Abstract
We evaluated changes in cardiovascular and renal functions as well as arginine vasopressin (AVP) secretion, with remifentanil and dexmedetomidine administration alone or in combination in sevoflurane-anesthetized dogs. Six healthy adult Beagle dogs received one of the following four treatments in a randomized crossover study: saline (C), remifentanil alone at successively increasing doses (R; 0.15, 0.60, and 2.40 µg/kg/min), dexmedetomidine alone (D; 0.5 µg/kg intravenously for initial 10 min followed by a constant rate infusion at 0.5 µg/kg/hr), and a combination of remifentanil and dexmedetomidine at the above-mentioned doses (RD). Sevoflurane doses were adjusted to 1.5 times of minimum alveolar concentration (MAC) equivalent according to MAC-sparing effects with remifentanil and dexmedetomidine as previously reported. Cardiovascular measurements, renal function data, and plasma AVP concentrations were determined before and every 60 min until 180 min after drug administration as per each treatment. In the R, D and RD, heart rate significantly decreased and mean arterial pressure significantly increased from baseline or with C. Cardiac index significantly decreased and systemic vascular resistance index increased with D and RD. Oxygen extraction ratio, renal blood flow, and glomerular filtration rate were not affected. The plasma AVP concentrations significantly decreased in D and RD, but increased in R. Only in D, the natriuresis was elicited. The combination of remifentanil and dexmedetomidine in sevoflurane-anesthetized dogs was acceptable in terms of the hemodynamics, oxygenation, and renal function. Remifentanil may interfere with dexmedetomidine-induced diuresis and inhibition of AVP secretion.
Keywords: arginine vasopressin, cardiovascular and renal effect, dexmedetomidine, dog, remifentanil
Dexmedetomidine, a dextro optical isomer of medetomidine, is an α2-adrenoreceptor agonist that induces sedation and analgesia [29]. Dexmedetomidine also has cardiovascular side effects, including bradycardia, decreased cardiac output (CO), and increased systemic vascular resistance [28, 29, 33]. In addition, dexmedetomidine and the other α2-adrenoreceptor agonists have been known to have a remarkable diuretic property; inhibition of arginine vasopressin (AVP) secretion has been proposed as one of the mechanisms for this diuretic property [6, 10, 36, 40, 49]. Dexmedetomidine constant rate infusions (CRI) have been applied perioperatively to consistently maintain its anesthetic-sparing and hemodynamic-stabilizing effects or to provide adjunctive analgesia in dogs [1, 32, 47, 48]. In the clinical setting, dexmedetomidine and medetomidine CRI are frequently co-administered with other analgesics, especially µ opioid agonists, during the perioperative period in dogs [12, 32, 37, 46,47,48].
Remifentanil is a very short-acting µ opioid analgesic that is rapidly metabolized by nonspecific esterases in blood and tissues, independent of hepatic and renal clearances [4, 8, 18]; this pharmacokinetic property of remifentanil may be beneficial for both predictivity and adjustability on the drug effects even in patients who have compromised in the liver and kidneys. Previous studies have demonstrated that remifentanil significantly decreases cardiac index (CI) accompanied by bradycardia in dogs anesthetized with inhaled anesthetics [9, 27]. In contrast to α2-adrenoreceptor agonists, remifentanil per se was related to an increase in the plasma AVP concentrations [27] and opioids tended to cause reduction in the urine flow [2, 38].
Recently, we reported that a combination of remifentanil and dexmedetomidine in CRI synergistically reduced the sevoflurane requirement in dogs; however, bradycardia with the concurrent CRI of these drugs is a concern [1]. It would be clinically beneficial to identify whether the hemodynamic depression under the co-administration of remifentanil and dexmedetomidine is acceptable enough to maintain tissue oxygenation and the functional blood flow in vital organs such as kidneys. Furthermore, although both remifentanil and dexmedetomidine potentially affect urine production and AVP secretion in different ways as noted above, no study has documented the combined effects of µ opioid and α2-adrenoreceptor agonists on renal functions.
This study aimed to experimentally evaluate the changes in cardiovascular and renal functions as well as AVP secretion with the CRI of remifentanil and dexmedetomidine alone and in combination in dogs anesthetized with sevoflurane.
MATERIALS AND METHODS
Animals
Six healthy, adult neutered Beagle dogs (three males and three females), aged 3.0 ± 0.7 years (mean ± standard deviation [SD]) and weighing 10.2 ± 1.2 kg (mean ± SD), were included in this study. Health status was assessed before the study by physical examination, complete blood count, serum biochemical analysis, and urinalysis. The dogs were housed in cages; food, but not water, was withheld for 12 hr before anesthesia. This study was approved by the Animal Research Committee of Tottori University (no. 16-T-19).
Study design
Each dog was randomly anesthetized on four occasions with a ≥ 4-week washout period and received one of the following four treatments: CRI of saline at 2 ml/kg/min as control (C treatment); CRI of remifentanil (Ultiva, Janssen Pharmaceutical K.K., Tokyo, Japan) at successive dose rates of 0.15, 0.60, and 2.40 µg/kg/min (R treatment); dexmedetomidine (Precedex, Maruishi Pharmaceutical Co., Ltd., Osaka, Japan), initially at a loading dose of 0.5 µg/kg intravenously (IV) over 10 min, followed by CRI at 0.5 µg/kg/hr (D treatment); and combined CRI of remifentanil and dexmedetomidine at the dose rates mentioned above (RD treatment).
Instrumentation
Anesthesia was induced by sevoflurane (vaporizer setting 8%; Sevoflo, DS Pharma Animal Health Co., Ltd., Osaka, Japan) in oxygen at 5 l/min, using a face mask connected to an anesthetic machine (Aestiva 7900, GE Healthcare Japan Corp., Tokyo, Japan) through a pediatric, semi-closed circle circuit. After endotracheal intubation, the dogs were placed in the left lateral recumbent position, and the anesthesia was maintained with 2.5 to 3.0% sevoflurane in oxygen at 2 l/min. Pressure-controlled mechanical ventilation with a peak inspiratory pressure of 8 to 15 cm H2O and a respiratory rate of 10 breaths/min (inspiration to expiration ratio: 1:2) were set to maintain normocapnia (end tidal partial pressure of carbon dioxide [ETCO2] between 35 and 45 mm Hg). Airway gases were continuously sampled at 200 ml/min through an 8-French catheter (Atom Multipurpose Tube, Atom Medical Corp., Tokyo, Japan) that was passed into the distal end of the endotracheal tube. The catheter was tightly inserted from the sampling port of the L-shaped adaptor, which connected the endotracheal tube to the breathing circuit. Prior to each experiment, the gas analyzer was calibrated using manufacturer-supplied calibration mixed gases. The esophageal temperature was controlled within 37.5 to 38.5°C using forced warm air (3MTM Bair HuggerTM Model 775 Patient Warming Unit, 3M Health Care Sales Ltd., Tokyo, Japan). The left and right cephalic veins were cannulated with 22-guage catheters (Surflo F&F, Terumo Corp., Tokyo, Japan) for the administration of drugs and lactated Ringer’s solution at a rate of 3 ml/kg/hr, respectively, throughout anesthesia. A 24-guage catheter (Surflo F&F, Terumo Corp.) was placed percutaneously into a dorsal pedal artery and connected to a transducer (DTXPlus, Merit Medical Devices Japan K.K., Tokyo, Japan) by a 120-cm extension tubing (TOP blood pressure monitoring tube, TOP Corp., Tokyo, Japan) for continuous measurements of systolic, mean, and diastolic arterial pressures (SAP, MAP, and DAP, respectively). After local anesthesia over the right jugular vein infiltrated with 1 mg of lidocaine (Xylocaine Injection 2%, Aspen Japan K.K. Tokyo, Japan), a 5-French, 75-cm thermodilution catheter (Swan-Ganz thermodilution catheter 132F5, Edwards Lifesciences Corp., Tokyo, Japan) was inserted through the right jugular vein using a 5-French catheter introducer (XEMEX Introducer Set, Zeon Medical Inc., Tokyo, Japan). The thermodilution catheter was advanced into the pulmonary artery, as confirmed by the transitions of characteristic pressure waveform patterns under fluoroscopic guidance (SXT-1000A, Canon Medical Systems Corp., Otawara, Japan). The proximal and distal ports of this catheter were placed in the right atrium and the pulmonary artery, respectively, and were connected to pressure transducers (DTXPlus, Merit Medical Devices Japan K.K.) for continuous measurements of right atrial pressure (RAP) and mean pulmonary artery pressure (MPAP), respectively. Pulmonary artery temperature (PAT) was obtained from the thermistor at the distal port of the catheter. Pulmonary artery occlusion pressure (PAOP) was measured intermittently at the distal port by inflating the balloon at the tip of the thermodilution catheter with 0.7 ml of air. All transducers were placed and zeroed at the manubrium of the dogs and were calibrated with a digital manometer before each anesthesia. The entire pressure measurement system was filled with heparinized (1 U/ml) saline and was continuously flushed. The CO was measured using a handy bolus injection of 3-ml cold (1 to 5°C) 5% dextrose solution (5w/v% Glucose Injection, Terumo Corp.) through the proximal port of the thermodilution catheter at end expiration. Each CO determination included five measurements; the maximum and minimum values were discarded, and the average of the three remaining measurements that differed within 10% of each other was recorded as the CO value. For urine sample collection, an 8-French urinary catheter, which is 55 cm long for male (Silicon Foley Catheter, Kirikan Ltd., Tokyo, Japan) or 34 cm long for female (All Silicon Foley Catheter, Create Medic Co., Ltd., Yokohama, Japan) was inserted through the urethra and was held in place in the bladder by inflating the balloon. Pulse rate, peripheral oxygen saturation by pulse oximetry, ETCO2, end tidal sevoflurane concentration (ETSEV), SAP, MAP, DAP, RAP, and esophageal temperature were monitored using a multiparametric device (BSM-5192, Nihon Kohden, Tokyo, Japan) throughout the anesthesia. Another monitor (BSM-5132, Nihon Kohden) was used to monitor lead II electrocardiogram (ECG), heart rate (HR) obtained by ECG, MPAP, PAT, and PAOP and for the measurement of CO.
