Abstract
Aim
To ascertain the changing incidence over time of the three commonest primary sarcomas of bone. Data obtained with particular reference to central chondrosarcoma from the annual referral rate to a large UK-based specialist orthopaedic oncology unit. To discuss how the “barnyard pen” analogy of cancers previously applied to certain commoner cancers can also be applicable to central chondrosarcoma (CS) of bone.
Materials and methods
A retrospective review was conducted of a computerised database identifying all central cartilage tumours (CCT) of bone, including enchondroma and CS subtypes, between 1985 and 2018. These were compared with the referrals of the other two commonest primary sarcomas of bone, osteosarcoma and Ewing sarcoma.
Results
There was a total of 1507 CS showing a 68% overall increase in annual referral rate/incidence over the study period. 68% cases were the borderline malignant lesions now known as atypical cartilaginous tumour (ACT). The annual referral rate/incidence of this entity increased by 194% over the 30 years. Whereas, the annual referral rate/incidence for osteosarcoma and Ewing sarcoma was static for the past 20 years.
Conclusion
The annual incidence of central CS of bone showed a marked increase over the 33-year period as compared with both osteosarcoma and Ewing sarcoma. This is especially in the ACT category and is thought to be due to the increased provision of MRI scanning flagging up a rise in incidental findings. The spectrum of CCTs from benign to highly malignant elegantly fits the “barn yard” pen analogy and could prove useful as an explanatory tool for patients and clinicians unfamiliar with these diseases.
Keywords: Enchondroma, Chondrosarcoma, Atypical cartilage tumour (ACT)
Highlights
-
•
There has been a steady increase in the incidence of central cartilage tumours of bone over the past thirty years.
-
•
This is particularly seen in the atypical cartilage tumour (ACT) category formerly known as low-grade or grade 1 chondrosarcoma.
-
•
This is attributed to the rise in use over the same period of MRI scanning in orthopaedics revealing previously undiagnosed cases
1. Introduction
Central cartilage tumours (CCT) of bone produce a chondroid matrix and are classified according to histological criteria as benign (enchondroma), borderline (atypical cartilaginous tumour (ACT) – formerly known as low-grade/grade 1 chondrosarcoma), and higher grades (grades 2, 3 and dedifferentiated chondrosarcoma).1 Reliable distinction and grading of CCTs of bone are a challenge for both radiologists and pathologists, particularly with the borderline lesions, due to the overlap of imaging and histological criteria.2, 3, 4, 5, 6 Recent publications have highlighted that, in the context of multidisciplinary deliberations, what was an occasional academic exercise, has now become an all too regular occurrence in specialist orthopaedic oncology units.7,8 This has been attributed largely to the increasing routine use of magnetic resonance imaging (MRI)7,9 with CCTs identified in the proximal humerus in 2.1%10 and around the knee in 2.8%11,12 on routine MRI studies. The purpose of this retrospective study was to identify the change in referral incidence of central chondrosarcoma (CS) of bone treated in a single large specialist orthopaedic oncology centre over 33 years. Comparison of the referral incidence over the same period was made with the other two commonest primary sarcomas of bone, osteosarcoma and Ewing sarcoma. In addition, the discussion endeavours to show how the spectrum of CCTs, from benign to highly malignant, neatly fits the “barnyard pen” analogy attributed to certain other cancers by the American physician, Gilbert Welch.13
2. Methods
This was a retrospective observational study institution approved as a service evaluation. The computer database of a large specialist orthopaedic oncology centre, with a catchment population of approximately 22 million, was reviewed to identify all CCTs of bone seen in a 33-year period from 1985 to 2018 inclusive. The annual referral incidence of the CCTs, together with a breakdown of subtypes (enchondroma, atypical cartilage tumour (ACT), grades 2, 3 and dedifferentiated CS) were recorded. In recognition of the current WHO classification1 all CCTs initially labelled as low-grade, grade 1 or “CLUMP” (cartilage lesion of unknown malignant potential) were included in the ACT category. Other CS subtypes including peripheral, clear cell and mesenchymal were excluded from the analysis. CCTs associated with a bone dysplasia (Ollier disease and Maffucci syndrome) were not excluded as the small numbers of cases involved were assumed not to significantly alter the results over time. The location of the CCTs and patient outcome was not recorded. All cases of primary bone tumours were diagnosed by histological diagnosis and/or an MDT consensus. Statistical analysis of the variance of the number of cases referred per year in the last two decades was calculated with a paired T-test, assuming a normal distribution and significant set with p = 0.05.
