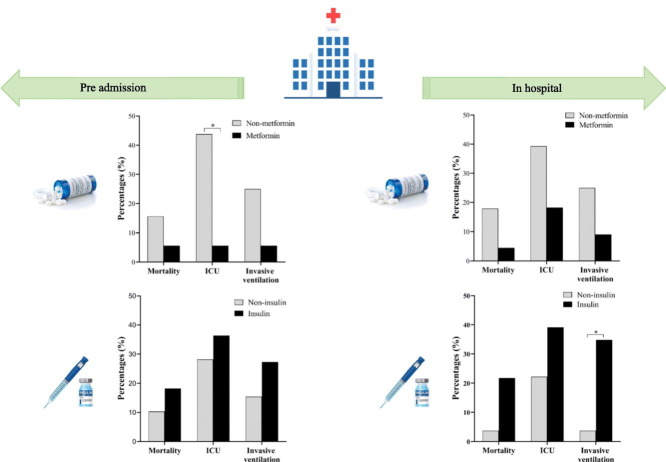
Abstract
Aims
Type 2 diabetes is considered to be one of the essential risks of adverse outcomes in coronavirus disease 2019 (COVID-19).1 Metformin and insulin were suggested to affect the outcomes. However, divergent views are still expressed. We aim to gain further insight into metformin and insulin in both pre-admission and in-hospital usage in COVID-19 patients with pre-existed type 2 diabetes.
Main methods
This is a multicentral retrospective study of the hospital confirmed COVID-19 patients between January 19 to April 09, 2020, who admitted to 3 main hospitals in Xiangyang city, China. The effect of type 2 diabetes, metformin, and insulin on COVID-19 were analyzed, respectively. Clinical characteristics, blood laboratory indices, clinical observational indices, and outcomes of these cases were collected.
Key findings
A total of 407 confirmed COVID-19 patients (including 50 pre-existed type 2 diabetes) were eligible in our study. COVID-19 patients with type 2 diabetes had more adverse outcomes than non-diabetes (OR2: mortality: 1.46 [95% CI3 1.11, 1.93]; P < 0.001). Pre-admission metformin usage showed a declined intensive care unit admission rate in a dose-dependent fashion (OR 0.04 [95% CI 0.00, 0.99]; adjust P = 0.049). While in-hospital insulin usage attempted to increase the invasive ventilation (8 [34.8%] vs. 1 [3.7%], adjust P = 0.043), independent of age and blood glucose.
Significance
Our study indicated that pre-admitted metformin usage may have beneficial effects on COVID-19 with pre-existed type 2 diabetes, insulin should be used sparingly in the hospital stay.
Abbreviations: ACE2, angiotensin converting enzyme 2; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, creatine kinase-myocardial band; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CI, confidence intervals; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IQR, interquartile range; LDH, lactic dehydrogenase; LOS, length of stay; MYO, myoglobin; OR, odds ratio; PCT, procalcitonin; r-GGT, r-gamma glutamyl transferase; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; T2DM, type 2 diabetes mellitus
Keywords: COVID-19, Type 2 diabetes, Metformin, Insulin
Graphical abstract
1. Introduction
To date, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has created a cosmopolitan pandemic. As of 2 March 2021, over 113 million COVID-19 cases and 2.5 million death have been reported globally by the World Health Organization since the start of the pandemic [1]. The numbers of confirmed infections and death cases are consistently increasing due to the lack of specific medicine for SARS-CoV-2 infection. Although the current large-scale vaccine production has brought dawn to mankind, the globalization of vaccination [2] and virus mutation [3] are still challenging to eliminate SARS-CoV-2.
Among them, patients with type 2 diabetes mellitus (T2DM) facing on the higher risks of adverse outcomes on COVID-19 than non-diabetes [4]–[6]. Besides, diabetes has been suggested as the risk factor of structural and pulmonary ventilation changes in the lungs in severe COVID-19 patients [7]. The antidiabetic medicine treatment regimen may have impacted the progression [8,9]. Metformin and insulin are the most commonly used antidiabetic medicine. Theoretical evidence has determined that metformin might have a beneficial on antivirus through reducing the proinflammatory, profibrotic states [10,11] and improve the immune response [12]. Insulin treatment may have the potential risk of poor prognosis with an increase in renal angiotensin converting enzyme 2 (ACE2) expression in mice with diabetes [13]. Several clinical studies have assessed the impact of metformin and insulin, whereas, their opinions were somehow discrepant [14]–[17]. The divergence of views may be attributed to the disparity objects, which was either in-history or in-hospital drug usage rather than both.
Our aims of this study were to carry out a retrospective analysis of the effect of metformin/insulin in COVID-19 patients with T2DM in both pre-admission and in-hospital usage. We considered the discharge or death as the primary endpoint; poor prognosis — either intensive care unit (ICU) admission or invasive ventilation — as the secondary study endpoint, to validate the role of T2DM in COVID-19 and to gain an insight into metformin and insulin treatment in COVID-19.
2. Materials and methods
2.1. Subjects
Other than Wuhan, Xiangyang city is another central traffic-hub-city in Hubei province, China, which led to the severe influence of SARS-CoV-2. The hospital confirmed patients with COVID-19 who were admitted from January 19 to April 09, 2020, were collected. To reduce the potential sources of bias, the confirmed patients were collected from Xiangyang No.1 People's Hospital, Xiangyang Central Hospital, and Xiangyang Xiangzhou People's Hospital, which are the three main hospitals for receiving and treating COVID-19 patients, and admitted in approximately half of the confirmed COVID-19 patients in Xiangyang city.
2.2. Study design
In-hospital confirmed COVID-19 patients were divided into T2DM and non-diabetes, T2DM defined by fasting venous or capillary glucose ≥ 7.0 mmol/L (≥126 mg/dL); two-hour postprandial venous plasma glucose or random plasma glucose ≥ 11.1 mmol/L (≥200 mg/dL) according to the World Health Organization diagnostic criteria [18].
Patients with pre-existed T2DM who historically had metformin till admission were included in the pre-admission metformin group. Patients who received metformin alone or with other antidiabetic medications after admission were considered into the hospital metformin group. The definitions of pre-admission and in-hospital insulin group were consistent with metformin. Due to the lack of the data of HbA1c, we use the median of random blood glucose levels, which were tested within 24 h of admission, to assess the preadmission blood glucose control status.
The baseline was defined by the time when patients were admitted to the hospital. For the endpoint, we considered the discharge or death as the primary endpoint, and poor prognosis as the secondary study endpoint, including ICU admission and invasive ventilation.
The study protocol was approved by the local ethics committee (Ethics Committee of Xiangyang No. 1 People's Hospital Affiliated to Hubei University of Medicine, 2020KY002), and waived informed consent from study participants because of the study's retrospective design. All clinical investigations were conducted in accordance with the guidelines of the Declaration of Helsinki.
2.3. Inclusion criteria
Referring to the diagnostic criteria by Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (6th) by the National Health Commission of China [19], confirmed COVID-19 patients were included in our study.
Confirmed cases were: a positive result of real-time reverse transcription-polymerase chain reaction detection of novel coronavirus nucleic acid in suspected cases, who having epidemiological history (having a history of contact with the Wuhan area or contact with the confirmed cases in the last 14 days), or clinical presentations (having fever and/or respiratory tract symptoms or having the novel coronavirus pneumonia features through chest computed tomography scan (CT)).
Confirmed patients were defined into four forms: mild form, moderate form, severe form, and critical form, in accordance with the Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (6th) [19].
2.4. Exclusion criteria
Patients were excluded who were re-detected positive, whose prognosis could not be tracked, patients with less data, or the hospital stay less than 3 days.