Experimental protocol
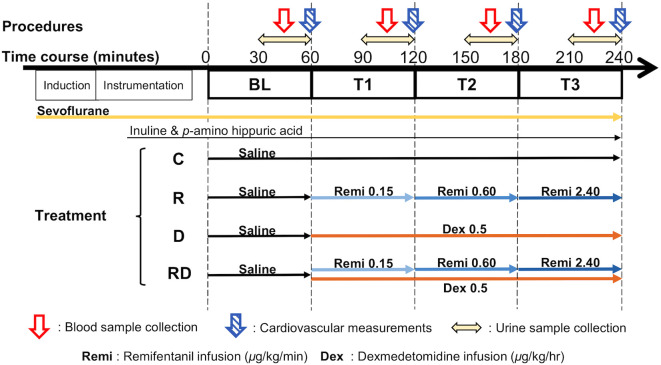
A schematic representation of procedures and drug administrations is shown in Fig. 1. After the instrumentation period, ETSEV was adjusted to maintain 3.2%, which was 1.5 times that of the previously reported sevoflurane minimum alveolar concentration (MAC) value in dogs [1], and was equilibrated for 60 min, simultaneously with an administration of lactated Ringer’s solution at a temporarily increased rate of 10 ml/kg/hr for hydration. Thereafter, 2 ml of the venous blood sample was collected. Then, inulin (Inulead Inj., Fuji Yakuhin Co., Ltd., Saitama, Japan) and p-amino hippuric acid (PAH) (Sodium Para-Aminohippurate Injection 10%, Daiichi Sankyo Co., Ltd., Tokyo, Japan) in saline solutions were given IV through the left cephalic vein at respective primary loading doses of 15 and 6 mg/kg, followed by CRI at 0.2 and 0.3 mg/kg/min throughout the experiment, respectively, allowing 30 min for equilibration [26, 31].
Fig. 1.
A schematic representation of procedures and drug administrations in each treatment in the present study. Measurements and sample collections were repeated every 60 min with saline as baseline (BL) and after drug administration assigned to each treatment until 240 min (T1, T2 and T3). Sevoflurane doses were adjusted to 1.5 times the minimum alveolar concentration (MAC) equivalent to sevoflurane alone during administration of remifentanil and/or dexmedetomidine infusions according to their MAC-sparing effects.
Following those equilibrations for anesthesia and infusions of inulin and PAH, the baseline measurement period (BL) was started at a set time of 0 min with the initiation of infusion of saline at 2 ml/kg/hr. The administration rate of lactated Ringer’s solution was lowered to 3 ml/kg/hr, and it was continued throughout the experiment. After 30-min equilibration for the infusion, the bladder was emptied, followed by continuous urine sample collection for 30 min (30−60 min). In the middle of the urine collecting period (45 min), 7 ml of the venous blood sample was collected from the proximal port of thermodilution catheter for the determination of plasma osmolality and electrolytes as well as plasma AVP, and serum inulin and PAH concentrations. An aliquot of 4 ml from the 7-ml blood sample was placed in a tube containing ethylene diamine tetra-acetic acid (EDTA) to obtain plasma. Another 2 ml was placed in a serum tube, and the remaining 1 ml was placed in a heparinized tube. At the end of the period (55−60 min), HR, ETCO2, ETSEV, SAP, MAP, DAP, RAP, MPAP, and PAT were recorded, and PAOP and CO were measured. Correspondingly, both arterial and mixed venous blood samples (0.5 ml, respectively) were anaerobically collected using 2-ml heparinized syringes (PICO 50; Radiometer K.K., Tokyo, Japan) and were immediately placed in the analyzer (ABL-5; Radiometer K.K.) for the determination of pH, oxygen partial pressure, and carbon dioxide partial pressure, in both arterial (pHa, PaO2, and PaCO2 respectively) and mixed venous blood (pHmv, PmvO2, and PmvCO2, respectively). An additional 0.5 ml of the arterial blood sample was collected into an EDTA tube for measurements of hemoglobin (Hb) and hematocrit (Hct) (pocH-100iV Diff, Sysmex TMC Co., Ltd., Kobe, Japan). The urine samples were collected (60 min) and stored on ice until centrifugation for separation of the supernatant after the measurement of urine volume using a plastic syringe and urine specific gravity (USG) using an analyzer (PAL-DOG&CAT, ATAGO Co., Ltd., Tokyo, Japan). All the samples were centrifuged at 2,000 g at 4°C for 15 min and stored until assay; plasma and serum samples were stored at −80°C and urine samples were stored at −30°C.
After completion of the procedures at BL (60 min), CRI of saline, remifentanil alone, dexmedetomidine alone, or remifentanil combined with dexmedetomidine according to treatment was initiated through the right cephalic vein. Considering the MAC-sparing effect, the ETSEV was reduced for each treatment to 1.5 times sevoflurane MAC with these CRI, as previously reported [1], to maintain sevoflurane doses that were equipotent to the requirement as that when sevoflurane was administered alone. The procedures of sample collection and measurements noted above were repeated every 60 min until 240 min (T1: 60−120 min; T2: 120−180 min; T3: 180−240 min). For the C treatment, an administration of saline (2 ml/kg/hr) with 3.2% ETSEV was continued from T1 to T3. For the D treatment, an infusion of dexmedetomidine (initiated with LD of 0.5 µg/kg for 10 min followed by 0.5 µg/kg/hr CRI) with 2.4% ETSEV was administered from T1 to T3. For the R treatment, remifentanil was administered in increments at 0.15 (T1), 0.6 (T2), and 2.4 (T3) µg/kg/min with 2.1, 1.5, and 1.1% ETSEV, respectively. For the RD treatment, dexmedetomidine was consistently administered through T1 to T3 and was combined with remifentanil at the following infusion rates (µg/kg/min) with ETSEV: 0.15 with 1.6%, 0.60 with 1.1%, and 2.40 with 0.7% at T1, T2, and T3, respectively. Solutions for CRI of remifentanil and dexmedetomidine, as well as a mixture of inulin and PAH, were made separately in saline to infuse at a rate of 1 ml/kg/hr using syringe pumps (TOP-551VC, TOP Corp.) that were calibrated before the study, in accordance with the manufacturer’s specifications. An additional infusion of saline (1 ml/kg/hr) was prepared for R and D treatments; thus, the volume for drug administration was equal to 2 ml/kg/hr between each treatment. After all the measurements were completed at T3, all drug administrations were discontinued, and all catheters were removed; the dog was then allowed to recover from anesthesia. Meloxicam (0.2 mg/kg; Metacam, Boehringer Ingelheim Vetmedica Japan Co., Ltd, Tokyo, Japan) was subcutaneously administered during the recovery period.
Sample collection and analysis
Urine (UOsm) and plasma osmolality (POsm) were measured using a vapor pressure osmometer (Wescor Vapro 5520, Phoenix Science Inc., Tokyo, Japan). The concentrations of electrolytes (i.e., sodium, potassium, and chloride) in both the urine and heparin plasma samples were measured using a clinical blood biochemical autoanalyzer (Fuji DRI-CHEM 7000V, Fujifilm Corp., Tokyo, Japan). In both the urine and serum samples, the inulin and PAH concentrations were determined using commercially available kits enzymatically (Diacolor Inulin Kit, Toyobo Co., Ltd., Osaka, Japan) and calorimetrically (PAH Assay Kit, Sigma-Aldrich Japan Co., Tokyo, Japan). The analysis of plasma AVP concentrations using EDTA plasma samples was outsourced to BML Inc. (Tokyo, Japan) and was conducted by radioimmunoassay.
Data analysis
The measured blood gas and hemodynamic values were used for the calculation of CI, stroke volume index (SVI), systemic vascular resistance index (SVRI), pulmonary vascular resistance index (PVRI), arterial (SaO2) and mixed vinous (SmvO2) hemoglobin saturation, arterial bicarbonate (HCO3−), base excess (BE), oxygen delivery index (DO2I), oxygen consumption index (VO2I), and oxygen extraction (O2ER), with reference to previously published formulae [14, 35]. Urine output (UO) was calculated from the 30-min urine volume. Using urine volume and the measured values of osmolality and electrolytes, osmolar clearance (CLOsm), free-water clearance (CLH2O), fractional clearance of sodium (FENa), potassium (FEK), and chloride (FECl) were calculated, as previously reported [40, 49]. Glomerular filtration rate (GFR) and renal blood flow (RBF) were obtained according to the clearances of inulin and PAH, respectively [16, 26].
Statistical analysis
The visual inspection of scatter plot and the Shapiro–Wilk test were used to verify the normality of distribution of data. Data with normal distribution are presented as mean ± SD and were analyzed by repeated-measures one-way analysis of variance (ANOVA) with post hoc Bonferroni multiple comparisons test to evaluate the effects between all time points within each treatment and using one-way ANOVA with post hoc Tukey–Kramer’s test to identify differences among the treatments at each time point. Data with non-normal distribution are presented as median with range (minimum, maximum) and were analyzed using Friedman tests for within-treatment analyses and Kruskal–Wallis test for between-treatment comparisons, with post hoc Dunn’s multiple comparisons test, respectively. Significance was established at P<0.05. All data were analyzed using commercially available software (Prism version 7.05, GraphPad Software, San Diego, CA, USA).
RESULTS
The cardiovascular and renal functional variables, and plasma AVP levels at BL did not differ among the treatments. For the C treatment, all the variables did not significantly different over time.
The HR was significantly decreased in the R, D, and RD treatments than in the respective BL values and C treatment (Table 1). At all the time points, the SVI significantly increased from BL only in the R treatment group. The CI significantly decreased from BL in the D and RD treatments. For comparisons among the treatment groups, the CI values were significantly lower in the RD treatment than C and R treatments. At some time points, a significant increase above the BL values was observed for SAP and MAP in R, D, and RD treatments and for DAP in D and RD treatments. The SVRI was significantly increased from BL with D and RD treatments and was significantly higher at all time points with RD treatment than with R treatment. The PAOP significantly increased from BL in the D and RD treatments, and was significantly higher in the RD treatment group at T3 than in the other three treatment groups. The RAP significantly increased at T3 from BL in R and RD treatments. The MPAP significantly increased in the RD treatment from BL, but PVRI did not change through the experimental period.