3. Results
The annual incidence, as measured from the referral rates, from 1985 to 2018 of all central CS, osteosarcoma and Ewing sarcoma referred to the unit are illustrated as a rolling 5 year average in Fig. 1. The gradual increase in all three categories in the earlier years is thought to be due to the increasing national reputation of the unit first set up in the mid 1970s. In the past 20 years the incidence of both osteosarcoma and Ewing sarcoma has slightly decreased with the mean number of osteosarcomas treated each year between 1999 and 2008 being 55 cases decreasing to 47 cases per year from 2009 to 2018 (p = 0.01). Whilst, the mean number of Ewing sarcoma treated each year was not significantly different over the same period with 28 cases per year from 1999 to 26 cases per year from 2009 to 2018 (p = 0.31). In contrast the incidence of central CS showed an increase of 68% from a mean of 44 cases per year from 1999 to 2008 to 74 cases per year from 2009 to 2018 (p < 0.0001). Fig. 1 also shows that at current referral rates CS is the commonest primary sarcoma treated in the unit comprising almost 50% of the top three diagnoses.
Fig. 1.
Annual referral rate to the orthopaedic oncology unit of the 3 commonest primary sarcomas of bone expressed as a 5-year rolling average. Note data included from 1985 onward so first data points correspond to 1985.
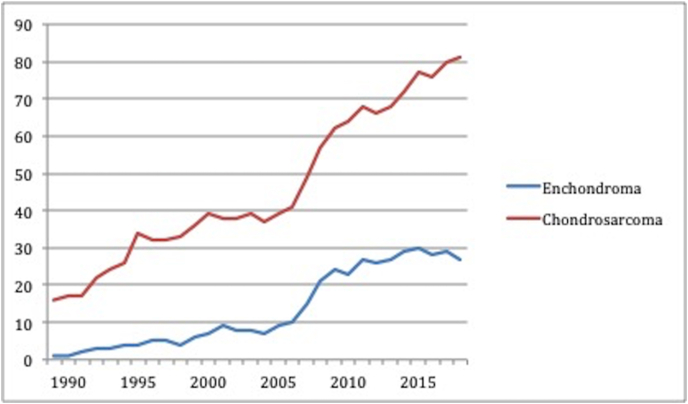
In Fig. 2 the annual incidence of all central CS (same data as included in the CS category in Fig. 1) is compared with the annual incidence of enchondromas referred to the unit. During the two decades the incidence of chondrosarcoma increases from a mean of 45 cases in 1999–2008 to a mean of 74 cases in 2009–2018 (increase by 66%) and the incidence of enchondromas increases in a similar manner to that of the CS with an annual mean in 1999–2008 of 14 cases increasing to 27 cases in 2009–2018 (85% increase). Fig. 2 also shows that at current referral rates approximately 115 CCTs are referred to the unit each year which equates to an average 2.2 cases per week.
Fig. 2.
Annual referral rate to the orthopaedic oncology unit of all central CS compared with enchondroma expressed as a 5-year rolling average. Note data included from 1985 onward so first data points correspond to 1985.
Fig. 3 shows the annual incidence of CS broken down by subtype ie. ACT, grades 2, 3 and dedifferentiated CS. A total of 1507 CS cases were referred in the past three decades of which 68% were diagnosed as ACTs. This category showed a 194% increase in the annual referral rate from a mean of 18 cases per year (1989–1998), to 31 per year (1999–2008) and finally 53 per year (2009–2018) (p = 0.024). Grade 2 CS made up 17% of the total and showed no change in referral rate over the past two decades with a mean of 10 referrals per year (p = 0.95). Grade 3 CS showed an increased referral rate of 91% over the three decades from a mean of 2.4–4.4 cases per year but this was not significant due to the small number of cases (7% of overall cases, p = 0.64). Dedifferentiated CS showed the largest increase in referral rate (466%) over the three decades with an increase in mean referrals from 1.5 to 3.8 and then 7 cases per year. Over the last two decades this increase was significant (p = 0.04) but again this category only comprised 8% of the total.
