Abstract
In addition to SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection itself, an increase in the incidence of antimicrobial resistance poses collateral damage to the current status of the COVID-19 (coronavirus disease 2019) pandemic. There has been a rapid increase in multidrug-resistant organisms (MDROs), including extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae, carbapenem-resistant New Delhi metallo-β-lactamase (NDM)-producing Enterobacterales, Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus (MRSA), pan-echinocandin-resistant Candida glabrata and multi-triazole-resistant Aspergillus fumigatus. The cause is multifactorial and is particularly related to high rates of antimicrobial agent utilisation in COVID-19 patients with a relatively low rate of co- or secondary infection. Appropriate prescription and optimised use of antimicrobials according to the principles of antimicrobial stewardship as well as quality diagnosis and aggressive infection control measures may help prevent the occurrence of MDROs during this pandemic.
Keywords: COVID-19, Antimicrobial resistance, Antibiotic usage, Multidrug-resistant organism
1. Introduction
Since the first case of COVID-19 (coronavirus disease 2019), initially named 2019 novel coronavirus (2019-nCoV) pneumonia, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in Wuhan, China, the disease has affected more than 32.7 million people, with over 1 million fatalities worldwide [1,2]. To combat this global health crisis, every health authority developed and implemented numerous infection control and prevention measures, including mask wearing, practicing good hand hygiene, social distancing, avoiding crowded areas, active identification and quarantine of close contacts, and rapid set up of shelter hospitals and lockdown strategies [3], [4], [5], [6], [7]. These aggressive management measures in response to COVID-19 brought additional benefits in terms of reducing other infections. Many studies have reported a decreased incidence of several infectious respiratory diseases, such as seasonal influenza, invasive pneumococcal disease and tuberculosis during the COVID-19 pandemic compared with the same period in previous years [8], [9], [10], [11]. However, we cannot neglect the increased incidence of antimicrobial resistance, which may be attributed to the excess use of antimicrobial agents during the COVID-19 pandemic [12].
2. Increasing incidence of antimicrobial resistance
Several recent reports have described an increase in multidrug-resistant organisms (MDROs) during the COVID-19 pandemic [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. A retrospective study found that the incidence of carbapenem-resistant Enterobacterales colonisation increased from 6.7% in 2019 to 50% in March–April 2020 [13]. The underlying reasons included the high intensity of care required for patients, the susceptibility of 4–5 healthcare workers (HCWs) with prolonged contact with the patient, and the presence of 32 new HCWs lacking work experience in an intensive care unit (ICU) setting [13]. In Wuhan, China, Li et al. demonstrated that among 159 strains of bacteria isolated form 102 hospitalised COVID-19 patients with acquired secondary bacterial infections, Acinetobacter baumannii was the most common pathogen (35.8%; n = 57), followed by Klebsiella pneumoniae (30.8%; n = 49) and Stenotrophomonas maltophilia (6.3%; n = 10) [14]. Moreover, the carbapenem resistance rate for A. baumannii and K. pneumoniae was 91.2% and 75.5%, respectively [14]. Another retrospective study in a French ICU found that 26 COVID-19 patients who were admitted to the ICU for acute respiratory failure were considered to be co-infected with a pathogenic bacterium, and two and five isolates were resistant to third-generation cephalosporins and amoxicillin/clavulanate, respectively [15]. Similarly, a cohort of 19 COVID-19 patients who required ICU admission had secondary infection due to 17 multidrug-resistant A. baumannii and 1 methicillin-resistant Staphylococcus aureus (MRSA) [16]. Fu et al. observed five cases of critical COVID-19 patients with secondary infections, in which extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae, S. maltophilia, Burkholderia cepacia and Pseudomonas aeruginosa were considered the causative pathogens [17]. Nori et al. reported five COVID-19 patients from New York (USA) who were co-infected with New Delhi metallo-β-lactamase (NDM)-producing Enterobacter cloacae [18]. Farfour et al. reported an outbreak of NDM-5-producing Escherichia coli involving six COVID-19 patients in a 12-bed ICU in France [19].
