Abstract
Terpenes are vital metabolites found in various plants and animals and known to be beneficial in the treatment of various diseases. Previously, our group identified terpenes that increased the survival of Alzheimer's disease (AD) model flies expressing human amyloid β (Aβ) and identified linalool as a neuroprotective terpene against Aβ toxicity. Linalool is a monoterpene that is commonly present as a constituent in essential oils from aromatic plants and is known to have anti-inflammatory, anticancer, antihyperlipidemia, antibacterial, and neuroprotective properties. Although several studies have shown the beneficial effect of linalool in AD animal models, the mechanisms underlying the beneficial effect of linalool on AD are yet to be elucidated. In the present study, we showed that linalool intake increased the survival of the AD model flies during development in a dose-dependent manner, while the survival of wild-type flies was not affected even at high linalool concentrations. Linalool also decreases Aβ-induced apoptosis in eye discs as well as the larval brain. Moreover, linalool intake was found to reduce neurodegeneration in the brain of adult AD model flies. However, linalool did not affect the total amount of Aβ42 protein or Aβ42 aggregation. Rather, linalool decreased Aβ-induced ROS levels, oxidative stress, and inflammatory response in the brains of AD model flies. Furthermore, linalool attenuated the induction of oxidative stress and gliosis by Aβ1-42 treatment in the rat hippocampus. Taken together, our data suggest that linalool exerts its beneficial effects on AD by reducing Aβ42-induced oxidative stress and inflammatory reactions.
1. Introduction
Alzheimer's disease (AD) refers to a neurodegenerative condition, which is recognized as the most common cause of dementia [1]. The hallmarks of AD are abnormal aggregation of amyloid β (Aβ) and tau protein, and the accumulation of the pathological forms of these proteins has been considered to be a cause of AD [2]. In particular, the accumulated Aβ forms toxic oligomers, which subsequently induce various pathophysiological events such as inflammation, reactive oxygen species (ROS) generation, and neuronal death during the progression of AD [3–6].
Currently, four drugs, including donepezil, galantamine, rivastigmine, and memantine, have been approved by the U.S. Food and Drug Administration for the treatment of AD [7]. However, these treatments are used as temporary memory enhancers to relieve symptoms rather than to modify disease progression. In addition, these treatments are associated with adverse effects related to the gastrointestinal tract and cardiovascular system [8, 9]. Therefore, studies on the neuroprotective effects of natural compounds that do not exhibit any pronounced toxicity are being conducted to replace the current treatments for AD.
Terpenes are natural compounds that have a potential to treat AD. They are volatile organic compounds that contain hydrocarbons with 5 carbon atoms as their building blocks. Terpenes are produced by various organisms, especially plants [10, 11]. Previous studies have shown that many terpenes have anti-inflammatory, antitumor, or neuroprotective properties [12]. Based on that, our prior screening identified terpenes that exhibit neuroprotective effects against Aβ cytotoxicity using a Drosophila AD model and identified six terpenes, namely, ρ-cymene, limonene (+), limonene (-), linalool, α-pinene (+), and β-pinene (-), as neuroprotective terpenes that can effectively suppress the Aβ phenotype in AD flies [13].
Linalool is a monoterpene that is commonly present as a constituent in essential oils from aromatic plants. Linalool is known to have anticancer, antihyperlipidemia, antibacterial, and neuroprotective properties and has a wide range of biological activities, including antioxidant and anti-inflammatory effects [14, 15]. It is a colorless and volatile monoterpene found in essential oils from more than 200 plants [16, 17]. Several studies have shown that linalool exerts a neuroprotective effect by inhibiting inflammation in vitro and in vivo [18–20]. Linalool also protects neurons by decreasing ROS levels in patients with carpal tunnel syndrome, which is a ROS-induced peripheral neuropathy [21]. Importantly, the safety of linalool is attested by the fact that it has been approved as a food flavoring by the European Commission [22]. Therefore, linalool is a promising natural compound for treating diseases related to ROS and inflammation [14, 15].
The beneficial effects of linalool on neurodegeneration were reported in two AD mouse models. Linalool reduced Aβ aggregation in a transgenic AD mouse model and reversed Aβ-induced memory loss in both transgenic and Aβ-injected AD mouse models [23, 24]. However, the detailed molecular mechanisms by which linalool protects the neurons from Aβ toxicity are not fully understood. Therefore, the use of linalool as a treatment for AD needs to be studied in many respects.
In the present study, we showed that linalool intake suppressed Aβ-induced neuronal cell death without affecting Aβ aggregation in the brains of flies expressing human Aβ. Linalool ameliorated AD-like phenotypes, including neurodegeneration, through its anti-inflammatory and antioxidative properties in fly and rat AD models. These results suggest that linalool is a potential therapeutic agent for AD treatment.
2. Materials and Methods
2.1. Drosophila Strains
The w1118, glass multimer reporter-GAL4 (GMR-GAL4; eye driver), embryonic lethal abnormal vision-GAL4 (elav-GAL4; pan-neuronal driver), and Drosomycin-tagged green fluorescent protein (Drs-GFP) strains were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). The UAS-Aβ422X strain was a gift from Dr. Fernandez-Funez (University of Florida, USA). The genotypes of the flies were elav>Aβ422X (elav-GAL4/+; UAS-Aβ422X/+) and GMR>Aβ422X (GMR-GAL4, UAS-Aβ422X/+).
2.2. Survival Assay
In order to determine the survival rate of Drosophila, 250 Drosophila embryos were collected on grape juice agar plates. Every 50 embryos were transferred into a vertical plastic vial and maintained at 25°C and 60% humidity. The survival rate of the female adults was estimated. DMSO-fed w1118 and DMSO-fed elav>Aβ422X were used as controls. This experiment was repeated three times (total of 750 embryos per group were used).
