Abstract
Johne’s disease, caused by Mycobacterium avium subsp. paratuberculosis (MAP), is a chronic enteritis of ruminants. Previous studies have shown that programmed death-ligand 1 (PD-L1) is associated with the disease progression, and PD-L1 blockade activates MAP-specific Th1 responses in vitro. Here, we performed anti-PD-L1 antibody administration using 2 MAP-infected cattle at the late subclinical stage of infection. After administration, bacterial shedding was reduced or maintained at a low level. Additionally, MAP-specific Th1 cytokine production was upregulated, and CD69 expression was increased in T cells. Collectively, the treatment has a potential as a novel control method against Johne’s disease.
Keywords: cattle, immunotherapy, Johne’s disease, programmed death-ligand 1, Th1 response
Johne’s disease (paratuberculosis) is a chronic enteritis of ruminants, and is caused by the bacteria Mycobacterium avium subsp. paratuberculosis (MAP). The clinical signs of Johne’s disease include chronic diarrhea, severe weight loss, reduced milk production, and mortality [17]. Johne’s disease is endemic in many countries, including Japan [13]. In an early stage of infection, MAP induces strong Th1 responses that cause the activation of macrophages to kill intracellular mycobacteria [4, 21, 22]. Th1 cytokines, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, are important for enhancing bactericidal activity of macrophages with the production of reactive oxygen and nitrogen species, T cell activation, and dendritic cell maturation [8, 12]. During the late subclinical stage, the Th1 response declines, which allows bacterial growth and progression to clinical disease [1, 2, 24]. Therefore, the Th1 response is essential for the prevention of the disease progression.
Programmed death (PD)-1 is one of the immunoinhibitory receptors expressed on T cells, and induces immunosuppression by binding to PD-ligand 1 (PD-L1) [11]. In chronic infections, the upregulation of PD-1 and PD-L1 expression is involved in the exhaustion of antigen-specific T cells which contributes to the disease progression [11, 25]. During human tuberculosis that is caused by Mycobacterium tuberculosis, the PD-1/PD-L1 pathway inhibits effector function of T cells [9], and PD-1 expression on M. tuberculosis-specific CD4+ T cells is associated with bacterial loads [5]. Previous studies have shown that the PD-1/PD-L1 pathway is involved in the suppression of Th1 responses in cattle infected with MAP [16]. The blockade of PD-L1 using a specific antibody (Ab) increases MAP-specific Th1 immune responses in vitro [18]. Thus, the PD-1/PD-L1 pathway is considered to have a therapeutic potential for Johne’s disease. In addition, previous studies have demonstrated that anti-PD-L1 rat-bovine chimeric antibody (chAb) has therapeutic effects against other chronic bovine infections, such as bovine leukemia virus (BLV) infection and Mycoplasma bovis infection [7, 15, 19]. However, there is no report which evaluates the function of PD-L1 blockade in MAP-infected animals. Therefore, in this study, we performed the administration of anti-PD-L1 chAb using 2 MAP experimentally-infected cattle to examine the responses to the antibody administration by immunological and bacteriological analyses.