Fungal co-infections in COVID-19 patients pose a common challenge for clinical treatment. Posteraro et al. reported a complicated 53-day clinical course of a COVID-19 patient with type 2 diabetes mellitus who developed three episodes of secondary bloodstream infection by MRSA, Morganella morganii and potentially fatal Candida glabrata [20]. The development of FKS-associated pan-echinocandin resistance was detected in C. glabrata isolated after 13 days of caspofungin treatment [20]. In New Delhi (India), candidaemia affected 15 critical COVID-19 patients during April–July 2020 and multidrug-resistant Candida auris accounted for 10 cases and 6 deaths [21]. Mohamed et al. reported on an Irish patient with severe COVID-19 pneumonia that was complicated by a fatal co-infection with a multi-triazole-resistant strain of Aspergillus fumigatus [22].
3. High rate of antibiotic utilisation
In light of the COVID-19 pandemic, it has been reported that the necessity for the use of antimicrobial agents has increased compared with previous years. In an early report of 99 cases in China, more than 70% of patients with COVID-19 had received antibiotic treatment, approximately 15% of whom received antifungal agents [24]. Another study including 138 hospitalised patients showed that moxifloxacin, ceftriaxone and azithromycin were prescribed in 89 (64.5%), 34 (24.6%) and 25 (18.1%) patients, respectively [25]. A large-scale study showed that 58% of 1099 patients received intravenous (i.v.) antibiotics [26], whereas in a smaller Brazilian cohort of 72 hospitalised patients 84.7% had received i.v. antibiotic therapy [27]. Additionally, a recent review showed that 72.1% (1450/2010) of hospitalised COVID-19 patients received antibiotics [28]. Overall, more than one-half of COVID-19 patients may receive an i.v. antibiotic, and this number can be higher in patients with severe disease.
This high rate of antibiotic utilisation for patients infected with SARS-CoV-2, particularly in severe COVID-19 cases, could be due to the following: (i) as the prevalent presentations of SARS-CoV-2 infection (cough, fever and radiological infiltrates) are also hallmarks of community-acquired bacterial pneumonia, clinicians empirically add a broad-spectrum antibiotic despite the suspicion of a viral origin; (ii) the anxiety and uncertainty regarding the COVID-19 outbreak as well as the absence of effective anti-SARS-CoV-2 treatments are potential drivers of widespread and excessive prescription of antibiotics; and (iii) co-bacterial, fungal or secondary infection with COVID-19 is possible; however, it is difficult to differentiate between sole SARS-CoV-2 infection and co- or secondary infections.
4. Variable rate of co-infection
Patients with COVID-19 could be vulnerable to other infections owing to multiple co-morbidities in patients with severe COVID-19, prolonged hospitalisation and SARS-CoV-2-associated immune dysfunction [29,30]. However, the prevalence of co-infection among COVID-19 patients is considerably lower due to the frequency of antibiotic use for patients with SARS-CoV-2 infection. In the UK, a retrospective study involving 836 patients with SARS-CoV-2 infection showed 3.2% of patients (n = 27) to have confirmed bacterial infection within the first 5 days after admission, the incidence of which rose to 6.1% (n = 55) for the duration of admission [16]. Li et al. showed that 6.8% (n = 102) of 1495 patients hospitalised with COVID-19 in Wuhan, China, had acquired secondary bacterial infection during hospitalisation [14]. A review by Rawson et al. noted that only 8% (n = 62) of 806 COVID-19 patients had superimposed bacterial or fungal co-infections [28]. Another review indicated that the prevalence of co-infection among COVID-19 patients varied; however, it could be up to 50% among non-survivors [31]. At present, many micro-organisms have been reported as the co-pathogens, including Streptococcus pneumoniae, S. aureus, K. pneumoniae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, E. coli, P. aeruginosa, S. maltophilia, A. baumannii, Mycobacterium tuberculosis, Candida spp., Aspergillus spp. and viruses such as influenza, coronavirus, rhinovirus/enterovirus, parainfluenza virus, metapneumovirus, influenza B virus and human immunodeficiency virus (HIV) [15,28,[31], [32], [33], [34]]. However, the majority of MDROs developed in patients with severe or critical COVID-19 and the incidence of co- or secondary infection may increase, resulting in prolonged hospitalisation [16,18,20,25,28,31].