Fig. 3.
Annual referral rate to the orthopaedic oncology unit of all central CS broken down by subtype expressed as a 5-year rolling average. Note data included from 1985 onward so first data points correspond to 1985. Note annual average for dedifferentiated CS prior to 1995 < 1. ACT = atypical cartilage tumour. Gr II = Grade 2, Gr III = Grade 3 and Dedifferentiated CS.
4. Discussion
This retrospective study confirms a marked increase in the overall incidence of CS over 33 years with a referral rate exceeding that of osteosarcoma (Fig. 1). Given the increase in incidence over the study period we believe that it should no longer be considered to be the second commonest primary malignancy of bone in the UK. The most frequent subtype was the ACT comprising 68% of the total with a 194% increase in referral rate over the past three decades (Fig. 3). This is in accordance with a recent Netherlands publication which attributes this change to an ageing population and a higher incidence of incidental findings the latter due to a tenfold increase in MRI scans in that country.9 We agree with this explanation as the static referral rates in the current study for the other two commonest primary sarcomas of bone, osteosarcoma and Ewing sarcoma, would not suggest any internal influences (e.g. the expansion of the orthopaedic oncology service) or external influences (e.g. changes in referral patterns to the unit). There was no significant increase in the referral rate for grade 2 CS but both grade 3 and dedifferentiated CS showed increases of 91% and 466% respectively but the numbers of cases involved were smaller comprising only 15% of the total cases. It is of note that the increase rather than decrease in these referrals would suggest that early identification and management of lower grade CCTs (i.e. ACT and grade 2 CS) does not affect the long-term incidence of these higher-grade categories. As with the Netherlands study there is, therefore, no evidence that treating more ACTs will influence over time the risk of malignant transformation of a CCT.9
Criticisms of this study include the fact that it is retrospective review of a prospectively maintained computerised database. Due to the large number of cases, the imaging and histology was not reviewed for each case and were derived from a consensus MDT diagnosis established on imaging features alone or a combination of imaging and histological findings, with not every case undergoing a biopsy. With one exception, the individuals providing the specialist diagnostic opinions over this period had changed several times including nine radiologists and eleven pathologists. There was no assessment as to how imaging and pathological diagnostic criteria might have changed over time or whether the spectre of litigation might impact on a current more risk averse practise. It should be noted however that most sarcoma related litigation is focussed on delayed rather than under-diagnosis.14 However importantly, had there been a tendency in the past decade to upgrade the final diagnosis, one would have expected to see a consequent decrease in the number of enchondromas but this also showed a greater increase in diagnosis with time (Fig. 2).
The American physicia, Gilbert Welch, acknowledging the work the American surgeon George Crile,15 has popularised both in the medical literature and the wider press the analogy of the barnyard pen (BP) concept of cancers.13 The pen comprises a space enclosed with some form of barrier be it a fence or wall to ensure that any livestock within it is unable to escape. The analogy envisages that there are three types of creature corralled within the pen (Fig. 4). First, birds that despite the best efforts of the farmer cannot be confined within the pen notwithstanding the size of the enclosure and height of the barrier but can at any time simply fly away. The birds represent the most aggressive types of cancer that frequently prove resistant to both medical and surgical treatment. Second, rabbits which are the intermediate cancers that with prompt diagnosis and appropriate treatment can be restricted within the pen representing a successful outcome for the patient. Third, turtles (referred to as tortoises in the UK) which are slow-moving reptiles that are not going anywhere irrespective of the height of the barrier of the pen. They represent the most indolent of cancers for which treatment may be at best unnecessary and at worst harmful to the patient both physically and mentally.
Fig. 4.
Schematic applying the “barnyard pen” (BP) analogy of cancers to the spectrum of central CS. 1 represents the most aggressive type of CS, dedifferentiated, with a high metastatic potential symbolised by the bird. The bird can fly out of the pen at any time irrespective of the height of the fence. 2 represents the locally aggressive CS, grade 2 and 3, with intermediate metastatic potential symbolised by the rabbit. The confinement of the rabbit within the BP will depend on prompt diagnosis and treatment. Failure to do so may result in the rabbit escaping over the fence. 3 represents the most indolent CS (ACT – atypical cartilage tumour) symbolised by the turtle. These will remain confined within the pen notwithstanding the height of the fence.
