consulting/speaking role with Genentech Roche, Exelixis, Genomic Health, and Sanofi Aventis; and serves on the consulting/advisory board for AstraZeneca, Bayer, Pfizer, Foundation Medicine, Janssen, Amgen, BMS, Calithera, Dendreon, and MJH Associates; stock ownership/employment (spouse) from Capio Biosciences, Archimmune Therapeutics, and Nanorobotics. AYZ has currently or during the past 2 years owned stock or held an ownership interest in Gilead Sciences. LZ reports personal fees from MERCK, outside the submitted work. All others have declared no conflicts of interest.
Footnotes
Note: An earlier version of this study was presented as two oral proffered papers (LBA71 and LBA72) at the 2020 ESMO Annual Meeting.
Supplementary data
References
- 1.World Health Organization (WHO) WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/table Available at: Accessed November 24, 2020.
- 2.Johns Hopkins Coronavirus Resource Center https://coronavirus.jhu.edu/ Available at: Accessed December 9, 2020.
- 3.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q., Berger N.A., Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garassino M.C., Whisenant J.G., Huang L.-C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L.Y., Cazier J.-B., Angelis V. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinato D.J., Zambelli A., Aguilar-Company J. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Discov. 2020;10(10):1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian J., Yuan X., Xiao J. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai M., Liu D., Liu M. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannakoulis V.G., Papoutsi E., Siempos I.I. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakouny Z., Hawley J.E., Choueiri T.K. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38(5):629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Estimating mortality from COVID-19. https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19 Available at: Accessed December 9, 2020.
- 15.Desai A., Warner J., Kuderer N. Crowdsourcing a crisis response for COVID-19 in oncology. Nat Cancer. 2020;1(5):473–476. doi: 10.1038/s43018-020-0065-z. [DOI] [PubMed] [Google Scholar]
- 16.Rivera D.R., Peters S., Panagiotou O.A. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discov. 2020;10(10):1514–1527. doi: 10.1158/2159-8290.CD-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albiges L., Foulon S., Bayle A. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat Cancer. 2020;1(10):965–975. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COVID-19 and Cancer Consortium A systematic framework to rapidly obtain data on patients with cancer and COVID-19: CCC19 governance, protocol, and quality assurance. Cancer Cell. 2020;38:761–766. doi: 10.1016/j.ccell.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker S.H., Duncan D.B. Estimation of the probability of an event as a function of several independent variables. Biometrika. 1967;54(1):167–179. [PubMed] [Google Scholar]
- 22.Harrell F.E., Lee K.L., Pollock B.G. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 23.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 24.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 25.Lex A., Gehlenborg N., Strobelt H., Vuillemot R., Pfister H. UpSet: visualization of intersecting sets. IEEE Trans Vis Comput Graph. 2014;20(12):1983–1992. doi: 10.1109/TVCG.2014.2346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conway J.R., Lex A., Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinforma Oxf Engl. 2017;33(18):2938–2940. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai A., Khaki A.R., Kuderer N.M. Use of real-world electronic health records to estimate risk, risk factors, and disparities for COVID-19 in patients with cancer. JAMA Oncol. 2021;7:227–229. doi: 10.1001/jamaoncol.2020.5461. [DOI] [PubMed] [Google Scholar]
- 29.Mackey K., Ayers C.K., Kondo K.K. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynants L., Calster B.V., Bonten M.M.J. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izcovich A., Ragusa M.A., Tortosa F. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11):e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng L., Li H., Li L. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34(10):e23618. doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coiffier B., Lepage E., Briere J. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 35.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glaría E., Valledor A.F. Roles of CD38 in the immune response to infection. Cells. 2020;9(1):228. doi: 10.3390/cells9010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munshi N.C., Anderson K.C. Don't compromise myeloma care due to COVID-19 pandemic! Blood Cancer Discov. 2020;1(3):218–220. doi: 10.1158/2643-3230.BCD-20-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chari A., Samur M.K., Martinez-Lopez J. Clinical features associated with COVID-19 outcome in MM: first results from International Myeloma Society Dataset. Blood. 2020;136:3033–3040. doi: 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hultcrantz M., Richter J., Rosenbaum C.A. COVID-19 infections and clinical outcomes in patients with multiple myeloma in New York City: a cohort study from five academic centers. Blood Cancer Discov. 2020;1(3):234–243. doi: 10.1158/2643-3230.BCD-20-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robilotti E.V., Babady N.E., Mead P.A. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy Drugs. JAMA Netw Open. 2019;2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montopoli M., Zumerle S., Vettor R. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai A.G., Pasea L., Banerjee A. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. doi: 10.1136/bmjopen-2020-043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17(5):268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeBoer R.J., Fadelu T.A., Shulman L.N., Van Loon K. Applying lessons learned from low-resource settings to prioritize cancer care in a pandemic. JAMA Oncol. 2020;6(9):1429–1433. doi: 10.1001/jamaoncol.2020.2976. [DOI] [PubMed] [Google Scholar]
- 47.Garrett-Mayer E., Rini B.I. To treat or not to treat-balancing benefits and risks of treatment delay among patients with cancer during the COVID-19 pandemic. JAMA Oncol. 2020;6:1868–1869. doi: 10.1001/jamaoncol.2020.4886. [DOI] [PubMed] [Google Scholar]
- 48.Pennell N.A., Dillmon M., Levit L.A. American Society of Clinical Oncology road to recovery report: learning from the COVID-19 experience to improve clinical research and cancer care. J Clin Oncol. 2021;39:155–169. doi: 10.1200/JCO.20.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brar G., Pinheiro L.C., Shusterman M. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol. 2020;38(33):3914–3924. doi: 10.1200/JCO.20.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall J.C., Murthy S., Diaz J. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jee J., Foote M.B., Lumish M. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38(30):3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.