5. The scenario in Taiwan
The increase in the incidence of antimicrobial resistance was reported in sites with a high burden of severe or critical COVID-19 cases [14], [15], [16], [17], [18], [19], including New York (USA), Wuhan (China), France, Iran and Brazil during the earlier pandemic period. In Taiwan, as of 10 October 2020, a total of 527 laboratory-confirmed COVID-19 cases, including 55 indigenous cases and 7 deaths, were reported (Taiwan Centers for Diseases Control and Prevention; https://www.cdc.gov.tw/En). The low incidence (2.2 per 100 000 population) of COVID-19 in Taiwan could be due to the implementation of many infection control and prevention measures [35]. Since the end of 2019, the healthcare authority made a quick response, including surveillance, laboratory diagnosis, border control, control of community transmission, medical system response and preparedness, stockpile and allocation of personal protective equipment and other medical supplies, health education and disinformation management. Furthermore, the government rapidly established a Central Epidemic Command Center (CECC) to co-ordinate inter-ministerial responses, to mobilise resources and to conduct daily press briefings. By the effort of the CECC, people can obtain enough masks to use and adequate antiseptics for hand hygiene, which can effectively prevent droplet and contact transmission [35].
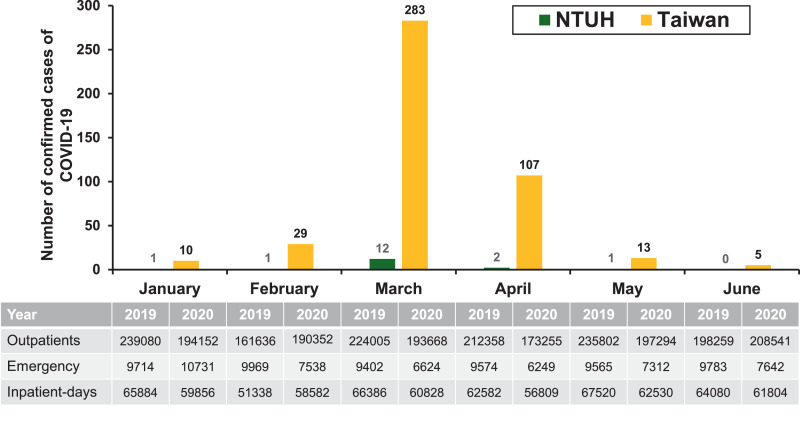
The National Taiwan University Hospital (NTUH) is a 2500-bed, academically-affiliated medical centre that provides primary and tertiary care in northern Taiwan. As of 10 October 2020, only 18 patients with COVID-19 were treated at the hospital, of whom 11 (61.1%) had pneumonia and 4 (22.2%) were admitted to the ICU. No HCWs at the hospital were reported to be infected with SARS-CoV-2. Fig. 1 shows the monthly number of confirmed cases of COVID-19 in Taiwan (n = 515; 12 were diagnosed between 1 July 2020 and 10 October 2020) and those treated at NTUH (n = 17; 1 was diagnosed in August 2020) during January–June 2020. The patient census between January–June 2019 and January–June 2020 was 1 271 140 and 1 157 262 for outpatient clinics, respectively, and 58 007 and 46 096 for the emergency department, respectively (Fig. 1). The numbers of inpatient-days at the hospital between these periods were 377 790 and 360 409, respectively. Overall, the number of patients hospitalised between these periods was similar. However, higher consumption of antibiotics, including β-lactam/β-lactamase inhibitor combinations, quinolones, carbapenems, colistin, tigecycline, fosfomycin, glycopeptides, linezolid and daptomycin, was observed during January–June 2020 compared with January–June 2019 (Table 1 ).
Fig. 1.
Monthly number of confirmed cases of COVID-19 in Taiwan (n = 447) and those treated at National Taiwan University Hospital (NTUH) (n = 17) during January–June 2020. The patient census of outpatient clinics, emergency department and inpatient-days of hospitalisation of NTUH during January–June 2019 and January–June 2020.