2.5. Criteria for hospital discharge
Patients considered for discharge need to achieve three criteria: 1) three or more than three days of normal body temperature with ameliorated respiratory symptoms, 2) substantially improved chest radiology of acute exudative lesions, 3) taken at least 24 h apart of two consecutive negative nucleic acid tests using respiratory tract samples.
2.6. Patient evaluation
Clinical characteristics, blood laboratory indices, chest CT images, clinical observational indices, and outcomes of these cases were collected. Blood laboratory indices included blood glucose, blood routine (white blood cells, lymphocytes, lymphocytes (%), monocytes, monocytes (%), neutrophils, neutrophils (%)), hepatic function (alanine aminotransferase (ALT), aspartate aminotransferase (AST), r-gamma-glutamyl transferase (r-GGT)), renal function (blood urea nitrogen (BUN), creatinine, urea), cardiac function (creatine kinase (CK), creatine kinase-myocardial band (CK-MB), lactate dehydrogenase (LDH), myoglobin (MYO), brain natriuretic peptide (BNP)), inflammation factors (C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), procalcitonin (PCT)), and coagulation function (d-dimer). SARS-CoV-2 infection virus nucleic acid test was taking respiratory specimens (including nasopharyngeal swabs, bronchoalveolar lavage, sputum, or bronchial aspiration). The severity of chest CT was judged by assessing the total lung severity score (hereinafter referred to as CT score), as shown in Table 1 .
Table 1.
Chest computed tomography score.
| Score | Unifocal | Multifocal |
|---|---|---|
| Peripheral distribution or unilateral involvement | 1 | 2 |
| Bilateral involvement | 2 | 4 |
2.7. Statistical analysis
The results are reported as the median (interquartile range [IQR]) for non-normal continuous variables and number [percentage] for categorical variables. χ2 test or the Fisher exact test, as appropriate, were performed in comparing categorical data, with Bonferroni correction in multiple comparisons. Mann-Whitney U test was performed in comparing non-normal continuous data. In all COVID-19 populations, univariable and multivariable logistic regression were performed to determine the odds ratios (OR) and 95% confidence intervals (CIs) for factors associated with clinical outcomes. To avoid bias, data less than half of the total cases in the cohort will not be performed in multiple regression and subgroup analysis. To perform the comorbidities status, considering each of the comorbidity was indicated a significant association with outcomes in univariable logistic regression, we included the numbers of comorbidities (T2DM, Hypertension, cardiovascular disease, chronic kidney disease) into the multivariable analysis, rather than each individual. Depending on the multivariable logistic regression in all patients, age and blood glucose were closely correlated with the outcomes, therefore, were chosen as confounders to adjust during the analysis of the T2DM cohort. The liner regression and logistic regression were performed in adjusting in continuous variables and categorical variables, respectively. P < 0.05 was considered statistically significant. Statistical analysis was carried out with SPSS (IBM Corp, Armonk, NY, USA), version 23.0.
3. Results
3.1. Demographics and characteristics of the study population
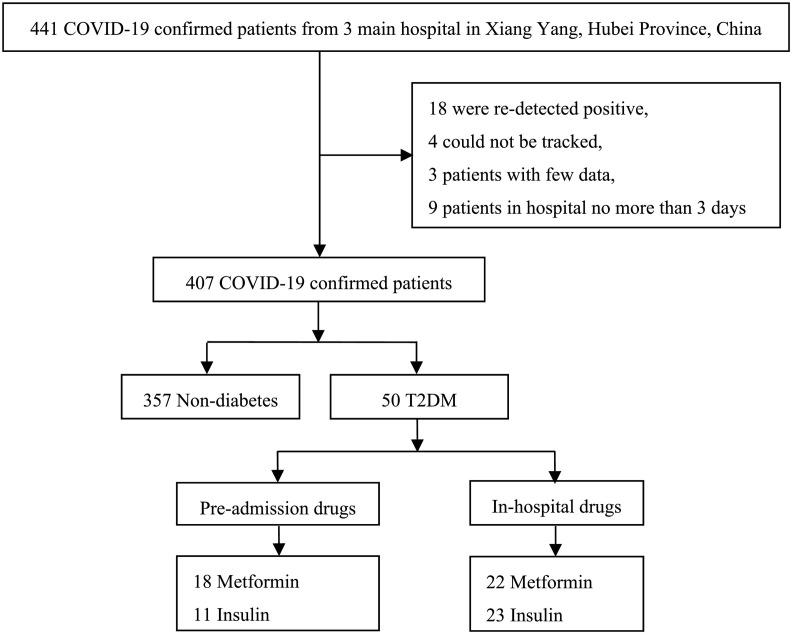
From January 19 to April 09, 2020, 441 cases were collected, among them, 34 were excluded for the following reasons: 18 patients who were re-detected positive, 4 patients whose prognosis could not be tracked, 3 patients with few data, and 9 patients in hospital stay no more than 3 days. Finally, 407 confirmed patients were eligible in our study (Fig. 1 ).
Fig. 1.
The flowchart of study procedure. COVID-19, coronavirus disease 2019; T2DM, type 2 diabetes mellitus.
Amount of the 407 confirmed patients, the median age was 48.0 years [IQR 36.0–58.0], 195 patients [47.9%] were male. The median body mass index (BMI) was 23.80 kg/m2 [IQR 22.04–25.82]. The distribution of comorbidities including: hypertension (65 [16.0%]) contributed the most, followed by pre-existed diabetes (50 [12.3%]), cardiovascular disease (21 [5.2%]) and chronic kidney diseases (18 [4.4%]). The proportion of diabetes was no different from the newest prevalence of diabetes ([12.3%] vs. [11.2%], P = 0.265, data not shown) recorded in mainland China [20]. Among these 50 patients with diabetes, 100% are T2DM. At hospital admission, the distribution of severity category was: 4.4% mild, 80.6% moderate, 4.9% severe, and 10.1% critical COVID-19 patients. The median in-hospital fever days and the median hospital length of stay (LOS) were 2.0 days [IQR 0–4.0] and 19.0 days [IQR 13.0–25.0], respectively. Among the patients, 13 [3.2%] died during hospitalization, 27 [6.6%] patients were admitted to ICU, and 15 [3.7%] had invasive ventilation (Table 2 ).
Table 2.
Characteristics, baseline laboratory indices, and clinical observational indices of patients with or without T2DM in COVID-19 cases.
