Abstract
Background
Osteonecrosis of the femoral head (ONFH) is a refractory disease due to its unclear pathomechanism. Neither conservative treatment nor surgical treatment during the early stage of ONFH achieves satisfactory results. Therefore, this study aims to explore the available evidence on the effect of zoledronic acid on early-stage ONFH.
Methods
For groups were established:the Normal group, model group, Normal saline group(NS group) and zoledronic acid-treated group. The blood supply to the femoral head of animals in the model group and zoledronic acid-treated group was interrupted via a surgical procedure, and zoledronic acid was then locally administered to the femoral head. Four weeks after surgery, all the hips were harvested and evaluated by micro-CT and histopathology(H&E staining, TRAP staining, Toluidine blue staining and masson staining).
Results
The values of BMD, BS/BV and Tb.Th in the Normal group and zoledronic acid-treated group were significantly higher than those in the model group and NS group (p < 0.05). The outcome of H&E staining, Toluidine blue staining and masson staining were consistent with that of micro-CT.
Conclusion
The local administration of zoledronic acid in the femoral head had positive effects on the bone structure of the femoral head in a modified rat model of traumatic ONFH and offered a promising therapeutic strategy during the early stage of ONFH.
The Translational potential of this article
This article could provide a choice for treating patients who have osteonecrosis of femora head and can be the basic research for advanced development over this disease
Keywords: Osteonecrosis of the femoral head, zoledronic acid, Local administration, Prevent, Early stage
Introduction
Until now, healing of osteonecrosis of the femoral head (ONFH) has been difficult due to the unclear pathomechanism. Many studies have shown that various etiologies are involved in the course of ONFH, and the main causes are trauma, glucocorticoid use and alcohol abuse. ONFH caused by glucocorticoid use and alcohol abuse is often called nontraumatic ONFH. In Japan, the annual incidence rate of nontraumatic ONFH is 1.91/100,000 [1]; every year, the number of patients with ONFH due to various causes is between 20,000 and 30,000 in America [2]. Furthermore, China has an estimated 8.12 million young people, especially those aged more than 15 years, diagnosed with nontraumatic ONFH [3]. ONFH is a severe disease that often occurs in young people and ultimately causes osteoarthritis and hip replacement without zoledronic acid treatment. Hence, it is crucial to intervene during the early stage to avoid and delay the progress of ONFH.
Currently, the traditional therapies before collapse include conservative treatment, such as pharmacologic or physical therapeutic regimens, and surgical treatment, such as core decompression, osteotomy and bone transplantation [4]. Unfortunately, the results of these methods are different due to many factors. Therefore, research regarding additional effective and stable procedures is necessary.
As we know, bisphosphonates can inhibit osteoclastic activity which have been used as the treatment of various clinical diseases [5]. Fabian von Knoch et al. [6]demonstrated that bisphosphonate may also promote proliferation and osteoblastic differentiation of human bone marrow stromal cells(BMSC) and further promote osteoblastic bone formation. As one of bisphosphonates, zoledronic acid is an effective drug for osteoporosis and has also been used to cure osteonecrosis. DG, L et al. [7]indicated that zoledronic acid treatment was beneficial for femoral head sphericity in a rat model of Perthes disease. Bisphosphonates have also demonstrated positive outcomes in patients with nonfemoral avascular necrosis and in a rabbit model of surgically induced ONFH [8,9]. However, controversy still exists. Lee, Y.-K., et al. [10]indicated that zoledronate was ineffective for preventing collapse of the femoral head, and reduction of hip arthroplasty did not occur. Therefore, further fundamental studies of zoledronic acid treatment are essential. In addition, an effective animal model of traumatic ONFH is still needed to investigate ONFH.
Materials and methods
Materials
Tianqing etazoledronic acid (made in China), 5 ml: 4 mg.
Animals
Twenty-two adult SPF Sprague–Dawley rats (purchased from the Animal Center of the General Hospital of the PLA, weighing between 350 and 400 g) were used. This experiment was approved by the Animal Care and Use Committee of the General Hospital of the PLA, according to the National Institutes of Health guidelines for the use of experimental animals. All rats were bred in the same environment (the same temperature and humidity) with unlimited access to food and water under a standard diet.
In total, in this study, four groups were established, and all animals were divided into three groups: the model group(6), normal saline group(NS group) (6) and the zoledronic acid-treated group(10). and the normal group consisted of 10 normal hips from the model group. All the samples were harvested at the fourth week after surgery.