Table 1.
Consumptions of broad-spectrum antimicrobial agents at National Taiwan University Hospital during two time periods: January–June 2019 and January–June 2020
| Antibiotic | Antibiotic consumption (DDDs/1000 patient-days) by indicated time period |
% change | |
|---|---|---|---|
| January–June 2019 | January–June 2020 | ||
| β-Lactam/β-lactamase inhibitor combinations a | 372.3 | 387.6 | 4.1 |
| Extended-spectrum cephalosporins b | 763.5 | 763.7 | 0.0 |
| Quinolones c | 182.3 | 201.8 | 10.7 |
| Carbapenems d | 330.4 | 376 | 13.8 |
| Aminoglycosides e | 237 | 221.3 | –6.6 |
| Colistin | 63.3 | 78.4 | 23.9 |
| Tigecycline | 56.3 | 89.7 | 59.3 |
| Fosfomycin | 23.7 | 41.4 | 74.7 |
| Glycopeptides f | 340.1 | 384 | 12.9 |
| Linezolid | 12.1 | 15.4 | 27.3 |
| Daptomycin | 77.3 | 95.1 | 23.0 |
DDD, defined daily doses.
Including cefoperazone/sulbactam, piperacillin/tazobactam and ceftazidime/avibactam.
Including cefotaxime, cefoperazone, flomoxef, ceftazidime, ceftriaxone and cefepime.
Including ciprofloxacin, moxifloxacin and levofloxacin.
Including imipenem, meropenem, doripenem and ertapenem.
Including gentamicin, amikacin and streptomycin.
Including vancomycin and teicoplanin.
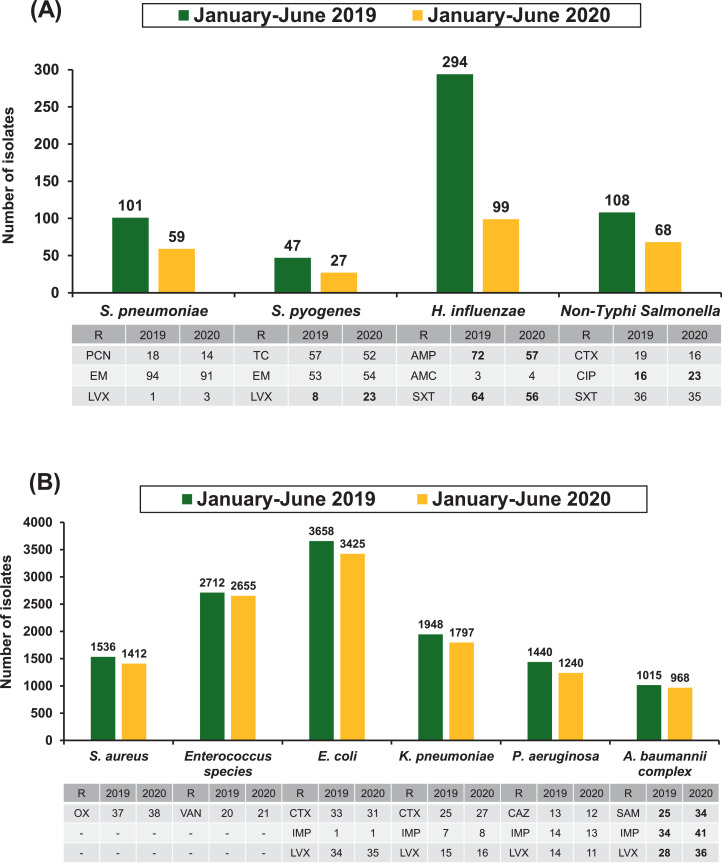
Fig. 2 illustrates the number and rate of antimicrobial-resistant infections of clinically important bacterial species recovered from patients who visited NTUH during the period January–June in 2019 and 2020. The number of isolates of S. pneumoniae, Streptococcus pyogenes, Haemophilus influenzae and non-Typhi Salmonella spp. notably decreased during these periods (Fig. 2A). These findings were partly consistent with those of other studies [9,11]. However, the number of isolates of S. aureus, Enterococcus spp., E. coli, K. pneumoniae, P. aeruginosa and A. baumannii complex remained stable (Fig. 2B). In general, the resistance rates of the selected antibiotics remained constant between the two time periods (≤5% difference), with the exception of a decline in ampicillin and trimethoprim/sulfamethoxazole resistance of H. influenzae (15% and 9% difference, respectively) and an increase in levofloxacin resistance of S. pyogenes (15% difference), ciprofloxacin resistance of non-Typhi Salmonella spp. (7% difference), and ampicillin/sulbactam, imipenem and levofloxacin resistance of A. baumannii complex isolates (9%, 7% and 8% difference, respectively). The rate (per 10 000 inpatient-days) of ampicillin/sulbactam-, imipenem- and levofloxacin-resistant A. baumannii complex infection was 43.7, 59.5 and 49.0, respectively, during January–June 2019 and was 71.4, 86.1 and 75.6, respectively, during January–June 2020. An increase in the incidence of antimicrobial resistance during the COVID-19 pandemic was observed for A. baumannii complex isolates at NTUH despite strict implementation of infection control measures and an antibiotic stewardship programme. However, further long-term study is warranted to investigate the association between the use of antibiotics and antimicrobial resistance in COVID-19 cases.
Fig. 2.