Welch’s thesis is that much of modern cancer medicine, driven in part by advances in imaging as well as financial incentives, is fixated on the identification of turtles resulting in overtreatment and skewing of outcome results.16 He and others have cited examples in prostate17,18 thyroid19 and breast cancers.20,21 This phenomenon of overdiagnosis, turning people into patients, so-called “pseudodisease”, is by no means just confined to cancers.22,23
The spectrum of central CS of bone fits neatly with the BP analogy of cancers (Fig. 4). The birds are the rapidly progressive dedifferentiated CS with a 5-year survival of only 18–24%.24, 25, 26 The rabbits are the grades 2 and 3 CS that, with early diagnosis and management, can be successfully confined within the pen as reflected by a 5-year survival of 63–92%27 and 50–77%27,28 respectively. Whereas the turtles are the enchondromas and low-grade CS (grade 1/ACT) where a watch-and wait policy may suffice or surgical intervention can be limited to simple curettage, with a 5-year survival of 95%.28,29
We would suggest that the BP analogy can be extended to consider the design of the barrier to the pen. The more substantial and higher the construction the more effective the confinement of the creatures with the proviso that the birds can still escape whenever they wish to do so. The size of the structure is a measure of the medical resources, frequently imaging, that is deemed necessary and therefore directly represents the cost over time to the health care system. Recent studies have specifically questioned the overutilization and cost effectiveness of surveillance of cartilaginous tumours of bone.30,31
While histological grade is a critical prognostic indicator in CCTs of bone, location is another important factor that has to be considered. The proportion of enchondromas to CS in the pelvis and proximal femur is more equivalent, as compared with the high ratio of benign to malignant diagnoses in the proximal humerus and around the knee. This may be in part due to the fact that, unlike the shoulder and the knee, these sites are less frequently imaged with MRI thereby reducing the likelihood of revealing large numbers of incidental lesions. Therefore, CCTs at these sites should be viewed with much greater circumspection, resulting in a lower threshold for undertaking image-guided biopsy or follow-up. Albeit recognizing that needle-biopsy can underestimate the grade of the CS in 36–86% cases as compared with histological examination of the resected specimen.2,32, 33, 34
CCTs may transform from a lower to a higher-grade lesion, which is analogous to a turtle in the barnyard pen metamorphosing into a rabbit or even a bird. This transmogrification is well recognised with a quoted occurrence in the literature of 2.5–6% cases28,35,36 but these figures are arguably biased, as they do not take into account the numerous asymptomatic cases in the population that, until the mass proliferation of MRI, never presented to medical care. Indeed, one recent study estimates the risk of malignant transformation of a CCT arising in the proximal humerus or around the knee to be <1%.8 Again, it is prudent to be wary of CCTs of the pelvis where a more aggressive surgical approach is recommended in view of the impact on potential local recurrence and disease-free survival.37, 38, 39
The increased incidence of CCTs is most likely due to the upsurge of MRI in healthcare, particularly in the field of orthopaedics. Recent studies have shown the demand for all types of MRI in the UK rising by between 6.7 and 13% per annum.40,41 If one takes a 10% year-on-year increase that equates to an overall rise in requirement of MRI over thirty years of 1740%. This is borne out by an industry-based study reporting a 54% increase in the installed base of MRI scanners in the European Union (+Switzerland and Norway) in the decade up to 2018.42 The resultant epidemic in CCTs creates a problem for those orthopaedic surgeons responsible for the assessment and management of these cases. Undoubtedly, cases with aggressive imaging features such as cortical destruction and soft tissue extension, along with those with possible malignant features such as generalised endosteal scalloping require prompt onward referral to a sarcoma service for consideration of image-guided biopsy. The remaining border-line cases, sometimes referred to as quiescent, will require some form of imaging follow-up usually with MRI although how often and for how long is contentious.4,6,43, 44, 45, 46 It is for this reason that the authors of this study have adopted an in-house protocol for the follow-up of CCTs in the proximal humerus and around the knee which attempts to be risk averse without a commitment to a never-ending cycle of MRI7,8,47 similar to subsequently published Danish guidelines.48 However, the threshold for follow-up imaging and biopsy of central and proximal femoral CCTs as stressed above needs to be lower as the risk of malignancy at these sites is higher.