For the experimental infection of MAP, 2 male Holstein calves, animals #80 (3 weeks of age) and #99 (a week of age), were orally inoculated with intestinal tissue homogenate from an infected cow containing MAP (#80: 1.36 × 108 CFU; #99: 2.50 × 108 CFU) which was measured by using Middlebrook 7H10 agar-based slants as described in a previous paper [10]. Both animals were sourced from farms with no history of paratuberculosis and confirmed negative by fecal quantitative polymerase chain reaction (qPCR) targeting MAP-specific gene IS900 as described previously [10] and by Pourquier ELISA (Institut Pourquier, Montpellier, France) prior to inoculation with MAP. Animals #80 (770 kg, 212 weeks post-infection) and #99 (320 kg, 47 weeks post-infection) were intravenously administered with 2 mg/kg of the purified anti-PD-L1 chAb (Boch4G12) [15] a time and three times at 2 week-intervals, respectively. Both animals were kept in a biosafety level 2 animal facility at the National Institute of Animal Health, Tsukuba, Japan. All experiments using these animals were approved by the National Institute of Animal Health Ethics Committee (approval No. 17-077-2 and 18-077). After the experimental infection, we collected blood and fecal samples at intervals of 2–4 weeks, and monitored IFN-γ production responded to Johnin purified protein derivative (J-PPD) by whole-blood cultures as described previously [16], the serum levels of Abs against MAP by Pourquier ELISA, and fecal shedding of MAP by qPCR. To examine the effects of anti-PD-L1 Ab in MAP-infected cattle, blood samples were collected from animal #80 on the day of administration (day 0), and on several points after administration (days 1, 3, 8, 15, 29, 43, 57, and 85). Blood samples on day 0 were obtained before administration. Blood samples were collected from animal #99 on days 0, 7, 14, 21, 28, 42, 56, 70, 84, 98, and 112. Blood samples on days 0, 14, and 28 were obtained before administration. Peripheral blood mononuclear cells (PBMCs) were purified from blood samples using density gradient centrifugation on Percoll (GE Healthcare, Little Chalfont, UK), and cultured with 2 µg/ml of J-PPD or 20 µg/ml of concanavalin A (Con A; Sigma-Aldrich, St. Louis, MO, USA). Phosphate buffered saline (PBS) and PPD from Mycobacterium bovis BCG strain (B-PPD) were used as a negative control and a control antigen, respectively. After 6 days, collected culture supernatants were assayed for IFN-γ and TNF-α by Bovine IFN-γ ELISA Development Kit (Mabtech, Nacka Strand, Sweden) and Bovine TNF alpha Do-It Yourself ELISA (Kingfisher Biotech, St. Paul, MN, USA), respectively. Additionally, the isolation of T cells (CD3+ cells) from PBMCs was performed according to a previous report [15], and the purity of CD3+ cells (>95%) was confirmed using FACS Verse (BD Biosciences, San Jose, CA, USA). To quantitate gene expression levels of IFN-γ, TNF-α, and CD69, an activation marker of lymphocytes, qPCR was conducted as described previously [18]. Primers for IFN-γ, TNF-α, and ACTB were shown in a previous report [18]. Primers for CD69 were 5′-ATA GCT CTC GTT GCT CTA TCA GTG-3′ and 5′-CCT TGT GTC CAA TCC AAT CA-3′.
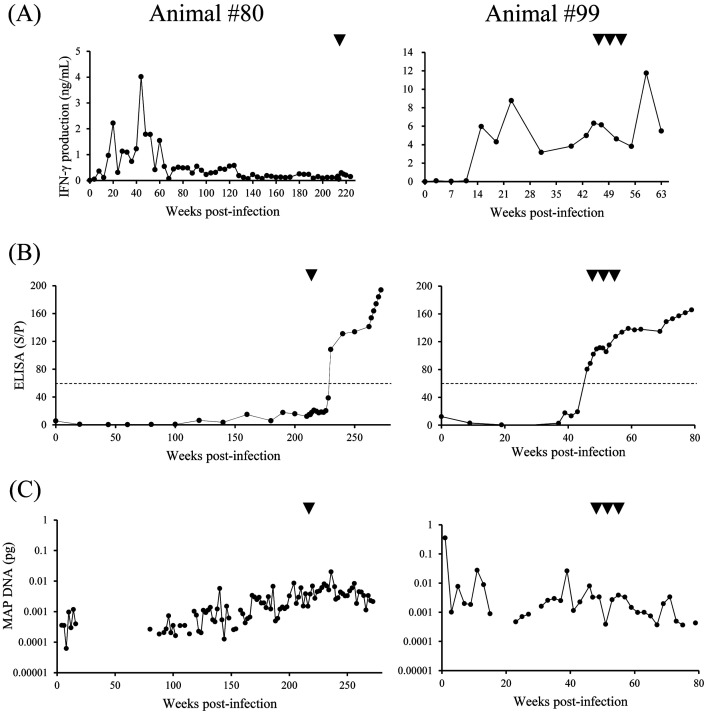
In animal #80, J-PPD-specific IFN-γ response peaked during the acute phase of infection (Fig. 1A). This response was gradually suppressed from approximately 60 weeks post-inoculation, and was maintained at a low level until the time of administration (Fig. 1A). In contrast, the serum levels of Abs against MAP were increased from 160 weeks post-infection (Fig. 1B). The tentative shedding of MAP was observed in feces of animal #80 from 4 to 16 weeks post-infection (Fig. 1C). Fecal shedding was not detected between 18–78 weeks, and then the MAP DNA quantity gradually increased from 80 weeks post-infection (Fig. 1C). In animal #99, J-PPD-specific IFN-γ response peaked at 23 weeks after the experimental infection, and was maintained at a high level (Fig. 1A). The serum levels of Abs against MAP were increased and then turned positive at 46 weeks post-infection, a week before the administration (Fig. 1B). We observed bacterial shedding intermittently until the time of administration (Fig. 1C). Although both animals #80 and #99 showed the bacterial shedding, these animals did not show clinical symptoms such as diarrhea. The serum levels of Abs against MAP was increasing before anti-PD-L1 Ab administration. A previous report has described that detectable levels of Abs against MAP return in mid- to late-stage subclinical infections [3]. From these data, we concluded that animals #80 and #99 were both in the late subclinical stage at the time of administration.