Comparison of the number and rate of antimicrobial-resistant infections of clinically important bacterial species recovered from various clinical sources of patients treated at National Taiwan University Hospital (NTUH) between January–June 2019 and January–June 2020: (A) Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae and non-Typhi Salmonella spp. (predominantly associated with community-acquired infections); and (B) Staphylococcus aureus, Enterococcus spp., Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii complex (causing community- and hospital-acquired infections). R, resistant to the indicated antibiotic; PCN, penicillin; EM, erythromycin; LVX, levofloxacin; TC, tetracycline; AMP, ampicillin; AMC, amoxicillin/clavulanate; SXT, trimethoprim/sulfamethoxazole; CTX, cefotaxime; CIP, ciprofloxacin; OX, oxacillin; VAN, vancomycin; IMP, imipenem; CAZ, ceftazidime; SAM, ampicillin/sulbactam. Differences in rates of resistance >5% between two time periods are indicated in boldface.
Of the 18 patients with COVID-19 treated at NTUH, 3 exhibited co-infections. Two patients with COVID-19 suffered complications with pneumonia and co-infection with a multidrug-resistant A. baumannii complex isolate (resistant to ampicillin/sulbactam, levofloxacin, amikacin, cefepime and imipenem but susceptible to colistin) and Streptococcus dysgalactiae, respectively, while the other patient was co-infected with influenza virus B.
6. The resolution
Combating an increase in the incidence of antimicrobial resistance during the COVID-19 pandemic remains a great challenge. First, reducing inappropriate antibiotic prescription remains the most effective way to tackle antimicrobial resistance, and international or local guidelines always advise restrictive antibiotic prescribing for respiratory tract infections. However, non-adherence to or poor uptake of local and international guidelines was commonly reported in previous studies [36], [37], [38], [39]. Moreover, unnecessary use of antimicrobial agents would be associated with a significant economic burden on healthcare systems, which could be directly caused by the drug itself and indirectly caused by healthcare costs for the management of drug-related adverse events. Although the ongoing pandemic is stretching the limits of optimal antibiotic stewardship, continuing this intervention to curb inappropriate antibiotic usage and surveying the reasons for guideline non-adherence should be conducted within hospitals [40]. Second, early detection of possible respiratory pathogens using newly developed syndromic multiplex panels that incorporate SARS-CoV-2 is fundamental [41]. However, diagnosis of other respiratory pathogens through old syndromic multiplex panels cannot definitively rule out COVID-19 at this stage of the pandemic. Third, due to most MDROs developing in critical COVID-19 patients with prolonged ICU admission or hospitalisation, every hospital should continue implementing bundle care for prevention of device-associated infections within the hospital, including ventilator-associated pneumonia, catheter-associated bloodstream infection and catheter-associated urinary tract infection. Fourth, infection prevention initiatives fostered among HCWs could increase awareness of effective hand washing, cleaning equipment after use and appropriate use of personal protective equipment, which could subsequently reduce healthcare-acquired infections with MDROs [42,43]. Finally, healthcare authorities and hospital administrators should be responsible for providing adequate personal protective equipment, particularly masks and gloves, and hand sanitiser and disinfection to prevent nosocomial transmission of MDROs. Concurrently, the infection control department should continue monitoring the prevalence of antibiotic-resistant bacteria and the possible outbreak of MDROs (Table 2 ).