2.3. Acridine Orange Staining
Acridine orange (AO) staining was performed to detect cell death. As previously described [13], the brain or eye discs of L3 larvae were dissected in phosphate buffer saline (PBS, pH 7.8, Bio Basic, Seoul, Republic of Korea) and incubated with 1.6 × 10−6 M AO (Sigma Aldrich, St. Louis, USA) for 5 min at room temperature. The samples were washed twice with PBS for 5 min each. The cells were examined under an Axiophot 2 fluorescence microscope (Carl Zeiss, Oberkochen, Germany), and then the number of stained cells was counted.
2.4. Brain Section
To measure neuronal loss, the fly heads were sectioned as previously reported [25]. Fly heads were fixed in Carnoy solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid) at 4°C for 4 d and embedded in paraffin. The embedded heads were sectioned (5 μm thick sections) and stained with hematoxylin and eosin. The stained samples were examined under a light microscope.
2.5. Immunohistochemical Analysis of Drosophila Brain
Fly brains were fixed in 4% paraformaldehyde (PFA) prepared in PBST (PBS + 0.5% Triton X‐100) for 3 h at room temperature. The samples were washed thrice with PBST for 10 min and incubated in blocking solution (2% normal goat serum + 2% bovine serum albumin (BSA) + 0.5% Triton X-100) for 3 h. Then, the samples were incubated with anti-Aβ42 antibody prepared in the blocking solution for 48 h at 4°C (blocking solution 1 : 200; Santa Cruz, CA, USA). Alexa Fluor 555 anti-rabbit antibody (PBST 1 : 200; Cell Signaling Technology, Danvers, USA) was used as the secondary antibody.
2.6. Measurement of ROS Levels
The ROS levels in the eye discs were measured using dihydroethidium (DHE; Invitrogen molecular probe, USA). As previously described [13], the eye discs of L3 larvae were dissected and incubated with Schneider's medium containing 40 μM DHE for 5 min and then washed twice in Schneider's medium for 5 min each time. The samples were observed under an Axiophot 2 fluorescence microscope (Carl Zeiss).
2.7. Measurement of Nitric Oxide Levels
As previously described [13], the heads of 3- to 5-day-old flies (n = 15) were resuspended in 10× PBS on ice. The samples were ground and centrifuged at 10,000 × g for 10 min at 4°C. The supernatants were mixed with Greiss reagent at 1 : 1 ratio (Sigma-Aldrich) and incubated for 15 min at 25°C. Nitrite oxide (NO) levels were measured at 550 nm using a spectrophotometer.
2.8. Measurement of Inflammation after Bacterial Infection
The DH5α strain of Escherichia coli (Dongin Biotech, Republic of Korea) was cultured in Luria Bertani medium at 37°C for 16–20 h and concentrated by centrifugation. Septic injury was induced by pricking the thorax of 3- to 5-day-old Drs-GFP adult females with a thin needle dipped into a concentrated bacterial pellet (4.4 × 1010 cfu/ml) [13].
2.9. Animals
All experimental procedures were in accordance with the guidelines of the Laboratory Animal Manual of the National Institutes of Health Guide to the Care and Use of Animals, which were approved by the Ethics Review Committee of Konkuk University for Animal Experiments (approval number: KU20170). Sprague-Dawley rats, 260 to 280 g at the time of surgery, were housed two to three per cage with ad libitum access to water and food during a 12-hour light/dark cycle.
2.10. Preparation of Aβ Peptide
Aβ1-42 (Invitrogen, Camarillo, CA) were prepared as described previously [26] by dissolving the peptide in a freshly prepared 35% acetonitrile solution with dilution to a final concentration of 0.5 mM in PBS (pH 7.4). The Aβ1-42 solution was incubated for 24 h at 37°C to prepare fibrillary aggregates. After incubation, Aβ1-42 were stored at -20°C until use.
2.11. Stereotaxic Surgery
Rats were anesthetized and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A midline sagittal incision was made in the scalp, and holes were drilled in the skull over the dorsal hippocampus using the following coordinates: 3.6 mm posterior to the bregma and 2.0 mm lateral to the midline for intrahippocampal injections according to the atlas of Paxinos and Watson [27]. The hole of the tip was directed down to 2.6 mm beneath the surface of the brain for the hippocampus. All injections were made using a Hamilton syringe equipped with a 30S gauge beveled needle and attached to a syringe pump (KD Scientific, New Hope, PA). Infusions were made at a rate of 0.2 μl/min for Aβ1-42 (1 nmol in 2 μl). After injection, the needle was left in place for an additional 5 min before being slowly retracted.
2.12. Linalool Injection
Linalool was administered intraperitoneally (i.p.) once a day for 7 days before surgery and 14 days after surgery. Linalool was dissolved in DMSO (vehicle) and injected at doses of 50 and 100 mg/kg. The control groups received the same volume of vehicle for 3 weeks.
2.13. Rat Brain Tissue Preparation and Immunostaining
Rats were anesthetized at the indicated time points after injection and were perfused transcardially with a 0.9% saline solution containing 0.5% sodium nitrate and heparin (10 U/ml), which was followed by fresh cold 4% PFA fixative (pH 7.4). Brains were removed from the skull, postfixed in 4% PFA for 24 h at 4°C, and then placed in a 30% sucrose solution until they sank. Brains were frozen sectioned using a sliding microtome into 35 μm coronal sections after they were embedded in optimum cutting temperature compound (Surgipath), and they were then collected into six separate series. Sections were stored free-floating in cryopreservative medium at −20°C. The tissue sections were washed in cold PBS for 15 min and incubated with a universal blocking solution in PBS (0.3% Triton X-100, 1% BSA, 0.05% Tween 20, 0.1% cold fish gelatin, and 0.05% sodium azide) for 1 h at room temperature. Brain sections were rinsed in PBS then incubated with the following primary antibodies: antiglial fibrillary acidic protein (GFAP, 1 : 500, mouse, Sigma Aldrich) for astrocytes and anti-4-hydroxynonenal (4-HNE, 1 : 300, mouse, abcam, Cambridge, UK) for lipid peroxidation. For fluorescence microscopy, the samples were incubated with Alexa 594-conjugated secondary antibodies (Cell Signaling Technology). For light microscopy, brain tissues were incubated with a biotin-conjugated secondary antibody followed by streptavidin-conjugated HRP (Vectastain ABC kit, Vector Laboratories). Immunostaining was visualized by incubating the samples in a 0.1 M-PB solution containing 0.05% diaminobenzidine-HCl and 0.003% hydrogen peroxide.