Establishment of traumatic ONFH and the zoledronic acid-treated group
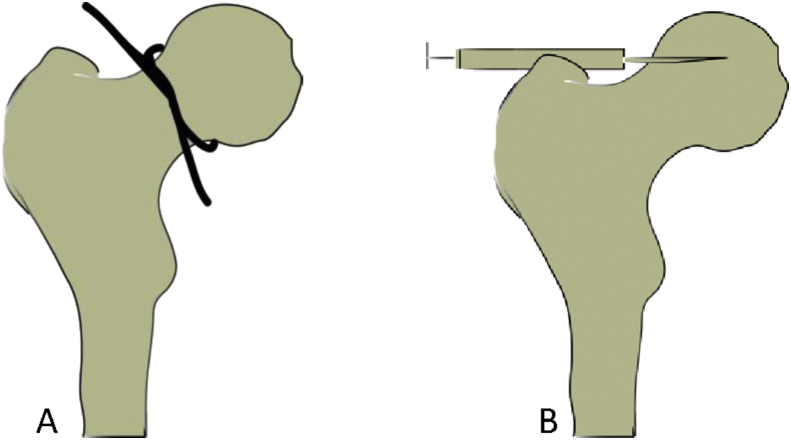
All 22 rats were anaesthetized by injecting 2% pentobarbital (0.3 ml/100 g) into the abdominal cavity; then, the rats were placed onto the operating table. Once the rats were deeply anesthetized, the fur was removed from the operative area; the skin was preserved, sterilized, and covered with a sterile sheet; and a right hip lateral incision was made from the center of the trochanter of approximately 2 cm in length. The muscle was separated and pulled along the direction of the muscle fibers, the hip joint capsule was cut to expose the femoral head, the hip joint was dislocated, the round ligament was cut off with a 3-0 suture closely rounding the base of the femoral head, and it was slid left and right 5 times to destroy all the blood supply nourishing the femoral head. Due to approximate 14 μL of the volume of the femoral head in rat and the consequence of preliminary experiment, in order to insure enough zoledronic acid keeping in the femoral head, in the zoledronic acid-treated group, approximately 100 μL(4mg/5 ml) zoledronic acid injection (Zhengda Tianqing) per rat was injected into the channel, which was approximately 0.2 cm long, and drilled with the syringe needle of a 2 ml injector. During the operation, the sciatic nerve was bypassed to avoid injury, the joint capsule was enhanced, and the integrity of the surrounding muscle of the hip joint was maintained to avoid dislocation of the hip joint. To prevent infection, the wound was rinsed with normal saline, and the incision was sutured in layers. After all the rats had awoken, they were active and fed without any abnormalities (Figure 1, Figure 2).
Figure 1.
Diagrams showing the establishment of the procedure of traumatic ONFH and the pathway of injecting zoledronic acid.
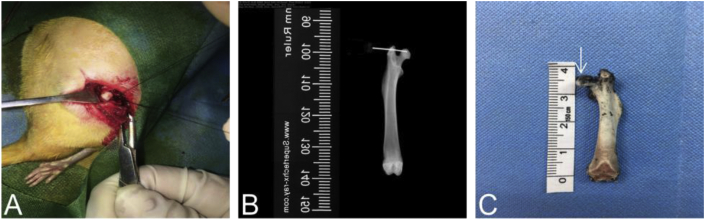
Figure 2.
Ink was injected into the normal femoral head to verify that zoledronic acid can be also successfully applied using the same way.A.during operation; B.under X ray; C. coronal plane of femora head after injection of ink.
Assessment of traumatic ONFH
Four weeks after the surgery, the femurs of rats in the model group were removed from the body and soaked in 10% neutral calcium formaldehyde. ONFH was evaluated by using micro-CT and H&E staining analyses.
Micro-CT evaluation
All the right femoral heads and 10 left femoral heads were scanned and reconstructed with a micro-CT scanner (GE e Xplore Locus, USA) to and assess the relevant bone parameters, such as the BMD(bone mineral density), BV(bone volume), BV/TV(bone volume/tissue volume), BS/BV(bone surface/bone volume), Tb. Sp(trabecular space) and Tb.Th (trabecular thickness). The ROI Size was X:2.8207; Y:2.8413; Z:2.2854 (millimeters), the scanning resolution was 27 μm, the scanning voltage was 74.01 kV, the current was 133.0 μA, and the threshold was 75–255. All the data were analyzed by GE Micro View software.
H&E staining, Toluidine blue staining and Masson staining
Specimens were soaked in 10% neutral calcium formaldehyde for 2 weeks and then put into 10% EDTA for decalcification. After decalcification and paraffin embedding, the samples were sectioned at a thickness of 5 μm in the coronary position, and the sections were stained with hematoxylin and eosin (H&E), Toluidine blue staining and Masson staining.
TRAP staining
TRAP staining suit (G1050,Servicebio), Hematoxylin dye(G1004,Servicebio), The differentiation of liquid (G1005-3,Servicebio), the blue liquid(G1005-4,Servicebio). All dyeing procedures follow the instructions of TRAP staing suit.
Statistical analyses
All statistical analyses were performed by IBM SPSS Statistics 20 software. Values are represented by the mean ± standard deviation, and the data conforming to P < 0.05 were considered statistically significant. All data satisfying the normal distribution and the test of homogeneity of variance were analyzed using ANOVA with multiple comparisons, and the data that did not satisfy the test of homogeneity of variance were analyzed by nonparametric tests.