Table 2.
The resolution for high rates of antimicrobial resistance and utilisation and unresolved issues during the COVID-19 pandemic
| Issue | Resolution | Unresolved |
|---|---|---|
| High antimicrobial resistance |
|
|
| High antibiotic utilisation |
|
|
COVID-19, coronavirus disease 2019; HCW, healthcare worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
7. Unresolved issues
The greatest barrier to appropriate use of antimicrobial agents is that clinicians cannot definitively diagnose a co-pathogen with a SARS-CoV-2 infection, or easily identify the precise co-pathogen in a severe COVID-19 patient (Table 1). Although we can use the newly developed SARS-CoV-2 incorporated syndromic multiplex panels to detect SARS-CoV-2 and other common respiratory pathogens in a short time, these kits are not available in most conventional laboratories. Additionally, although procalcitonin (PCT) has been proposed as one of the promising biomarkers for differentiating bacteria and other pathogens, the usefulness of PCT in COVID-19 patients remains unclear [44]. Among patients with severe COVID-19, an elevated level of PCT was common and could be associated with adverse outcomes [44], [45], [46]. However, a previous meta-analysis of 32 randomised controlled trials showed that the use of PCT to guide initiation and duration of antibiotic treatment in patients with acute respiratory tract infection could result in a lower risk of death, antibiotic utilisation and antibiotic-associated adverse events [47,48]. Therefore, the use of PCT in the context of antibiotic stewardship may be applied when caring for severe COVID-19 patients. Based on the experience of influenza patients, PCT has a high sensitivity, particularly in ICU patients. However, it has a low specificity for identifying secondary bacterial infections and may not be sufficient as a stand-alone marker for discontinuing antibiotics [49,50]. Thus far, there is little associated research regarding the use of PCT in COVID-19 patients [48,51]. Further study is warranted to define the role of PCT in COVID-19 patients.
8. Conclusions
The emergence of antimicrobial resistance is an unforeseen and unavoidable consequence of the COVID-19 pandemic. The cause is multifactorial, particularly a high rate of antimicrobial agent utilisation in COVID-19 patients with relatively low rates of co- or secondary infections. Appropriate prescription and optimised use of antimicrobials according to the principles of antimicrobial stewardship, together with quality diagnosis and aggressive infection control measures, may prevent the occurrence of MDROs during this pandemic.
Funding
None.
Competing interests: None declared.
Ethical approval: Not required.
Editor: Professor Geoffrey Coombs
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19) weekly epidemiological update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [accessed 5 October 2020].