2.14. Statistical Analysis
Data were quantitatively analyzed for significance using either Student's t-test (two-tailed) or one-way ANOVA followed by Tukey-Kramer multiple comparison test. Student's t-test was used for comparisons between two groups. GraphPad Prism 8.0 (GraphPad Software Inc., USA) was used for performing statistical analyses. P values < 0.05 indicated a significant difference.
3. Results
3.1. Linalool Intake Increases Survival during the Development of the Drosophila AD Model
It is known that the Drosophila AD model used in this study shows decreased survival compared to the wild-type Drosophila during development [13]. To confirm the effect of linalool on AD, we used the Drosophila AD model (elav-GAL4>UAS-Aβ422X) that expresses Aβ42 in the neurons. Donepezil (Done), a current AD treatment, was used as a positive control (Figure 1(a)). As expected, donepezil increased the survival of Aβ42-expressing flies during development (Figure 1(a)). Linalool also showed protective effects against Aβ42 toxicity in a dose-dependent manner. More importantly, linalool did not affect the survival of wild-type flies at the concentrations used in AD model flies (Figure 1(b)). These results demonstrate that linalool has a protective effect against Aβ toxicity and has no apparent harmful effects even at high concentrations.
Figure 1.
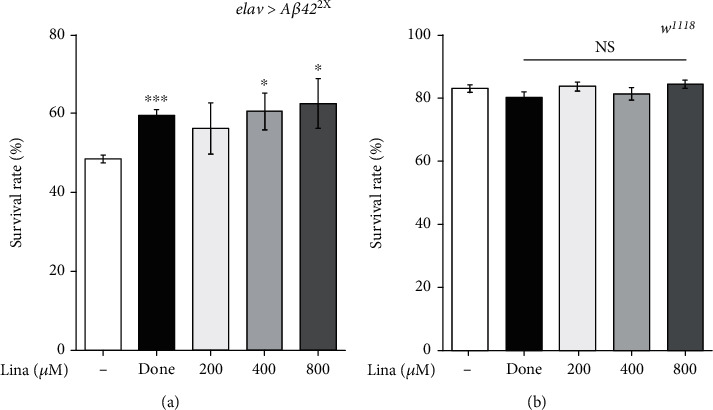
Linalool intake increases survival of the Drosophila AD model during development. The graphs show the effect of linalool on the survival of Drosophila expressing Aβ42 (elav>Aβ422X) (a) and w1118 (b) during development (n = 750). Flies fed with donepezil (Done) were used as positive control. All data in the graphs are expressed as mean ± standard error of mean (SEM). ∗∗∗P < 0.001, ∗P < 0.05 vs. DMSO-fed elav>Aβ422X (a) or DMSO-fed w1118 (b); Student t-test. Done: 0.1 μg/ml donepezil; Lina: linalool; NS: not significant.
3.2. Linalool Intake Suppressed Aβ42-Induced Cell Death
As linalool reduced Aβ toxicity during development, we investigated whether linalool intake suppressed Aβ42-induced cell death in the developing eyes and the brain using AO staining, which detects dead cells. Similar to a previous report [13], ectopic expression of human Aβ42 in the developing eyes using GMR-GAL4 driver (GMR>Aβ422X) strongly induced cell death (Figures 2(a) and 2(b)). Linalool and donepezil significantly reduced Aβ42-induced cell death in the developing eye discs (Figures 2(a) and 2(b)). Consistently, linalool and donepezil treatment also suppressed Aβ42-induced cell death in the larval brain (Figures 2(c) and 2(d)). We also examined whether linalool intake suppresses neurodegeneration in the brains of Aβ42-expressing flies. Neurodegeneration leads to vacuolation in the brains of AD model flies [28], which is not observed in the brains of wild-type flies (Figure 2(e)). The vacuole area in the brains of flies fed with linalool was significantly decreased compared to that in the brains of control (DMSO-fed elav>Aβ422X) flies (Figures 2(e) and 2(f)), demonstrating that feeding with linalool reduced neurodegeneration. Collectively, our results suggest that linalool protects neurons from neurodegeneration by inhibiting neuronal cell death in AD model flies.
Figure 2.