Results
The assessment of the success of the traumatic ONFH model
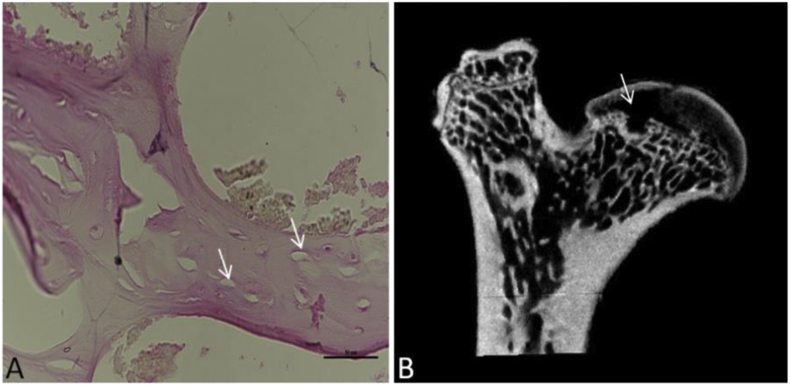
Four right femurs from the model group were abandoned because of infection. Micro-CT and H&E staining were valid methods to evaluate the success of the traumatic ONFH model. All the right femurs from the model group were analyzed by these two means. In the micro-CT images, the trabecular continuity of the subchondral bone tissue was interrupted and disordered, and the local trabecula was sparse and thin. Furthermore, the outcome of H&E staining also showed typical features of ONFH, in which many empty lacunae appeared (Fig. 3).
Figure 3.
H&E staining and micro-CT assessment of the model group: (A) Image in the coronal plane of the femoral head stained by H&E at 40× magnification. The black arrow indicates empty lacunae, the trabecula was disordered, and the local trabecula was sparse and thin. (B) Image in the coronal plane of the femoral head scanned by micro-CT. The white arrow indicates that bones became less dense and more porous and that the trabecular continuity was interrupted, disordered and thin.
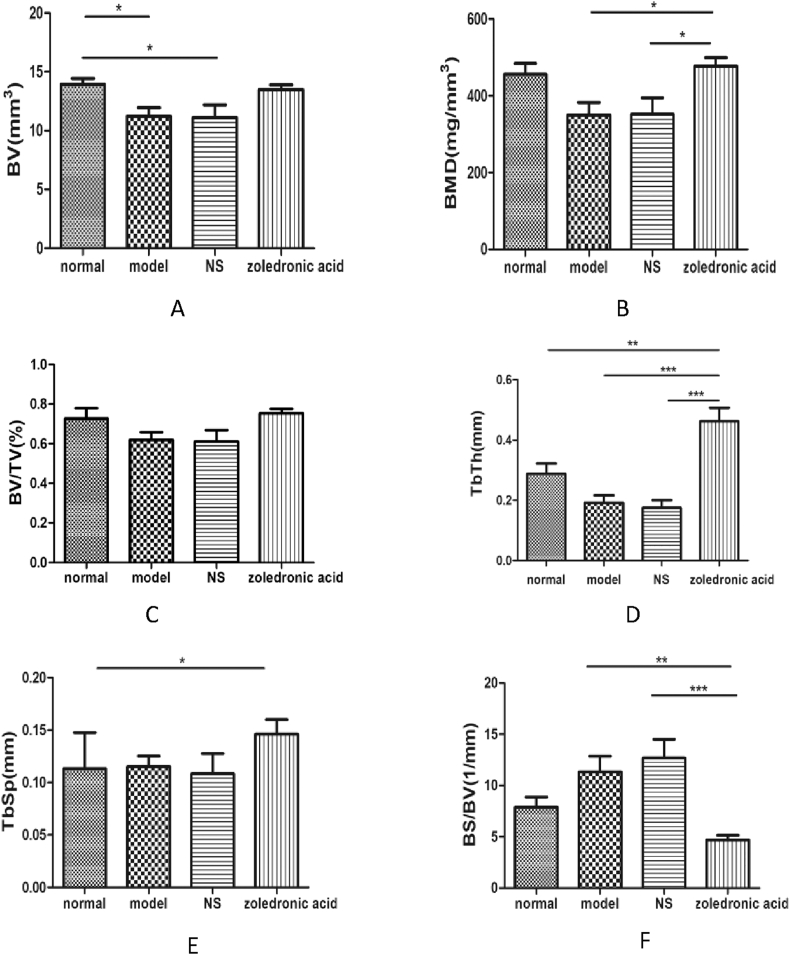
Results of the micro-CT analysis
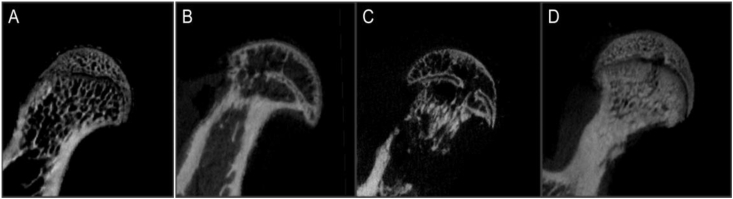
All 32 samples from the four groups were scanned by micro-CT. After comparing the results between each group among the four groups, the values of the BMD (B), Tb.Th(D) and BS/BV(F) were significantly different among zoledronic acid-treated group, NS group and model group (p < 0.05) (Figure 4, Figure 5).