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Georgeo M.R., De Georgeo J.M., Egan T.M., Klee K.P., Schwemm M.S., Bye-Kollbaum H., et al. Containing SARS-CoV-2 in hospitals facing finite PPE, limited testing, and physical space variability: navigating resource constrained enhanced traffic control bundling. J Microbiol Immunol Infect. 2021;54:4–11. doi: 10.1016/j.jmii.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H.T., Chen T.C., Liu T.Y., Chiu C.F., Hsieh W.C., Yang C.J., et al. How to prevent outbreak of a hospital-affiliated dementia day-care facility in the pandemic COVID-19 infection in Taiwan. J Microbiol Immunol Infect. 2020;53:394–395. doi: 10.1016/j.jmii.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C.C., Wang J.H., Ko W.C., Yen M.Y., Lu M.C., Lee C.M., et al. COVID-19 in long-term care facilities: an upcoming threat that cannot be ignored. J Microbiol Immunol Infect. 2020;53:444–446. doi: 10.1016/j.jmii.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Chen Q., Feng L., Rodewald L., Xia Y., Yu H., et al. Active case finding with case management: the key to tackling the COVID-19 pandemic. Lancet. 2020;396:63–70. doi: 10.1016/S0140-6736(20)31278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S., Zhang Z., Yang J., Wang J., Zhai X., Bärnighausen T., et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto H., Ishikane M., Ueda P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. JAMA. 2020;323:1969–1971. doi: 10.1001/jama.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juan H.C., Chao C.M., Lai C.C., Tang H.J. Decline in invasive pneumococcal disease during COVID-19 pandemic in Taiwan. J Infect. 2021;82:282–327. doi: 10.1016/j.jinf.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai C.C., Yu W.L. The COVID-19 pandemic and tuberculosis in Taiwan. J Infect. 2020;81:e159–e161. doi: 10.1016/j.jinf.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh C.C., Lin C.H., Wang W.Y.C., Pauleen D.J., Chen J.V. The outcome and implications of public precautionary measures in Taiwan—declining respiratory disease cases in the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17:4877. doi: 10.3390/ijerph17134877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossato L., Negrão F.J., Simionatto S. Could the COVID-19 pandemic aggravate antimicrobial resistance? Am J Infect Control. 2020;48:1129–1130. doi: 10.1016/j.ajic.2020.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiri B., Sensi E., Marsiliani V., Cantarini M., Priante G., Vernelli C., et al. Antimicrobial stewardship program, COVID-19, and infection control: spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J Clin Med. 2020;9:E2744. doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Wang J., Yang Y., Cai P., Cao J., Cai X., et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9:153. doi: 10.1186/s13756-020-00819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contou D., Claudinon A., Pajot O., Micaëlo M., Longuet Flandre P., Dubert M., et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-Ardakani R., Koohpaei A., et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dis. 2020;20:646. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y., Yang Q., Xu M., Kong H., Chen H., Fu Y., et al. Secondary bacterial infections in critical ill patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7:ofaa220. doi: 10.1093/ofid/ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nori P., Szymczak W., Puius Y., Sharma A., Cowman K., Gialanella P., et al. Emerging co-pathogens: New Delhi metallo-β-lactamase producing Enterobacteriaceae infections in New York City COVID-19 patients. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farfour E., Lecuru M., Dortet L., Guen M.L., Cerf C., Karnycheff F., et al. Carbapenemase-producing Enterobacterales outbreak: another dark side of COVID-19. Am J Infect Control. 2020;48:1533–1536. doi: 10.1016/j.ajic.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posteraro B., Torelli R., Vella A., Leone P.M., De Angelis G., De Carolis E., et al. Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. J Fungi (Basel) 2020;6:163. doi: 10.3390/jof6030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg Infect Dis. 2020;26:2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamed A., Hassan T., Trzos-Grzybowska M., Thomas J., Quinn A., O'Sullivan M., et al. Multi-triazole-resistantAspergillus fumigatus and SARS-CoV-2 co-infection: a lethal combination. Med Mycol Case Rep. 2021;31:11–14. doi: 10.1016/j.mmcr.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teich V.D., Klajner S., Santiago de Almeida F.A., Batista Dantas A.C., Laselva C.R., Torritesi M.G., et al. Epidemiologic and clinical features of patients with COVID-19 in Brazil. Einstein (Sao Paulo) 2020;18:eAO6022. doi: 10.31744/einstein_journal/2020AO6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrill A., Tsao T., Dong V., Huy N.T. SARS-CoV-2-induced immunodysregulation and the need for higher clinical suspicion for co-infection and secondary infection in COVID-19 patients. J Microbiol Immunol Infect. 