| Normal range | No. of patients tested | All patients (N = 407) | Non-diabetes (N = 357) | T2DM (N = 50) | P-value | |
|---|---|---|---|---|---|---|
| Characteristics | ||||||
| Age (years) | – | 407 | 48.0 (36.0–58.0) | 47.0 (35.0–57.0) | 56.0 (45.8–64.2) | <0.001 |
| Male (n, %) | – | 407 | 195 (47.9) | 166 (46.5) | 29 (54.0) | 0.365 |
| BMI | 18.5–25.0 kg/m2 | 207 | 23.80 (22.04–25.82) | 23.51 (22.04–25.51) | 25.56 (22.68–26.92) | 0.040 |
| Comorbidities (n, %) | ||||||
| Hypertension | – | 407 | 65 (16.0) | 47 (13.2) | 18 (36.0) | <0.001 |
| Cardiovascular disease | – | 407 | 21 (5.2) | 15 (4.2) | 6 (12.0) | 0.032 |
| Chronic kidney disease | – | 407 | 18 (4.4) | 1 (0.3) | 17 (34.0) | <0.001 |
| Severity categories (n, %) | – | 407 | – | – | – | <0.001 |
| Mild | – | – | 18 (4.4) | 15 (4.2) | 3 (6.0) | – |
| Moderate | – | – | 328 (80.6) | 301 (84.0) | 27 (54.0)* | – |
| Severe | – | – | 20 (4.9) | 16 (4.5) | 4 (8.0) | – |
| Critical | – | – | 41 (10.1) | 25 (7.0) | 16 (32.0)* | – |
| Baseline laboratory indices and CT score | ||||||
| Blood glucose | 3.60–11.10 mmol/L | 344 | 5.21 (4.80–5.94) | 5.09 (4.76–5.55) | 9.66 (6.94–12.24) | <0.001 |
| White blood cells | 3.50–9.50 ∗ 109/L | 366 | 4.75 (3.76–6.04) | 4.53 (3.71–5.87) | 5.81 (4.63–6.49) | <0.001 |
| Lymphocytes | 1.10–3.20 ∗ 109/L | 359 | 1.26 (0.98–1.67) | 1.27 (0.99–1.67) | 1.25 (0.87–1.68) | 0.266 |
| Lymphocytes % | 20.0–50.0% | 369 | 28.2 (20.5–35.9) | 29.40 (21.70–36.30) | 23.00 (15.15–30.40) | 0.001 |
| Monocytes | 0.12–1.20 ∗ 109/L | 351 | 0.42 (0.30–0.53) | 0.42 (0.30–0.53) | 0.37 (0.29–0.52) | 0.617 |
| Monocytes % | 3.0–12.0% | 351 | 8.60 (6.40–10.80) | 8.80 (6.60–11.10) | 7.45 (5.33–9.63) | 0.002 |
| Neutrophils | 1.80–6.30 ∗ 109/L | 351 | 2.70 (2.10–4.09) | 2.89 (2.01–3.79) | 3.96 (2.64–5.15) | <0.001 |
| Neutrophils % | 40.0–75.0% | 352 | 61.40 (52.30–69.18) | 60.20 (51.20–68.30) | 67.30 (56.10–76.70) | <0.001 |
| ALT | 9.00–50.00 IU/L | 372 | 20.00 (14.00–29.98) | 19.00 (14.00–29.65) | 22.32 (16.25–31.95) | 0.119 |
| AST | 15.00–40.00 IU/L | 370 | 23.24 (18.82–31.62) | 23.00 (18.96–31.00) | 25.90 (16.25–33.36) | 0.607 |
| r-GGT | 10.00–60.00 IU/L | 370 | 24.06 (16.24–41.00) | 23.00 (16.00–38.00) | 38.13 (22.01–62.06) | <0.001 |
| BUN | 3.60–9.50 mmol/L | 367 | 4.10 (3.20–5.10) | 4.06 (3.20–4.91) | 5.15 (3.81–6.31) | <0.001 |
| Creatinine | 57.00–97.00 μmol/L | 369 | 60.90 (50.40–75.20) | 60.50 (50.60–73.89) | 65.00 (49.12–86.50) | 0.244 |
| Urea | 155.00–357.00 μmol/L | 332 | 269.50 (215.40–331.00) | 266.00 (213.60–325.00) | 312.00 (243.42–358.00) | 0.016 |
| CK | 40.00–200.00 U/L | 321 | 66.00 (45.00–106.00) | 65.46 (45.00–103.03) | 69.35 (40.25–133.00) | 0.345 |
| CK-MB | 0.00–25.00 U/L | 309 | 9.00 (5.18–14.00) | 9.00 (6.00–13.48) | 9.00 (4.61–17.00) | 0.920 |
| LDH | 120.00–250.00 U/L | 313 | 215.40 (180.00–270.40) | 212.35 (179.80–269.85) | 231.40 (186.30–321.00) | 0.168 |
| MYO | 0–154.90 ng/mL | 139 | 30.00 (11.90–34.40) | 28.35 (11.29–33.10) | 30.00 (13.56–45.77) | 0.252 |
| BNP | 0–100.00 pg/mL | 45 | 115.00 (54.80–229.80) | 107.50 (53.28–222.55) | 173.00 (131.00–1521.00) | 0.079 |
| CRP | 0–8.00 mg/L | 345 | 10.60 (4.47–29.35) | 9.71 (4.20–24.50) | 29.29 (5.00–50.30) | 0.007 |
| ESR | 0–15.00 mm/h | 300 | 20.50 (12.00–38.00) | 20.00 (12.00–34.00) | 39.00 (18.25–70.25) | <0.001 |
| PCT | <0.10 ng/mL | 238 | 0.10 (0.05–0.17) | 0.09 (0.05–0.17) | 0.10 (0.05–0.20) | 0.507 |
| D-dimer | 0–0.55 μg/mL | 337 | 0.20 (0.07–0.36) | 0.20 (0.09–0.35) | 0.14 (0.03–0.40) | 0.610 |
| Baseline CT score | 0–4 | 401 | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 0.910 |
| COVID-19 treatment protocols | ||||||
| Corticosteroid (n, %) | – | 394 | 93 (23.6) | 72 (20.9) | 21 (42.0) | 0.002 |
| Antivirals (n, %) | – | 397 | 368 (92.7) | 325 (93.7) | 43 (86.0) | 0.074 |
| Antibiotics (n, %) | – | 394 | 315 (79.9) | 277 (80.3) | 38 (77.6) | 0.703 |
| Clinical observational indices | ||||||
| Fever days (≥37.3 °C) | – | 256 | 2.0 (0–4.0) | 2.0 (0–4.0) | 2.0 (0–6.0) | 0.144 |
| Hospital LOS | – | 407 | 19.0 (13.0–25.0) | 19.0 (13.0–25.0) | 21.0 (15.0–29.3) | 0.094 |
| Outcomes | ||||||
| Mortality (n, %) | – | 407 | 13 (3.2) | 7 (2.0) | 6 (12.0) | 0.002 |
| Poor prognosis (n, %) | ||||||
| ICU admission | – | 407 | 27 (6.6) | 12 (3.4) | 15 (30.0) | <0.001 |
| Invasive ventilation | – | 407 | 15 (3.7) | 6 (1.7) | 9 (18.0) | <0.001 |
T2DM, type 2 diabetes mellitus; COVID-19, coronavirus disease 2019; BMI, body mass index; CT, computed tomography; ALT, alanine aminotransferase; AST, aspartate aminotransferase; r-GGT, r-gamma-glutamyl-transferase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; CK, creatine kinase, CK-MB, creatine kinase-myocardial band, MYO, myoglobin; BNP, brain natriuretic peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin; LOS, length of stay; ICU, intensive care unit. Data were n (%) or median (IQR). P-values were shown as χ2 test or Fisher's Exact Test (*: significant difference in multiple comparisons, corrected by Bonferroni) or Mann–Whitney U test. P < 0.05 was considered significant (bolded) between non-diabetes vs. T2DM.
At the time of admission, compared with non-diabetes cohort, T2DM cohort were older age (56.0 years [IQR 45.8–64.2] vs. 47.0 years [IQR 35.0–57.0], P < 0.001), with higher BMI (25.56 kg/m2 [IQR 22.68–26.92] vs. 23.51 kg/m2 [IQR 22.04–25.51], P = 0.040), greater proportion of comorbidities including hypertension (18 [36.0%] vs. 47 [13.2%], P < 0.001), cardiovascular disease (6 [12.0%] vs. 15 [4.2%], P = 0.032) and chronic kidney disease (17 [34.0%] vs. 1 [0.3%], P < 0.001). None of T2DM had acute diabetic complications including diabetic ketoacidosis, hyperglycemia hyperosmolar state, or hypoglycemia during hospital stay (data not shown). Besides, higher proportion of critical COVID-19 cases (16 [32.0%] vs. 25 [7.0%], P < 0.001) was seen in T2DM cohort. Proportion of gender had no difference between two groups (P > 0.05) (Table 2).