Table 1. Cardiovascular variables (mean ± standard deviation) in six Beagle dogs before (baseline: BL) and during an infusion of saline as control (C), remifentanil at incremented doses of 0.15 (T1), 0.60 (T2) and 2.40 (T3) μg/kg/min (R), dexmedetomidine at 0.5 μg/kg/hr (D), or the combination of remifentanil and dexmedetomidine (RD) under sevoflurane anesthesia equipotent to 1.5 times of minimum alveolar concentration.
| Variable | Treatment | BL | T1 | T2 | T3 |
|---|---|---|---|---|---|
| HR (beats/min) | C | 100.3 ± 18.0 | 101.5 ± 18.0d) | 103.5 ± 16.2cd) | 103.8 ± 16.1cd) |
| R | 102.7 ± 19.4 | 82.8 ± 12.0a) | 76.8 ± 10.9ab) | 71.5 ± 12.8ab) | |
| D | 101.2 ± 14.3 | 79.3 ± 10.6ab) | 79.5 ± 12.5ab) | 80.0 ± 14.7ab) | |
| RD | 104.5 ± 17.9 | 71.8 ± 7.8ab) | 66.7 ± 8.4ab) | 60.0 ± 11.2ab) | |
| SAP (mmHg) | C | 100.3 ± 8.8 | 98.7 ± 11.2 | 97.5 ± 8.3cd) | 100.8 ± 9.2cd) |
| R | 104.0 ± 10.6 | 109.0 ± 12.1 | 124.5 ± 13.5ab) | 136.7 ± 14.7ab) | |
| D | 100.0 ± 13.3 | 123.5 ± 14.1a) | 121.5 ± 16.4ab) | 124.0 ± 11.9ab) | |
| RD | 97.8 ± 10.0 | 126.0 ± 25.5b) | 130.8 ± 16.6ab) | 145.3 ± 18.9ab) | |
| MAP (mmHg) | C | 70.5 ± 6.2 | 69.5 ± 6.0 | 69.7 ± 4.5 | 71.0 ± 4.3c) |
| R | 72.7 ± 9.4 | 70.7 ± 9.3 | 81.3 ± 10.3 | 92.8 ± 12.2ab) | |
| D | 70.0 ± 6.3 | 91.0 ± 13.6 | 88.5 ± 14.6a) | 90.0 ± 11.5a) | |
| RD | 67.7 ± 2.2 | 93.2 ± 23.3b) | 97.3 ± 22.4b) | 109.2 ± 19.8ab) | |
| DAP (mmHg) | C | 58.7 ± 5.9 | 57.5 ± 5.4 | 58.0 ± 3.2 | 58.5 ± 3.4 |
| R | 59.3 ± 8.7 | 56.0 ± 7.9 | 64.7 ± 8.4 | 75.5 ± 10.3 | |
| D | 58.0 ± 4.8 | 77.5 ± 11.7a) | 76.2 ± 14.3a) | 77.5 ± 11.9a) | |
| RD | 56.8 ± 2.6 | 80.7 ± 23.3bc) | 82.8 ± 22.9b) | 93.3 ± 19.8ab) | |
| RAP (mmHg) | C | 2.7 ± 0.5 | 2.7 ± 0.5 | 2.3 ± 0.5 | 2.7 ± 1.0c) |
| R | 2.5 ± 0.8 | 4.2 ± 1.8 | 4.2 ± 1.8 | 5.2 ± 1.7ab) | |
| D | 2.7 ± 1.0 | 4.0 ± 1.1 | 3.7 ± 1.2 | 4.0 ± 1.7 | |
| RD | 2.5 ± 1.0 | 4.2 ± 2.0 | 4.8 ± 1.8b) | 6.7 ± 1.4abd) | |
| MPAP (mmHg) | C | 11.5 ± 1.5 | 11.5 ± 1.4 | 11.3 ± 1.0 | 11.5 ± 1.0 |
| R | 11.2 ± 0.4 | 11.8 ± 2.7 | 12.5 ± 2.2 | 12.8 ± 1.7 | |
| D | 11.5 ± 1.0 | 12.3 ± 0.5 | 12.2 ± 1.2 | 12.0 ± 1.3 | |
| RD | 11.0 ± 1.1 | 12.3 ± 1.4a) | 13.0 ± 0.9a) | 14.0 ± 1.4ab) | |
| PAOP (mmHg) | C | 5.2 ± 1.2 | 5.3 ± 1.2 | 5.0 ± 1.1 | 5.0 ± 1.7 |
| R | 5.2 ± 1.2 | 5.7 ± 1.5 | 6.3 ± 1.6 | 6.8 ± 1.9 | |
| D | 4.8 ± 0.8 | 6.8 ± 1.5a) | 6.5 ± 1.4a) | 6.3 ± 0.8a) | |
| RD | 5.0 ± 0.9 | 6.5 ± 2.1 | 8.0 ± 2.4ab) | 9.7 ± 1.0abcd) | |
| CI (l/min/m2) | C | 2.90 ± 0.46 | 2.89 ± 0.30 | 2.90 ± 0.38 | 3.03 ± 0.39 |
| R | 3.05 ± 0.34 | 2.97 ± 0.48 | 3.09 ± 0.85 | 2.80 ± 0.88 | |
| D | 3.03 ± 0.37 | 2.28 ± 0.47a) | 2.26 ± 0.43a) | 2.36 ± 0.55a) | |
| RD | 2.99 ± 0.50 | 2.14 ± 0.45abc) | 2.12 ± 0.48ac) | 1.92 ± 0.60ab) | |
| SVI (ml/beat/m2) | C | 29.1 ± 5.9 | 29.0 ± 5.2 | 28.3 ± 4.2 | 29.5 ± 3.7c) |
| R | 30.2 ± 3.5 | 36.4 ± 6.9a) | 39.8 ± 6.4abd) | 38.5 ± 6.3abd) | |
| D | 30.5 ± 6.5 | 29.1 ± 7.0 | 28.7 ± 6.0c) | 29.5 ± 6.0c) | |
| RD | 28.9 ± 4.4 | 29.7 ± 5.7 | 31.4 ± 4.1 | 31.4 ± 4.5 | |
| SVRI (dynes·sec/cm5/m2) | C | 1,929 ± 355 | 1,861 ± 195d) | 1,879 ± 270d) | 1,826 ± 297 |
| R | 1,838 ± 135 | 1,822 ± 359d) | 2,105 ± 542 | 2,744 ± 1,078 | |
| D | 1,789 ± 202 | 3,109 ± 529abc) | 3,067 ± 617ab) | 3,054 ± 787a) | |
| RD | 1,790 ± 314 | 3,406 ± 882abc) | 3,623 ± 970abc) | 4,631 ± 1,575abc) | |
| PVRI (dynes·sec/cm5/m2) | C | 177 ± 18 | 171 ± 29 | 175 ± 45 | 172 ± 48 |
| R | 159 ± 32 | 171 ± 69 | 169 ± 54 | 182 ± 48 | |
| D | 180 ± 42 | 194 ± 29 | 201 ± 34 | 198 ± 35 | |
| RD | 166 ± 42 | 226 ± 56 | 200 ± 81 | 179 ± 30 | |
HR, heart rate; SAP, systolic arterial pressure; MAP, mean arterial pressure; DAP, diastolic arterial pressure; RAP, right atrial pressure; MPAP, mean pulmonary artery pressure; PAOP, pulmonary artery occlusion pressure; CI, cardiac index; SVI, stroke volume index; SVRI, stroke vascular resistance index; PVRI, pulmonary vascular resistance index. a) Significantly differ from respective baseline value (P<0.05); b) significantly differ from C treatment at this time point (P<0.05); c) significantly differ from R treatment at this time point (P<0.05); d) significantly differ from D treatment at this time point (P<0.05).
In the RD treatment at T3, pHa significantly decreased and manifested as mild acidemia (7.32 ± 0.04) as compared to that with the C treatment (Table 2). The HCO3− and BE significantly decreased from BL in the R and RD treatments at T3. The Hct and Hb significantly increased from BL with D and RD treatments in certain time points. Furthermore, both PmvO2 and SmvO2 significantly decreased from BL in the D treatment at some time points and in the RD treatment at T3. The values of PaO2, PaCO2, pHmv, DO2I, VO2I, and O2ER did not change significantly over time and among treatments. The values of SaO2, PmvCO2, and PAT remained unchanged throughout the study (data not shown).
