Abstract
We evaluated the postsurgical outcomes of cutaneous or subcutaneous mast cell tumors (MCTs, n=25) in 23 dogs, resected with lateral surgical margins proportional to the widest tumor diameter, including at least one facial plane. The tumor diameter range was 0.3–2.6 cm (median: 0.9 cm), and all were histologically diagnosed as Kiupel’s low-grade MCT. Resection was histologically complete in 20, close (deep margin) in three, and incomplete (deep margin) in two. No dogs developed local recurrence at the site of initial surgery during follow-up of 161–2,219 days (median: 976 days). These results suggest that resection of low-grade, relatively small MCTs with surgical margins proportional to the tumor diameter is a practical procedure with high success rates.
Keywords: dog, margin proportional to tumor diameter, mast cell tumor, outcome
Mast cell tumors (MCTs) are the most common type of cutaneous malignancy in dogs. Complete resection with a wide margin of normal tissue is widely accepted as the best treatment for localized MCTs [1, 9]. Recently, it has been recognized that the traditional 3-cm lateral margin is not always required; a 2-cm lateral margin with one fascial plane being sufficient when resecting most grade 1−2 MCTs [2, 4, 17]. However, the optimal margin for grade 3 MCT has not been well investigated [15].
With increasing owner interest in their dogs’ health, the opportunities to treat small MCT nodules detected early have increased in primary care hospitals, raising questions regarding the need for lateral 2-cm margins for all MCT nodules. Such wide resection is often difficult when the MCT is in a location with limited normal skin, such as on the face or extremities [9, 15]. Furthermore, the histological grade of the MCT is usually unknown before surgery [2].
Generally, small MCT nodules are more likely to be grade 1–2 [6], for which the narrower surgical margin may be optimal, whereas larger or grade 3 MCTs could require wider margins. Recently, Pratschke et al. [15] reported a practical policy of widening surgical margins proportional to tumor diameter for all grades of cutaneous or subcutaneous MCTs, resulting in a local recurrence rate of only 2% during a median follow-up period of 420 days. This study was performed in a university hospital and did not report the number of cases in which this approach could not be applied [15]. Although no further studies of this method have been reported, its applicability and validity are worth evaluating in MCTs encountered in primary care settings with longer follow-up. The purpose of this study was to examine the long-term postsurgical outcomes of MCTs resected in accordance with this approach and to evaluate its applicability.
We retrospectively reviewed the medical records and pathology reports of dogs with cutaneous or subcutaneous MCTs resected in Aoba Animal Hospital between November 2013 and September 2018. Inclusion criteria were MCT cases resected with surgical margins proportional to tumor diameter (proportional margin resection: PM resection) and examined pathologically; however, the clinicopathologic data of the excluded cases were also recorded. The following data were retrieved: signalment, clinical features of the tumor, histological grade (Patnaik’s classification [14], Kiupel’s classification [7]), histological site (cutaneous or subcutaneous), treatments, and prognosis. The grade of subcutaneous MCTs was classified only by the Kiupel’s classification. Regarding postsurgical outcomes, we evaluated histological completeness of resection, local recurrence at or near the surgical site, new-MCT development at cutaneous or subcutaneous sites distant to the surgical site, metastasis, and cause of death. One dog’s prognosis was determined by telephone interview with the owner.
All MCTs were resected after a preoperative cytological diagnosis of MCT. PM resection was performed as described in a previous report [15]. Briefly, the maximal tumor diameter (MTD) was measured using a caliper and several points around the tumor at the same distance from the tumor margin marked, after which the planned resection line was drawn. The skin was incised along the planned resection line, and the MCT resected, including at least one fascial plane as a deep margin, and by placing tacking sutures to connect the deep margin to the skin, as needed. Regional lymph node resection was performed in one dog, in which MCT at the lateral knee and popliteal lymph node was resected en bloc. The same PM resection was performed for newly developed MCTs that occurred during follow-up.
Both the lateral and deep margins of the resected lesion were marked with colored inks (Davidson Marking System®; Bradley Products, Bloomington, MN, USA) prior to submission for histopathological analysis. The completeness of the resection was histologically determined to be complete, close (tumor cells within 1 mm of the margin), or incomplete (tumor cells in the margin), as reported previously [1, 4, 17], by re-evaluating the slides of the operative specimens.
