Abstract
This study evaluated the virucidal efficacy of acidic electrolyzed water (AEW) against African swine fever virus (ASFV) and avian influenza virus (AIV), according to the Animal and Plant Quarantine Agency (APQA) guidelines for efficacy testing of veterinary disinfectants. AEW (pH 5.0–6.5) was prepared using a commercially available “Electrolyzed Water Generator” with a free chlorine concentration (FCC) of 5–140 ppm, and its efficiency in reducing the titer of ASFV and AIV was tested in a suspension under low- and high-level organic soiling. Under low-level organic soiling conditions, AEW with FCC ≥40 ppm was effective against ASFV; under high-level organic soiling conditions, AEW with FCC ≥80 ppm was effective against ASFV. Under low-level organic soiling conditions, AEW with FCC ≥60 ppm was effective against AIV; under high-level organic soiling conditions, AEW with FCC ≥100 ppm was effective against AIV. The virucidal effect of AEW seemed dependent on the FCC and the presence of organic soiling. Based on these data, we recommend the following minimum FCCs in AEW treatment for routine disinfection in veterinary field under low- and high-level organic soiling conditions: for ASFV, 50 ppm and 100 ppm; and for AIV, 75 ppm and 125 ppm, respectively. In conclusion, the virucidal effects of AEW against ASFV and AIV emphasize its potential utility as a disinfectant, and we suggest considering organic soiling conditions while using AEW for implementing effective control measures for field applications.
Keywords: acidic electrolyzed water, African swine fever virus, avian influenza virus, disinfection, virucidal efficacy
In South Korea, African swine fever virus (ASFV) was first detected at a farrow-to-finish farm, and it caused outbreaks of African swine fever (ASF) in 2019 [16]. In addition, a continuously evolving highly pathogenic avian influenza virus (AIV) has been identified since the first outbreak of the H5N1 highly pathogenic avian influenza (AI) in 2003 [14]. The emergence of infectious animal disease viruses has had devastating economic consequences. Between 2013 and 2016, approximately 48 million livestock were killed in Korea in an attempt to prevent the spread of AI [8].
ASFV and AIV are pathogenic, enveloped viruses, that are highly detrimental to the pig and poultry industries. They spread via direct contact with infected animals and are capable of spreading via the environment, including through contact with materials contaminated by virus-containing matter. Currently, there are no adequately successful disease control measures against ASFV and AIV, and the mass culling of infected animals and animals that may have had contact with them is an essential strategy as prevention and control is largely dependent on effective biosecurity [12, 13, 18, 19, 33]. Disinfection is an important strategy for reducing the risk of contaminating environments, and disinfectants are important tools for biosecurity programs [5, 12].
Chlorine-based disinfectants, such as sodium hypochlorite, are widely used owing to their high efficacy and low cost. As a disinfectant, chlorine is a strong oxidant and is used to control against a broad spectrum of bacteria and viruses in the clinical, agricultural, and food industries [4, 11, 25, 27,28,29]. However, a common concern is that it is potentially toxic, corrosive, and volatile and leaves toxic residues [1, 10, 27]. Advances in technology have allowed large-scale production of stable chlorinated water through the electrolysis of diluted salt solution, which is also referred to as “electrolyzed water (EW)” or “electrochemically activated (ECA) water” [4, 25, 30].
Notably, numerous studies have demonstrated the advantages of acidic EW (AEW); these include, broad-spectrum antimicrobial activity, non-environmental hazards, reduced corrosion, non-toxicity, low cost, and ease of onsite production, which indicate its usefulness and biocompatibility as a disinfectant [9, 10, 17, 22, 25, 29].
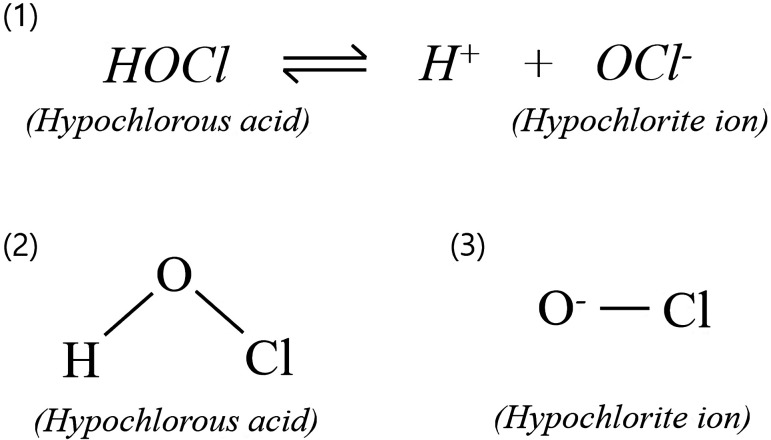
Hypochlorous acid (HOCl) is a form of free chlorine and an active component of AEW. AEW is generated by passing an aqueous salt (NaCl) or acid solution (HCl) through an electrolytic cell where the electrolysis reaction takes place. The aqueous salt solution is supplied to the electrolytic cell and the subsequently produced chlorine ion is electrolyzed to chlorine which in turn undergoes reaction with water to generate HOCl. As shown in Fig. 1, HOCl further dissociates into hypochlorite ions (OCl−), which is also referred as free chlorine, and hydrogen ions (H+). The reaction is reversible and pH dependent [20]. These three species exist in equilibrium, and the pH of the solution dictates the predominant chlorine species; undissociated HOCl predominates at lower pH, whereas dissociated OCl− predominates at higher pH [20]. AEW is considered to have a pH of 5.0–6.8 and contains HOCl as the predominant species (approximately 95%), which is a more effective, and stronger oxidant than OCl− [1, 10, 20, 30].