Fig. 1.
Interferon (IFN)-γ responses in blood, antibody (Ab) levels in sera, and fecal shedding of Mycobacterium avium subsp. paratuberculosis (MAP). (A) Whole-blood cells were cultured with Johnin purified protein derivative for 24 hr. IFN-γ concentrations in culture supernatants were determined by ELISA. (B) The serum levels of Abs against MAP were examined by ELISA. Results were indicated as percentage S/P, and the dotted lines indicated cutoff as recommended by the manufacturer. (C) MAP DNA quantity in feces detected by qPCR. (A–C) Arrowheads show the points of anti-programmed death-ligand 1 Ab administration.
To evaluate anti-bacterial effects of Boch4G12 in MAP-infected cattle, fecal shedding of MAP was monitored by qPCR for 60 weeks for animal #80 and 34 weeks for #99 after administration. The bacterial loads in feces from #80 and #99 were maintained at a low level after administration (Fig. 1C). Remarkably, in animal #99, MAP DNA was not detected in feces at 30 and 34 weeks after Boch4G12 administration (correspondence to 77 and 81 weeks-post infection, respectively) (Fig. 1C). A previous report has shown that persistent shedding patterns related on ELISA-positive samples rarely reverse to negativity [14]. In addition, cattle detected more than 1.0 × 10−2 pg MAP DNA in feces are associated with progressing to severe disease [23]. These data suggest that anti-PD-L1 Ab treatment has a possibility to regulate the bacterial shedding in MAP-infected cattle.
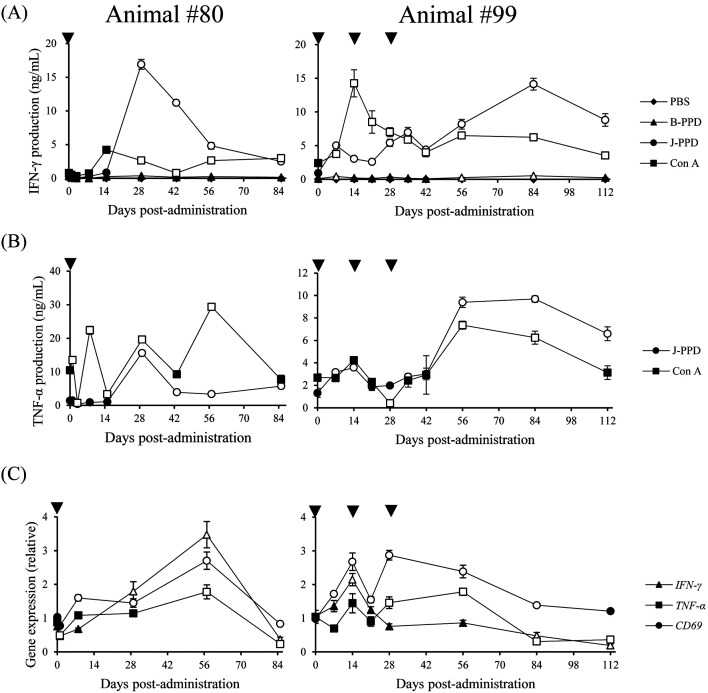
We then examined the effects of Boch4G12 on Th1 responses in vitro. IFN-γ and TNF-α production from PBMCs was significantly increased in the presence of J-PPD and Con A (Fig. 2A and 2B). TNF-α was not detected from culture supernatants of groups stimulated with PBS and B-PPD. In addition, the expression levels of IFN-γ, TNF-α, and CD69 in T cells of #80 were significantly upregulated after Boch4G12 administration (Fig. 2C). CD69 upregulation in T cells was also observed in animal #99 (Fig. 2C). Collectively, these results demonstrated that treatment with anti-PD-L1 chAb activated Th1 responses in both animals, suggesting a therapeutic potential for Johne’s disease.