Figure 4.
Morphometric evaluation of micro-CT images among the four groups: (A = normal group) trabeculae were dense, continuous, thick and arranged in order; (B=model group), (C=nomal saline group) the local structure disappeared, trabeculae in other areas of the femoral head were discontinuous and disordered, they also became thin, and necrosis of the femoral head was more severe than it in the other groups; (D = zoledronic acid-treated group) the trabeculae in the same area appeared denser and more continuous than those in the model group and NS group but less than those in the normal group. (NS = normal saline).
Figure 5.
3D reconstruction results of the four groups: (A) there was no difference for the value of BV in zoledronic acid-treated group comparing with other groups; (B) the value of the BMD had difference in zoledronic acid-treated group comparing NS group and model group ; (C) There was no difference for BV/TV among the four groups; (D) There was significant difference for Tb.Th in zoledronic acid-treated group comparing with other three groups; (E) TbSp had difference between zoledronic acid-treated group and normal group; (F) BS/BV were significantly different among zoledronic acid-treated group, NS group and model group (p < 0.05). (NS = normal saline).
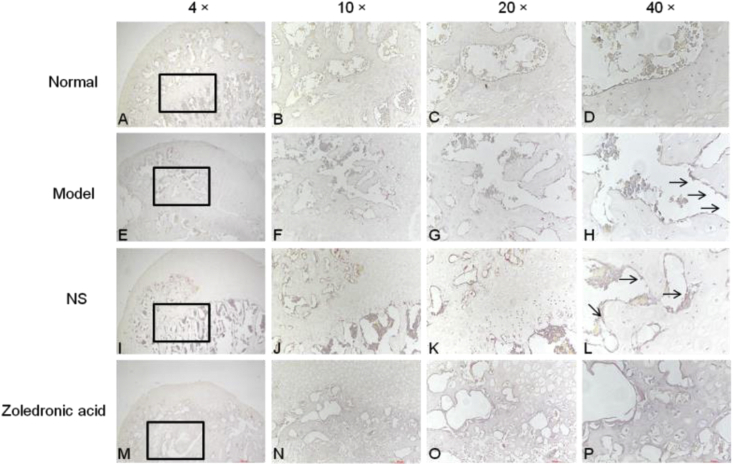
Outcome of H&E staining, Toluidine blue staining, Masson staining and TRAP staining
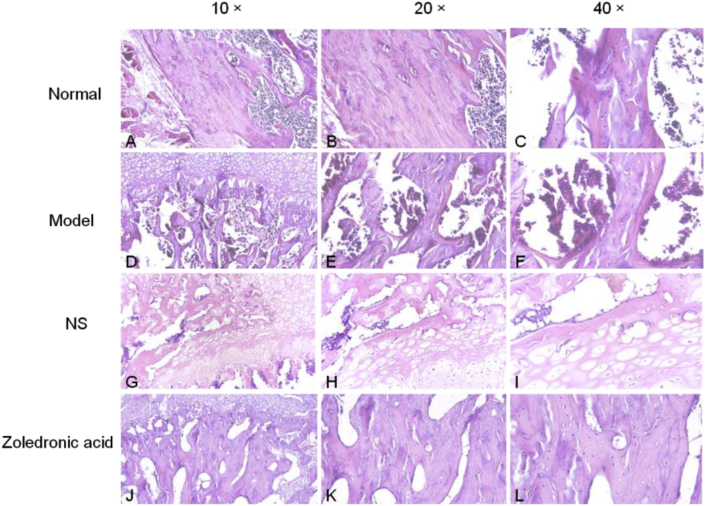
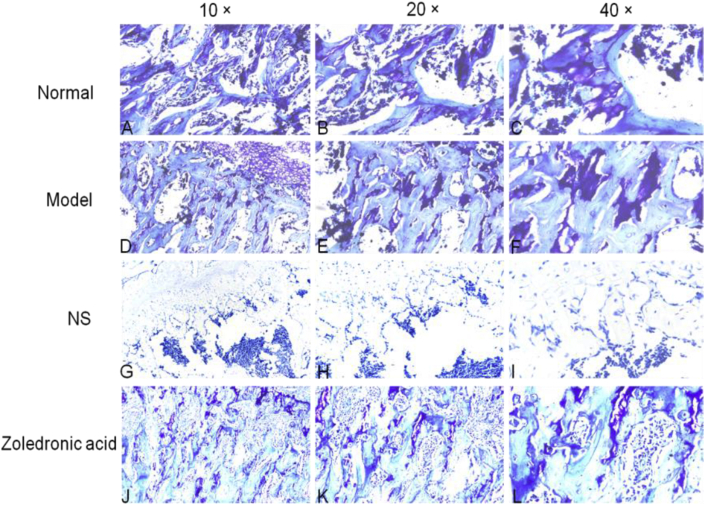
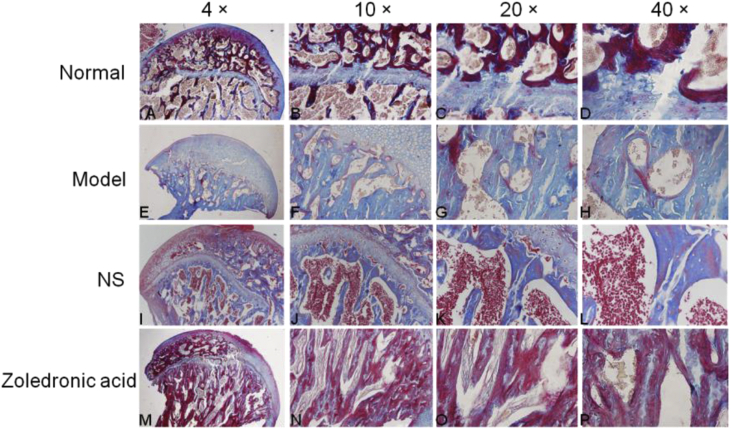
In the model group, osteocyte death on trabeculae was observed. At the magnifications of 40×,20× and 10×, H&E staining also displayed more empty lacunae in the model group than in the normal group and zoledronic acid-treated group. The trabeculae were sparser and thinner in the model group than in the other two groups. The Toluidine blue staining were not only evaluated cartilage tissues but also assessed bone [11,12], the outcome of Toluidine bue staining revealed that calcified cartilage, subchondral bone plate, and subchondral trabecular bone in normal group and zoledronic acid-treated group were better than them in model group. Masson staining showed that in the normal group, blue staining representing inmature bone tissue and collagen were seldom; Compared with the normal group, the model group showed a large amount of blue-stained collagen in the trabecular bone, and the inmature collagen area was significantly increased. Compared with the model group and NS group, the zoledronic acid-treated group displayed significantly reduced area of inmature bone tissue (blue staining) and the tissue was mostly stained red. The area of blue-stained collagen was significantly decreased in the zoledronic acid-treated group. TRAP staining showed that there were less osteoclasts(the osteoclasts were wine-red and the nuclei were blue) on the surface of trabeculae in normal group and zoledronic acid-treated group than them in other groups (Figure 6, Figure 7, Figure 8, Figure 9).
Figure 6.