Fig. 1.
Main chemical reaction in electrolysis and structures of free chlorines. (1) The ratio of hypochlorous acid (HOCl) to hypochlorite ions (OCl−) varies in dependence of the pH. The reaction is reversible and both species are referred to as free chlorine. (2) Structural formula of HOCl. (3) Structural formula of OCl−.
In South Korea, the disinfectants used and their effective concentrations are officially approved when they are tested according to the Animal and Plant Quarantine Agency (APQA) guidelines for efficacy testing of veterinary disinfectants. As the critical evaluation of the specific efficacy and toxicity of AEW on ASFV and AIV is lacking, we aimed to evaluate the virucidal efficacy of AEW against ASFV and AIV according to the APQA guidelines. In addition, we aimed to optimize its effective free chlorine concentration (FCC) under various organic soiling conditions to confirm the virucidal effect.
MATERIALS AND METHODS
Preparation of the treatment solution
AEW was prepared using a commercially available “Electrolyzed Water Generator” (HAS-1560; Enputech Co., Ltd., Gyeonggi-do, Korea) in our laboratory. Following manufacturer’s instructions, a provided electrolyzing agent and tap water (temperature 15–25°C, hardness ≤80 ppm) were simultaneously pumped into the generator at 4.0–12.0 l/min with a current of 50 A. AEW with FCC was obtained by modulating the flow rate through the generator, and it was adjusted to pH 5.0–6.5. The FCC was measured using a free chlorine and chlorine ultra-high range meter (HI 96771; Hanna Instruments, Woonsocket, RI, USA) and pH was measured using a pH meter (Seven Compact; Mettler Toledo, Columbus, OH, USA) according to the manufacturer’s instructions. The working AEWs were prepared on the day of use and stored at 4°C until use.
Viruses and cultures
ASFV strain BA71V (KVCC VR1900048) and low pathogenic avian influenza virus (LPAIV) H9N2 strain A/chicken/Korea/MS96/1996 (KVCC VR 1100013) were provided by the Korean Veterinary Culture Collection (KVCC, Gyeongsangbuk-do, Korea). The ASFV was propagated and titrated using a Vero cell line (Korean Cell Line Bank [KCLB] no. 10081) provided by KCLB (Seoul, Korea). Vero cells were maintained and propagated in a Dulbecco’s modified Eagle’s medium (DMEM) (Corning Inc., Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Corning Inc.), 1% MEM non-essential amino acids (NEAA) (Gibco, Grand Island, NY, USA), and 0.1% gentamicin sulfate solution (GS) (Corning Inc.). ASFV infection was identified by the cytopathic effect (CPE) after 5 days of infection. The LPAIV was propagated and titrated using 9- to 11-day-old embryonated chicken eggs. LPAIV infection was determined using a hemagglutination (HA) assay as described in the World Organisation for Animal Health (OIE) manual [32].
Virucidal efficacy assays
The experiments were performed according to the APQA guidelines for efficacy testing of veterinary disinfectants [3], and were carried out in triplicate.
Preparation of the virus inoculum
Viruses were mixed 1:19 with each appropriate diluent in the following conditions:
● For the low-level organic soiling (LS) conditions, we used hard water (CureBio, Seoul, Korea) as the virus diluent
● For the high-level organic soiling (HS) conditions, we used hard water containing 5% FBS as the virus diluent
For the virus control condition, we used hard water without disinfectant. In addition, hard water was used instead of the virus-diluent mixture as a control for determining the toxicity caused by the AEW (Table 1).
Table 1. Treatment groups for the determination of disinfectant effective dilution factors.
| Treatment | Composition of each treatment group | |||
|---|---|---|---|---|
| Hard water | Soiling | Disinfectant | Virus | |
| Low-level organic soiling | + | - | + | + |
| High-level organic soiling | + | + | + | + |
| Virus control | + | - | - | + |
| Disinfectant toxicity control | + | - | + | - |
+, presence; -, absence.