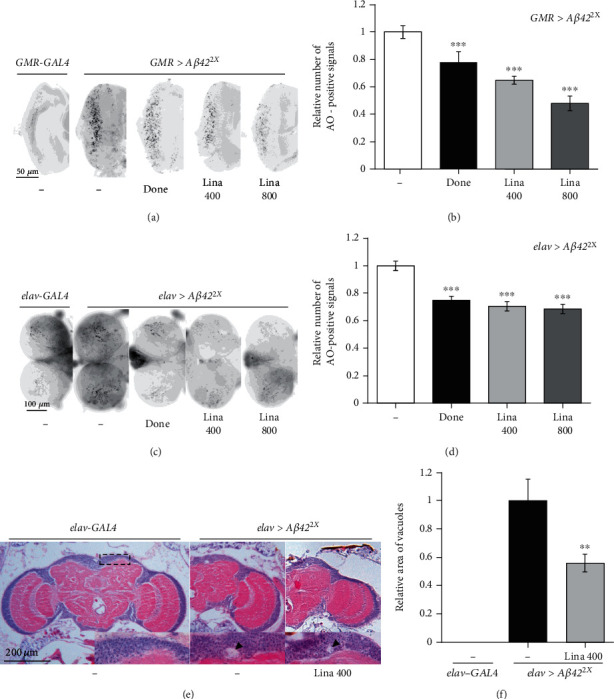
Linalool intake suppresses Aβ42-induced cell death and neurodegeneration. Linalool inhibited cell death in the Drosophila eye discs (a, b) and the larval brains (c, d) expressing Aβ42. (a, c) Representative images of acridine orange- (AO-) stained larval eye discs (a) and brains (c) of indicated groups. (b, d) The graphs show the relative number of AO-positive signals in the larval eye discs and brains of indicated groups (n ≥ 20). All data in the graphs are expressed as mean ± SEM. ∗∗∗P < 0.001 vs. DMSO-fed elav>Aβ422X (b) or DMSO-fed GMR>Aβ422X (d); Student's t-test. (e, f) Linalool inhibited neurodegeneration in the brains expressing Aβ42. (e) Representative optical microscopic images of the adult brains of control (elav-GAL4) and the AD model (elav>Aβ422X). The dotted box is the region in which vacuoles were observed. Insets are magnified views of dorsomedial region of the brain in which vacuoles appeared. Arrowheads indicate vacuoles caused by neurodegeneration. (f) The graph shows the relative size of vacuole areas (n ≥ 7). All data in the graphs are expressed as mean ± SEM. ∗∗P < 0.01 vs. DMSO-fed elav>Aβ422X (f); Tukey–Kramer test. Done: 0.1 μg/ml donepezil; Lina 400: 400 μM linalool; Lina 800: 800 μM linalool.
3.3. Linalool Did Not Affect Aβ42 Protein Levels or Aggregation
As Aβ42 aggregation and deposition are closely related to neuronal cell death, we investigated whether the inhibitory effect of linalool on cell death is due to a decrease in the amount of Aβ42 deposition or aggregation. Therefore, we compared the amount of Aβ42 protein accumulation in the brains of AD flies fed with linalool to that in brains of DMSO-fed control flies. The accumulated Aβ42 was visualized using immunohistochemistry. As shown in Figures 3(a) and 3(b), linalool intake did not alter Aβ42 levels in the brains of AD flies. We also examined the levels of Aβ42 aggregates by thioflavin S staining, which stains oligomeric fibrils or plaques of Aβ42 [29]. Similar to accumulated Aβ42 levels, aggregation of Aβ42 was not altered by linalool (Figures 3(c) and 3(d)). These results indicate that the neuroprotective effect of linalool is not achieved by inhibiting Aβ42 accumulation or aggregation.
Figure 3.
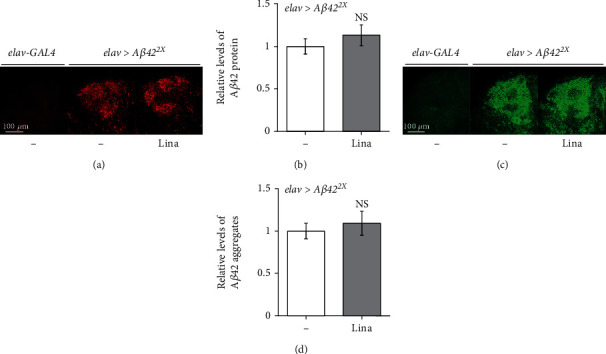
Linalool intake does not affect Aβ42 peptide accumulation and aggregation. The levels of Aβ42 peptide (a, b) and aggregation (c, d) in the Drosophila brains expressing Aβ42 (elav>Aβ422X) were not altered by linalool intake. (a, c) Representative confocal images of the Kenyon cell body region in the adult fly brains. The Aβ42 protein accumulation (a) and aggregation (c) in the brains of flies fed with linalool (elav>Aβ422X, Lina) were compared to those in the brains of control (elav>Aβ422X, DMSO) flies. Adult brains were stained with anti-Aβ42 antibody (a) or thioflavin S (c). (b, d) The graphs show the relative intensity of Aβ42-antibody staining (b) and thioflavin S staining (d) (n ≥ 20). All data in the graphs are expressed as mean ± SEM; Student's t-test. Lina: 400 μM linalool; NS: not significant.
3.4. Linalool Decreased Aβ42-Induced ROS Generation
Given that linalool has an antioxidant effect [12], and that ROS is an important mediator of Aβ42 toxicity [30], ROS was measured by DHE staining to determine whether linalool prevents ROS production in AD flies through its antioxidant activity. Similar to a previous report [13], ectopic expression of Aβ42 in the developing eyes (GMR>Aβ422X) clearly induced ROS levels, as detected by DHE staining, while DHE-positive signals were not observed in control flies without Aβ42 (GMR-GAL4) (Figure 4). Linalool and donepezil significantly decreased ROS levels (Figure 4), suggesting that the protective effects of linalool against Aβ42 toxicity are related to its antioxidant effect, at least in part.
Figure 4.
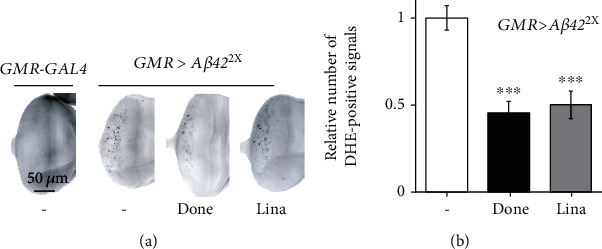
Linalool intake decreases ROS levels in the eye discs expressing Aβ42. (a) Representative DHE-staining images of larval eye discs expressing Aβ42 (GMR>Aβ422X). DHE-positive signals in the eye discs of linalool-fed flies were compared to those in the eye discs of control (GMR>Aβ422X, DMSO fed) flies. (b) The graph shows the relative number of DHE-positive signals (n ≥ 10). All data in the graph are expressed as mean ± SEM. ∗∗∗P < 0.001 vs. DMSO-fed GMR>Aβ422X (b); Student's t-test. Done: 0.1 μg/ml donepezil; Lina: 400 μM linalool.