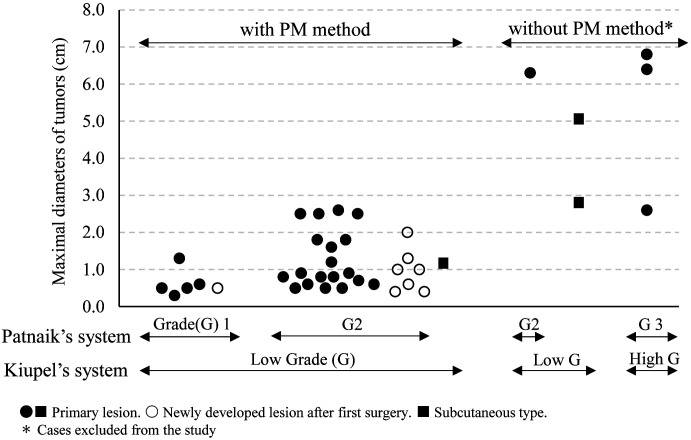
Twenty-nine dogs had undergone resection of MCTs during the study period. Six MCTs (MTD: 2.8–5.1 cm, median 5.7 cm) of six dogs, including four cutaneous MCTs (Kiupel’s low grade: one MCT, high grade: three MCTs) and two subcutaneous MCTs (both Kiupel’s low grade), were excluded because the margins were narrower or wider than specified for PM resection (Fig. 1). As a result, 23 dogs, from which 25 MCTs had been resected in the first surgery, were included.
Fig. 1.
Histological grades and maximal diameters of mast cell tumors that were resected with or without implementation of the proportional margin (PM) method.
Toy poodles were the most common breed (n=8, 35%), other breeds including three mixed-breed dogs, two Chihuahuas, two Labrador retrievers, and one each of Miniature dachshund, Shiba, Sikoku, Pug, Jack Russell Terrier, French bulldog, Boston terrier, and Bernese mountain dog. The dogs weighed 2.2–32.6 kg (median: 6.9 kg). Female dogs (18 dogs, 12 spayed) were more common than male dogs (five dogs, four castrated). The age range at the time of surgery was 4–15 years (median: 10 years), and the site of origin was the trunk in 12, extremities in seven, head in two, vagina/perineum in three, and tail in one tumor. The MTD ranged from 0.3 cm to 2.6 cm (median: 0.9 cm) (Fig. 1). Some MCTs (MTD: 0.4–1.2 cm) occurred in areas where a 2-cm margin could not be secured for anatomical reasons, such as the head, distal extremities, and tail; however, they were easily removed by the PM method. Two dogs had undergone simultaneous resection of two different MCTs.
Histologically, all 24 cutaneous MCTs were diagnosed as Kiupel’s low grade (Patnaik’s grade 1: five MCTs and grade 2: 19 MCTs), and the one subcutaneous MCT was diagnosed as Kiupel’s low grade. Surgical margins were determined to be complete in 20 cases, close (deep margin) in three (2 cutaneous, 1 subcutaneous), and incomplete (deep margin) in two. Lymph node metastases were found on histological examination in one dog (grade 2 MCT) that had undergone resection of the popliteal lymph node. This dog had no evidence of recurrence during 990 days of follow-up.
No dogs received postoperative adjuvant therapy, including prednisolone. Two dogs died from non-MCT-related causes 161–248 days postoperatively, and the remaining 21 dogs were followed for 410–2,219 days (median: 990 days). During the follow-up period (161–2,219 days, median: 976 days), no dogs developed local recurrences at the site of initial surgery. However, seven dogs (30%) developed new MCTs at distant sites 15–1,148 days (median: 399 days) postoperatively, for which the same PM resections were performed, achieving complete removal in all cases. Five of these dogs were disease-free for additional 577–1,722 days (median: 672 days) after their second surgeries. The remaining two dogs developed local recurrences near the second surgical site; one developing a grade 1 MCT (MTD: 0.4 cm) 588 days after complete resection of the second grade 1 MCT, and the other a subcutaneous low-grade MCT (MTD: 2.8 cm) 385 days after complete resection of the second cutaneous low-grade MCT. These recurrent lesions were re-excised with 2-cm and 3-cm lateral margins, respectively, after which the dogs remained disease-free for 618 days and 249 days after their third surgeries, respectively.