Virus-disinfectant reaction
The virus inoculum was mixed at a ratio of 1:1 with AEW containing 5, 10, 20, 40, 60, 80, 100, 120, and 140 ppm of free chlorine. Subsequently, the virus-AEW mixtures were incubated at 4°C for 30 min (vortexed every 10 min) and further diluted with an equal amount of DMEM containing 10% FBS for the ASFV mixtures and phosphate-buffered saline (PBS) (Corning Inc.) containing 10% FBS for the LPAIV mixtures to quench AEW activity.
Virucidal efficacy against ASFV
The mixture of AEW-treated ASFV were serially diluted 10-fold in microtiter plates with DMEM containing 2% FBS, 1% NEAA, and 0.1% GS up to 10−7. Aliquots of 0.1 ml of the diluted and undiluted mixtures were transferred into six wells of a microtiter plate containing Vero cell cultures and the virus-induced cytopathic effect (CPE) was evaluated after 5 days of incubation. The viral titer was calculated using the Spearman-Karber method [24] and was expressed as 50% tissue culture infectious dose per 0.1 ml (TCID50/0.1 ml) in log units. All viral experiments were conducted in a biosafety level 3 (BSL-3) facility at APQA in South Korea.
Virucidal efficacy against AIV
The mixture of AEW-treated AIV were serially diluted 10-fold in PBS containing 1% GS up to 10−5. Five embryonated chicken eggs (10-day embryos) were inoculated into allantoic cavity with 0.2 ml of the diluted and undiluted mixtures and were incubated at 37°C for 5 days. For virus control, 0.2 ml of the diluted mixture starting with 10−3 was inoculated into five embryonated chicken eggs. The allantoic fluid was harvested from the embryonated chicken eggs and the viral titer was determined using the HA assay as described in the OIE manual [32]. Viral titer was calculated using the Spearman-Karber method [24] and expressed as 50% egg infectious dose per 0.2 ml (EID50/0.2 ml) in log units.
Inactivation analysis
Inactivation efficacy against the viruses was determined by subtracting the titer obtained from the AEW treatment from the titer of the corresponding virus control in log units. The final viral titer was determined as the median value of the triplicates within a 20% (± 10%) error range. If the difference was ≥4 log, indicating a more than 10,000 times reduction of the viral titer, the corresponding FCC of AEW was considered to be an effective concentration against the virus. Based on the study data, a recommended concentration was suggested as a preventative measure for field application, which was calculated as 125% of the corresponding effective concentration as described in APQA guidelines for efficacy testing of veterinary disinfectants [3].
Experimental control measures
It was crucial that the minimum post-exposure/neutralization titer for the virus control group was at least 2 × 105 TCID50/ml or EID50/ml to enable measurements of more than 4 log reduction in titer by AEW treatment. It was also essential to verify that the AEW itself was free of toxic effects on cells and embryos.
RESULTS
Determination of the effective concentration of AEW against ASFV
The titer of ASFV was measured after the virus was incubated with AEW. ASFV-induced CPE was observed after 5 days of infection, and the viral titers were compared with control viral titers under the corresponding organic soiling conditions. Table 2 shows the log reduction values obtained under the two separate experimental conditions, LS and HS. Under LS conditions, AEW treatments of 20 ppm or lower FCC did not exhibit enough virucidal efficacy against ASFV to meet the 4-log reduction, while a ≥4.3-log reduction was obtained with 40 and 60 ppm FCC. Under HS conditions, a 4.6-log reduction was obtained with an AEW treatment of 80 ppm FCC, and a higher FCC generated a ≥4.8-log reduction for this virus.
Table 2. Virucidal efficacy of acidic electrolyzed water under low- and high-level organic soiling conditions against African swine fever virus.