Table 2. Blood gas and oxygenation variables (mean ± standard deviation) in six Beagle dogs before (baseline: BL) and during an infusion of saline as control (C), remifentanil at incremented doses of 0.15 (T1), 0.60 (T2) and 2.40 (T3) μg/kg/min (R), dexmedetomidine at 0.5 μg/kg/hr (D), or the combination of remifentanil and dexmedetomidine (RD) under sevoflurane anesthesia equipotent to 1.5 times of minimum alveolar concentration.
| Variable | Treatment | BL | T1 | T2 | T3 |
|---|---|---|---|---|---|
| pHa | C | 7.39 ± 0.02 | 7.37 ± 0.02 | 7.39 ± 0.01 | 7.38 ± 0.02 |
| R | 7.37 ± 0.03 | 7.37 ± 0.03 | 7.38 ± 0.04 | 7.35 ± 0.04 | |
| D | 7.37 ± 0.03 | 7.38 ± 0.02 | 7.39 ± 0.05 | 7.37 ± 0.03 | |
| RD | 7.37 ± 0.02 | 7.35 ± 0.04 | 7.34 ± 0.04 | 7.32 ± 0.04a) | |
| PaO2 (mmHg) | C | 562 ± 39 | 561 ± 47 | 564 ± 48 | 570 ± 47 |
| R | 560 ± 25 | 558 ± 30 | 559 ± 71 | 562 ± 35 | |
| D | 575 ± 40 | 578 ± 17 | 595 ± 23 | 573 ± 31 | |
| RD | 550 ± 45 | 571 ± 42 | 564 ± 40 | 559 ± 36 | |
| PaCO2 (mmHg) | C | 39.2 ± 3.7 | 39.8 ± 2.0 | 38.2 ± 2.5 | 38.3 ± 3.1 |
| R | 39.5 ± 3.3 | 40.0 ± 3.0 | 38.2 ± 5.9 | 39.8 ± 3.4 | |
| D | 39.3 ± 2.1 | 38.5 ± 1.2 | 37.0 ± 3.3 | 36.7 ± 2.2 | |
| RD | 40.7 ± 1.2 | 41.2 ± 3.3 | 41.2 ± 2.8 | 40.3 ± 2.3 | |
| HCO3− (mEq/l) | C | 22.7 ± 1.6 | 22.3 ± 1.0 | 22.2 ± 1.0 | 22.1 ± 1.4 |
| R | 22.2 ± 1.1 | 22.2 ± 1.1 | 21.4 ± 1.7 | 21.0 ± 1.2a) | |
| D | 22.2 ± 1.3 | 21.9 ± 0.9 | 21.4 ± 0.8 | 20.7 ± 1.3 | |
| RD | 22.6 ± 1.3 | 22.0 ± 1.5 | 21.5 ± 1.4 | 20.1 ± 2.1a) | |
| Base excess (mEq/l) | C | –2.3 ± 1.6 | –2.9 ± 1.2 | –2.8 ± 0.9 | –3.0 ± 1.4 |
| R | –3.0 ± 1.1 | –3.1 ± 1.3 | –3.8 ± 1.4 | –4.7 ± 1.5a) | |
| D | –3.1 ± 1.7 | –3.2 ± 1.2 | –3.6 ± 1.4 | –5.0 ± 2.5 | |
| RD | –2.8 ± 1.7 | –3.6 ± 2.0 | –4.2 ± 1.8 | –6.1 ± 2.7a) | |
| pHmv | C | 7.33 ± 0.02 | 7.32 ± 0.02 | 7.34 ± 0.02 | 7.33 ± 0.02 |
| R | 7.33 ± 0.02 | 7.32 ± 0.02 | 7.32 ± 0.04 | 7.32 ± 0.04 | |
| D | 7.33 ± 0.02 | 7.33 ± 0.03 | 7.33 ± 0.03 | 7.31 ± 0.04 | |
| RD | 7.32 ± 0.02 | 7.31 ± 0.06 | 7.30 ± 0.04 | 7.29 ± 0.05 | |
| PmvO2 (mmHg) | C | 63.7 ± 3.2 | 64.3 ± 1.5 | 66.0 ± 5.1 | 68.2 ± 5.5cd) |
| R | 67.5 ± 5.6 | 62.3 ± 3.9 | 61.8 ± 9.1 | 58.0 ± 8.0b) | |
| D | 65.5 ± 2.7 | 58.0 ± 4.5a) | 56.7 ± 5.4a) | 56.7 ± 6.1ab) | |
| RD | 65.7 ± 2.7 | 56.2 ± 6.6b) | 57.8 ± 4.6 | 53.3 ± 3.0ab) | |
| SmvO2 (%) | C | 87.6 ± 1.5 | 88.0 ± 0.7 | 88.5 ± 2.0 | 89.4 ± 2.0 |
| R | 89.1 ± 2.4 | 86.8 ± 2.3 | 85.9 ± 4.1 | 83.6 ± 5.5 | |
| D | 88.5 ± 1.2 | 84.2 ± 3.0a) | 83.1 ± 4.3a) | 82.9 ± 5.2 | |
| RD | 88.5 ± 1.3 | 82.6 ± 4.8b) | 84.1 ± 3.0 | 80.9 ± 2.3ab) | |
| Hct (%) | C | 32.3 ± 3.4 | 32.5 ± 3.8 | 32.2 ± 3.5 | 31.8 ± 4.0 |
| R | 32.8 ± 3.2 | 30.2 ± 0.4d) | 31.2 ± 2.6d) | 30.5 ± 3.3d) | |
| D | 32.5 ± 3.4 | 36.2 ± 2.9ac) | 37.2 ± 3.1ac) | 38.2 ± 3.0abc) | |
| RD | 32.5 ± 2.4 | 35.8 ± 3.3ac) | 37.0 ± 3.7c) | 37.8 ± 4.5c) | |
| Hb (g/dl) | C | 10.4 ± 0.8 | 10.8 ± 1.3 | 11.1 ± 1.3 | 11.2 ± 2.0 |
| R | 10.5 ± 0.8 | 10.1 ± 0.3d) | 10.3 ± 0.8d) | 10.3 ± 1.3 | |
| D | 10.8 ± 1.2 | 12.1 ± 1.2ac) | 12.5 ± 1.0ac) | 12.7 ± 1.1a) | |
| RD | 10.9 ± 0.6 | 12.0 ± 1.1c) | 12.3 ± 1.3c) | 12.5 ± 1.5 | |
| DO2I (ml/min/m2) | C | 443 ± 60 | 461 ± 48 | 479 ± 86 | 505 ± 90 |
| R | 481 ± 70 | 451 ± 79 | 484 ± 179 | 443 ± 192 | |
| D | 488 ± 41 | 404 ± 64 | 417 ± 76 | 437 ± 95 | |
| RD | 484 ± 75 | 378 ± 77 | 386 ± 104 | 357 ± 135 | |
| VO2I (ml/min/m2) | C | 91.5 ± 11.5 | 92.7 ± 11.2 | 91.9 ± 14.0 | 92.5 ± 11.4 |
| R | 99.2 ± 14.9 | 92.0 ± 10.5 | 102.5 ± 17.9 | 100.8 ± 19.5 | |
| D | 96.4 ± 8.8 | 92.0 ± 7.6 | 96.4 ± 10.1 | 97.9 ± 14.5 | |
| RD | 92.8 ± 12.9 | 90.0 ± 10.5 | 85.3 ± 11.0 | 85.3 ± 28.1 | |
| O2ER (%) | C | 20.7 ± 1.5 | 20.1 ± 1.0 | 19.4 ± 2.9 | 18.7 ± 2.9 |
| R | 19.1 ± 2.2 | 20.8 ± 3.4 | 22.2 ± 3.8 | 24.5 ± 5.3 | |
| D | 19.8 ± 1.1 | 23.1 ± 2.4 | 24.0 ± 3.7 | 23.8 ± 4.6 | |
| RD | 19.3 ± 1.3 | 24.5 ± 4.6 | 22.8 ± 3.4 | 24.3 ± 4.5 | |
pHa, arterial blood pH; PaO2, arterial blood oxygen partial pressure; PaCO2, arterial blood carbon dioxide partial pressure; HCO3−, arterial bicarbonate; pHmv, mixed venous blood pH; PmvO2, mixed venous blood oxygen partial pressure; SmvO2, mixed venous hemoglobin saturation; Hct, Hematocrit; Hb, Hemoglobin; DO2I, oxygen delivery index; VO2I, oxygen consumption index; O2ER, oxygen extraction. a) Significantly differ from respective baseline value (P<0.05); b) significantly differ from C treatment at this time point (P<0.05); c) significantly differ from R treatment at this time point (P<0.05); d) significantly differ from D treatment at this time point (P<0.05).
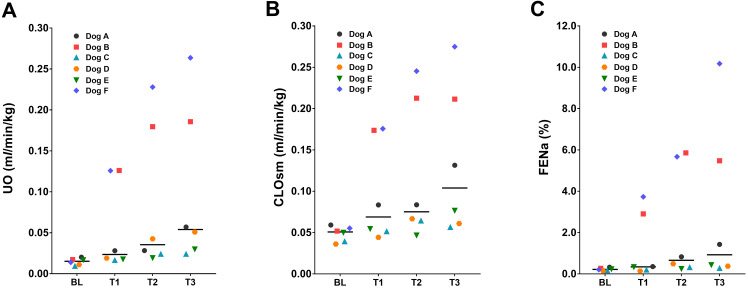
No significant changes in GFR and RBF were detected among the time points or treatments (Table 3). For the R treatment, none of the renal function variables significantly differed from that at BL and with C treatment. The renal function variables changed mainly in the D treatment: UO (Fig. 2A), CLOsm (Fig. 2B), and FENa (Fig. 2C) significantly increased and UOsm and USG decreased as compared to the BL. Individually, two dogs (dogs B and F) showed a marked increase in UO to >10 times the BL value with urine dilution (300–400 mOsm/kg for UOsm). In the other two dogs (dogs A and D), a moderate increase in UO (three to four times of the values at BL) was observed; the remaining two dogs did not show polyuria. Increases in the values of CLOsm and FENa compared with BL were observed only in dogs B and F, both of which showed a marked UO increase. A moderate increase in UO from BL was also observed in RD treatment in some dogs, although the changes were not statistically significant. No significant changes in CLH2O, POsm, FEK, and FECl did not change though the experiment, except for the FECl value in the RD treatment group at T1.