Regarding laboratory indices, higher blood glucose (9.66 mmol/L [IQR 6.94–12.24] vs. 5.09 mmol/L [IQR 4.76–5.55], P < 0.001) was seen in T2DM cohort. Besides, impaired immunity (decreased blood lymphocytes (%) and monocytes (%)), more deteriorated inflammation (increased white blood cells, neutrophils, neutrophils (%), CRP, ESR), exacerbated hepatic-renal function (increased r_GGT, BUN, urea levels) were observed in T2DM cohort (P < 0.05), as shown in Table 2.
For the clinical observational indices, even greater rate of corticosteroid was utilized in T2DM cohort (21 [42.0%] vs. 72 [20.9%], P = 0.002), no difference was seen in hospital fever days (2.0 days [IQR 0–6.0] vs. 2.0 days [IQR 0–4.0], P = 0.074) or hospital length of stay (LOS) (21.0 days [IQR 15.0–29.3] vs. 19.0 days [IQR 13.0–25.0], P = 0.094) between two groups. Notably, higher rate of mortality (6 [12.0%] vs. 7 [2.0%], P = 0.002), greater rate of ICU admissions (15 [30.0%] vs. 12 [3.4%], P < 0.001), and invasive ventilation (9 [18.0%] vs. 6 [1.7%], P < 0.001) were observed in T2DM cohort (Table 2).
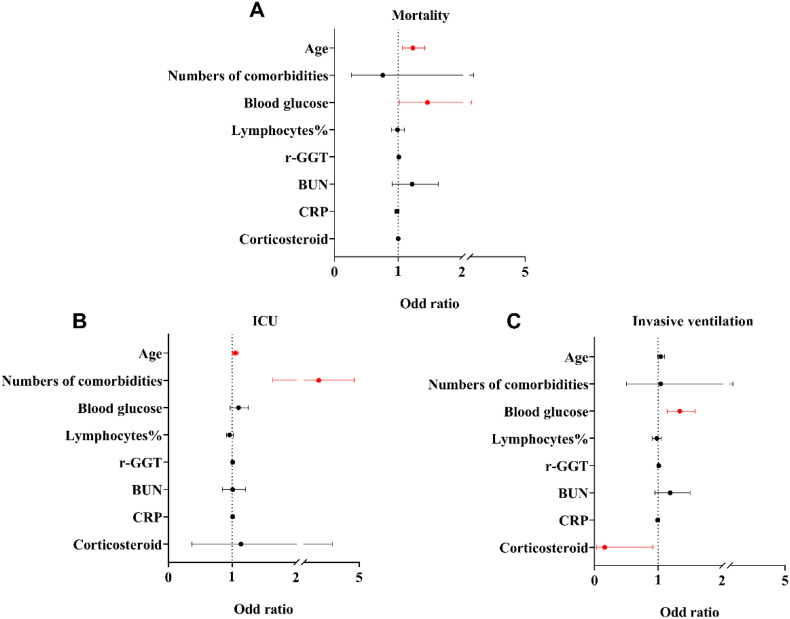
Based on the aforementioned significant difference parameters between groups, the numbers of comorbidities and further seven factors had chosen into multivariable analysis, which contains: age, blood glucose, lymphocytes%, r-GGT, BUN, CRP, and the use of corticosteroid (yes/no). In multivariable analysis, age and blood glucose were still significantly associated with mortality (age: OR 1.23 [95% CI 1.07, 1.42]; P = 0.003; blood glucose: OR 1.46 [95% CI 1.02, 2.07]; P = 0.037). Besides, regarding the poor prognosis, the age (OR 1,05 [95% CI 1.01, 1.09]; P = 0.022), numbers of comorbidities (OR 2.78 [95% CI 1.64, 4.72]; P < 0.001) associated with ICU admission. Whereas, blood glucose (OR 1.34 [95% CI 1.14, 1.58]; P < 0.001), use of corticosteroid (OR 0.16 [95% CI 0.03, 0.92]; P = 0.041) were associated with invasive ventilation (Fig. 2 ).
Fig. 2.
Multiple regression of hospital mortality (A), ICU admission (B), and invasive ventilation (C) with risk factors in COVID-19 patients. r-GGT, r-Gamma-glutamyl-transferase; BUN, blood urea nitrogen; CRP, C-reactive protein; ICU, intensive care unit. Data were odds ratios and 95% confidence intervals. Lines in red color mean P < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Pre-admission metformin or insulin usage
Before hospitalization, in T2DM cohort, 18 of 50 had metformin treatment. Patients with pre-admission metformin with a median dose of 1.0 g per day [IQR 0.89–1.22]. Compared with non-metformin users, patients who utilizing metformin were younger (48.0 years [IQR 40.5–56.5] vs. 58.0 years [IQR 49.5–66.5], P = 0.024). After adjusting the age and blood glucose, there was no difference in gender, BMI, or the number of other comorbidities (P > 0.05) between the two groups. In admitted laboratory indices, pre-admission metformin cases had higher lymphocytes (1.51 ∗ 109/L [IQR 1.12–1.95] vs. 1.08 ∗ 109/L [IQR 0.67–1.34], adjust P = 0.008), lymphocytes (%) (26.05% [IQR 21.50–35.80] vs. 21.30% [IQR 11.20–27.15], adjust P = 0.011) and lower neutrophil (%) (65.50% [IQR 63.90–68.70] vs. 67.90% [IQR 63.43–83.33], adjust P = 0.009), and lower LDH (218.00 U/L [IQR 153.15–235.70] vs. 244.10 U/L [IQR 198.25–349.85], adjust P = 0.020) than non-metformin. There were 11 of 50 had insulin treatment insulin users. Patients had pre-admission insulin with a median of 36.0 unit per day [IQR 28.7–48.2]. Whereas no difference of characteristics, laboratory indices, and COVID-19 treatment protocol were found with respect to non-insulin group. (Table 3 ).
Table 3.
Characteristics, baseline laboratory indices, clinical observational indices, and outcomes in pre-admission metformin or insulin users.