2021;54:105–108. doi: 10.1016/j.jmii.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai C.C., Wang C.Y., Hsueh P.R. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020;53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai C.C., Yu W.L. COVID-19 associated with pulmonary aspergillosis: a literature review. J Microbiol Immunol Infect. 2021;54:46–53. doi: 10.1016/j.jmii.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilbrun S.C., Mathurin L., Pape J.W., Fitzgerald D., Walsh K.F. Case report: Multidrug-resistant tuberculosis and COVID-19 coinfection in Port-au-Prince, Haiti. Am J Trop Med Hyg. 2020;103:1986–1988. doi: 10.4269/ajtmh.20-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yousaf Z., Khan A.A., Chaudhary H.A., Mushtaq K., Parengal J., Aboukamar M., et al. Cavitary pulmonary tuberculosis with COVID-19 coinfection. IDCases. 2020;22:e00973. doi: 10.1016/j.idcr.2020.e00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai C.C., Yen M.Y., Lee P.I., Hsueh P.R. How to keep COVID-19 at bay: a Taiwan perspective. J Epidemiol Glob Health. 2020;11:1–5. doi: 10.2991/jegh.k.201028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanovska V., Hek K., Mantel Teeuwisse A.K., Leufkens H.G., Nielen M.M., van Dijk L. Antibiotic prescribing for children in primary care and adherence to treatment guidelines. J Antimicrob Chemother. 2016;71:1707–1714. doi: 10.1093/jac/dkw030. [DOI] [PubMed] [Google Scholar]
- 37.Saust L.T., Bjerrum L., Siersma V., Arpi M., Hansen M.P. Quality assessment in general practice: diagnosis and antibiotic treatment of acute respiratory tract infections. Scand J Prim Health Care. 2018;36:372–379. doi: 10.1080/02813432.2018.1523996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouwels K.B., Dolk F.C.K., Smith D.R.M., Robotham J.V., Smieszek T. Actual versus ‘ideal’ antibiotic prescribing for common conditions in English primary care. J Antimicrob Chemother. 2018;73(Suppl 2):19–26. doi: 10.1093/jac/dkx502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulliford M.C., Dregan A., Moore M.V., Ashworth M., Staa T.V., McCann G., et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Getahun H., Smith I., Trivedi K., Paulin S., Balkhy H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020;98:442. doi: 10.2471/BLT.20.268573. –442A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai C.C., Wang C.Y., Ko W.C., Hsueh P.R. In vitro diagnostics of coronavirus disease 2019: technologies and application. J Microbiol Immunol Infect. 2020 Jun 5 doi: 10.1016/j.jmii.2020.05.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole J., Barnard E. The impact of the COVID-19 pandemic on healthcare acquired infections with multidrug resistant organisms. Am J Infect Control. 2020 Oct 1 doi: 10.1016/j.ajic.2020.09.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell B.G., Russo P.L., Kiernan M., Curryer C. Nurses’ and midwives’ cleaning knowledge, attitudes and practices: an Australian study. Infect Dis Health. 2021;26:55–62. doi: 10.1016/j.idh.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vazzana N., Dipaola F., Ognibene S. Procalcitonin and secondary bacterial infections in COVID-19: association with disease severity and outcomes. Acta Clin Belg. 2020 Sep 23 doi: 10.1080/17843286.2020.1824749. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Mori S., Ai T., Otomo Y. Characteristics, laboratories, and prognosis of severe COVID-19 in the Tokyo metropolitan area: a retrospective case series. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krause M., Douin D.J., Tran T.T., Fernandez-Bustamante A., Aftab M., Bartels K. Association between procalcitonin levels and duration of mechanical ventilation in COVID-19 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuetz P., Wirz Y., Sager R., Christ-Crain M., Stolz D., Tamm M., et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10 doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, d-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937175. 1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu M.H., Lin C.C., Huang S.L., Shih H.M., Wang C.C., Lee C.C., et al. Can procalcitonin tests aid in identifying bacterial infections associated with influenza pneumonia? A systematic review and meta-analysis. Influenza Other Respir Viruses. 2013;7:349–355. doi: 10.1111/j.1750-2659.2012.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfister R., Kochanek M., Leygeber T., Brun-Buisson C., Cuquemelle E., Machado M.B., et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care. 2014;18:R44. doi: 10.1186/cc13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2020 Sep 15 doi: 10.1136/bmjebm-2020-111536. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]