Potential benefits of PM resection include minimal invasiveness [2, 15], feasibility in sites where wide margins cannot be achieved for anatomical reasons [15], and applicability regardless of histologic site (cutaneous or subcutaneous) and grade [15]. Six of the original MCTs in six dogs were excluded from our study because the margins were too large or too small; we were able to perform PM resection on the remaining 33 of 39 MCTs (85%) that were resected during the study period (Fig. 1), suggesting high applicability of this method. None of the 25 MCTs that were initially managed by PM resection had tumor cells in the lateral margins, and no local recurrences occurred in any of these 25 cases. In all 33 MCTs including newly-developed MCTs, the local recurrence rate at surgical sites after PM resection was 6.1% (2/33 MCTs), despite the lack of postoperative medical therapy. These results suggest that PM resection can lead to local control of most small MCTs, as reported previously [15].
One potential disadvantage of PM resection is an increased risk of incomplete MCT excision, which is of particular concern for high grade MCTs [2]. It should be noted that all MCTs managed by PM resection in the present study were Kiupel’s low grade MCTs. PM resection is not applicable to large MCTs, including high grade (grade 3) cases; therefore, only small MCTs were included in the present study. The results of our previous study [6] suggested that small nodular MCTs are unlikely to be grade 3, which is supported by the results of the present study. More than 80% of canine cutaneous MCTs resected before the age of 12 years are low grade [11], and 30 of 33 MCTs in our study were in that age category. This fact, together with our selection of small tumors, may have contributed to all participating dogs having low-grade tumors and in turn, good outcomes in the present study.
Histologically, the deep margin, which was not affected by PM method, was determined to be close for three MCTs and as incomplete for two MCTs. Close margins have often been defined as ≤1 mm of histological tumor-free margin (HTFM) [1, 4, 17]; however, there is no evidence to support the prognostic significance of this assessment [8, 10]. It has recently been proposed that all tumors with HTFM >0 mm be classified as complete [8]. Using this criterion, the rate of incomplete resection (HTFM=0 mm) with PM resection was 15% (7/47) in a cohort of grade 1–3 MCTs (MTD: 0.5–6 cm, 4-cm of maximal lateral margin) [15] and 6.5% (3/46) in a cohort of grade 1–2 MCTs (MTD: 0.1–4 cm [median 1.0 cm], 2-cm of maximal lateral margin) [2], the latter being consistent with our results (6.1% [2/33], low-grade MCTs, MTD: 0.3–2.6 cm [median 0.9 cm]).
Two dogs with MCTs judged as incomplete in the present study showed no local recurrence, as often observed in previous studies [4, 15, 16,17,18,19]. In these two cases, there were scattered mast cells at the surgical margin of the muscle far away from the tumor foci; these cells may have been non-neoplastic mast cells [4, 15]. In one study of low-grade MCTs, the recurrence rate was low (4%, 2/51) despite 30% of the MCTs having close margins [3]. In another recent study of 56 incompletely-resected grade 2 MCTs (tumor infiltration, n=10; close margins, n=46), only nine MCTs (16%) were found to have tumor cells in the re-excised tissue [19]. Therefore, basing additional treatment on histological evaluation of the surgical margins should be considered carefully for low-grade MCTs [15, 19].
Development of new MCTs at different sites has been reported in 11–19% of grade 2 MCTs [16, 18], in 19–24% of grade 1–2 MCTs [4, 17], and in 23% of subcutaneous MCTs [5]. Such new MCTs are generally regarded as de novo tumors because of the favorable prognosis [5, 12, 13, 16, 18], the exception being grade 3 or large (>3cm) MCTs, which may have cutaneous metastasis with poor prognoses [13]. In the present study, 30% of dogs (7/23) developed new MCTs, which is a higher proportion than previously reported [4, 16,17,18]. This discrepancy is likely attributable to longer follow-up or case composition, rather than the surgical procedure. The median time from surgery to new-MCT development was longer in our study (399 days) than in other studies (54–362 days) [4, 16, 17]. It has been suggested that female dogs, which accounted for 78% (18/23 dogs) of our cases, have a higher risk of developing multiple cutaneous MCTs than male dogs [12, 13]. Toy poodles (n=8) were the most common breed of dog in the present study, four of which developed new MCTs and another one had a previous MCT, suggesting that this breed may have a high incidence of new-MCT development (63%, 5/8 dogs). The present results suggest that multiple or new MCTs may not be uncommon in dogs, and that PM resection can be a practical and effective option for such lesions, as suggested previously [15].