3.5. Linalool Decreased Inflammation Induced by Aβ42 and Bacterial Infection
Previous studies have shown that neuroinflammation plays a key role in the pathology of AD [5]. As linalool has been reported to exert anti-inflammatory effects [31, 32], we investigated whether the beneficial effect of linalool is owing to its suppressive function in inflammation. First, to examine whether linalool can reduce inflammation in Drosophila, we used a microbial infection model, in which inflammatory response can be measured by an antimicrobial peptide reporter system after septic injury that infects the microorganism. In this system, the levels of inflammatory response can be measured based on the expression levels of GFP-labeled Drosomycin (Drs-GFP) in Drosophila. As expected, GFP levels increased in the thorax of Drosophila following DH5α infection when compared to those in the thorax of sucrose-injected control Drosophila (Figures 5(a) and 5(b)). Interestingly, linalool intake significantly reduced the expression of DH5α infection-induced Drs-GFP (Figures 5(a) and 5(b)). Then, we assessed whether linalool intake reduced the inflammation caused by Aβ42 by measuring NO levels in the fly brains. Similar to a previous report [13], Aβ42 expression in neurons increased NO levels in the brain of flies (Figure 5(c)). Moreover, linalool decreased NO levels in the AD flies to the levels of the control flies (Figure 5(c)). These results indicate that linalool suppresses inflammation in AD flies through its anti-inflammatory effect.
Figure 5.
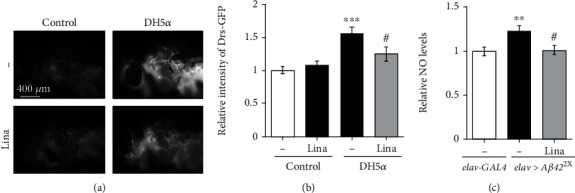
Linalool intake reduces inflammation induced by bacterial infection or Aβ42 expression. (a) Fluorescent microscopic images of linalool- or DMSO-fed Drs-GFP flies that express GFP controlled by the Drs promoter. Sucrose is used for a negative control. (b) The graph shows the relative levels of GFP in the thorax of flies injected with sucrose or DH5α (n ≥ 10). (c) The effect of linalool intake on the elevated NO levels in Aβ42-expressing fly heads. The graph shows relative NO levels in the heads of adult flies (n ≥ 9). NO levels in the heads of flies expressing Aβ42 (elav>Aβ422X) were compared to those in the heads of control (elav-GAL4) flies. All data in the graphs are expressed as mean ± SEM. ∗∗∗P < 0.001, ∗∗P < 0.01 vs. DMSO-fed elav-GAL4 (b) or DMSO-fed Drs-GFP injected with sucrose (c), #P < 0.05 vs. DMSO-fed elav>Aβ422X (b) or DMSO-fed Drs-GFP injected with DH5α (c); Tukey–Kramer test. Lina: 400 μM linalool; Drs-GFP: GFP-tagged Drosomycin.
3.6. Linalool Reduced Aβ1-42-Induced Oxidative Stress and Gliosis in the Rat Hippocampus
To determine whether these finding extend to a mammalian model of AD, the rat unilateral Aβ1-42 lesion model of AD was used [26]. Aβ1-42 and vehicle were stereotaxically injected into the hippocampus. Linalool was administered intraperitoneally once a day for 7 days before Aβ1-42 injection and for 14 days after Aβ1-42 injection (Figure 6(a)). To investigate effects of linalool on Aβ1-42-induced oxidative damage, we performed immunostaining with an anti-4-HNE antibody in the hippocampal tissues. Immunohistochemical analysis showed that 4-HNE levels were significantly increased in the CA1 layer of the hippocampus of Aβ1-42-treated rats compared to vehicle-treated rats (Figures 6(b) and 6(c)). This Aβ1-42-induced increase of 4-HNE levels was significantly attenuated at 21 days after linalool treatment (50 and 100 mg/kg/day) (Figures 6(b) and 6(c)). Next, we used a GFAP antibody to examine glial reactivity in the hippocampus of Aβ1-42-induced rats. Increased GFAP immunostaining was detected in the hippocampus of Aβ1-42-injected rats as compared to the control. Hypertrophic astrocytes were apparent in the hippocampus of the Aβ1-42-injected rats, while linalool treatment (50 and 100 mg/kg/day) reduced the number of GFAP+ immunoreactive astrocytes (Figures 6(d) and 6(e)). Taken together, these results provide evidence that linalool can prevent Aβ1-42-induced oxidative stress and gliosis in the hippocampus of rats.
Figure 6.
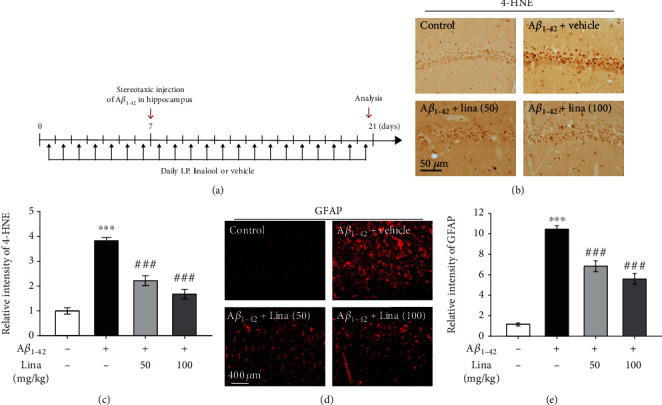
Linalool reduces oxidative stress and gliosis in the hippocampus of Aβ1-42-injected rats. (a) Experimental scheme. (b) Representative images of 4-HNE immunostaining, an indicator of lipid peroxidation showing oxidative stress. (c) Quantification of the intensity of 4-HNE immunostaining in the hippocampal CA1 region (n = 5). Data are presented as mean ± SEM. ∗∗∗P < 0.001 vs. vehicle-treated rats, ###P < 0.001 vs. Aβ1-42-injected rats; Tukey's multiple comparison test. (d) Representative images of GFAP immunostaining in hippocampal CA1 region. (e) Quantification of the intensity of GFAP immunostaining in the hippocampal CA1 region (n = 5). Data are presented as mean ± SEM. ∗∗∗P < 0.001 vs. vehicle-treated rats, ###P < 0.001 vs. Aβ1-42-injected rats; Tukey's multiple comparison test. I.P.: intraperitoneal injection; Lina (50): linalool 50 mg/kg I.P.; Lina (100): linalool 100 mg/kg I.P.