| Free chlorine concentration (ppm) | Experiments | Low-level organic soiling | High-level organic soiling | ||||
|---|---|---|---|---|---|---|---|
| Virus titer (log10 TCID50/0.1 ml) | Log reduction | Median value | Virus titer (log10 TCID50/0.1 ml) | Log reduction | Median value | ||
| Control | 1 | 4.7 | - | - | 5.3 | - | - |
| 2 | 4.8 | 5.0 | |||||
| 3 | 5.2 | 5.3 | |||||
| 5 | 1 | 4.3 | 0.4 | 0.4 | Not performed | ||
| 2 | 4.5 | 0.3 | |||||
| 3 | 4.0 | 1.2 | |||||
| 10 | 1 | 3.3 | 1.4 | 1.4 | |||
| 2 | 3.9 | 0.9 | |||||
| 3 | 3.2 | 2.0 | |||||
| 20 | 1 | 3.3 | 1.4 | 1.4 | |||
| 2 | 3.8 | 1.0 | |||||
| 3 | 2.3 | 2.9 | |||||
| 40 | 1 | ≤0.5 | ≥4.2 | ≥4.3 | |||
| 2 | ≤0.5 | ≥4.3 | |||||
| 3 | ≤0.5 | ≥4.7 | |||||
| 60 | 1 | ≤0.5 | ≥4.2 | ≥4.3 | 2.0 | 3.3 | 3.3 |
| 2 | ≤0.5 | ≥4.3 | 1.8 | 3.2 | |||
| 3 | ≤0.5 | ≥4.7 | 1.3 | 4.0 | |||
| 80 | 1 | Not performed | 0.7 | 4.6 | 4.6 | ||
| 2 | ≤0.5 | ≥4.5 | |||||
| 3 | ≤0.5 | ≥4.8 | |||||
| 100 | 1 | ≤0.5 | ≥4.8 | ≥4.8 | |||
| 2 | ≤0.5 | ≥4.5 | |||||
| 3 | ≤0.5 | ≥4.8 | |||||
| 120 | 1 | ≤0.5 | ≥4.8 | ≥4.8 | |||
| 2 | ≤0.5 | ≥4.5 | |||||
| 3 | ≤0.5 | ≥4.8 | |||||
| 140 | 1 | ≤0.5 | ≥4.8 | ≥4.8 | |||
| 2 | ≤0.5 | ≥4.5 | |||||
| 3 | ≤0.5 | ≥4.8 | |||||
TCID50, 50% tissue culture infectious dose.
Determination of the effective concentration of AEW against AIV
The titer of AIV was measured after the virus was incubated with AEW. The HA assay was performed after 5 days of infection to determine the titer values, and the viral titers were compared with control viral titers under the corresponding organic soiling conditions. Table 3 shows the log reduction values obtained under the two separate experimental conditions, LS and HS. Under LS conditions, the reduction was limited to 3.4 logs with AEW of 40 ppm FCC; however, a 4.4-log reduction was obtained with the AEW treatment of 60 ppm FCC and a higher FCC generated a ≥5.2-log reduction for this virus. Under HS conditions, AEW treatments with 80 ppm or lower FCC showed insufficient virucidal efficacy against AIV to meet the 4-log reduction, while a ≥4.2-log reduction was obtained with 100 and 120 ppm FCC.
Table 3. Virucidal efficacy of acidic electrolyzed water under low- and high-level organic soiling conditions against avian influenza virus.
| Free chlorine concentration (ppm) | Experiments | Low-level organic soiling | High-level organic soiling | ||||
|---|---|---|---|---|---|---|---|
| Virus titer (log10 EID50/0.2 ml) | Log reduction | Median value | Virus titer (log10 EID50/0.2 ml) | Log reduction | Median value | ||
| Control | 1 | 6.7 | - | - | 6.9 | - | - |
| 2 | 5.7 | 6.1 | |||||
| 3 | 5.3 | 6.3 | |||||
| 40 | 1 | 3.3 | 3.4 | 3.4 | 5.5 | 1.4 | 1.4 |
| 2 | 1.9 | 3.8 | 5.1 | 1.0 | |||
| 3 | 1.9 | 3.4 | 4.9 | 1.4 | |||
| 60 | 1 | 1.7 | 5.0 | 4.4 | 4.7 | 2.2 | 1.8 |
| 2 | 1.3 | 4.4 | 4.7 | 1.4 | |||
| 3 | 0.9 | 4.4 | 4.5 | 1.8 | |||
| 80 | 1 | ≤0.5 | ≥6.2 | ≥5.2 | 2.7 | 4.2 | 2.6 |
| 2 | ≤0.5 | ≥5.2 | 3.7 | 2.4 | |||
| 3 | ≤0.5 | ≥4.8 | 3.7 | 2.6 | |||
| 100 | 1 | ≤0.5 | ≥6.2 | ≥5.2 | 2.3 | 4.6 | 4.2 |
| 2 | ≤0.5 | ≥5.2 | 2.3 | 3.8 | |||
| 3 | ≤0.5 | ≥4.8 | 2.1 | 4.2 | |||
| 120 | 1 | ≤0.5 | ≥6.2 | ≥5.2 | 1.9 | 5.0 | 5.0 |
| 2 | ≤0.5 | ≥5.2 | 1.1 | 5.0 | |||
| 3 | ≤0.5 | ≥4.8 | 0.9 | 5.4 | |||
EID50, 50% egg infectious dose.