| No. of patients tested | Non-metformin (N = 32) | Metformin (N = 18) | P-value | Adjust P-value | Non-insulin (N = 39) | Insulin (N = 11) | P-value | Adjust P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Concentrations (per day) | – | – | 1.00 (0.89–1.22)a | NA | NA | – | 36.0 (28.7–48.2)b | NA | NA |
| Characteristics | |||||||||
| Age (years) | 50 | 58.0 (49.5–66.5) | 48.0 (40.5–56.5) | 0.024 | NA | 52.0 (44.0–65.0) | 58.0 (54.0–64.0) | 0.325 | NA |
| Gender (M, %) | 50 | 15 (46.9) | 12 (66.7) | 0.241 | 0.380 | 21 (53.8) | 6 (54.5) | 1.000 | 0.798 |
| BMI | 24 | 24.51 (21.30–28.04) | 25.87 (24.72–26.87) | 0.462 | 0.535 | 26.03 (23.78–27.18) | 24.80 (19.94–25.56) | 0.095 | 0.095 |
| Comorbidities (n, %) | |||||||||
| Hypertension | 50 | 14 (43.8) | 4 (22.2) | 0.219 | 0.252 | 15 (38.5) | 3 (27.3) | 0.724 | 0.420 |
| Cardiovascular disease | 50 | 3 (9.4) | 3 (16.7) | 0.654 | 0.418 | 5 (12.8) | 1 (9.1) | 1.000 | 0.798 |
| Chronic kidney disease | 50 | 12 (37.5) | 5 (27.8) | 0.548 | 0.449 | 14 (35.9) | 3 (27.3) | 0.728 | 0.653 |
| Baseline laboratory indices and CT score | |||||||||
| Blood glucose | 50 | 9.40 (6.89–11.47) | 9.95 (6.77–13.56) | 0.652 | NA | 9.66 (6.83–12.64) | 10.15 (7.80–12.90) | 0.585 | NA |
| White blood cells | 47 | 5.82 (4.68–6.68) | 5.80 (4.06–6.40) | 0.673 | 0.207 | 5.80 (4.78–6.57) | 6.19 (4.05–7.15) | 0.735 | 0.954 |
| Lymphocytes | 47 | 1.08 (0.67–1.34) | 1.51 (1.12–1.95) | 0.006 | 0.008 | 1.26 (0.85–1.56) | 1.13 (0.74–1.79) | 0.938 | 0.736 |
| Lymphocytes % | 49 | 21.30 (11.20–27.15) | 26.05 (21.50–35.80) | 0.044 | 0.011 | 23.35 (15.23–29.70) | 21.40 (13.90–36.30) | 0.914 | 0.810 |
| Monocytes | 46 | 0.35 (0.26–0.51) | 0.42 (0.32–0.53) | 0.360 | 0.364 | 0.37 (0.27–0.51) | 0.39 (0.30–0.67) | 0.480 | 0.244 |
| Monocytes % | 46 | 7.40 (4.60–9.20) | 7.70 (6.50–9.90) | 0.286 | 0.129 | 7.45 (5.18–9.50) | 8.10 (5.53–10.23) | 0.594 | 0.288 |
| Neutrophils | 46 | 4.09 (2.72–5.29) | 3.93 (2.54–4.50) | 0.219 | 0.053 | 3.96 (2.60–5.06) | 3.63 (2.64–5.98) | 0.100 | 0.892 |
| Neutrophils % | 47 | 67.90 (63.43–83.33) | 65.50 (63.90–68.70) | 0.046 | 0.009 | 67.30 (58.35–76.90) | 66.00 (54.98–79.80) | 0.640 | 0.575 |
| ALT | 48 | 22.73 (16.00–35.69) | 21.00 (17.00–28.96) | 0.694 | 0.083 | 22.73 (17.00–32.69) | 18.00 (11.26–33.68) | 0.292 | 0.456 |
| AST | 48 | 26.51 (19.50–33.36) | 21.00 (14.88–36.47) | 0.186 | 0.068 | 26.51 (16.00–33.23) | 21.50 (18.18–38.11) | 0.839 | 0.687 |
| r-GGT | 48 | 47.84 (22.77–62.06) | 30.60 (22.00–62.32) | 0.630 | 0.837 | 36.10 (22.02–64.51) | 38.13 (19.75–68.28) | 0.859 | 0.880 |
| BUN | 46 | 5.33 (3.85–6.78) | 4.59 (3.42–5.40) | 0.206 | 0.349 | 5.10 (3.66–6.29) | 5.15 (4.36–6.86) | 0.699 | 0.656 |
| Creatinine | 49 | 64.00 (48.93–89.64) | 65.20 (49.00–74.50) | 0.443 | 0.467 | 71.80 (78.74–87.90) | 53.45 (49.50–65.49) | 0.259 | 0.410 |
| Urea | 43 | 286.57 (222.25–378.16) | 325.00 (296.95–347.50) | 0.321 | 0.338 | 312.00 (246.63–355.60) | 313.07 (211.72–381.55) | 0.931 | 0.806 |
| CK | 40 | 85.00 (58.95–138.75) | 57.00 (30.50–133.75) | 0.175 | 0.383 | 77.35 (40.25–146.84) | 64.00 (41.94–110.31) | 0.748 | 0.898 |
| CK-MB | 38 | 9.44 (6.62–17.00) | 5.00 (2.50–17.00) | 0.186 | 0.521 | 9.00 (4.02–17.00) | 9.45 (5.01–17.00) | 0.777 | 0.876 |
| LDH | 39 | 244.10 (198.25–349.85) | 218.00 (153.15–235.70) | 0.069 | 0.020 | 228.80 (187.23–286.00) | 355.70 (182.00–376.00) | 0.323 | 0.328 |
| MYO | 25 | 30.00 (13.88–52.26) | 30.00 (11.90–30.00) | 0.538 | 0.925 | 30.00 (29.34–48.09) | 12.54 (8.83–26.31) | 0.659 | 0.681 |
| CRP | 46 | 31.06 (6.92–50.17) | 13.60 (3.02–47.43) | 0.129 | 0.092 | 30.77 (5.00–49.70) | 19.80 (3.93–53.49) | 0.787 | 0.729 |
| ESR | 36 | 40.00 (26.00–58.50) | 38.00 (7.00–89.00) | 0.618 | 0.557 | 39.00 (14.00–66.75) | 41.50 (29.00–79.25) | 0.671 | 0.868 |
| PCT | 33 | 0.08 (0.05–0.20) | 0.13 (0.04–0.20) | 0.894 | 0.415 | 0.11 (0.05–0.17) | 0.08 (0.05–0.25) | 0.947 | 0.622 |
| D–dimer | 45 | 0.20 (0.03–0.71) | 0.13 (0.06–0.36) | 0.547 | 0.320 | 0.14 (0.03–0.33) | 0.41 (0.08–1.35) | 0.117 | 0.059 |
| Baseline-CT score | 50 | 4.0 (3.0–4.0) | 4.0 (4.0–4.0) | 0.692 | 0.883 | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 0.698 | 0.578 |
| COVID-19 treatment protocols | |||||||||
| Corticosteroid (n, %) | 50 | 14 (43.8) | 7 (38.9) | 0.774 | 0.851 | 17 (43.6) | 4 (36.4) | 0.741 | 0.520 |
| Antivirals (n, %) | 50 | 27 (84.4) | 16 (88.9) | 1.000 | 0.592 | 33 (84.6) | 10 (90.9) | 1.000 | 0.583 |
| Antibiotics (n, %) | 49 | 26 (83.9) | 12 (66.7) | 0.286 | 0.130 | 30 (76.9) | 8 (80.0) | 1.000 | 0.937 |
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; r-GGT, r-Gamma-glutamyl-transferase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, creatine kinase-myocardial band; MYO, myoglobin; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin; CT, computed tomography; COVID-19, coronavirus disease 2019. Data were n (%) or median (IQR). P-values were shown as χ2 test or Fisher's exact test or Mann–Whitney-U test. P < 0.05 was considered significant (bolded).
The concentration of metformin presenting in “gram” per day.
The concentration of insulin presenting in “unit” per day.
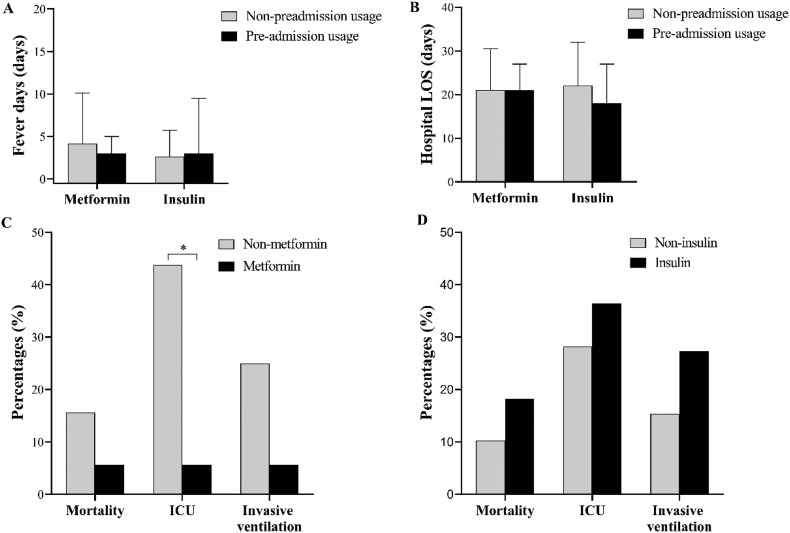
After adjusting the age and blood glucose levels, no difference in fever days, hospital LOS, mortality, and invasive ventilation utilization between groups, but with a dramatically lower rate of ICU admission (1 [5.6%] vs. 14 [43.8%], adjust P = 0.005) in metformin patients compared with the non-metformin group. Interestingly, the dose of metformin (per day) in pre-admission showed a negative association with the rate of ICU admission after controlling (OR 0.04 [95% CI 0.00, 0.99]; adjust P = 0.049), as shown in Table S1. Whereas, no pronounced difference in clinical observational indices and outcomes between insulin and non-insulin usage before admission (Fig. 3 ).