4. Discussion
In our previous study, we discovered linalool as one of the major monoterpenes secreted in forests and that it has the potential to alleviate the cytotoxicity of Aβ [13]. Based on that, in this study, we investigated how linalool exhibits neuroprotective effects using Drosophila models that express human Aβ in neurons or developing eyes as well as an Aβ1-42-injected rat model. As a result, we found that linalool suppressed ROS production and inflammatory response induced by Aβ expression and reduced neurodegeneration. These results are consistent with the results of previous studies showing that linalool has antioxidant and anti-inflammatory properties in mouse models and in vitro assay [17, 33], which suggests that these properties are the main mechanisms underlying the neuroprotective effect of linalool.
Several previous studies have reported the neuroprotective effect of linalool [20, 34]. In fact, linalool mitigates cognitive impairment and brain damage caused by Aβ in a transgenic AD mouse model [23]. Moreover, linalool decreases the amount of Aβ in the brains of mice, but it is unclear whether linalool decreases Aβ production or increases Aβ clearance [23]. The amount of Aβ accumulation is determined by the sum of Aβ production and Aβ clearance in the triple transgenic AD mouse model expressing PS1M146V, tauP301L, and APPswe. However, the Drosophila model used in the present study does not undergo β-amyloidosis and directly expresses Aβ42; therefore, in our model, we only observed whether linalool affects Aβ clearance. As linalool did not affect the accumulation and aggregation of Aβ42 in our model, we believe that linalool does not influence Aβ clearance. Herein, linalool seems to inhibit Aβ production rather than its clearance. Further studies are needed to determine how linalool inhibits Aβ production in the future.
In addition, linalool inhibits not only Aβ accumulation but also free radical formation and inflammatory reaction induced by Aβ [23]. However, from these results alone, it is unknown whether linalool inhibits Aβ formation and consequently inhibits free radical formation and inflammatory responses or whether it inhibits Aβ formation while simultaneously inhibiting free radical formation and inflammatory responses. In other words, it is unclear whether the neuroprotective effects of linalool against Aβ toxicity were entirely attributable to Aβ reduction itself or whether the neuroprotective effects were also affected by antioxidant and anti-inflammatory properties of linalool as well as Aβ reduction. However, as Aβ reduction was not observed in our model, our results clearly demonstrate that linalool acts as a neuroprotective agent regardless of Aβ levels. Similar to our results, linalool reduced apoptosis and oxidative stress and attenuated cognitive deficits in a mouse model in which Aβ was directly injected without β-amyloidosis [24]. Collectively, linalool is expected to inhibit AD progression through various physiological activities.
We found that linalool protects neurons by reducing free radical production and inflammatory responses caused by Aβ42, thus reducing apoptosis. However, the mechanism through which linalool acted to suppress free radical formation and the inflammatory response induced by Aβ42 was unknown. Linalool appeared to regulate ROS through Nrf2 and p21 and suppress NF-κB expression, which is increased by ROS [35, 36]. Linalool is also reported to prevent mitochondrial apoptosis by inhibiting ROS via suppressing the interaction between SIRT3 and SOD2 [37], suggesting that linalool may prevent the production of free radicals by regulating the interaction between SIRT3 and SOD2 and controlling Nrf2 expression [38]. Additionally, a study reported that linalool alleviates the lipopolysaccharide-stimulated inflammatory response through the activation of the Nrf2/HO-1 pathway in BV2 microglia [32]. Linalool not only enhances the expression of Nrf2 and HO-1 but also suppresses the expression of inflammatory mediators such as TNF-α, IL-1β, NO, and PGE 2 [32]. Linalool also showed an inhibitory effect on inflammation via the inhibition of NF-κB activity in mice with allergic asthma caused by ovalbumin exposure [39]. Future studies are warranted to confirm whether linalool reduces the Aβ42-stimulated inflammatory response through the regulation of the Nrf2/HO-1 pathway and NF-κB activity. Moreover, changes in the expression levels of inflammatory mediators TNF-α, IL-1β, and PGE 2 by linalool treatment in AD models should also be addressed.
AD is often accompanied by depression [40], and depression is reported to increase fibrous tangles in AD [41]. Linalool is known to have a beneficial effect on depression, which is one of the main symptoms of AD. In fact, linalool activates GABAA receptors in the brain through smell and reduces depression [42]. When the sense of smell is blocked, linalool cannot activate the GABAA receptor, preventing its antidepressant effect [42]. Further, it was confirmed that there were fewer functional GABAA receptors in the AD brain than in the normal brain [43]. Depression medications have no effect on depression associated with AD, and to date, no medication is available that treats both diseases [44]. However, linalool can suppress depression through the GABAA receptor and AD progression via its antioxidant and anti-inflammatory properties; thus, it may have an effect on AD-related depression.
Recently, many AD drugs have been developed, among which therapeutic agents developed by immunologic approaches targeting Aβ clearing have attracted attention [45, 46]. Among them, both monoclonal antibodies and intravenous immunoglobulin showed significant results in the reduction of Aβ plaques in clinical trials, but the AD patient's cognitive improvement effect was insufficient [47–50]. Therefore, in the treatment of AD, it is possible that therapeutic agents that act on pathological mechanisms, such as inflammatory response and ROS production increased by Aβ in addition to removal of Aβ, may be effective. In particular, for the treatment of multifactorial diseases such as AD, the development of therapeutic agents using natural products such as linalool, which regulates various biological targets, should be considered.