Two dogs developed local recurrences after second complete resections of MCT. One of them had a long interval (588 days) from the second resection (grade 1) to the local recurrence (grade 1), and the other developed a different type of MCT (second: cutaneous, third: subcutaneous). Neither dogs had a recurrence or metastasis after their third surgeries. Therefore, these locally recurrent MCTs may have been de novo MCTs rather than true recurrences as a result of incomplete resection.
The limitations of this study include its retrospective nature and small number of cases. Both surgical margin assessment (recommended as five directions of interest [10]) and clinical staging (evaluation of nodal and visceral metastases) were incomplete. However, the follow-up period of this study (median: 976 days) was longer than that of other reports (median: 351–540 days) [4, 15,16,17]; therefore, there was a minimal likelihood of missing local recurrence or visceral metastases. We performed PM resection regardless of histologic grade, location (cutaneous or subcutaneous), and numbers (single or multiple) of MCTs, as in a previous study [15], but were unable to determine its validity for subcutaneous (n=1), high-grade (n=0), or large (>2.6 cm, n=0) MCTs owing to insufficient numbers of cases in these categories. Further larger studies are needed to evaluate the validity of PM resection for subcutaneous, high-grade, and large MCTs.
In conclusion, PM resection is a practical method applicable to relatively small MCTs and can achieve local control with high success rates in small, low-grade MCTs.
POTENTIAL CONFLICTS OF INTEREST. The authors have nothing to disclose.
REFERENCES
- 1.Blackwood L., Murphy S., Buracco P., De Vos J. P., De Fornel-Thibaud P., Hirschberger J., Kessler M., Pastor J., Ponce F., Savary-Bataille K., Argyle D. J.2012. European consensus document on mast cell tumours in dogs and cats. Vet. Comp. Oncol. 10: e1–e29. doi: 10.1111/j.1476-5829.2012.00341.x [DOI] [PubMed] [Google Scholar]
- 2.Chu M. L., Hayes G. M., Henry J. G., Oblak M. L.2020. Comparison of lateral surgical margins of up to two centimeters with margins of three centimeters for achieving tumor-free histologic margins following excision of grade I or II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 256: 567–572. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly L., Mullin C., Balko J., Goldschmidt M., Krick E., Hume C., Brown D. C., Sorenmo K.2015. Evaluation of histological grade and histologically tumour-free margins as predictors of local recurrence in completely excised canine mast cell tumours. Vet. Comp. Oncol. 13: 70–76. [DOI] [PubMed] [Google Scholar]
- 4.Fulcher R. P., Ludwig L. L., Bergman P. J., Newman S. J., Simpson A. M., Patnaik A. K.2006. Evaluation of a two-centimeter lateral surgical margin for excision of grade I and grade II cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 228: 210–215. doi: 10.2460/javma.228.2.210 [DOI] [PubMed] [Google Scholar]
- 5.Gill V., Leibman N., Monette S., Craft D. M., Bergman P. J.2020. Prognostic indicators and clinical outcome in dogs with subcutaneous mast cell tumors treated with surgery alone: 43 cases. J. Am. Anim. Hosp. Assoc. 56: 215–225. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T., Nishi A., Uchida K., Kushima K., Shii H.2014. Clinical findings and prognoses of 58 cases of canine cutaneous mast cell tumor. J. Vet. Med. 67: 839–843 (in Japanese). [Google Scholar]