Table 3. Renal function variables (mean ± standard deviation or median with range [minimum, maximum]) in six Beagle dogs before (baseline: BL) and during an infusion of saline as control (C), remifentanil at incremented doses of 0.15 (T1), 0.60 (T2) and 2.40 (T3) μg/kg/min (R), dexmedetomidine at 0.5 μg/kg/hr (D), or the combination of remifentanil and dexmedetomidine (RD) under sevoflurane anesthesia equipotent to 1.5 times of minimum alveolar concentration.
| Variable | Treatment | BL | T1 | T2 | T3 |
|---|---|---|---|---|---|
| UO (ml/min/kg) | C | 0.015 (0.010, 0.022) | 0.021 (0.011, 0.045) | 0.017 (0.012, 0.032) | 0.020 (0.013, 0.052) |
| R | 0.014 (0.011, 0.032) | 0.015 (0.010, 0.019) | 0.014 (0.010, 0.030)d) | 0.017 (0.014, 0.040) | |
| D | 0.015 (0.009, 0.020) | 0.024 (0.017, 0.126) | 0.035 (0.019, 0.228)ac) | 0.054 (0.024, 0.264)a) | |
| RD | 0.015 (0.012, 0.027) | 0.024 (0.015, 0.060) | 0.018 (0.012, 0.070) | 0.017 (0.013, 0.029)d) | |
| USG | C | 1.041 ± 0.009 | 1.037 ± 0.014 | 1.041 ± 0.013 | 1.036 ± 0.014d) |
| R | 1.038 ± 0.012 | 1.046 ± 0.009d) | 1.044 ± 0.010d) | 1.037 ± 0.009d) | |
| D | 1.039 ± 0.009 | 1.025 ± 0.012c) | 1.019 ± 0.011abc) | 1.017 ± 0.010abc) | |
| RD | 1.038 ± 0.010 | 1.030 ± 0.010 | 1.034 ± 0.014 | 1.040 ± 0.010d) | |
| UOsm (mOsm/kg H2O) | C | 1,051 ± 179 | 1,013 ± 270 | 1,068 ± 192 | 964 ± 272 |
| R | 1,018 ± 231 | 1,093 ± 199d) | 1,120 ± 132d) | 1,021 ± 210d) | |
| D | 996 ± 156 | 698 ± 240c) | 574 ± 231abc) | 510 ± 201abc) | |
| RD | 996 ± 203 | 732 ± 206 | 854 ± 331 | 1,054 ± 201d) | |
| POsm (mOsm/kg H2O) | C | 295.3 ± 5.4 | 294.4 ± 3.3 | 295.1 ± 5.2 | 294.6 ± 4.2 |
| R | 294.9 ± 4.7 | 291.8 ± 2.4 | 289.0 ± 3.2 | 289.0 ± 3.6 | |
| D | 294.2 ± 2.4 | 293.3 ± 2.2 | 290.6 ± 3.6 | 289.8 ± 3.1 | |
| RD | 292.7 ± 2.9 | 291.0 ± 2.3 | 292.1 ± 3.2 | 290.8 ± 3.7 | |
| CLOsm (ml/min/kg) | C | 0.056 (0.044, 0.066) | 0.064 (0.051, 0.093) | 0.060 (0.048, 0.090) | 0.070 (0.054, 0.083) |
| R | 0.052 (0.048, 0.071) | 0.055 (0.044, 0.066) | 0.056 (0.042, 0.099) | 0.063 (0.052, 0.106) | |
| D | 0.051 (0.041, 0.059) | 0.069 (0.044, 0.176) | 0.075 (0.047, 0.245)a) | 0.104 (0.057, 0.275)a) | |
| RD | 0.050 (0.042, 0.075) | 0.059 (0.045, 0.081) | 0.055 (0.042, 0.073) | 0.058 (0.048, 0.075) | |
| CLH2O (ml/min/kg) | C | –0.037 (−0.047, −0.034) | –0.046 (−0.058, −0.039) | –0.042 (−0.058, −0.036) | –0.044 (−0.054, −0.031) |
| R | –0.037 (−0.047, −0.037) | –0.041 (−0.047, −0.027) | –0.042 (−0.070, −0.031) | –0.048 (−0.066, −0.036) | |
| D | –0.034 (−0.041, −0.025) | –0.042 (−0.055, −0.025) | –0.030 (−0.055, −0.017) | –0.029 (−0.074, −0.010) | |
| RD | –0.036 (−0.049, −0.026) | –0.035 (−0.044, −0.020) | –0.037 (−0.050, 0.017) | –0.044 (−0.050, −0.035) | |
| GFR (ml/min/kg) | C | 3.39 (2.82, 4.12) | 2.84 (2.64, 4.27) | 3.06 (2.78, 4.39) | 3.19 (2.40, 5.52) |
| R | 3.56 (2.26, 4.42) | 3.18 (2.60, 3.79) | 2.90 (2.47, 4.50) | 3.14 (2.10, 3.54) | |
| D | 3.72 (2.63, 3.96) | 3.18 (2.48, 4.57) | 3.20 (2.65, 4.03) | 3.37 (2.43, 4.72) | |
| RD | 3.32 (2.73, 3.93) | 3.45 (2.75, 4.20) | 3.08 (2.63, 4.37) | 3.39 (3.09, 4.30) | |
| RBF (ml/min/kg) | C | 12.4 (9.6, 19.8) | 16.6 (10.6, 26.3) | 19.6 (11.4, 26.3) | 15.0 (11.0, 19.0) |
| R | 14.8 (7.4, 19.7) | 12.6 (8.6, 15.1) | 13.3 (10.3, 21.7) | 17.0 (11.7, 25.5) | |
| D | 11.7 (10.7, 19.8) | 16.3 (10.2, 26.8) | 18.0 (9.3, 19.7) | 19.4 (14.5, 29.6) | |
| RD | 12.0 (9.6, 15.9) | 19.7 (9.3, 24.8) | 19.5 (8.4, 22.6) | 14.1 (10.0, 25.6) | |
| FENa (%) | C | 0.32 (0.15, 0.48) | 0.77 (0.16, 0.98) | 0.38 (0.11, 1.58) | 0.67 (0.12, 1.43) |
| R | 0.27 (0.13, 0.41) | 0.21 (0.16, 0.30) | 0.25 (0.11, 0.42) | 0.32 (0.17, 0.88) | |
| D | 0.22 (0.16, 0.32) | 0.34 (0.14, 3.73) | 0.66 (0.24, 5.86)a) | 0.93 (0.28, 10.18)a) | |
| RD | 0.24 (0.15, 0.52) | 0.27 (0.16, 0.36) | 0.31 (0.10, 0.44) | 0.29 (0.17, 0.61) | |
| FEK (%) | C | 27.6 (22.3, 56.0) | 37.6 (28.4, 51.5) | 31.9 (15.4, 38.7) | 32.5 (9.2, 47.5) |
| R | 37.3 (18.9, 55.5) | 28.8 (16.5, 40.3) | 36.0 (12.5, 42.0) | 41.7 (17.9, 47.2) | |
| D | 27.9 (19.5, 41.0) | 38.1 (15.7, 74.4) | 33.7 (21.1, 44.4) | 43.4 (16.5, 69.6) | |
| RD | 33.5 (18.3, 57.2) | 27.3 (16.1, 32.5) | 26.6 (17.2, 57.1) | 28.6 (21.5, 44.8) | |
| FECl (%) | C | 0.66 (0.27, 0.95) | 0.92 (0.57, 1.30) | 0.58 (0.33, 1.97) | 0.89 (0.18, 1.92) |
| R | 0.54 (0.29, 1.40) | 0.48 (0.40, 0.76) | 0.54 (0.30, 1.50) | 0.86 (0.39, 2.28) | |
| D | 0.50 (0.25, 0.89) | 0.75 (0.16, 3.39) | 0.67 (0.51, 6.05) | 1.26 (0.32, 10.17) | |
| RD | 0.53 (0.28, 1.97) | 0.34 (0.20, 0.74)b) | 0.58 (0.088, 1.69) | 0.92 (0.48, 1.71) | |
UO, urine output; USG, urine specific gravity; UOsm, urine osmolality; POsm, plasma osmolality; CLOsm, osmolar clearance; CLH2O, free-water clearance; GFR, glomerular filtration rate; RBF, renal blood flow; FENa, fractional clearance of sodium; FEK, fractional clearance of potassium; FECl, fractional clearance of chloride. a) Significantly differ from respective baseline value (P<0.05); b) significantly differ from C treatment at this time point (P<0.05); c) significantly differ from R treatment at this time point (P<0.05); d) significantly differ from D treatment at this time point (P<0.05).
Fig. 2.
The median (bar) and individual values of (A) urine output (UO), (B) osmolar clearance (CLOsm), and (C) fractional clearance of sodium (FENa) in six Beagle dogs that before (baseline: BL) and after administration of dexmedetomidine infused at 0.5 µg/kg/hr (T1–T3).
Plasma potassium concentration (mEq/l) decreased slightly but significantly in the D treatment at T3 (4.1 ± 0.4) and in the RD treatment at T2 (4.5 ± 0.4) and T3 (4.3 ± 0.3) from BL. Both sodium and chloride concentrations in plasma were within normal ranges and were not significantly changed through this experiment (data not shown).
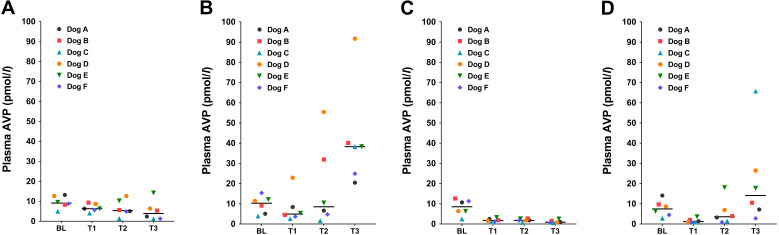
The plasma AVP concentrations significantly increased from BL to T3 with R treatment (P=0.042; Fig. 3B); however, the concentration significantly decreased at T3 with D treatment (P=0.0002; Fig. 3C) and at T1 with RD treatment (P=0.011; Fig. 3D) compared with BL, respectively. In the comparisons among the treatments at T3, plasma AVP concentration was significantly higher with RD treatment than with D treatment (P=0.029).
Fig. 3.
The median (bar) and individual values of plasma arginine vasopressin (AVP) concentrations in six Beagle dogs that before (baseline: BL) and after an infusion of (A) saline (C treatment), (B) remifentanil at incremented doses of 0.15 (T1), 0.60 (T2) and 2.40 (T3) µg/kg/min (R treatment), (C) dexmedetomidine at 0.5 µg/kg/hr consistently through T1–T3 (D treatment), and (D) the combination of dexmedetomidine and remifentanil (RD treatment) under sevoflurane anesthesia equipotent to 1.5 times of minimum alveolar concentration.