Virucidal efficacy of AEW against ASFV and AIV
The effective and recommended concentrations against the viruses examined in this study are summarized in Table 4. As shown in Tables 2 and 3, the efficacy of AEW in this study was dependent on FCC, and the presence of HS reduced the efficacy of AEW, requiring higher effective concentrations. The effective FCCs of AEW against ASFV under LS and HS conditions were 40 ppm and 80 ppm, respectively, whereas those against AIV were 60 ppm and 100 ppm, respectively. Based on these results, we recommend using AEW with the following FCC, which are suggested concentrations indicating 125% of the corresponding effective concentrations as described in the APQA guidelines for efficacy testing of veterinary disinfectants for routine veterinary field applications depending on organic soiling conditions: for ASFV, 50 ppm and 100 ppm, and for AIV, 75 ppm and 125 ppm under LS and HS conditions, respectively. None of the FCC of AEW showed toxicity to the cell cultures and embryos (data not shown).
Table 4. Summary of virucidal efficacy of acidic electrolyzed water examined in this study.
| Conditions | ASFV | AIV | ||
|---|---|---|---|---|
| Free chlorine concentration (ppm) | ||||
| Effective | Recommended | Effective | Recommended | |
| Low-level organic soiling | 40 | 50 | 60 | 75 |
| High-level organic soiling | 80 | 100 | 100 | 125 |
ASFV, African swine fever virus; AIV, avian influenza virus.
DISCUSSION
Disinfection is crucial for stopping further spread of disease during outbreaks and to sanitize contaminated agricultural and veterinary facilities, especially in farm settings. Several chemical substances are generally accepted as disinfectants for inactivating enveloped viruses. FAO-recommended disinfectants effective against enveloped viruses belonging to OIE category A, including ASFV and AIV, are groups of detergents, oxidizing agents, alkalis, and glutaraldehyde [7]. Among them, sodium hypochlorite is the most widely used chlorine-based oxidizing disinfectant and many studies have confirmed its virucidal effect is the main active substance in disinfectants against ASFV and AIV [7, 13, 18, 19, 35]. However, recent studies have suggested that HOCl is more reactive than hypochlorite, although both are strong oxidizing agents [30].
Here, we aimed to evaluate the efficacy of AEW (pH 5.0–6.5) as a disinfectant against ASFV and AIV. We carried out experiments according to the APQA guidelines [3]. Increasing the FCC of AEW increased the efficacy of AEW on the viruses, indicating that the virucidal effect of AEW was solely dependent on the FCC in the solution.
The predominant active oxidizing agent in AEW at pH 5.0–6.8 is considered to be HOCl. Several previous studies investigating the general efficacy of HOCl reported its favorable antimicrobial effects against a wide range of bacteria, fungi, and viruses, including foot-and-mouth disease virus, and AIV [4, 9, 10, 15, 25, 26, 29,30,31]. To our knowledge, this is the first study to investigate the virucidal effect of AEW on ASFV. In the present study, under LS conditions, AEW with FCCs ≥40 ppm and ≥60 ppm resulted in a ≥4-log reduction against ASFV and AIV, respectively. Under HS conditions, AEW with FCCs ≥80 ppm and ≥100 ppm resulted in a ≥4-log reduction against ASFV and AIV, respectively. Consistent with our results, previous studies have also observed a significant reduction in AIV by AEW. Tamaki et al. [29] reported that EW (pH 6.4) containing approximately 40 ppm FCC showed virucidal effects against AIV. Hakim et al. [9] also reported the virucidal ability of EW (pH 6) containing 50 ppm FCC (≥5.2-log reduction), and higher efficacy when treated with 100 and 200 ppm FCC (5.3- and 5.5-log reduction, respectively). In their studies, all solutions lost the disinfection efficacy for reactions in the presence of organic soiling. Kim et al. [15] observed similar levels of efficacy of AEW (pH 4.0–6.5). Moreover, they performed the same test used in this study, and their results also showed virucidal effects against AIV using the method in the APQA guidelines. They observed that AEW with an FCC ≥100 ppm resulted in a 3-log reduction against AIV under HS conditions, and 120 ppm FCC showed toxicity to embryos. A possible reason for the difference in the detailed effective FCC is attributable to the difference in pH values of AEW and/or initial titers of the viruses within certain ranges. It has been reported that decreasing the pH in AEW increases the virucidal potential of AEW [21, 29]. Moreover, Yilmaz et al. [34] reported that the initial viral titer strongly influences the results of a disinfectant test, and a high initial titer can influence results to the disadvantage of the tested disinfectants.