In conclusion, the referrals to this orthopaedic oncology unit over a 33-year period mirror that of the Netherlands experience.9 Namely a major increase in the incidence of central chondrosarcoma of bone, while referrals for the other main primary sarcomas of bone remain static. This is likely to be due to the mass proliferation of MRI scanning as seen in the majority of modern health care systems. The continuum of CCTs from benign to highly malignant elegantly fits the “barnyard pen” analogy of cancers. This analogy could prove useful as an explanatory tool for both clinicians and patients unfamiliar with this spectrum of cancers.
Conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
No financial disclosures.
No funding to declare.
Acknowledgments
Dr Gilbert Welch who’s lecture on the REAL course organised by the Royal College of Radiologists in Birmingham, UK on February 3, 2020 stimulated this study.
References
- 1.Hogendoorn P.C.W., Bovee J.V.M.G., Nielsen G.P. Chondrosarcoma (grades I-III) In: Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., editors. WHO Classification of Tumours of Soft Tissue and Bone. International Agency for Research on Cancer; Lyon: 2013. pp. 264–268. [Google Scholar]
- 2.Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group Reliability of histopathologic and radiologic grading of cartilaginous neoplasms of long bones. J Bone Joint Surg. 2007;89A:2113–2123. doi: 10.2106/JBJS.F.01530. [DOI] [PubMed] [Google Scholar]
- 3.Eefting D., Schrage Y.M., Geirnaerdt M.J. EuroBoneNet consortium. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading central cartilaginous tumors. Am J Surg Pathol. 2009;33:50–57. doi: 10.1097/PAS.0b013e31817eec2b. [DOI] [PubMed] [Google Scholar]
- 4.Crim J., Schmidt R., Layfield L. Can imaging criteria distinguish enchondroma from grade I chondrosarcoma? Eur J Radiol. 2015;84:2222–2230. doi: 10.1016/j.ejrad.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Fritz B., Muller D.A., Sutter R. Magnetic resonance imaging-based grading of cartilaginous bone tumors: added value of quantitative texture analysis. Invest Radiol. 2018;53:663–672. doi: 10.1097/RLI.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 6.Afonso P.D., Isaac A., Villagran J.M. Chondroid tumors as incidental findings and differential diagnosis between enchondromas and low-grade chondrosarcomas. Semin Muscoskel Radiol. 2019;23:3–18. doi: 10.1055/s-0038-1675550. [DOI] [PubMed] [Google Scholar]
- 7.Patel A., Davies A.M., Botch R., James S. A pragmatic approach to the imaging and follow-up of solitary central cartilage tumours of the proximal humerus and knee. Clin Radiol. 2019;74:517–526. doi: 10.1016/j.crad.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Davies A.M., Patel A., James S.L., Botchu R. A retrospective validation of an imaging protocol for the management of solitary central cartilage tumours of the proximal humerus and around the knee. Clin Radiol. 2019;74:962–971. doi: 10.1016/j.crad.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 9.van Praag V.M., Rueten-Budde A.J., Ho V. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surgical Oncology. 2018;27:402–408. doi: 10.1016/j.suronc.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Hong E.D., Carrino J.A., Weber K.L. Prevalence of shoulder enchondromas on routine MR imaging. Clin Imag. 2011;35:378–384. doi: 10.1016/j.clinimag.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Walden M.J., Murphey M.D., Vidal J.A. Incidental enchondromas of the knee. AJR Am J Roentgenol. 2008;190:1611–1615. doi: 10.2214/AJR.07.2796. [DOI] [PubMed] [Google Scholar]