Fig. 3.
Fever days (A), hospital stay (B), and outcomes (C, D) in pre-admission metformin or insulin users. ICU, intensive care unit; LOS, length of stay. Data were n (%) or median (IQR). *, adjust P < 0.05.
3.3. Hospital metformin or insulin usage
During hospitalization, 22 of 50 cases with T2DM had metformin. Patients had in-hospital metformin with a median of 1.0 g per day [IQR 0.92–1.27]. After adjusting the age and blood glucose, there is no difference in all characteristics at admission (adjust P > 0.05) (Table 4 ). However, after the non-divergent baseline, lower CK-MB, LDH, and d-dimer, but higher urea levels were observed in hospital metformin users at the endpoint (Table S2).
Table 4.
Characteristics, baseline laboratory indices, clinical observational indices, and outcomes in hospital metformin or insulin users.
| No. of patients tested | Non-metformin (N = 28) | Metformin (N = 22) | P-value | Adjust P-value | Non-insulin (N = 27) | Insulin (N = 23) | P-value | Adjust P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Concentrations (per day) | – | – | 1.00 (0.92–1.27)a | NA | NA | – | 22.0 (20.0–33.9)b | NA | NA |
| Characteristics | |||||||||
| Age (years) | 50 | 57.5 (48.0–64.8) | 51.0 (43.8–64.3) | 0.162 | NA | 51.0 (44.0–65.0) | 57.0 (48.0–64.0) | 0.311 | NA |
| Gender (M, %) | 50 | 14 (50.0) | 13 (59.1) | 0.577 | 0.747 | 14 (51.9) | 13 (56.5) | 0.782 | 0.689 |
| BMI | 24 | 25.31 (22.20–26.87) | 25.56 (23.23–27.65) | 0.682 | 0.722 | 24.22 (23.05–28.12) | 25.71 (21.88–26.87) | 0.706 | 0.496 |
| Comorbidities (n, %) | |||||||||
| Hypertension | 50 | 12 (42.9) | 6 (27.3) | 0.374 | 0.318 | 12 (44.4) | 6 (26.1) | 0.241 | 0.221 |
| Cardiovascular disease | 50 | 3 (10.7) | 3 (13.6) | 1.000 | 0.843 | 5 (18.5) | 1 (4.3) | 0.199 | 0.276 |
| Chronic kidney disease | 50 | 10 (35.7) | 7 (31.8) | 1.000 | 0.711 | 9 (33.3) | 8 (34.8) | 1.000 | 0.623 |
| Baseline laboratory indices and CT score | |||||||||
| Blood glucose | 49 | 9.95 (7.05–12.64) | 9.26 (6.77–11.21) | 0.463 | NA | 8.30 (6.48–10.16) | 11.03 (9.06–15.74) | 0.003 | NA |
| White blood cells | 47 | 5.83 (4.63–6.74) | 5.62 (4.53–6.47) | 0.771 | 0.586 | 5.62 (4.72–6.24) | 6.16 (4.08–705) | 0.500 | 0.639 |
| Lymphocytes | 47 | 1.15 (0.87–1.37) | 1.34 (0.73–1.77) | 0.407 | 0.385 | 1.32 (0.89–1.69) | 1.07 (0.80–1.56) | 0.392 | 0.972 |
| Lymphocytes % | 49 | 22.75 (15.55–27.90) | 23.00 (14.25–34.15) | 0.777 | 0.413 | 24.35 (15.38–34.93) | 21.80 (13.90–26.40) | 0.362 | 0.913 |
| Monocytes | 46 | 0.37 (0.26–0.62) | 0.39 (0.30–0.50) | 0.991 | 0.975 | 0.39 (0.30–0.51) | 0.36 (0.25–0.58) | 0.667 | 0.848 |
| Monocytes % | 46 | 7.35 (5.20–9.63) | 7.95 (5.28–9.60) | 0.715 | 0.580 | 7.60 (5.40–9.65) | 7.40 (4.50–9.55) | 0.834 | 0.868 |
| Neutrophils | 46 | 4.04 (2.68–5.22) | 3.94 (2.58–4.74) | 0.634 | 0.406 | 3.93 (2.58–4.67) | 4.09 (2.68–5.58) | 0.667 | 0.749 |
| Neutrophils % | 47 | 67.20 (63.10–76.70) | 67.50 (54.23–76.90) | 0.691 | 0.346 | 67.30 (54.68–77.03) | 67.20 (64.90–76.35) | 0.600 | 0.912 |
| ALT | 48 | 22.00 (15.00–35.34) | 23.00 (18.00–31.90) | 0.499 | 0.268 | 22.82 (16.00–29.90) | 22.00 (16.50–37.02) | 0.950 | 0.545 |
| AST | 48 | 26.00 (16.00–35.17) | 25.80 (16.50–34.65) | 0.533 | 0.081 | 26.00 (16.00–33.42) | 25.41 (19.17–34.17) | 0.533 | 0.035 |
| r-GGT | 48 | 28.00 (18.00–52.06) | 48.90 (24.00–90.47) | 0.045 | 0.089 | 48.90 (23.00–68.27) | 28.00 (22.00–58.38) | 0.371 | 0.242 |
| BUN | 46 | 5.30 (3.59–6.44) | 4.80 (4.13–625) | 0.973 | 0.658 | 4.70 (3.49–5.89) | 5.33 (4.36–8.54) | 0.118 | 0.608 |
| Creatinine | 49 | 65.00 (48.74–80.50) | 65.10 (49.65–89.65) | 0.952 | 0.468 | 65.00 (47.70–80.50) | 66.14 (50.27–89.11) | 0.463 | 0.575 |
| Urea | 43 | 28,300 (220.61–344.76) | 330.50 (281.98–368.07) | 0.218 | 0.504 | 316.45 (244.09–349.14) | 312.00 (236.82–371.23) | 0.974 | 0.366 |
| CK | 40 | 69.00 (41.00–115.75) | 90.40 (34.64–155.52) | 0.880 | 0.309 | 85.00 (40.00–149.53) | 69.00 (48.00–107.00) | 0.880 | 0.604 |
| CK-MB | 38 | 9.44 (5.01–17.00) | 7.00 (2.50–15.00) | 0.188 | 0.161 | 8.82 (3.64–15.26) | 9.45 (4.86–17.00) | 0.679 | 0.741 |
| LDH | 39 | 232.15 (189.08–327.73) | 226.20 (175.20–293.45) | 0.755 | 0.563 | 226.20 (170.00–320.00) | 232.15 (192.75–353.75) | 0.549 | 0.569 |
| MYO | 25 | 30.00 (9.51–52.26) | 30.00 (29.27–30.00) | 1.000 | 0.423 | 30.00 (18.73–45.98) | 30.00 (9.17–45.77) | 0.565 | 0.436 |
| CRP | 46 | 28.94 (4.73–55.37) | 29.40 (5.17–47.68) | 0.816 | 0.855 | 27.45 (4.68–48.28) | 30.95 (5.88–53.14) | 0.825 | 0.198 |
| ESR | 36 | 38.00 (13.75–57.75) | 50.00 (32.00–78.50) | 0.249 | 0.476 | 38.00 (23.00–62.00) | 44.00 (17.00–76.00) | 0.949 | 0.972 |
| PCT | 33 | 0.08 (0.05–0.18) | 0.13 (0.06–0.21) | 0.437 | 0.304 | 0.07 (0.05–0.17) | 0.12 (0.05–0.23) | 0.469 | 0.174 |
| D–dimer | 45 | 0.20 (0.03–0.35) | 0.13 (0.02–0.42) | 0.275 | 0.299 | 0.13 (0.03–0.37) | 0.25 (0.06–0.55) | 0.208 | 0.441 |
| Baseline-CT score | 50 | 4.0 (2.5–4.0) | 4.0 (4.0–4.0) | 0.725 | 0.892 | 4.0 (2.0–4.0) | 4.0 (4.0–4.0) | 0.326 | 0.235 |
| COVID-19 treatment protocols | |||||||||
| Corticosteroid (n %) | 50 | 13 (46.4) | 8 (36.4) | 0.569 | 0.591 | 9 (33.3) | 12 (52.2) | 0.252 | 0.449 |
| Antivirals (n %) | 50 | 25 (89.3) | 18 (81.8) | 0.684 | 0.455 | 23 (85.2) | 20 (87.0) | 1.000 | 0.647 |
| Antibiotics (n %) | 49 | 22 (81.5) | 16 (72.7) | 0.510 | 0.573 | 20 (74.1) | 18 (81.8) | 0.732 | 0.823 |
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; r-GGT, r-gamma-glutamyl-transferase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, creatine kinase-myocardial band; MYO, myoglobin; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin; CT, computed tomography; COVID-19, coronavirus disease 2019. Data were n (%) or median (IQR). P-values were shown as χ2 test or Fisher's exact test or Mann–Whitney-U test. P < 0.05 was considered significant (bolded).