Fig. 2.
Activation of Mycobacterium avium subsp. paratuberculosis (MAP) specific Th1 responses by anti- programmed death-ligand 1 chimeric antibody. (A and B) PBMCs were cultured with Phosphate buffered saline (PBS), purified protein derivative from Mycobacterium bovis BCG strain (B-PPD), Johnin-PPD (J-PPD), or concanavalin A (Con A) for 6 days. Interferon (IFN)-γ (A) and tumor necrosis factor (TNF)-α (B) concentrations in culture supernatants were determined by ELISA. (A and B) The symbols represent the means of three independent culture wells, and white symbols show P<0.05 compared with the value on day 0. (C) Gene expression levels of IFN-γ, TNF-α, and CD69 in CD3+ cells were quantitated by qPCR. The symbols represent the means of three independent experiments, and white symbols show P<0.05 compared with the value on day 0. (A–C) Statistical significance was determined by the Dunnett’s test.
In this study, the effects of anti-PD-L1 Ab treatment on bacterial shedding were different between #80 and #99. Animal #80 was administrated with anti-PD-L1 Ab a time, whereas animal #99 was administrated three times. Before anti-PD-L1 Ab administration, IFN-γ production responded to J-PPD in animal #80 had been suppressed for more than 100 weeks, whereas IFN-γ production in animal #99 had not been suppressed. These differences might be factors to determine the effect of anti-PD-L1 Ab treatment on fecal shedding. Further clinical studies using a number of MAP-infected animals are required to examine the influence of these factors, such as the experimental conditions (administration time, interval, and dosage) and examined animals (disease stage, response to J-PPD, and age), on the clinical efficacy of anti-PD-L1 Ab.
Th1 responses, especially IFN-γ production, are considered to be essential for the prevention of the disease progression. Our previous and present studies have shown that treatment with anti-PD-L1 Ab enhanced MAP-specific Th1 responses both in vitro and in vivo [18]. Therefore, the observed effect of anti-PD-L1 Ab on the bacterial shedding is presumably due to the activation of Th1 responses. Additional experiments are required to elucidate the underlying mechanism of the anti-bacterial effect of anti-PD-L1 Ab on MAP-infected cattle. Further, recent studies have described that the combined treatment of anti-PD-L1 Ab with other medicines has a potential to enhance therapeutic effects of PD-1/PD-L1 blockade in several chronic bovine infections [6, 7, 18,19,20]. A previous study on Johne’s disease revealed that the dual blockade of the PD-1/PD-L1 pathway and prostaglandin E2 production enhanced MAP-specific Th1 responses in vitro [18]. Hence, the combination with other medicines could be a strategy to enhance the anti-bacterial effect of anti-PD-L1 Ab in cattle infected with MAP.
In conclusion, we showed a potential of anti-PD-L1 Ab treatment for the regulation of bacterial shedding. Additionally, anti-PD-L1 Ab treatment also activated MAP-specific Th1 cytokine production in MAP-infected cattle. To our best knowledge, this is the first study which shows immune activating effects of the PD-L1 blockade in cattle infected with MAP. Although the number of experimentally-infected cattle used in this study was limited, the observations in the present study could play an important role to establish a novel therapeutic strategy against Johne’s disease.
CONFLICT OF INTEREST. The authors declare that they have no competing interests.
Acknowledgments
ACKNOWLEDGMENTS. This work was supported by JSPS KAKENHI grant number 19KK0172 [to S.K.], grants from the Project of the NARO, Bio-oriented Technology Research Advancement Institution (Research Program on Development of Innovative Technology 26058 BC [to S.K.] and Special Scheme Project on Regional Developing Strategy, Grant 16817557 [to S.K.]), regulatory research projects for food safety, animal health and plant protection (JPJ008617.17935709) funded by the Ministry of Agriculture, Forestry and Fisheries of Japan, and AMED under grant number JP20am0101078 [to Y.K.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful to Dr. Hideyuki Takahashi and Dr. Tomio Ibayashi for valuable advice and discussions.