- 12.Stomp W., Reijnierse M., Kloppenburg M. NEO study group. Prevalence of cartilaginous tumours as an incidental finding on MRI of the knee. Eur Radiol. 2015;25:3480–3487. doi: 10.1007/s00330-015-3764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch H.G. Beacon Press; Boston: 2015. Less Medicine, More Health: 7 Assumptions that Drive Too Much Medical Care; pp. 60–71. [Google Scholar]
- 14.Harrison W.D., Sargazi N., Yin Q., Chandrasekar C.R. Delayed diagnosis in primary care – the main cause of sarcoma litigation in the United Kingdom. J Surg Oncol. 2016;113:361–363. doi: 10.1002/jso.24149. [DOI] [PubMed] [Google Scholar]
- 15.Crile G.A. A plea against the blind fear of cancer: an experienced surgeon says that excessive worry leads to costly tests, undue suffering and unnecessary operations. LIFE Magazine. 1955:128–142. [Google Scholar]
- 16.Welch H.G., Fisher E.S. Income and cancer overdiagnosis – when too much care is harmful. N Engl J Med. 2017;376:2208–2209. doi: 10.1056/NEJMp1615069. [DOI] [PubMed] [Google Scholar]
- 17.Hinman F. Screening for prostatic carcinoma. J Urol. 1991;145:126–130. doi: 10.1016/s0022-5347(17)38267-8. [DOI] [PubMed] [Google Scholar]
- 18.Kattan M.W. The hypothetical rabbit. Front Oncol. 2016;6:123–124. doi: 10.3389/fonc.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn H.S., Kim H.J., Welch H.G. Koreas’s thyroid-cancer “epidemic” – screening and overdiagnosis. N Engl J Med. 2014;371:1765–1766. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 20.Bleyer A., Welch H.G. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 21.Welch H., Prorok P.C., O’Malley A.J., Kramer B.S. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 22.Moynihan R., Doust J., Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:19–23. doi: 10.1136/bmj.e3502. [DOI] [PubMed] [Google Scholar]
- 23.Brodersen J., Kramer B.S., Macdonald H., Schwartz L., Woloshin S. Editorial: overdiagnosis: a driver of too much medicine. BMJ. 2018 Aug 17;362:k3494. doi: 10.1136/bmj.k3494. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell A.D., Ayoub K., Mangham D.C., Grimer R.J., Carter S.R., Tillman R.M. Experience in the treatment of dedifferentiated chondrosarcoma. J Bone Joint Surg [Br] 2000 82B:55–61. doi: 10.1302/0301-620x.82b1.9020. [DOI] [PubMed] [Google Scholar]
- 25.Grimer R.J., Gosheger G., Taminiau A. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Canc. 2007;43:2060–2065. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Strotman P.K., Reif T.J., Kliethermes S.A., Sandhu J.K., Nystrom L.M. Dedifferentiated chondrosarcoma: a survival analysis of 159 cases from the SEER database (2001-2011) J Surg Oncol. 2017;116:252–257. doi: 10.1002/jso.24650. [DOI] [PubMed] [Google Scholar]
- 27.Angelini A., Guerra G., Mavrogenis A.F. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929–937. doi: 10.1002/jso.23173. [DOI] [PubMed] [Google Scholar]
- 28.Andreou D., Gilg M.M., Gosheger G., Werner M. Metastatic potential of Grade I chondrosarcoma of bone: results of a multi-institutional study. Ann Surg Oncol. 2016;23:120–125. doi: 10.1245/s10434-015-4852-1. [DOI] [PubMed] [Google Scholar]
- 29.Fromm J., Klein A., Baur-Melnyk A. Survival and prognostic factors in conventional G1 chondrosarcoma. World J Surg Oncol. 2019 Sep 3;17(1):155. doi: 10.1186/s12957-019-1695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson R.J., Zumsteg J.W., Hartley K.A. Overutilization and cost of advanced imaging for long-bone cartilaginous lesions. Ann Surg Oncol. 2015;22:3466–3473. doi: 10.1245/s10434-014-4325-y. [DOI] [PubMed] [Google Scholar]