H&E staining analysis of coronal plane sections of the femoral head in the 3 groups with different magnifications: (A-C=normal group) trabeculae were dense, continuous and thick, and almost no empty lacunae or cases of osteocyte death were detected; (D-F=model group,G-I=normal saline group) osteocyte death on the trabeculae was shown, and the sections also displayed more empty lacunae than in the normal group and zoledronic acid-treated group; (J-L = zoledronic acid-treated group) osteonecrosis was less than in the model group and there were more empty lacunae than in the normal group.(NS = normal saline); (bar:10× = 100 μm, 20× = 100 μm, 40× = 50 μm).
Figure 7.
Toluidine blue staining analysis of coronal plane sections of the femoral head in the 3 groups with different magnifications: calcified cartilage, subchondral bone plate, and subchondral trabecular bone in Normal group and zoledronic acid-treated group were better than them in other groups (A-C=Normal group; D-F=model group; G-I= Normal saline group; J-L = zoledronic acid-treated group); (NS=Normal saline) (bar: 10× = 100 μm, 20× = 100 μm, 40× = 50 μm).
Figure 8.
Effects of the zoledronic acid on Masson staining of the femoral head in rats with osteonecrosis of the femoral head. In the Normal group, inmature bone tissue and collagen were seldom; Compared with the model group and NS group, the zoledronic acid-treated group displayed significantly reduced area of inmature bone tissue (blue staining) and the tissue was mostly stained red(mature bone). (A-D = Normal group; E-H=model group; I-L= Normal saline group; M-P = zoledronic acid-treated group); (NS = normal saline); (bar:4× = 500 μm, 10× = 100 μm, 20× = 100 μm, 40× = 50 μm).
Figure 9.
TRAP(tartrate-resistant acid phosphatase) staining showed that there were less osteoclasts(black arrow) on the surface of trabeculae in normal group and zoledronic acid-treated group than them in other groups. (A-D = Normal group; E-H=model group; I-L= Normal saline group; M-P = zoledronic acid-treated group); (NS= Normal saline); (bar:4× = 500 μm, 10× = 100 μm, 20× = 100 μm, 40× = 50 μm).
Discussion
Numerous studies have demonstrated that the process of ONFH is caused by cofounding factors, which include metabolic disorders, genetic factors, and interrupted blood supply of the femoral head induced by comprehensive causes ranging from high intraosseous pressure, mechanical injury and impaired blood vessels [13,14]. The mechanisms of ONFH have been thought to likely be due to primary procedures associated with osteonecrosis [15]. For example, the large doses of glucocorticoids used by many patients with autoimmune diseases and alcohol abuse have been closely related to changes in blood fat, which then result in thrombi in the vessel supplying the femoral head [16]. Trauma is another etiology associated with ONFH. These pathological processes lead to bone cell death and eventual disruption of the structure of the femoral head. Moreover, it is difficult to identify an ideal therapeutic schedule to manage patients with ONFH before collapse; to obtain an effective consequence of preserving the hip joint, treatment for ONFH at the early stage of the disease is extremely vital [15]. In general, the main treatments for ONFH during the early stage are nonsurgical treatment and surgical treatment, while the outcomes vary.