DISCUSSION
In the present study, we outlined the changes in cardiovascular and renal functions, along with evaluations of AVP secretion with remifentanil and dexmedetomidine infusion, alone or in combination, in sevoflurane-anesthetized dogs. In this study, the remifentanil infusion led to a dose-dependent decrease in HR, although CI was maintained because of increased SVI, compared with BL. These properties were not consistent with results reported previously with remifentanil CRI at 0.15 to 0.90 µg/kg/min in mixed-breed dogs under isoflurane anesthesia equipotent to 1.3 MAC [27], as the previous study has demonstrated a significant decrease in CI with greater decreases in HR than those observed in the current study. The cardiovascular changes with dexmedetomidine, HR-dependent decrease in CI with increased SVRI and blood pressure, were typical to α2-adrenoceptor agonists as reported previously [21, 25, 28, 33], but CI reduction in this study slightly varied from that in previous reports showing reduction values of 1 to 17% with dexmedetomidine CRI at 0.5 µg/kg/hr [33] and of 27.1% with medetomidine at 1.0 µg/kg/hr (equivalent to 0.5 µg/kg/hr of dexmedetomidine) [21], in isoflurane-anesthetized dogs. The differences in CI changes between the present and previous studies may be attributed to individual differences in dogs or variation in the inhaled anesthetics.
In the study reported here, the decreases in HR and CI with dexmedetomidine alone were not significantly augmented by an additional administration of remifentanil. These results coincided with previous reports that did fentanyl CRI combined with dexmedetomidine (0.1 to 3.0 µg/kg IV) in enflurane-anesthetized dogs [42] and that used fentanyl IV bolus in dogs sedated with medetomidine (1.5 µg/kg/hr) [11]. Both remifentanil and dexmedetomidine can decrease HR and increase vagal tone; these changes are resulted by vagotonic effect with remifentanil through the stimulation of central and peripheral µ opioid receptors [43]; however, they are associated with the baroreceptor reflex with dexmedetomidine in response to peripheral vasoconstriction resulting from the activation of α2b adrenoceptors in vascular smooth muscle, or the sympatholytic effect through central α2 adrenoceptor in the brainstem [29]. It is suggested that the influences in cardiovascular system with dexmedetomidine are more direct and are greater than those with remifentanil.
The highest infusion rate (2.4 µg/kg/min) of remifentanil was included in this study to clarify the effects when the rate was excessively high above the clinically used range (0.15 and 0.60 µg/kg/min). In RD treatment, SVRI was significantly elevated according to increased remifentanil rates particularly at T3, with concurrent increases in RAP and PAOP. The sympatholytic activity with dexmedetomidine can reduced inotropy and thus limit stroke volume in the face of increased afterload [29]. Although there was no statistically significant difference in CI between D and RD treatments, the increased RAP and PAOP are likely to represent blood stasis in the heart as a result of increasingly enhanced vasoconstriction by the combined CRI of remifentanil and dexmedetomidine. Moreover, pHa was significantly decreased at T3 in RD treatment; this was similar to the reported effects of remifentanil alone accompanied with significant decreases in DO2I and increases in SVRI [27]. We did not measure the lactate concentration in blood, but the concomitant decrease in HCO3− and BE at a relatively low pHa indicated metabolic acidosis, suggesting decreased oxygenation in some tissues. These results suggested that the remifentanil particularly at excessively high rates during a dexmedetomidine infusion causes undesirable effects that result from excessive vasoconstriction and thus should be avoided.
Sevoflurane also induces dose-dependent cardiopulmonary depression and decreases arterial pressure primarily via systemic vasodilation [30]. In this study, sevoflurane doses in each treatment or time point were reduced to be equipotent to 1.5 MAC according to the MAC-sparing effects of remifentanil and dexmedetomidine [1]. Therefore, the increased SVRI during administrations of remifentanil and dexmedetomidine CRI would be also induced by the modulation of peripheral vasodilation associated with reduced sevoflurane doses, similar to those in a previous study using remifentanil CRI during isoflurane anesthesia equipotent to 1.3 MAC in dogs [27].
This study showed that dexmedetomidine CRI obviously increased Hct and Hb by approximately 5% and 2 g/dl, respectively, in mean values in both alone and combined with remifentanil in dogs. Although the mechanisms have not been elucidated, the increased Hct and Hb were similarly reported in some studies with dexmedetomidine CRI in anesthetized dogs [25, 33]. The recruitment of red blood cells from the spleen could cause these increases; however, this idea has been controversial because the dexmedetomidine administration did not induce marked splenic contraction in dogs [3]. Another possible cause is a blood concentrating activity resulted by the movement of intravascular fluid to the interstitium because of increased hydrostatic pressure in micro circulation related to vasoconstriction.
The present study demonstrated that DO2I and O2ER values were unchanged with dexmedetomidine, either alone and in combination with remifentanil, despite the decreased CI, presumably because of an increase in oxygen content (Hct and Hb). Further, changes in PmvO2 and SmvO2 were within the physiologically normal ranges [14] although these values were decreased in D and RD treatments in parallel with the CI changes. Thus, we considered that the cardiovascular changes are acceptable for healthy dogs in terms of maintenance of global oxygenation during combined infusions of remifentanil and dexmedetomidine.
The present study showed that despite the effect on CI due to the administration of remifentanil and dexmedetomidine, GFR and RBF were not significantly altered. These results were consistent with those in a previous study that dexmedetomidine alone were infused at 1.0 and 2.0 µg/kg/hr in isoflurane-anesthetized dogs [49]. Also, it has been demonstrated that GFR and RBF were not decreased by fentanyl administration even when the doses were sufficiently high to decrease blood pressure in dogs [7]. Dexmedetomidine decreases CO but did not eventually cause a reduction in the perfusion of vital organs, including the kidneys because functional redistribution of blood flow may occur [24]. Our results indicated that the hemodynamic changes with dexmedetomidine at 0.5 µg/kg/hr were enough to preserve functional renal perfusions even when used in combination with remifentanil.
In clinical relevance, the use of anticholinergic drugs to recover the decreased HR is debatable with the combined administration of remifentanil and dexmedetomidine during sevoflurane anesthesia. We suggest that aggressive recovery of HR in healthy dogs is not required when arterial blood pressure is maintained, because of global oxygenation and renal function as shown in the current study. In addition, anticholinergic drugs may lead to further hypertension particularly during dexmedetomidine administration [41]. Drugs that can attenuate systemic vasoconstriction, such as a peripheral α2-adrenoceptor antagonist, would be an alternative for alleviating the bradycardia [41].
The plasma AVP levels at BL were higher than the physiologic reference range of 1 to 6 pmol/l in dogs [50]; possibly due to anesthetic conditions [15] or noxious stimuli such as intubation and catheter instrumentation. Remifentanil increased the plasma AVP concentrations only at a relatively high infusion rate of 2.4 µg/kg/min compared from BL. The magnitude of the increase in plasma AVP concentrations with remifentanil was less, compared with those in a previous study reporting that remifentanil CRI at 0.15 to 0.90 µg/kg/min significantly increased the plasma AVP concentrations by 10- to 20-fold from that at baseline [27]. Monteiro et al. [27] also reported that the increase in plasma AVP levels correlated well with the significant increase in SVRI accompanied by CI reductions. In this present study, we did not find any significant changes in SVRI and CI with remifentanil alone. The increase in AVP secretion with pure µ opioid agonists possibly occurs as a compensatory response to a decrease in blood pressure secondary to bradycardia [39] or as a direct stimulation via µ opioid receptors in the neurohypophysis [17]. Simultaneously, the increase in SVRI with opioids was reported as part of the physiologic compensation for decreased blood pressure by bradycardia [20]. Therefore, the less increase in SVRI and plasma AVP concentrations in this study than in the previous report might have been related to the small CI changes. Interestingly, in this present study, 2.4 µg/kg/min of remifentanil significantly increased plasma AVP levels despite maintained CI. The result suggests the direct effect of the at least high rate of remifentanil on AVP secretion.
In contrast, plasma AVP levels were lowered by dexmedetomidine at 0.5 µg/kg/hr in accordance with previously published results on isoflurane-anesthetized dogs that received IV medetomidine at 20 and 40 µg/kg [40] and dexmedetomidine CRI at 1.0 to 2.0 µg/kg/hr [49]. Furthermore, the present study demonstrated that dexmedetomidine attenuated AVP secretion, even when given in combination with low-dose (0.15 µg/kg/min) remifentanil, whereas the attenuation disappeared under remifentanil infusion above 0.6 µg/kg/min. These results indicated dose-dependent interference activity between remifentanil and dexmedetomidine on AVP secretion. The mechanisms responsible for the decrease in AVP secretion with dexmedetomidine were not addressed in the current study, but it was presumably secondary to increased blood pressure [19] or was the result of the direct inhibition of neurosecretory cells in the hypothalamus [6, 22].
The UO significantly increased only with D treatment but was unchanged in C, R and RD treatments as compared to respective baseline values. UO with remifentanil CRI did not significantly differ from baseline, although the antidiuretic effects of opioids have been recognized in dogs [2, 38]. Anderson et al. [2] reported that the UO was significantly reduced to 0.012 ml/kg/min with a fentanyl CRI in healthy dogs compared from the control value of 0.021 ml/kg/min with an infusion of lactated Ringer’s solution, which was higher than our baseline values. The relatively lower UO values at BL in the present study might cause ambiguity in the antidiuretic effect.
Previous reports have shown that α2-adrenoceptor agonists produced diuretic effects in dogs, regardless of whether or not they received general anesthesia, including both aquaretic and natriuretic conditions [5, 6, 40, 44, 45, 49]. In our experiment, dexmedetomidine CRI induced diuresis that was concomitant with increased CLOsm and FENa, which represents the natriuresis. The diuretic condition was different from results in previous studies demonstrating that dexmedetomidine CRI (1.0 and 2.0 µg/kg/hr) and medetomidine IV (20 and 40 µg/kg) resulted in aquaresis (increases in UO and CLH2O) in isoflurane-anesthetized dogs, although concurrent decreases in plasma AVP levels was consistently observed in the present and previous studies [40, 49]. The cause of variations in diuretic conditions between the present and previous studies were still unknown but might have included the differences in administration doses or underlying hydration state of dogs. Considerable individual variation between dogs in this study also might have been caused by a lower dose rate (0.5 µg/kg/hr) of dexmedetomidine that was presumably too low to reveal the effect eventually in all dogs.