- 31.Akoh C.C., Craig E., Troester A.M., Miller B.J. Radiographic enchondroma surveillance: assessing clinical outcomes and cost effectiveness. Iowa Orthop J. 2019;39:185–193. [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings R., Riley N., Rose B. An evaluation of the diagnostic accuracy of the grade of preoperative biopsy compared to surgical excision in chondrosarcoma of the long bones. Int J Surg Oncol. 2010;2010:270195. doi: 10.1155/2010/270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roitman P.D., Farfalli G.L., Ayerza M.A. Is needle biopsy clinically useful in preoperative grading of central chondrosarcoma of the pelvis and long bones? Clin Orthop Relat Res. 2017;475:808–814. doi: 10.1007/s11999-016-4738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laitinen M.K., Stevenson J.D., Parry M.C., Sumathi V., Grimer R.J., Jeys L.M. The role of grade in local recurrence and the disease-free survival in chondrosarcomas. Bone Joint Lett J. 2018;100(B):662–666. doi: 10.1302/0301-620X.100B5.BJJ-2017-1243.R1. [DOI] [PubMed] [Google Scholar]
- 35.Altay M., Bayrakci K., Yildiz Y. Secondary chondrosarcomas in cartilage bone tumors: report of 32 patients. J Orthop Sci. 2007;12:415–423. doi: 10.1007/s00776-007-1152-z. [DOI] [PubMed] [Google Scholar]
- 36.Schwab J.H., Wenger D., Unni K. Does local recurrence impact on survival in low-grade chondrosarcoma of the long bones? Clin Orthop Relat Res. 2007;462:178–180. doi: 10.1097/BLO.0b013e3180caac2c. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson J.D., Laitinen M.K., Parry M.C. The role of surgical margins in chondrosarcoma. Eur J Surg Oncol. 2018;44:1412–1418. doi: 10.1016/j.ejso.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Bus M.P.A., Campanacci D.A., Albergo J.I. Conventional primary central chondrosarcoma of the pelvis: prognostic factors and outcome of surgical treatment in 162 patients. J Bone Joint Surg. 2018;100A:316–325. doi: 10.2106/JBJS.17.00105. [DOI] [PubMed] [Google Scholar]
- 39.Laitinen M.K., Parry M.C., Le Nail L.R. Locally recurrent chondrosarcoma of the pelvis and limbs can only be controlled by wide local excision. Bone Joint Lett J. 2019;101B:266–271. doi: 10.1302/0301-620X.101B3.BJJ-2018-0881.R1. [DOI] [PubMed] [Google Scholar]
- 40.www.smartsurvey.co.uk/s/CIBMREquip/ last accessed 24/1/21.
- 41.MRI Equipment, Operations and Planning in the NHS: Report from the Clinical Imaging Board. IPEM, CoR & RCR; 2017. accessed. [Google Scholar]
- 42.COCIR (The European Coordination Committee of the Radiological, Electromedical & Healthcare IT Industry) 2019. Medical Imaging Equipment: age profile & density (last accessed 24/1/21) [Google Scholar]
- 43.Parler-Cuau C., Bousson V., Ogilvie C.M. When should we biopsy a solitary central cartilaginous tumor of long bones? Literature review and management proposal. Eur J Radiol. 2011;77:6–12. doi: 10.1016/j.ejrad.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 44.Campanacci D.A., Scoccianti F., Franchi A. Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol. 2013;14:101–107. doi: 10.1007/s10195-013-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampath Kumar S.V., Tyrrell P.N.M., Singh J. Surveillance of intramedullary cartilage tumours in long bones. Bone Jt J. 2016;98B:1542–1547. doi: 10.1302/0301-620X.98B11.37864. [DOI] [PubMed] [Google Scholar]
- 46.Deckers C., Schreuder B.H.W., Hanninck G. Radiological follow-up of untreated enchondroma and atypical cartilaginous tumors in the long bones. J Surg Oncol. 2016;114:987–991. doi: 10.1002/jso.24465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies A.M., Patel A., James S.L., Azzopardi C., Botchu R. An imaging protocol for the management of central cartilage tumours of the proximal fibula. Clin Radiol. 2020;75(9):714e1–714e6. doi: 10.1016/j.crad.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 48.Jurik A.G., Hansen B.H., Weber K. Solitary enchondromas – diagnosis and surveillance. Danish guidelines. Radiologe. 2020;60(1):26–32. doi: 10.1007/s00117-020-00681-7. [DOI] [PubMed] [Google Scholar]