The APQA guidelines classify the objects of disinfection according to the level of organic soiling content. Generally, livestock barn floors, filth, carcasses, animal transport vehicles, and animal transport equipment are classified as objects with high levels of organic soiling; livestock barns, animal drinking water, livestock surfaces, appliances, fish farms, and general transport vehicles are classified as objects with low levels of organic soiling [3]. Disinfectants need to be effective under dirty conditions; therefore, the present study used FBS as organic soiling to simulate dirty conditions. It has been demonstrated that increased exposure to organic soiling decreases the FCC, resulting in a reduction in the virucidal effects of chlorine-based disinfectants, including EW [1, 9, 10, 30]. This is because HOCl is reduced by reacting with nitrogen compounds in organic soiling to form chloramines before interacting with any virus that might be present [6, 30].
To evaluate the virucidal efficacy of AEW in the presence of underlying organic soiling in the reaction, we carried out an evaluation under different organic soiling conditions. Our results showed a reduction in the virucidal effects caused by protein load (organic soiling), and were consistent with those reported by previous studies, wherein decreased disinfection efficacy was observed in viruses exposed to organic soiling [6, 9, 10, 29, 30, 34]. Therefore, it was confirmed that a highly effective concentration of free chlorine in AEW is required to disinfect viruses in the presence of high organic soiling.
As expected, in this study, AEW at pH 5.0–6.5 showed virucidal efficacy (as disinfectants) against ASFV and AIV. Moreover, its virucidal efficacy was dependent on the FCC in the reaction. However, it has been reported that the virucidal efficacy of highly acidic EW (pH ≤2.5) did not require the presence of free chlorine [29]. In our study, no relevant toxicity of any tested AEW was observed, verifying its high biocompatibility.
AEW has been regarded as a new disinfectant in recent years and applied in various fields including clinical settings, agriculture and food industries, livestock management, and other fields. Considering the large environmental burden of chemical disinfectants for the control and prevention of infectious diseases, it is worth noting that disinfection with EW has favorable ecological features; it reverts to normal water or diluted salt water after use, requiring no toxic waste disposal management [2, 4, 29]. The main advantage of AEW is the simplicity of production and application. It is a disinfectant that can be produced on site, thus avoiding problem associated with handling of dangerous chlorine including transport and storage [23]. Additionally, EW is cost effective because the electricity charges, cost of chemical salts, and water are the major operating expenses involved in running the EW production system, besides the initial investment of equipment [23]. According to data from the AEW generator manufacturer in this study, usage of AEW as a disinfectant enables significant cost savings on disinfection by almost 80 percent annually. It includes expenses for purchasing electrolyzing agent, disposal of wastewater generated. Rahman et al. [23] also reported cost effectiveness of EW against its counterpart glutaraldehyde.
In conclusion, the virucidal efficacy of AEW against ASFV and AIV emphasizes its potential utility as a disinfectant. Several environmental factors such as temperature, contact time, and protein load are found to govern the efficacy of disinfectants in field applications [9, 23, 35]. Efficacy of most disinfectants is negatively influenced by protein load, low temperature and short contact time. The importance of the factors has already been recognized in the APQA guidelines by requiring 4°C and 30 min as obligatory and −10, −5 and 10°C and 1, 5 and 15 min as optional test conditions depending on the characteristics of the disinfectants and the objects of disinfection for official approval. Therefore, we suggest considering the feasible disinfection conditions of AEW for implementing effective control measures in field applications. Furthermore, this highlights the necessity for future studies to establish the efficacy profiles of newly introduced disinfectant substances.
CONFLICT OF INTEREST. Enputech Co., Ltd. supplied the “Electrolyzed Water Generator” used in this study. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
This work was supported by the Animal and Plant Quarantine Agency (I-1543073-2020-22-01), Republic of Korea and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Animal Disease Management Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116098-03-3-HD020).
REFERENCES
- 1.Abadias M., Usall J., Oliveira M., Alegre I., Viñas I.2008. Efficacy of neutral electrolyzed water (NEW) for reducing microbial contamination on minimally-processed vegetables. Int. J. Food Microbiol. 123: 151–158. doi: 10.1016/j.ijfoodmicro.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 2.Al-Haq M. I., Sugiyama J., Isobe S.2005. Applications of Electrolyzed Water in Agriculture & Food Industries. Food Sci. Technol. Res. 11: 135–150. doi: 10.3136/fstr.11.135 [DOI] [Google Scholar]
- 3.Animal and Plant Quarantine Agency (APQA). 2018. Guidelines for efficacy testing of veterinary disinfectants. APQA notice 2018–16 (May 31, 2018) (in Korean).