REFERENCES
- 1.Bassey E. O., Collins M. T.1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 65: 4869–4872. doi: 10.1128/IAI.65.11.4869-4872.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrells C., Clarke C. J., Colston A., Kay J. M., Porter J., Little D., Sharp J. M.1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne’s disease). Vet. Immunol. Immunopathol. 68: 139–148. doi: 10.1016/S0165-2427(99)00022-7 [DOI] [PubMed] [Google Scholar]
- 3.Coussens P. M.2001. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2: 141–161. doi: 10.1079/AHRR200134 [DOI] [PubMed] [Google Scholar]
- 4.Coussens P. M.2004. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect. Immun. 72: 3089–3096. doi: 10.1128/IAI.72.6.3089-3096.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day C. L., Abrahams D. A., Bunjun R., Stone L., de Kock M., Walzl G., Wilkinson R. J., Burgers W. A., Hanekom W. A.2018. PD-1 expression on Mycobacterium tuberculosis-specific CD4 T cells is associated with bacterial load in human tuberculosis. Front. Immunol. 9: 1995. doi: 10.3389/fimmu.2018.01995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto S., Konnai S., Hirano Y., Kohara J., Okagawa T., Maekawa N., Sajiki Y., Watari K., Minato E., Kobayashi A., Gondaira S., Higuchi H., Koiwa M., Tajima M., Taguchi E., Uemura R., Yamada S., Kaneko M. K., Kato Y., Yamamoto K., Toda M., Suzuki Y., Murata S., Ohashi K.2020. Upregulation of PD-L1 expression by prostaglandin E2 and the enhancement of IFN-γ by anti-PD-L1 antibody combined with a COX-2 inhibitor in Mycoplasma bovis infection. Front. Vet. Sci. 7: 12. doi: 10.3389/fvets.2020.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto S., Konnai S., Hirano Y., Kohara J., Okagawa T., Maekawa N., Sajiki Y., Watari K., Minato E., Kobayashi A., Gondaira S., Higuchi H., Koiwa M., Tajima M., Taguchi E., Uemura R., Yamada S., Kaneko M. K., Kato Y., Yamamoto K., Toda M., Suzuki Y., Murata S., Ohashi K.2020. Clinical efficacy of the combined treatment of anti-PD-L1 rat-bovine chimeric antibody with a COX-2 inhibitor in calves infected with Mycoplasma bovis. Jpn. J. Vet. Res. 68: 77–90. [Google Scholar]
- 8.Hussain T., Shah S. Z., Zhao D., Sreevatsan S., Zhou X.2016. The role of IL-10 in Mycobacterium avium subsp. paratuberculosis infection. Cell Commun. Signal. 14: 29. doi: 10.1186/s12964-016-0152-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurado J. O., Alvarez I. B., Pasquinelli V., Martínez G. J., Quiroga M. F., Abbate E., Musella R. M., Chuluyan H. E., García V. E.2008. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 181: 116–125. doi: 10.4049/jimmunol.181.1.116 [DOI] [PubMed] [Google Scholar]
- 10.Kawaji S., Nagata R., Mori Y.2014. Detection and confirmation of Mycobacterium avium subsp. paratuberculosis in direct quantitative PCR positive fecal samples by the manual fluorescent MGIT culture system. J. Vet. Med. Sci. 76: 65–72. doi: 10.1292/jvms.13-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keir M. E., Butte M. J., Freeman G. J., Sharpe A. H.2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26: 677–704. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalifeh M. S., Al-Majali A. M., Stabel J. R.2009. Role of nitric oxide production in dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 131: 97–104. doi: 10.1016/j.vetimm.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 13.Li L., Katani R., Schilling M., Kapur V.2016. Molecular Epidemiology of Mycobacterium avium subsp. paratuberculosis on Dairy Farms. Annu. Rev. Anim. Biosci. 4: 155–176. doi: 10.1146/annurev-animal-021815-111304 [DOI] [PubMed] [Google Scholar]