As an antiresorptive drug, zoledronic acid-treated group can conserve bone mineral density and strengthen bones by inhibiting bone resorption [17]. Fabian von Knoch et al. [6] thought that bisposphonates were benefit to higher bone density, stimulation of bone formation, and decreased wear debris mediated osteolysisIn. lieu of this property, zoledronic acid has been considered the basic,first-line drug that is most commonly applied in the treatment of osteoporosis [18]. For the same reason, many researchers have introduced this kind of agent in their studies of ONFH and other diseases to explore the effect of treatment. An article published by Friedl, G, et al. demonstrated that the use of zoledronic in patients with ONFH requiring total hip arthroplasty was associated with a positive clinical result [19]. Zoledronate was also successfully applied for improving the morphology of the femoral head due to the inhibition of osteoclast activity in a rat model of traumatic ONFH [20]. In a clinical trial, in children with Legg-Calve-Perthes disease, intravenous zoledronic acid treatment obtained improved efficacy [21]. M, R. et al. [22] indicated that intravenous bisphosphonate treatment for traumatic ONFH in teenagers generated good outcomes. However, the common methods in most of these studies associated with zoledronic acid were intravenous or oral routes. Bisphosphonate therapy through blood circulation for improving the shape of ONFH has not appeared to determine outcomes in clinical practice [[22], [23], [24]]. Local application of ibandronate in the femoral head in a pig model of osteonecrosis showed a beneficial effect [25]. Local administration can ensure as much zoledronic acid as possible in the femoral head because of the high affinity of zoledronate acid for mineralized bone [26]; therefore, we decided to apply local administration in a rat model of ONFH in our study. Zheng LZ et al. [27] showed a good outcome using steroid-associated osteonecrosis model of rat. Traumatic ONFH was also common in clinical and the model of ONFH has the advantages of being fast and stable, so we ameliorated a rat model of traumatic ONFH to evaluate the efficacy of zoledronic acid treatment. To maintain homogeneity of all the animal models, we used a 3-0 suture closely rounding the base of the femoral head and then made a circle and slid the suture left and right 5 times to destroy all the blood supply nourishing the femoral head. With the use of this method, all the animal models of ONFH showed small heterogeneity. In the zoledronic acid-treated group, approximately 100 μL(4mg/5 ml) eta zoledronic acid was injected into the channel, which was approximately 0.2 cm long and entered at the base of the femoral head after drilling with the syringe needle of a 2 ml injector despite high pressure in the channel. The intervention began after surgery was achieved. According to micro-CT, the BMD, BS/BV, and Tb.Th., representing osteogenic parameters, showed significant differences among the model group, NS group and the zoledronic acid-treated group. The outcome of H&E staining, Toluidine blue staining and Masson staining were also parallel to that of micro-CT. TRAP staining showed that osteoclast activity was lower in zoledronic acid-treated, group than in other groups. Our results were also accord with the results which were demonstrated in Fabian von Knoch et al. [6] We confirmed that local administration of zoledronic acid in the femoral head had positive effects on the bone structure of the femoral head in a modified rat model of traumatic ONFH and offered a promising therapeutic strategy during the early stage of ONFH to delay the progression of this disease.
In fact, our study still has a limitation, as we did not identify the exact amount of zoledronic acid used to treat the femoral head and the efficacy of the treatment with different amount and concentration of zoledronic acid. The mechanisms that generated this outcome should also be further investigated. In subsequent studies, we will attempt to quantify zoledronic acid treatment and therapeutic range and verify the related mechanisms through various experiments. In addition, medication-related osteonecrosis of the jaws (MRONJ), a severe side effect of bisphosphonate, was reported in a series of studies [28]. Many studies in regard to this aspect will be performed in our following work. In recent years, combination therapies and tissue-engineered treatments have been increasing and have offered positive results [[29], [30], [31], [32]], and stem cell therapy has also been viewed as a promising treatment [33]. For that reason, the efficacy of stem cells combined with zoledronic acid. Lastly, if ZOL injection could treat already happened osteonecrosis needs to be investigated in our following studies.[]
Declaration of competing interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgement
This research was funded by National Natural Science Foundation of China(81972047); Project of Science and Technology assistance to Xinjiang (2017E0274).
Contributor Information
Jun Zhao, Email: 15801208995@163.com.
Tian Yue, Email: 66937763@sina.com.
Shibi Lu, Email: lushibi301@126.com.
Haoye Meng, Email: menghaoye@126.com.
Qiuxia Lin, Email: qiuxialin0803@163.com.