- 4.Bui V. N., Nguyen K. V., Pham N. T., Bui A. N., Dao T. D., Nguyen T. T., Nguyen H. T., Trinh D. Q., Inui K., Uchiumi H., Ogawa H., Imai K.2017. Potential of electrolyzed water for disinfection of foot-and-mouth disease virus. J. Vet. Med. Sci. 79: 726–729. doi: 10.1292/jvms.16-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae W.S., Cha C.N., Yoo C.Y., Kim S., Lee H.J.2018. Virucidal efficacy of a disinfectant solution composed of citric acid, malic acid and phosphoric acid against avian influenza virus. J. Prev. Vet. Med. 42: 16–21. doi: 10.13041/jpvm.2018.42.1.16 [DOI] [Google Scholar]
- 6.de Oliveira T. M. L., Rehfeld I. S., Coelho Guedes M. I., Ferreira J. M. S., Kroon E. G., Lobato Z. I. P.2011. Susceptibility of Vaccinia virus to chemical disinfectants. Am. J. Trop. Med. Hyg. 85: 152–157. doi: 10.4269/ajtmh.2011.11-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geering W. A., Penrith M. L., Nyakahuma D.2001. Manual on procedures for disease eradication by stamping-out: Part 3: Decontamination procedures. FAO Animal Health Manual 12. http://www.fao.org/3/Y0660E/Y0660E03.htm#ch3 [accessed on November 10, 2020].
- 8.Gyeonggi Research Institute (GRI).2017. Issue & Analysis (number 272), 2017.03.29. https://www.gri.re.kr/%ec%9d%b4%ec%8a%88-%ec%a7%84%eb%8b%a8/?ptype2=303&sc=&sv=&limit=10&searchcode=&pcode=&pageno=17 (in Korean) [accessed on November 10, 2020].
- 9.Hakim H., Thammakarn C., Suguro A., Ishida Y., Kawamura A., Tamura M., Satoh K., Tsujimura M., Hasegawa T., Takehara K.2015. Evaluation of sprayed hypochlorous acid solutions for their virucidal activity against avian influenza virus through in vitro experiments. J. Vet. Med. Sci. 77: 211–215. doi: 10.1292/jvms.14-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao X. X., Li B. M., Zhang Q., Lin B. Z., Ge L. P., Wang C. Y., Cao W.2013. Disinfection effectiveness of slightly acidic electrolysed water in swine barns. J. Appl. Microbiol. 115: 703–710. doi: 10.1111/jam.12274 [DOI] [PubMed] [Google Scholar]
- 11.Harada Y., Lekcharoensuk P., Furuta T., Taniguchi T.2015. Inactivation of foot-and-mouth disease virus by commercially available disinfectants and cleaners. Biocontrol Sci. 20: 205–208. doi: 10.4265/bio.20.205 [DOI] [PubMed] [Google Scholar]
- 12.Juszkiewicz M., Walczak M., Woźniakowski G.2019. Characteristics of selected active substances used in disinfectants and their virucidal activity against ASFV. J. Vet. Res. (Pulawy) 63: 17–25. doi: 10.2478/jvetres-2019-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juszkiewicz M., Walczak M., Mazur-Panasiuk N., Woźniakowski G.2019. Virucidal effect of chosen disinfectants against African swine fever virus (ASFV) - preliminary studies. Pol. J. Vet. Sci. 22: 777–780. [DOI] [PubMed] [Google Scholar]
- 14.Kang H. M., Lee E. K., Song B. M., Jeong J., Choi J. G., Jeong J., Moon O. K., Yoon H., Cho Y., Kang Y. M., Lee H. S., Lee Y. J.2015. Novel reassortant influenza A(H5N8) viruses among inoculated domestic and wild ducks, South Korea, 2014. Emerg. Infect. Dis. 21: 298–304. doi: 10.3201/eid2102.141268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J.W., Yun D.S., Lee H.Y., Jeong W.S., Park S.C.2019. Establishment of optimal disinfection condition of weak acid hypochlorous solution for prevention of avian influenza and foot-and-mouth disease virus transmission. Korean J. Vet. Res. 59: 101–104. [Google Scholar]
- 16.Kim H. J., Cho K. H., Lee S. K., Kim D. Y., Nah J. J., Kim H. J., Kim H. J., Hwang J. Y., Sohn H. J., Choi J. G., Kang H. E., Kim Y. J.2020. Outbreak of African swine fever in South Korea, 2019. Transbound. Emerg. Dis. 67: 473–475. doi: 10.1111/tbed.13483 [DOI] [PubMed] [Google Scholar]
- 17.Koide S., Takeda J.i., Shi J., Shono H., Atungulu G. G.2009. Disinfection efficacy of slightly acidic electrolyzed water on fresh cut cabbage. Food Control 20: 294–297. doi: 10.1016/j.foodcont.2008.05.019 [DOI] [Google Scholar]
- 18.Krug P. W., Larson C. R., Eslami A. C., Rodriguez L. L.2012. Disinfection of foot-and-mouth disease and African swine fever viruses with citric acid and sodium hypochlorite on birch wood carriers. Vet. Microbiol. 156: 96–101. doi: 10.1016/j.vetmic.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 19.Krug P. W., Lee L. J., Eslami A. C., Larson C. R., Rodriguez L.2011. Chemical disinfection of high-consequence transboundary animal disease viruses on nonporous surfaces. Biologicals 39: 231–235. doi: 10.1016/j.biologicals.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 20.LeChavallier M. W., Au K. -K.2004. Water treatment and pathogen control: Process efficiency in achieving safe drinking water. WHO IWA Publishing. pp. 1–107. https://apps.who.int/iris/bitstream/handle/10665/42796/9241562552.pdf [accessed on November 10, 2020].