The concurrent administration of remifentanil seemed to make the dexmedetomidine-induced diuresis unclear in our experiment; to our knowledge, this was the first report representing the interference activity of an µ opioid of remifentanil on the diuretic properties of dexmedetomidine. Although the experimental study has exhibited the diuretic effect with α2-adrenoceptor agonists, clinically it has never been well demonstrated in veterinary practice. In human medicine, few studies have reported dexmedetomidine-induced polyuria including both aquaresis and natriuresis; however, the occurrence is likely to be rare despite its widespread use [23, 34]. Our study suggests that the existence of co-administered opioid analgesics in clinical setting could be a cause for the rare appearance of diuresis with α2-adrenoceptor agonists. Further studies on mechanisms and the clinical significance for its diuretic effect are needed.
In the present study, it is controversial whether the changes in plasma AVP levels predominantly determined the effects on urine production with a CRI of remifentanil and/or dexmedetomidine. The decreased plasma AVP concentrations may contribute to diluted urine (decreased UOsm and USG) in D treatment, but the natriuretic condition represents functional alternation in mechanisms that are related to sodium reabsorption other than water reabsorption. Moreover, our data on individual dogs indicated that UO increased alongside decreased plasma AVP concentrations in some dogs but not in other dogs even with similar plasma AVP levels with D treatment. This independence was supported by a previous study on human patients according to which oral administration of 2.5 or 5.0 µg/kg clonidine apparently induced natriuresis without significant changes in the plasma AVP levels during isoflurane-nitrous oxide anesthesia [13]. In addition, it is difficult to explain using the changes in AVP secretion that UO did not increase at T1 in RD treatment despite there was low plasma AVP concentrations (<5 pg/ml), similar to those in D treatment that represented diuresis. A previous study has reported the absence of a relationship between AVP secretion and lower urine production after administration of morphine as a preanesthetic agent in dogs that underwent surgery [38]. Considering these results, we assumed that the changes in plasma AVP concentrations might not be the only factor responsible for both diuretic and antidiuretic properties with dexmedetomidine and remifentanil, respectively.
This study has several limitations. First, sevoflurane doses were altered to prioritize simulating the surgical anesthetic depth expected to be used clinically, with reference to previous studies evaluating cardiovascular effects of remifentanil alone and dexmedetomidine alone in isoflurane-anesthetized dogs [27, 33]. To clarify the intrinsic effects of each drug, especially the complicated interactions on urine production and AVP secretion, another study with a constant sevoflurane dose is required. Second, we did not measure the drug concentrations in blood; these measurements could have given information on the pharmacokinetic interactions of both drugs and the adequacy of the equilibration periods of the drugs. Third, the current study did not evaluate other hormones that are strongly associated with AVP secretion and renal functions. Talukder and Hikasa [45] reported that medetomidine increased atrial natriuretic peptide along with a decrease in plasma AVP levels in dogs. Francis et al. [9] demonstrated a concomitant increase in angiotensin II and AVP by the administration of remifentanil in isoflurane-anesthetized dogs. The changes in these hormones might have influenced our data, and these measurements would have helped us understand the mechanisms.
In conclusion, the present study for the first time demonstrated the following: (1) remifentanil co-administered with dexmedetomidine under sevoflurane anesthesia in dogs, was acceptable from the viewpoint of its cardiovascular effects, oxygenation, acid-base balance, and renal function, (2) 0.5 µg/kg/hr of dexmedetomidine significantly decrease plasma AVP concentrations and can elicit natriuresis, (3) remifentanil may interfere with the dexmedetomidine-induced diuresis and inhibition of AVP secretion.
POTENTIAL CONFLICTS OF INTEREST. The authors have nothing to disclose.
Acknowledgments
This work was supported by the JSPS KAKENHI (grant number JP15K18794).
REFERENCES
- 1.Akashi N., Murahata Y., Kishida H., Hikasa Y., Azuma K., Imagawa T.2020. Effects of constant rate infusions of dexmedetomidine, remifentanil and their combination on minimum alveolar concentration of sevoflurane in dogs. Vet. Anaesth. Analg. 47: 490–498. doi: 10.1016/j.vaa.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 2.Anderson M. K., Day T. K.2008. Effects of morphine and fentanyl constant rate infusion on urine output in healthy and traumatized dogs. Vet. Anaesth. Analg. 35: 528–536. doi: 10.1111/j.1467-2995.2008.00413.x [DOI] [PubMed] [Google Scholar]
- 3.Baldo C. F., Garcia-Pereira F. L., Nelson N. C., Hauptman J. G., Shih A. C.2012. Effects of anesthetic drugs on canine splenic volume determined via computed tomography. Am. J. Vet. Res. 73: 1715–1719. doi: 10.2460/ajvr.73.11.1715 [DOI] [PubMed] [Google Scholar]
- 4.Bürkle H., Dunbar S., Van Aken H.1996. Remifentanil: a novel, short-acting, mu-opioid. Anesth. Analg. 83: 646–651. doi: 10.1213/00000539-199609000-00038 [DOI] [PubMed] [Google Scholar]
- 5.Burton S., Lemke K. A., Ihle S. L., Mackenzie A. L.1998. Effects of medetomidine on serum osmolality; urine volume, osmolality and pH; free water clearance; and fractional clearance of sodium, chloride, potassium, and glucose in dogs. Am. J. Vet. Res. 59: 756–761. [PubMed] [Google Scholar]
- 6.Cabral A. D., Kapusta D. R., Kenigs V. A., Varner K. J.1998. Central α2-receptor mechanisms contribute to enhanced renal responses during ketamine-xylazine anesthesia. Am. J. Physiol. 275: R1867–R1874. [DOI] [PubMed] [Google Scholar]
- 7.Castiglia Y. M. M., Braz J. R. C., Vianna P. T. G., Lemonica L., Vane L. A.1997. Effect of high-dose fentanyl on renal function in dogs. Sao Paulo Med. J. 115: 1433–1439. doi: 10.1590/S1516-31801997000300006 [DOI] [PubMed] [Google Scholar]
- 8.Chism J. P., Rickert D. E.1996. The pharmacokinetics and extra-hepatic clearance of remifentanil, a short acting opioid agonist, in male beagle dogs during constant rate infusions. Drug Metab. Dispos. 24: 34–40. [PubMed] [Google Scholar]
- 9.Francis R. C., Reyle-Hahn M. S., Höhne C., Klein A., Theruvath I., Donaubauer B., Busch T., Boemke W.2008. The haemodynamic and catecholamine response to xenon/remifentanil anaesthesia in Beagle dogs. Lab. Anim. 42: 338–349. doi: 10.1258/la.2007.007048 [DOI] [PubMed] [Google Scholar]
- 10.Gellai M.1990. Modulation of vasopressin antidiuretic action by renal α 2-adrenoceptors. Am. J. Physiol. 259: F1–F8. [DOI] [PubMed] [Google Scholar]
- 11.Grimm K. A., Tranquilli W. J., Gross D. R., Sisson D. D., Bulmer B. J., Benson G. J., Greene S. A., Martin-Jimenez T.2005. Cardiopulmonary effects of fentanyl in conscious dogs and dogs sedated with a continuous rate infusion of medetomidine. Am. J. Vet. Res. 66: 1222–1226. doi: 10.2460/ajvr.2005.66.1222 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Blanco E., Victoria-Mora J. M., Ibancovichi-Camarillo J. A., Sauri-Arceo C. H., Bolio-González M. E., Acevedo-Arcique C. M., Marin-Cano G., Steagall P. V.2013. Evaluation of the isoflurane-sparing effects of fentanyl, lidocaine, ketamine, dexmedetomidine, or the combination lidocaine-ketamine-dexmedetomidine during ovariohysterectomy in dogs. Vet. Anaesth. Analg. 40: 599–609. doi: 10.1111/vaa.12079 [DOI] [PubMed] [Google Scholar]
- 13.Hamaya Y., Nishikawa T., Dohi S.1994. Diuretic effect of clonidine during isoflurane, nitrous oxide, and oxygen anesthesia. Anesthesiology 81: 811–819. doi: 10.1097/00000542-199410000-00007 [DOI] [PubMed] [Google Scholar]
- 14.Haskins S., Pascoe P. J., Ilkiw J. E., Fudge J., Hopper K., Aldrich J.2005. Reference cardiopulmonary values in normal dogs. Comp. Med. 55: 156–161. [PubMed] [Google Scholar]
- 15.Hauptman J. G., Richter M. A., Wood S. L., Nachreiner R. F.2000. Effects of anesthesia, surgery, and intravenous administration of fluids on plasma antidiuretic hormone concentrations in healthy dogs. Am. J. Vet. Res. 61: 1273–1276. doi: 10.2460/ajvr.2000.61.1273 [DOI] [PubMed] [Google Scholar]
- 16.Heiene R., Moe L.1998. Pharmacokinetic aspects of measurement of glomerular filtration rate in the dog: a review. J. Vet. Intern. Med. 12: 401–414. doi: 10.1111/j.1939-1676.1998.tb02143.x [DOI] [PubMed] [Google Scholar]