Haiyang Ma, Email: mahaiyang822@163.com.
Guangbo Liu, Email: dr_liuguangbo@126.com.
Huo Li, Email: lihuo301@126.com.
Qiang Lu, Email: 13911595980@163.com.
Aiyuan Wang, Email: wangaiyuan301@126.com.
Wenjing Xu, Email: wenjingkitty@163.com.
Jing Feng, Email: 178002849@qq.com.
Yiqun Wan, Email: wanyiqun@301hopital.com.cn.
Sida Liao, Email: 764815290@qq.com.
Xuefeng Zhou, Email: zhouxuefeng306@sina.com.
Jiang Peng, Email: pengjdxx@126.com, pengjiang301@126.com.
References
- 1.Ikeuchi Kazuma, Hasegawa Yukiharu, Seki Taisuke, Takegami Yasuhiko, Amano Takafumi, Ishiguro Naoki. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod Rheumatol. 2015;25(2):278–281. doi: 10.3109/14397595.2014.932038. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman Jay, Berry Daniel J., Mont Michael A., Aaron Roy K. vol. 52. 2003. pp. 337–355. (Osteonecrosis of the hip: management in the 21st century). [PubMed] [Google Scholar]
- 3.Zhao De-Wei, Yu Mang, Hu Kai, Wang Wei, Yang Lei, Wang Ben-Jie. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative. Survey. 2015;128(21):2843–2850. doi: 10.4103/0366-6999.168017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mont Michael A., Cherian Jeffrey J., Sierra Rafael J., Jones Lynne C., Lieberman Jay R. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A Ten-Year Update. 2015;97(19):1604–1627. doi: 10.2106/JBJS.O.00071. [DOI] [PubMed] [Google Scholar]
- 5.Rodan G.A., Martin T.J. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 6.von Knoch Fabian, Jaquiery Claude, Kowalsky Marc, Schaeren Stefan, Alabre Claude, Martin Ivan. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26(34):6941–6949. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 7.Graham Little David, Mcdonald Michelle, Sharpe Ian T., Peat Rachel. zoledronic acid improves femoral head sphericity in a rat model of perthes disease. J Orthoped Res. 2005;23(4):862–868. doi: 10.1016/j.orthres.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Gharanizadeh Kaveh, Aminizade Sinah, Molavi Nima, Darbandi Amir, Nadjafi Shabnam, Fadavighaffari Mahsa. Effects of zoledronic acid and vitamin E on surgical- induced osteonecrosis of the femoral head in rabbit. Archives Bone Jt Surg. 2018;6(6):547–553. [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwala S., Vijayvargiya M. Bisphosphonate combination therapy for non-femoral avascular necrosis. J Orthop Surg Res. 2019;14(1) doi: 10.1186/s13018-019-1152-7. 112-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Young-Kyun, Ha Yong-Chan, Cho Yoon Je, Suh Kuen Tak, Kim Shin-Yoon, Won Ye-Yeon. Does zoledronate prevent femoral head Collapse from osteonecrosis? A prospective, randomized, open-label, multicenter study. J Bone Jt Surg Am Vol. 2015;97(14):1142–1148. doi: 10.2106/JBJS.N.01157. [DOI] [PubMed] [Google Scholar]
- 11.Sun Xiaolei, Li Xueping, Qi Hongzhao, Hou Xin, Zhao Jin, Yuan Xubo. MiR-21 nanocapsules promote early bone repair of osteoporotic fractures by stimulating the osteogenic differentiation of bone marrow mesenchymal stem cells. J Orthop Translat. 2020;24:76–87. doi: 10.1016/j.jot.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dailiana Zoe H, Stefanou Nikolaos, Khaldi Lubna, Dimakopoulos Georgios, Bowers James R, Fink Cristian. Vascular endothelial growth factor for the treatment of femoral head osteonecrosis: an experimental study in canines. World J Orthoped. 2018;9(9):120–129. doi: 10.5312/wjo.v9.i9.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mankin H.J. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326(22):1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 14.Chang Christopher C., Greenspan Adam, ErickGershwin M. Osteonecrosis: current perspectives on pathogenesis and treatment. Seminars Arthritis Rheumat. 1993;23(1):47–69. doi: 10.1016/s0049-0172(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 15.Moya-Angeler Joaquin, Gianakos Arianna L., Villa Jordan C., Ni Amelia, Lane Joseph M. Current concepts on osteonecrosis of the femoral head. World J Orthoped. 2015;6(8):590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Jagt Dick, Mokete Lipalo, Pietrzak Jurek, Zalavras Charalampos, Lieberman Jay R. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2015;23(2):69–70. doi: 10.5435/JAAOS-D-14-00431. [DOI] [PubMed] [Google Scholar]
- 17.Dhillon Sohita. zoledronic acid (reclast, aclasta): a review in osteoporosis. Drugs. 2016;76(17):1683–1697. doi: 10.1007/s40265-016-0662-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen Jian Sheng, Sambrook Philip N. Antiresorptive therapies for osteoporosis: a clinical overview. Endocrinology. 2011;8(2):81–91. doi: 10.1038/nrendo.2011.146. [DOI] [PubMed] [Google Scholar]