- 21.Park H., Hung Y. C., Chung D.2004. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 91: 13–18. doi: 10.1016/S0168-1605(03)00334-9 [DOI] [PubMed] [Google Scholar]
- 22.Rahman S. M. E., Ding T., Oh D.H.2010. Inactivation effect of newly developed low concentration electrolyzed water and other sanitizers against microorganisms on spinach. Food Control 21: 1383–1387. doi: 10.1016/j.foodcont.2010.03.011 [DOI] [Google Scholar]
- 23.Rahman S. M. E., Khan I., Oh D.H.2016. Electrolyzed water as a novel sanitizer in the food industry: current trends and future perspectives. Compr. Rev. Food Sci. Food Saf. 15: 471–490. doi: 10.1111/1541-4337.12200 [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan M. A.2016. Determination of 50% endpoint titer using a simple formula. World J. Virol. 5: 85–86. doi: 10.5501/wjv.v5.i2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Severing A. L., Rembe J. D., Koester V., Stuermer E. K.2019. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): antimicrobial efficacy, cytotoxic impact and physicochemical parameters in vitro. J. Antimicrob. Chemother. 74: 365–372. doi: 10.1093/jac/dky432 [DOI] [PubMed] [Google Scholar]
- 26.Shin S. P., Kim M. S., Cho S. H., Kim J. H., Choresca C. H., Jr., Han J. E., Jun J. W., Park S. C.2013. Antimicrobial effect of hypochlorous acid on pathogenic microorganisms. J. Prev. Vet. Med. 37: 49–52. [Google Scholar]
- 27.Shirai J., Kanno T., Tsuchiya Y., Mitsubayashi S., Seki R.2000. Effects of chlorine, iodine, and quaternary ammonium compound disinfectants on several exotic disease viruses. J. Vet. Med. Sci. 62: 85–92. doi: 10.1292/jvms.62.85 [DOI] [PubMed] [Google Scholar]
- 28.Tagawa M., Yamaguchi T., Yokosuka O., Matsutani S., Maeda T., Saisho H.2000. Inactivation of a hepadnavirus by electrolysed acid water. J. Antimicrob. Chemother. 46: 363–368. doi: 10.1093/jac/46.3.363 [DOI] [PubMed] [Google Scholar]
- 29.Tamaki S., Bui V. N., Ngo L. H., Ogawa H., Imai K.2014. Virucidal effect of acidic electrolyzed water and neutral electrolyzed water on avian influenza viruses. Arch. Virol. 159: 405–412. doi: 10.1007/s00705-013-1840-2 [DOI] [PubMed] [Google Scholar]
- 30.Veasey S., Muriana P. M.2016. Evaluation of Electrolytically-Generated Hypochlorous Acid (‘Electrolyzed Water’) for Sanitation of Meat and Meat-Contact Surfaces. Foods 5: 5. doi: 10.3390/foods5020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Bassiri M., Najafi R., Najafi K., Yang J., Khosrovi B., Hwong W., Barati E., Belisle B., Celeri C., Robson M. C.2007. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J. Burns Wounds 6: e5. [PMC free article] [PubMed] [Google Scholar]
- 32.World Organisation for Animal Health (OIE).2019. Chapter 3.3.4. Avian influenza (infection with avian influenza viruses). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.03.04_AI.pdf [accessed on November 10, 2020].
- 33.World Organisation for Animal Health (OIE).2020. Update on avian influenza in animals (types H5 and H7). https://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2020 [accessed on November 10, 2020].
- 34.Yilmaz A., Kaleta E. F.2003. Evaluation of virucidal activity of three commercial disinfectants and formic acid using bovine enterovirus type 1 (ECBO virus), mammalian orthoreovirus type 1 and bovine adenovirus type 1. Vet. J. 166: 67–78. doi: 10.1016/S1090-0233(02)00269-1 [DOI] [PubMed] [Google Scholar]
- 35.Zou S., Guo J., Gao R., Dong L., Zhou J., Zhang Y., Dong J., Bo H., Qin K., Shu Y.2013. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol. J. 10: 289. doi: 10.1186/1743-422X-10-289 [DOI] [PMC free article] [PubMed] [Google Scholar]