- 17.Hellebrekers L. J., van den Brom W. E., Mol J. A.1989. Plasma arginine vasopressin response to intravenous methadone and naloxone in conscious dogs. J. Pharmacol. Exp. Ther. 248: 329–333. [PubMed] [Google Scholar]
- 18.Hoke J. F., Cunningham F., James M. K., Muir K. T., Hoffman W. E.1997. Comparative pharmacokinetics and pharmacodynamics of remifentanil, its principle metabolite (GR90291) and alfentanil in dogs. J. Pharmacol. Exp. Ther. 281: 226–232. [PubMed] [Google Scholar]
- 19.Humphreys M. H., Reid I. A., Chou L. Y. N.1975. Suppression of antidiuretic hormone secretion by clonidine in the anesthetized dog. Kidney Int. 7: 405–412. doi: 10.1038/ki.1975.58 [DOI] [PubMed] [Google Scholar]
- 20.Ilkiw J. E., Pascoe P. J., Haskins S. C., Patz J. D., Jaffe R.1994. The cardiovascular sparing effect of fentanyl and atropine, administered to enflurane anesthetized dogs. Can. J. Vet. Res. 58: 248–253. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaartinen J., Pang D., Moreau M., Vainio O., Beaudry F., del Castillo J., Lamont L., Cuvelliez S., Troncy E.2010. Hemodynamic effects of an intravenous infusion of medetomidine at six different dose regimens in isoflurane-anesthetized dogs. Vet. Ther. 11: E1–E16. [PubMed] [Google Scholar]
- 22.Kimura T., Share L., Wang B. C., Crofton J. T.1981. The role of central adrenoreceptors in the control of vasopressin release and blood pressure. Endocrinology 108: 1829–1836. doi: 10.1210/endo-108-5-1829 [DOI] [PubMed] [Google Scholar]
- 23.Kirschen G. W., Kim E., Adsumelli R. S. N.2019. Dexmedetomidine-induced massive diuresis in a patient undergoing spinal fusion surgery: a case report and synthesis of the literature. A A Pract. 12: 112–114. doi: 10.1213/XAA.0000000000000860 [DOI] [PubMed] [Google Scholar]
- 24.Lawrence C. J., Prinzen F. W., de Lange S.1996. The effect of dexmedetomidine on nutrient organ blood flow. Anesth. Analg. 83: 1160–1165. doi: 10.1213/00000539-199612000-00005 [DOI] [PubMed] [Google Scholar]
- 25.Lin G. Y., Robben J. H., Murrell J. C., Aspegrén J., McKusick B. C., Hellebrekers L. J.2008. Dexmedetomidine constant rate infusion for 24 hours during and after propofol or isoflurane anaesthesia in dogs. Vet. Anaesth. Analg. 35: 141–153. doi: 10.1111/j.1467-2995.2007.00365.x [DOI] [PubMed] [Google Scholar]
- 26.Mann W. A., Kinter L. B.1993. Characterization of the renal handling of p-aminohippurate (PAH) in the beagle dog (Canis familiaris). Gen. Pharmacol. 24: 367–372. doi: 10.1016/0306-3623(93)90318-R [DOI] [PubMed] [Google Scholar]
- 27.Monteiro E. R., Neto F. J. T., Campagnol D., Garofalo N. A., Alvaides R. K.2010. Hemodynamic effects in dogs anesthetized with isoflurane and remifentanil-isoflurane. Am. J. Vet. Res. 71: 1133–1141. doi: 10.2460/ajvr.71.10.1133 [DOI] [PubMed] [Google Scholar]
- 28.Moran-Muñoz R., Valverde A., Ibancovichi J. A., Acevedo-Arcique C. M., Recillas-Morales S., Sanchez-Aparicio P., Osorio-Avalos J., Chavez-Monteagudo J. R.2017. Cardiovascular effects of constant rate infusions of lidocaine, lidocaine and dexmedetomidine, and dexmedetomidine in dogs anesthetized at equipotent doses of sevoflurane. Can. Vet. J. 58: 729–734. [PMC free article] [PubMed] [Google Scholar]
- 29.Murrell J. C., Hellebrekers L. J.2005. Medetomidine and dexmedetomidine: a review of cardiovascular effects and antinociceptive properties in the dog. Vet. Anaesth. Analg. 32: 117–127. doi: 10.1111/j.1467-2995.2005.00233.x [DOI] [PubMed] [Google Scholar]
- 30.Mutoh T., Nishimura R., Kim H. Y., Matsunaga S., Sasaki N.1997. Cardiopulmonary effects of sevoflurane, compared with halothane, enflurane, and isoflurane, in dogs. Am. J. Vet. Res. 58: 885–890. [PubMed] [Google Scholar]
- 31.Nishida M., Uechi M., Kono S., Harada K., Fujiwara M.2012. Estimating glomerular filtration rate in healthy dogs using inulin without urine collection. Res. Vet. Sci. 93: 398–403. doi: 10.1016/j.rvsc.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 32.Pascal M., Allison A., Kaartinen J.2020. Opioid-sparing effect of a medetomidine constant rate infusion during thoraco-lumbar hemilaminectomy in dogs administered a ketamine infusion. Vet. Anaesth. Analg. 47: 61–69. doi: 10.1016/j.vaa.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 33.Pascoe P. J.2015. The cardiopulmonary effects of dexmedetomidine infusions in dogs during isoflurane anesthesia. Vet. Anaesth. Analg. 42: 360–368. doi: 10.1111/vaa.12220 [DOI] [PubMed] [Google Scholar]
- 34.Pratt A., Aboudara M., Lung L.2013. Case report: polyuria related to dexmedetomidine. Anesth. Analg. 117: 150–152. doi: 10.1213/ANE.0b013e3182917c86 [DOI] [PubMed] [Google Scholar]
- 35.Pypendop B. H., Verstegen J. P.1998. Hemodynamic effects of medetomidine in the dog: a dose titration study. Vet. Surg. 27: 612–622. doi: 10.1111/j.1532-950X.1998.tb00539.x [DOI] [PubMed] [Google Scholar]
- 36.Reid I. A., Nolan P. L., Wolf J. A., Keil L. C.1979. Suppression of vasopressin secretion by clonidine: effect of alpha-adrenoceptor antagonists. Endocrinology 104: 1403–1406. doi: 10.1210/endo-104-5-1403 [DOI] [PubMed] [Google Scholar]
- 37.Rioja E., Gianotti G., Valverde A.2013. Clinical use of a low-dose medetomidine infusion in healthy dogs undergoing ovariohysterectomy. Can. Vet. J. 54: 864–868. [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson S. A., Hauptman J. G., Nachreiner R. F., Richter M. A.2001. Effects of acetylpromazine or morphine on urine production in halothane-anesthetized dogs. Am. J. Vet. Res. 62: 1922–1927. doi: 10.2460/ajvr.2001.62.1922 [DOI] [PubMed] [Google Scholar]
- 39.Rockhold R. W., Crofton J. T., Wang B. C., Share L.1983. Effect of intracarotid administration of morphine and naloxone on plasma vasopressin levels and blood pressure in the dog. J. Pharmacol. Exp. Ther. 224: 386–390. [PubMed] [Google Scholar]
- 40.Saleh N., Aoki M., Shimada T., Akiyoshi H., Hassanin A., Ohashi F.2005. Renal effects of medetomidine in isoflurane-anesthetized dogs with special reference to its diuretic action. J. Vet. Med. Sci. 67: 461–465. doi: 10.1292/jvms.67.461 [DOI] [PubMed] [Google Scholar]
- 41.Salla K. M., Tuns C. I., Bennett R. C., Raekallio M. R., Scheinin M., Kuusela E., Vainio O. M.2017. Cardiovascular effects of premedication with medetomidine alone and in combination with MK-467 or glycopyrrolate in dogs subsequently anesthetized with isoflurane. Am. J. Vet. Res. 78: 1245–1254. doi: 10.2460/ajvr.78.11.1245 [DOI] [PubMed] [Google Scholar]
- 42.Salmenperä M. T., Szlam F., Hug C. C., Jr.1994. Anesthetic and hemodynamic interactions of dexmedetomidine and fentanyl in dogs. Anesthesiology 80: 837–846. doi: 10.1097/00000542-199404000-00017 [DOI] [PubMed] [Google Scholar]
- 43.Shinohara K., Aono H., Unruh G. K., Kindscher J. D., Goto H.2000. Suppressive effects of remifentanil on hemodynamics in baro-denervated rabbits. Can. J. Anaesth. 47: 361–366. doi: 10.1007/BF03020954 [DOI] [PubMed] [Google Scholar]
- 44.Strandhoy J. W., Morris M., Buckalew V. M., Jr.1982. Renal effects of the antihypertensive, guanabenz, in the dog. J. Pharmacol. Exp. Ther. 221: 347–352. [PubMed] [Google Scholar]
- 45.Talukder M. H., Hikasa Y.2009. Diuretic effects of medetomidine compared with xylazine in healthy dogs. Can. J. Vet. Res. 73: 224–236. [PMC free article] [PubMed] [Google Scholar]
- 46.Tayari H., Bell A.2019. Dexmedetomidine infusion as perioperative adjuvant in a dog undergoing craniotomy. Vet. Rec. Case Rep. 7: e000727. doi: 10.1136/vetreccr-2018-000727 [DOI] [Google Scholar]
- 47.Uilenreef J. J., Murrell J. C., McKusick B. C., Hellebrekers L. J.2008. Dexmedetomidine continuous rate infusion during isoflurane anaesthesia in canine surgical patients. Vet. Anaesth. Analg. 35: 1–12. doi: 10.1111/j.1467-2995.2007.00344.x [DOI] [PubMed] [Google Scholar]
- 48.Valtolina C., Robben J. H., Uilenreef J., Murrell J. C., Aspegrén J., McKusick B. C., Hellebrekers L. J.2009. Clinical evaluation of the efficacy and safety of a constant rate infusion of dexmedetomidine for postoperative pain management in dogs. Vet. Anaesth. Analg. 36: 369–383. doi: 10.1111/j.1467-2995.2009.00461.x [DOI] [PubMed] [Google Scholar]
- 49.Villela N. R., do Nascimento Júnior P., de Carvalho L. R., Teixeira A.2005. Effects of dexmedetomidine on renal system and on vasopressin plasma levels. Experimental study in dogs. Rev. Bras. Anestesiol. 55: 429–440. [DOI] [PubMed] [Google Scholar]
- 50.van Vonderen I. K., Wolfswinkel J., Oosterlaken-Dijksterhuis M. A., Rijnberk A., Kooistra H. S.2004. Pulsatile secretion pattern of vasopressin under basal conditions, after water deprivation, and during osmotic stimulation in dogs. Domest. Anim. Endocrinol. 27: 1–12. doi: 10.1016/j.domaniend.2004.01.007 [DOI] [PubMed] [Google Scholar]