5. Conclusions
In conclusion, the results of this study demonstrate that linalool prevents the neurotoxicity of Aβ by inhibiting free radical production and inflammatory response. Therefore, linalool is expected to be used as a preventive or therapeutic agent for AD.
Acknowledgments
We thank the Bloomington Drosophila Stock Center (Bloomington, IN, USA) and Dr. Fernandez-Funez (University of Florida, USA) for providing Drosophila strains used in this study. We also would like to thank Editage (http://www.editage.co.kr) for English language editing. This work was supported by the Konkuk University Research Fund in 2017 [2017-A019-0215 (K.S.C)].
Contributor Information
So-Yoon Won, Email: sywon@konkuk.ac.kr.
Kyoung Sang Cho, Email: kscho@konkuk.ac.kr.
Data Availability
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest, including any financial, personal, or other relationships, with other people or organizations.
Authors' Contributions
Chunyu Yuan and Myeongcheol Shin performed the experiments and wrote the manuscript; Youngjae Park, Byoungyun Choi, Seokhui Jang, and Chaejin Lim performed the experiments and contributed to the data analysis; Hye Sup Yun and Im-Soon Lee contributed to designing the experiment and the data analysis; and So-Yoon Won and Kyoung Sang Cho designed and supervised the study, wrote the manuscript, and read the final manuscript. Chunyu Yuan, Myeongcheol Shin, and Youngjae Park contributed equally to this work.
References
- 1.Patterson C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers. Alzheimer's Disease International; 2018. [Google Scholar]
- 2.Hardy J. A., Higgins G. A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield D. A., Drake J., Pocernich C., Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends in Molecular Medicine. 2001;7(12):548–554. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 4.Benilova I., Karran E., de Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nature Neuroscience. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 5.Heneka M. T., Carson M. J., Khoury J. E., et al. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe D., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Molecular Medicine. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tariot P. N., Federoff H. J. Current treatment for Alzheimer disease and future prospects. Alzheimer Disease & Associated Disorders. 2003;17(SUPPLEMENT 4):S105–S113. doi: 10.1097/00002093-200307004-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hansen R. A., Gartlehner G., Webb A. P., Moore C. G., Jonas D. E., Jonas D. E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. Clinical Interventions in Aging. 2008;3(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- 9.Howes L. G. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Safety. 2014;37(6):391–395. doi: 10.1007/s40264-014-0161-z. [DOI] [PubMed] [Google Scholar]
- 10.Aharoni A., Jongsma M., Bouwmeester H. Volatile science? Metabolic engineering of terpenoids in plants. Trends in Plant Science. 2005;10(12):594–602. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Loreto F., Bagnoli F., Fineschi S. One species, many terpenes: matching chemical and biological diversity. Trends in Plant Science. 2009;14(8):416–420. doi: 10.1016/j.tplants.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Cho K. S., Lim Y. R., Lee K., Lee J., Lee J. H., Lee I. S. Terpenes from forests and human health. Toxicological Research. 2017;33(2):97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin M., Liu Q. F., Choi B., et al. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila model of Alzheimer’s disease. Biological and Pharmaceutical Bulletin. 2020;43(3):409–417. doi: 10.1248/bpb.b19-00495. [DOI] [PubMed] [Google Scholar]
- 14.Letizia C. S., Cocchiara J., Lalko J., Api A. Fragrance material review on linalool. Food and Chemical Toxicology. 2003;41(7):943–964. doi: 10.1016/S0278-6915(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 15.Pereira I., Severino P., Santos A. C., Souto E. B., Souto E. B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids and Surfaces B: Biointerfaces. 2018;171:566–578. doi: 10.1016/j.colsurfb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Casabianca H., Graff J., Faugier V., Fleig F., Grenier C. Enantiomeric distribution studies of linalool and linalyl acetate. A powerful tool for authenticity control of essential oils. Journal of High Resolution Chromatography. 1998;21(2):107–112. doi: 10.1002/(SICI)1521-4168(19980201)21:2<107::AID-JHRC107>3.0.CO;2-A. [DOI] [Google Scholar]
- 17.Aprotosoaie A. C., Hăncianu M., Costache I. I., Miron A. Linalool: a review on a key odorant molecule with valuable biological properties. Flavour and Fragrance Journal. 2014;29(4):193–219. doi: 10.1002/ffj.3197. [DOI] [Google Scholar]
- 18.Peana A. T., Marzocco S., Popolo A., Pinto A. (−)-linalool inhibits in vitro NO formation: probable involvement in the antinociceptive activity of this monoterpene compound. Life Science. 2006;78(7):719–723. doi: 10.1016/j.lfs.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 19.Kannappan R., Gupta S. C., Kim J. H., Aggarwal B. B., Aggarwal B. B. Neuroprotection by spice-derived nutraceuticals: you are what you eat! Molecular Neurobiology. 2011;44(2):142–159. doi: 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehri S., Meshki M. A., Hosseinzadeh H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug and Chemical Toxicology. 2015;38(2):162–166. doi: 10.3109/01480545.2014.919585. [DOI] [PubMed] [Google Scholar]