The concentration of metformin presenting in “gram” per day.
The concentration of insulin presenting in “unit” per day.
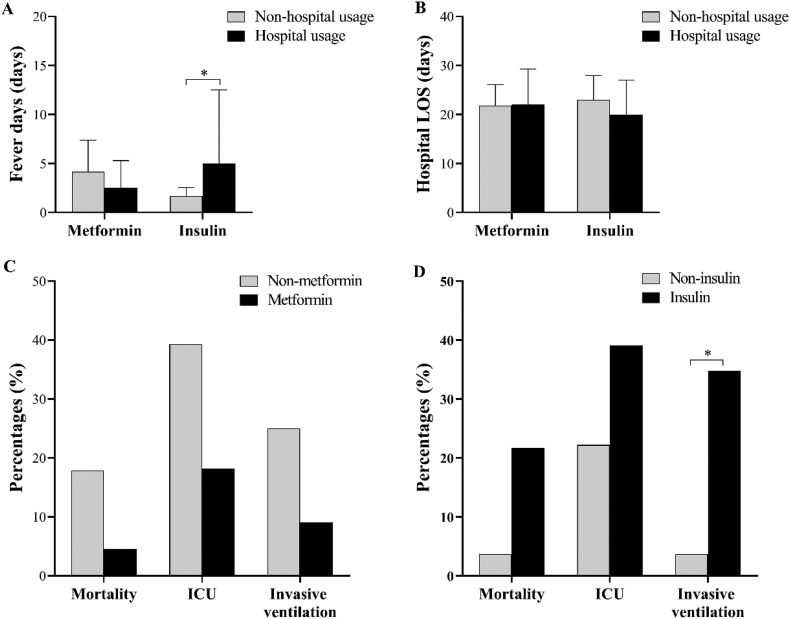
There were 23 of 50 had insulin after admission. In-hospital insulin users with a median of 22.0 unit per day [IQR 20.0–33.9]. Higher blood glucose (11.03 mmol/L, [IQR 9.06–15.74] vs. 8.30 mmol/L [IQR 6.48–10.16], adjust P = 0.003) was seen in insulin users. After adjusting the age and blood glucose, patients in the insulin group had lower AST levels than those of the non-insulin group (25.41 IU/L, [IQR 19.17–34.17] vs. 26.00 IU/L, [IQR 16.00–33.42], adjust P = 0.035), with no difference in general characteristics, other laboratory indices, and CT scores at admission (Table 4). However, under the comparable COVID-19 treatment protocols, higher LDH, more deteriorated inflammation including higher CRP; ESR, and CT scores, but lower r-GGT and urea levels were seen in hospital insulin users compared with non-insulin users at the endpoint (Table S2).
Regarding the clinical observations and outcomes, after adjusting the age and blood glucose, no difference was seen in fever days, hospital LOS and outcomes between in-hospital non-metformin and metformin users (adjust P > 0.05). In comparison, longer fever days (5.0 days [IQR 1.0–12.5] vs. 2.0 days [IQR 0.0–3.0], adjust P = 0.010), higher rate of invasive ventilation (8 [34.8%] vs. 1 [3.7%], adjust P = 0.043) were observed in in-hospital insulin users (Fig. 4 ). However, the dose of in-hospital insulin usage had positively association with the rate of invasive ventilation (OR 1.05 [95% CI 0.01, 1.10]; P = 0.027), but no association was found after controlling the age and blood glucose (OR 1.04 [95% CI 0.99, 1.09]; adjust P = 0.088), as shown in Table S1.
Fig. 4.
Fever days (A), hospital stay (B), and outcomes (C, D) in in-hospital metformin or insulin users. ICU, intensive care unit; LOS, length of stay. Data were n (%) or median (IQR). *, adjust P < 0.05.
4. Discussion
This retrospective study is aiming to explore the effects of both pre-admission and in-hospital use of metformin and insulin in COVID-19 patients with pre-existed T2DM. We validated the risk of T2DM in COVID-19 patients, which had increased mortality and poor prognosis (ICU admission and invasive ventilation) than non-diabetes. Age and blood glucose were indicated as the risk factors of poor outcomes after multivariable regression. Notable, after controlling the age and blood glucose, pre-admission metformin instructive for declining the ICU admission rate in a dose-dependent fashion in COVID-19 patients with pre-existed T2DM; whereas, in-hospital insulin usage inversely increased the rate of invasive ventilation.
Regarding the mortality in COVID-19 patients with diabetes, several lines of evidence have suggested that diabetes is one of the important risks of mortality in COVID-19 patients [4,21,22]. The underlying mechanism supposed to be the microvascular disease, endothelial dysfunction, severe pneumonia, and inflammatory storm underlies the adverse consequences of COVID-19 [5,23]. Older age and increased CRP were considered contribute to the increased mortality in COVID-19 patients with diabetes [16]. Uniformly, in our study, older age and more deteriorated inflammation were seen in T2DM. Moreover, exacerbated hepatic-renal functions were observed in T2DM, which may also contribute to the dramatically increased mortality and poor prognosis in comparison with non-diabetes. It was suggested that un-well glycemic control was associated with poor outcomes compared with those of well-controlled patients [24,25]. In accordance, we demonstrated that age and admission blood glucose were the independent risk factors of mortality and poor prognosis in COVID-19 patients. In order to eliminate the influence of these two factors on the outcomes, we had controlled the age and blood glucose in the analysis of metformin and insulin subgroups.