- 14.Navarro-Gonzalez N., Fourichon C., Blanquefort P., Delafosse A., Joly A., Ngwa-Mbot D., Biet F., Boichard D., Schibler L., Journaux L., Meens E., Guatteo R.2019. Longitudinal study of Mycobacterium avium ssp. paratuberculosis fecal shedding patterns and concurrent serological patterns in naturally infected dairy cattle. J. Dairy Sci. 102: 9117–9137. doi: 10.3168/jds.2018-15897 [DOI] [PubMed] [Google Scholar]
- 15.Nishimori A., Konnai S., Okagawa T., Maekawa N., Ikebuchi R., Goto S., Sajiki Y., Suzuki Y., Kohara J., Ogasawara S., Kato Y., Murata S., Ohashi K.2017. In vitro and in vivo antivirus activity of an anti-programmed death-ligand 1 (PD-L1) rat-bovine chimeric antibody against bovine leukemia virus infection. PLoS One 12: e0174916. doi: 10.1371/journal.pone.0174916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okagawa T., Konnai S., Nishimori A., Ikebuchi R., Mizorogi S., Nagata R., Kawaji S., Tanaka S., Kagawa Y., Murata S., Mori Y., Ohashi K.2015. Bovine immunoinhibitory receptors contribute to suppression of Mycobacterium avium subsp. paratuberculosis-specific T-cell responses. Infect. Immun. 84: 77–89. doi: 10.1128/IAI.01014-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathnaiah G., Zinniel D. K., Bannantine J. P., Stabel J. R., Gröhn Y. T., Collins M. T., Barletta R. G.2017. Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne’s disease. Front. Vet. Sci. 4: 187. doi: 10.3389/fvets.2017.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sajiki Y., Konnai S., Okagawa T., Nishimori A., Maekawa N., Goto S., Ikebuchi R., Nagata R., Kawaji S., Kagawa Y., Yamada S., Kato Y., Nakajima C., Suzuki Y., Murata S., Mori Y., Ohashi K.2018. Prostaglandin E2 induction suppresses the Th1 immune responses in cattle with Johne’s disease. Infect. Immun. 86: e00910–e00917. doi: 10.1128/IAI.00910-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajiki Y., Konnai S., Okagawa T., Nishimori A., Maekawa N., Goto S., Watari K., Minato E., Kobayashi A., Kohara J., Yamada S., Kaneko M. K., Kato Y., Takahashi H., Terasaki N., Takeda A., Yamamoto K., Toda M., Suzuki Y., Murata S., Ohashi K.2019. Prostaglandin E2-induced immune exhaustion and enhancement of antiviral effects by anti-PD-L1 antibody combined with COX-2 inhibitor in bovine leukemia virus infection. J. Immunol. 203: 1313–1324. doi: 10.4049/jimmunol.1900342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajiki Y., Konnai S., Okagawa T., Maekawa N., Nakamura H., Kato Y., Suzuki Y., Murata S., Ohashi K.2021. A TLR7 agonist activates bovine Th1 response and exerts antiviral activity against bovine leukemia virus. Dev. Comp. Immunol. 114: 103847. doi: 10.1016/j.dci.2020.103847 [DOI] [PubMed] [Google Scholar]
- 21.Sohal J. S., Singh S. V., Tyagi P., Subhodh S., Singh P. K., Singh A. V., Narayanasamy K., Sheoran N., Singh Sandhu K.2008. Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology 213: 585–598. doi: 10.1016/j.imbio.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Stabel J. R.2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim. Health Res. Rev. 7: 61–70. doi: 10.1017/S1466252307001168 [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi Y., Sakakibara S. I., Fujihara M., Yagi A., Fujiyoshi S.2020. The association between detection of Mycobacterium avium subsp. paratuberculosis DNA in feces and histopathological classification. J. Vet. Med. Sci. 82: 541–545. doi: 10.1292/jvms.18-0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss D. J., Evanson O. A., Souza C. D.2006. Mucosal immune response in cattle with subclinical Johne’s disease. Vet. Pathol. 43: 127–135. doi: 10.1354/vp.43-2-127 [DOI] [PubMed] [Google Scholar]
- 25.Wherry E. J.2011. T cell exhaustion. Nat. Immunol. 12: 492–499. doi: 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]