- 7.Kiupel M., Webster J. D., Bailey K. L., Best S., DeLay J., Detrisac C. J., Fitzgerald S. D., Gamble D., Ginn P. E., Goldschmidt M. H., Hendrick M. J., Howerth E. W., Janovitz E. B., Langohr I., Lenz S. D., Lipscomb T. P., Miller M. A., Misdorp W., Moroff S., Mullaney T. P., Neyens I., O’Toole D., Ramos-Vara J., Scase T. J., Schulman F. Y., Sledge D., Smedley R. C., Smith K., W Snyder P., Southorn E., Stedman N. L., Steficek B. A., Stromberg P. C., Valli V. E., Weisbrode S. E., Yager J., Heller J., Miller R.2011. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet. Pathol. 48: 147–155. doi: 10.1177/0300985810386469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liptak J. M.2020. Histologic margins and the residual tumour classification scheme: Is it time to use a validated scheme in human oncology to standardise margin assessment in veterinary oncology? Vet. Comp. Oncol. 18: 25–35. doi: 10.1111/vco.12555 [DOI] [PubMed] [Google Scholar]
- 9.London C. A., Thamm D. D.2020. Mast cells tumors. pp. 382–403. In: Withrow & MacEwen’s Small Animal Clinical Oncology, 6th ed. (Vail, D. M., Thamm, D. J. and Liptak, J. M. eds.), Elsevier, St. Louis. [Google Scholar]
- 10.Milovancev M., Russell D. S.2017. Surgical margins in the veterinary cancer patient. Vet. Comp. Oncol. 15: 1136–1157. doi: 10.1111/vco.12284 [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki H., Motsinger-Reif A., Bettini C., Moroff S., Breen M.2017. Association of breed and histopathological grade in canine mast cell tumours. Vet. Comp. Oncol. 15: 829–839. [DOI] [PubMed] [Google Scholar]
- 12.Mullins M. N., Dernell W. S., Withrow S. J., Ehrhart E. J., Thamm D. H., Lana S. E.2006. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004). J. Am. Vet. Med. Assoc. 228: 91–95. doi: 10.2460/javma.228.1.91 [DOI] [PubMed] [Google Scholar]
- 13.O’Connell K., Thomson M.2013. Evaluation of prognostic indicators in dogs with multiple, simultaneously occurring cutaneous mast cell tumours: 63 cases. Vet. Comp. Oncol. 11: 51–62. doi: 10.1111/j.1476-5829.2011.00301.x [DOI] [PubMed] [Google Scholar]
- 14.Patnaik A. K., Ehler W. J., MacEwen E. G.1984. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet. Pathol. 21: 469–474. doi: 10.1177/030098588402100503 [DOI] [PubMed] [Google Scholar]
- 15.Pratschke K. M., Atherton M. J., Sillito J. A., Lamm C. G.2013. Evaluation of a modified proportional margins approach for surgical resection of mast cell tumors in dogs: 40 cases (2008–2012). J. Am. Vet. Med. Assoc. 243: 1436–1441. doi: 10.2460/javma.243.10.1436 [DOI] [PubMed] [Google Scholar]
- 16.Séguin B., Leibman N. F., Bregazzi V. S., Ogilvie G. K., Powers B. E., Dernell W. S., Fettman M. J., Withrow S. J.2001. Clinical outcome of dogs with grade-II mast cell tumors treated with surgery alone: 55 cases (1996–1999). J. Am. Vet. Med. Assoc. 218: 1120–1123. doi: 10.2460/javma.2001.218.1120 [DOI] [PubMed] [Google Scholar]
- 17.Simpson A. M., Ludwig L. L., Newman S. J., Bergman P. J., Hottinger H. A., Patnaik A. K.2004. Evaluation of surgical margins required for complete excision of cutaneous mast cell tumors in dogs. J. Am. Vet. Med. Assoc. 224: 236–240. doi: 10.2460/javma.2004.224.236 [DOI] [PubMed] [Google Scholar]
- 18.Smith J., Kiupel M., Farrelly J., Cohen R., Olmsted G., Kirpensteijn J., Brocks B., Post G.2017. Recurrence rates and clinical outcome for dogs with grade II mast cell tumours with a low AgNOR count and Ki67 index treated with surgery alone. Vet. Comp. Oncol. 15: 36–45. doi: 10.1111/vco.12140 [DOI] [PubMed] [Google Scholar]
- 19.Vincenti S., Findji F.2017. Influence of treatment on the outcome of dogs with incompletely excised grade-2 mast cell tumors. Schweiz. Arch. Tierheilkd. 159: 171–177. doi: 10.17236/sat00109 [DOI] [PubMed] [Google Scholar]