- 21.Seol G. H., Kang P., Lee H. S., Seol G. H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurology. 2016;16(1):p. 17. doi: 10.1186/s12883-016-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapczynski A., Letizia C. S., Api A. M. Fragrance material review on d-linalool. Food and Chemical Toxicology. 2008;46(11):S193–S194. doi: 10.1016/j.fct.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 23.Sabogal-Guáqueta A. M., Osorio E., Cardona-Gómez G. P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer's mice. Neuropharmacology. 2016;102:111–120. doi: 10.1016/j.neuropharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P., Wang K., Lu C., et al. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Science. 2017;174:21–27. doi: 10.1016/j.lfs.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Iijima K., Iijima-Ando K. Drosophila models of Alzheimer's amyloidosis: the challenge of dissecting the complex mechanisms of toxicity of amyloid-β 42. Journal of Alzheimer's Disease. 2008;15(4):523–540. doi: 10.3233/JAD-2008-15402. [DOI] [PubMed] [Google Scholar]
- 26.Ryu J. K., Franciosi S., Sattayaprasert P., Kim S. U., McLarnon J. G. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48(1):85–90. doi: 10.1002/glia.20051. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G., Watson C. The rat brain in sterotaxic coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- 28.Haddadi M., Jahromi S. R., Sagar B. K. C., Shivanandappa T., Ramesh S. R., Ramesh S. R. Brain aging, memory impairment and oxidative stress: A study in Drosophila melanogaster. Behavioural Brain Research. 2014;259:60–69. doi: 10.1016/j.bbr.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Urbanc B., Cruz L., le R., et al. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadowaki H., Nishitoh H., Urano F., et al. Amyloid _β_ induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death & Differentiation. 2005;12(1):19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- 31.Huo M., Cui X., Xue J., et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. Journal of Surgical Research. 2013;180(1):e47–e54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Lv O., Zhou F., Wu Z., Zheng Y., Zheng Y. Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochemical Research. 2015;40(7):1520–1525. doi: 10.1007/s11064-015-1629-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu K., Chen Q., Liu Y., Wang X., Wang X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. Journal of Food Science. 2012;77(11):C1156–C1161. doi: 10.1111/j.1750-3841.2012.02924.x. [DOI] [PubMed] [Google Scholar]
- 34.Park H., Seol G. H., Ryu S., Choi I. Y. Neuroprotective effects of (-)-linalool against oxygen-glucose deprivation-induced neuronal injury. Archives of Pharmacal Research. 2016;39(4):555–564. doi: 10.1007/s12272-016-0714-z. [DOI] [PubMed] [Google Scholar]
- 35.Tekeli I. O., Ateşşahin A., Sakin F., Aslan A., Çeribaşı S., Yipel M. Protective effects of conventional and colon-targeted lycopene and linalool on ulcerative colitis induced by acetic acid in rats. Inflammopharmacology. 2019;27(2):313–322. doi: 10.1007/s10787-018-0485-x. [DOI] [PubMed] [Google Scholar]
- 36.Jana S., Patra K., Sarkar S., et al. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: an in vivo study in sarcoma-180 solid tumor model. Nutrition and Cancer. 2014;66(5):835–848. doi: 10.1080/01635581.2014.904906. [DOI] [PubMed] [Google Scholar]
- 37.Kim H., Lee Y. D., Kim H. J., Kim H. H., Kim H. H. SOD2 and Sirt3 control osteoclastogenesis by regulating mitochondrial ROS. Bone and Mineral Research. 2017;32(2):397–406. doi: 10.1002/jbmr.2974. [DOI] [PubMed] [Google Scholar]
- 38.Cheng Y., Dai C., Zhang J. SIRT3-SOD2-ROS pathway is involved in linalool-induced glioma cell apoptotic death. Acta Biochimica Polonica. 2017;64(2):343–350. doi: 10.18388/abp.2016_1438. [DOI] [PubMed] [Google Scholar]
- 39.Kim M. G., Kim S. M., Min J. H., et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. International Immunopharmacology. 2019;74, article 105706 doi: 10.1016/j.intimp.2019.105706. [DOI] [PubMed] [Google Scholar]
- 40.Wragg R. E., Jeste D. V. Overview of depression and psychosis in Alzheimer's disease. The American Journal of Psychiatry. 1989;146(5):577–587. doi: 10.1176/ajp.146.5.577. [DOI] [PubMed] [Google Scholar]
- 41.Rapp M. A., Schnaider-Beeri M., Grossman H. T., et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Archives of General Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 42.Harada H., Kashiwadani H., Kanmura Y., Kuwaki T. Linalool odor-induced anxiolytic effects in mice. Frontiers in Behavioral Neuroscience. 2018;12:p. 241. doi: 10.3389/fnbeh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rissman R. A., de Blas A. L., Armstrong D. M. GABAAreceptors in aging and Alzheimer’s disease. Journal of Neurochemistry. 2007;103(4):1285–1292. doi: 10.1111/j.1471-4159.2007.04832.x. [DOI] [PubMed] [Google Scholar]
- 44.Cassano T., Calcagnini S., Carbone A., et al. Pharmacological treatment of depression in Alzheimer's disease: a challenging task. Frontiers in Pharmacology. 2019;10, article 1067 doi: 10.3389/fphar.2019.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panza F., Lozupone M., Logroscino G., Imbimbo B. P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nature Reviews Neurology. 2019;15(2):73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- 46.Delrieu J., Ousset P. J., Caillaud C., Vellas B. Clinical trials of intravenous immunoglobulin for Alzheimer's disease. Journal of Clinical Immunology. 2012;120(1):186–193. [Google Scholar]
- 47.Yiannopoulou K. G., Anastasiou A. I., Zachariou V., Pelidou S. H. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines. 2019;7(4):p. 97. doi: 10.3390/biomedicines7040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodel R., Neff F., Noelker C., et al. Intravenous immunoglobulins as a treatment for Alzheimerʼs disease. Drugs. 2010;70(5):513–528. doi: 10.2165/11533070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Prins N. D., Scheltens P. Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. Alzheimer's Research & Therapy. 2013;5(6):p. 56. doi: 10.1186/alzrt220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Relkin N. Clinical trials of intravenous immunoglobulin for Alzheimer’s disease. Journal of Clinical Immunology. 2014;34(1):S74–S79. doi: 10.1007/s10875-014-0041-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