- 19.Friedl Gerald, Radl Roman, Stihsen Christoph, Rehak Peter, Aigner Reingard, Windhager Reinhard. The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty. A randomized, double-blind, controlled trial. J Bone Jt Surg Am Vol. 2009;91(2):274–281. doi: 10.2106/JBJS.G.01193. [DOI] [PubMed] [Google Scholar]
- 20.Fan Meng, Jiang Wen-xue, Wang Ai-yuan, Wang Yu, Peng Jiang, Zhang Li. Effect and mechanism of zoledronate on prevention of collapse in osteonecrosis of the femoral head. Zhongguo yi xue ke xue yuan xue bao. Acta Acad Med Sin. 2012;34(4):330–336. doi: 10.3881/j.issn.1000-503X.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Johannesen Jesper, Briody Julie, McQuade Mary, Little David G, Cowell Christopher T, Munns Craig F. Systemic effects of zoledronic acid in children with traumatic femoral head avascular necrosis and Legg-Calve-Perthes disease. Bone. 2009;45(5):898–902. doi: 10.1016/j.bone.2009.04.255. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran Manoj, Ward Kate, Brown Richard R., Munns Craig F., Cowell Christopher T., Little David G. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am. 2007;89(8):1727–1734. doi: 10.2106/JBJS.F.00964. [DOI] [PubMed] [Google Scholar]
- 23.Kotecha Rishi, Powers Neil, Lee Senq-J, Murray Kevin. Use of bisphosphonates for the treatment of osteonecrosis as a complication of therapy for childhood acute lymphoblastic leukaemia (ALL) Pediatr Blood Canc. 2010;54(7):934–940. doi: 10.1002/pbc.22428. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen ThiThuyAn, Zacharin Margaret R. Pamidronate treatment of steroid associated osteonecrosis in young patients treated for acute lymphoblastic leukaemia--two-year outcomes. J Pediatr Endocrinol Metab. 2006;19(2):161–167. doi: 10.1515/jpem.2006.19.2.161. [DOI] [PubMed] [Google Scholar]
- 25.Vandermeer Jacob S., Kamiya Nobuhiro, Aya-ay James, Garces Amanda, Browne Richard, Kim Harry K.W. Local administration of ibandronate and bone morphogenetic protein-2 after ischemic osteonecrosis of the immature femoral head: a combined therapy that stimulates bone formation and decreases femoral head deformity. J Bone Joint Surg Am. 2011;93(10):905–913. doi: 10.2106/JBJS.J.00716. [DOI] [PubMed] [Google Scholar]
- 26.Nancollas G.H., Tang R., Phipps R.J., Henneman Z., Gulde S., Wu W. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Li-Zhen, Wang Jia-Li, Xu Jian-Kun, Zhang Xiao-Tian, Liu Bao-Yi, Huang Le. Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model. Biomaterials. 2020;238 doi: 10.1016/j.biomaterials.2020.119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo Sook-Bin, Hellstein John W., Kalmar John R. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama Masahiro, Nabeshima Akira, Pan Chi-Chun, Behn Anthony W., Thio Timothy, Lin Tzuhua. The effects of a functionally-graded scaffold and bone marrow-derived mononuclear cells on steroid-induced femoral head osteonecrosis. Biomaterials. 2018;187:39–46. doi: 10.1016/j.biomaterials.2018.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Wu-Xun, Wang Lei. Adenovirus-mediated expression of BMP-2 and BFGF in bone marrow mesenchymal stem cells combined with demineralized bone matrix for repair of femoral head osteonecrosis in beagle dogs. Cell Physiol Biochem. 2017;43(4):1648–1662. doi: 10.1159/000484026. [DOI] [PubMed] [Google Scholar]
- 31.López-Fernández Alba, Barro Víctor, Ortiz-Hernández Mònica, Manzanares Maria Cristina, Vivas Daniel, Vives Joaquim. Effect of allogeneic cell-based tissue engineered treatments in a sheep osteonecrosis model. Tissue Eng Part A. 2020;26(17–18):993–1004. doi: 10.1089/ten.TEA.2019.0339. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Dewei, Cheng Liangliang, Yang Lei, Wang Benjie, Liu Baoyi. The combined therapy of tantalum rod implantation and vascularized bone transplantation for femoral head osteonecrosis: a retrospective long-term follow-up survival analysis. Surg Technol Int. 2019;35:406–409. [PubMed] [Google Scholar]
- 33.Zhang Chaofan, Fang Xinyu, Huang Zida, Li Wenbo, Zhang Wenming, Lee Gwo-Chin. Addition of bone marrow stem cells therapy achieves better clinical outcomes and lower rates of disease progression compared with core decompression alone for early stage osteonecrosis of the femoral head: a systematic review and meta-analysis. J Am Acad Orthop Surg. 2020 doi: 10.5435/JAAOS-D-19-00816. [DOI] [PubMed] [Google Scholar]