Metformin is considered the first-line T2DM treatment agents nowadays [26]. Initially, it was found from the plant Galega officinalis and used for treating flu and malaria [26]. In recent years, metformin has attracted attention again for its antiviral effect; several studies have suggested its anti-inflammation roles in several pathology situations [11,12]. Recently, Sharma et al. suggested that the possible machination of metformin on COVID-19 might ascribe to the declining of proinflammatory and profibrotic states, release acute lung injury via activation of adenosine monophosphate-activated protein kinase [10].
Notwithstanding, in our study, analogous to the study of Chen et al. [16], pre-admission metformin usage had no impact on mortality. Whereas, the attenuated inflammatory response (decreased neutrophils (%)) with benefits on immune-modulatory effects (increased lymphocytes, lymphocytes (%)), decreased LDH were observed in pre-admission metformin in our study. Besides, pre-admission metformin was negatively associated with ICU admission in a dose dependent fashion, independent of age and blood glucose. These results implicate that pre-admission metformin usage may have the protecting effects of COVID-19 patients with T2DM without affecting the mortality.
In comparison, in-hospital metformin had no impact on mortality or poor prognosis in our study. Whereas, the decreased LDH, CK-MB at the endpoint were observed, may involve in the cardiac protecting function, which has been raised in other literature [27]. Besides, a decreased d-dimer in hospital metformin cases was found, which was considered instructive for ameliorated outcomes of COVID-19 patients [28]. However, it is worth noting that the urea levels were increased in in-hospital metformin users, which could be a risk of acidosis, especially in patients with a history of nephrotic disease. Regarding this, X. Cheng et al. have turned out that in-hospital metformin usage could have the risk of increasing the incidence of acidosis [15]. Hence, the in-hospital metformin usage may have cardiac protecting and ameliorated coagulating function, but the monitoring of renal function is needed for metformin patients, and severe nephrotic patients should use metformin sparingly.
As for the COVID-19 patients with diabetes who utilized the insulin. A study from New York City implicated that an increased risk of mortality in confirmed COVID-19 cases with diabetes who had prior admission insulin treatment [29]. Similar results were found in 120 COVID-19 patients with diabetes in Wuhan China [16]. However, instead of pre-admission insulin usage, we found in-hospital insulin usage was closely associated with a greater rate of invasive ventilation. And the greater rate of invasive ventilation might among the consequences of deteriorated inflammation (greater CT score, higher CRP, ESR levels) and higher LDH at the endpoint in insulin users compared with those of non-insulin users. A diabetic mice model study indicated that the ACE2 expression increased under insulin treatment [13], which might result in an exacerbated outcome. Interestingly, the r-GGT and urea levels were lower in-hospital insulin group, which suggests that insulin might have less risk of haptic-renal impairment, but the mechanisms are still unclear.
The limitations of this study should be noted. First, due to the limitation of diabetes samples, our results may have been obtained by chance; the results must be confirmed in a more extensive study. Second, the complexity of anti-diabetic agents, purely single antidiabetic medicine groups or purely pre-admission/in-hospital antidiabetic medicine groups were hard to achieve, and the results might be confounded by other antidiabetic medicine. Last, data of HbA1c were not enough to analyze and the confounders we included were limited owing to the sample sizes, may also influence the outcomes. Large and long-term randomized controlled trials with follow-up evaluation are needed to confirm the present results.
5. Conclusion
In this study, the hospital confirmed COVID-19 patients with T2DM had adverse outcomes, which might among the consequences of deteriorated inflammation, impaired immunity, and exacerbated hepatic-renal function than non-diabetes. Among COVID-19 patients with T2DM, pre-admission metformin usage was associated with declining the rate of ICU admission, whereas, in-hospital insulin usage had the risk of increasing the invasive ventilation after controlling age and blood glucose. In aggregate, metformin might achieve greater benefit for the COVID-19 patients with T2DM than insulin. Further studies are needed to confirm the present results.
CRediT authorship contribution statement
Xueqi Cheng, Xingjian Zhou designed the study. Xueqi Cheng, Siyi Xin, analyzed and interpreted the data and completed the final manuscript. Xingjian Zhou revising it critically for important intellectual content. Yaqi Chen, Leyu Li, Wanjun Chen, Wenjia Li, Baoan Zhou, Chenxia Li, Gong Yu, Fei Li, Peng Duan, collected and sorted the data and drafting the article. All authors read and approved the final manuscript. Xinjian Zhou has primary responsibility for the final content.
Declaration of competing interest
No financial or commercial conflict of interest exists.
Acknowledgments
Acknowledgments
We greatly appreciate the whole group of frontline medical staff in the Xiangyang city for their dedication to defending against SARS-CoV-2 infection.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81901567) the Hubei Provincial Natural Science Foundation of China (2018CFB111) and the Cultivating Project for Young Scholar at Hubei University of Medicine (2017QDJZR07).
Footnotes
COVID-19, coronavirus disease 2019.
OR, odds ratio.
CI, confidence intervals.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2021.119371.
Appendix A. Supplementary data
Supplementary tables
References
- 1.WHO Coronavirus disease (COVID-19) dashboard. https://covid19.who.int
- 2.Wang W., et al. Global, regional, and national estimates of target population sizes for covid-19 vaccination: descriptive study. BMJ. Dec. 2020;371 doi: 10.1136/bmj.m4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. Nov. 2020;11(1) doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet Lond. Engl. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. Apr. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Y., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res. Clin. Pract. Apr. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penlioglou T., Papachristou S., Papanas N. COVID-19 and diabetes mellitus: may old anti-diabetic agents become the new philosopher’s stone? Diabetes Ther. May 2020:1–3. doi: 10.1007/s13300-020-00830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ursini F., Ciaffi J., Landini M.P., Meliconi R. COVID-19 and diabetes: is metformin a friend or foe? Diabetes Res. Clin. Pract. 2020;164 doi: 10.1016/j.diabres.2020.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res. Clin. Pract. Jun. 2020;164 doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menendez J.A. Metformin and SARS-CoV-2: mechanistic lessons on air pollution to weather the cytokine/thrombotic storm in COVID-19. Aging. 2020;12(10):8760–8765. doi: 10.18632/aging.103347. 10.18632/aging.103347 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78(7):1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem E.S.B., Grobe N., Elased K.M. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am. J. Physiol. Ren. Physiol. Mar. 2014;306(6):F629–F639. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y., et al. Risk of metformin in type 2 diabetes patients with COVID-19: a preliminary retrospective report. Clin. Transl. Sci. Sep. 2020 doi: 10.1111/cts.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. Oct. 2020;32(4):537–547. doi: 10.1016/j.cmet.2020.08.013. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. May 2020 doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 17.Luo P., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. J. Br. Diabet. Assoc. Jul. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.“Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 6th Edition).” http://www.nhc.gov.cn/xcs/zhengcwj/202002/8334a8326dd94d329df351d7da8aefc2.shtml (accessed April 06, 2020).
- 20.Li Y., et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. Apr. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 23.Whyte M.B., Vas P., Heiss C., Feher M.D. The contribution of diabetic micro-angiopathy to adverse outcomes in COVID-19. Diabetes Res. Clin. Pract. Jun. 2020;164:108217. doi: 10.1016/j.diabres.2020.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. Jun. 2020;31(6):1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z., et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19. J. Diabetes. Jul. 2020 doi: 10.1111/1753-0407.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin S., Lux A., O’Callaghan F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br. J. Clin. Pharmacol. 2019;85(1):37–46. doi: 10.1111/bcp.13780. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalan R. Metformin, neutrophils and COVID-19 infection. Diabetes Res. Clin. Pract. Jun. 2020;164 doi: 10.1016/j.diabres.2020.108230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S., Schechter C., Southern W., Crandall J.P., Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. Aug. 2020 doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables