Abstract
Increased interferon-α (IFN-α) production is a critical component in the pathophysiology of systemic lupus erythematosus (SLE) and other rheumatic autoimmune diseases. Herein, we report the characterization of S95021, a fully human IgG1 anti–IFN–α monoclonal antibody (mAb) as a novel therapeutic candidate for targeted patient populations. S95021 was expressed in CHOZN GS−/− cells, purified by chromatography and characterized by using electrophoresis, size exclusion chromatography and liquid chromatography-mass spectrometry. High purity S95021 was obtained as a monomeric entity comprising different charge variants mainly due to N-glycosylation. Surface plasmon resonance kinetics experiments showed strong association rates with all IFN-α subtypes and estimated KDs below picomolar values. Pan–IFN–α-binding properties were confirmed by immunoprecipitation assays and neutralization capacity with reporter HEK-Blue IFN-α/β cells. S95021 was IFN-α-selective and exhibited superior potency and broader neutralization profile when compared with the benchmark anti–IFN–α mAbs rontalizumab and sifalimumab. STAT-1 phosphorylation and the type I IFN gene signature induced in human peripheral blood mononuclear cells by recombinant IFN-α subtypes or plasmas from selected autoimmune patients were efficiently reduced by S95021 in a dose-dependent manner. Together, our results show that S95021 is a new potent, selective and pan IFN-α-neutralizing mAb. It is currently further evaluated as a valid therapeutic candidate in selected autoimmune diseases in which the IFN-α pro-inflammatory pathway is dysregulated.
Keywords: Interferon-alpha, Biological therapy, Autoimmune diseases, Systemic lupus erythematosus, Primary Sjögren syndrome
Highlights
-
•
Interferon-α (IFN-α) is a key pathogenic player in several autoimmune diseases.
-
•
S95021 is a new, natural, fully human, selective and anti-pan IFN-α monoclonal antibody (mAb).
-
•
S95021 has high binding affinity to its target, reaching picomolar KD values for all IFN-α subtypes.
-
•
S95021 potently inhibits the type I IFN gene signature in immune cells from autoimmune patients.
-
•
S95021 is proposed as a promising candidate to treat autoimmune diseases driven by IFN-α.
1. Introduction
Human type I interferons (IFNs) [1] encompass several structurally related cytokines including 13 IFN-α subtypes, IFN-β, IFN-ε, IFN-κ, and IFN-ω. All of them signal through a common type I IFN receptor called IFNAR [2] to induce the expression of gene transcripts and proteins with antiviral and immunomodulatory properties, known as the type I IFN gene signature (IGS) [3]. Among type I IFNs, IFN-α has been widely studied in light of its documented pro-inflammatory activity [4,5]. During the course of treatment with IFN-α, patients suffering from malignancies or chronic infections often developed autoimmune manifestations resembling the clinical features of systemic lupus erythematosus (SLE), with occurrence of autoantibodies to nuclear antigens, suggesting that type I IFN can trigger autoimmunity [[6], [7], [8]]. Consistent with this hypothesis, a large proportion of SLE patients exhibit high expression of type I IFN regulated genes [[9], [10], [11], [12]] most often associated with high circulating IFN levels and disease activity [[13], [14], [15]].
There is now substantial evidence that the type I IFN pathway is prominently upregulated in patients suffering from various autoimmune diseases beyond SLE, including dermatomyositis, primary Sjögren syndrome (pSS), systemic sclerosis and type I interferonopathies [16,17]. Such conditions for which a constitutive up-regulation of the type I IFN pathway is documented may benefit from therapies that reduce the levels of IFN-α or its effects [[18], [19], [20]]. While the implication of type I IFNs distinct from IFN-α has been poorly investigated in these diseases, one therapeutic approach consists in targeting their common receptor. Recent positive phase 3 outcomes with the anti IFNAR monoclonal antibody (mAb), anifrolumab [21] suggested potential therapeutic benefits in SLE patients for which the IGS is markedly increased [10,22]. While the TULIP-1 phase 3 trial did not meet its primary endpoint on the SLE responder index [23], the TULIP-2 trial showed positive outcome on the BILAG-Based Composite Lupus Assessment score after one year [24].
A more direct strategy for therapeutic inhibition of the type I IFN pathway consists in neutralizing IFN-α directly. Sifalimumab a human anti–IFN–α mAb demonstrated good tolerability and safety in phase I studies [25,26], and clinical efficacy in a phase IIb study of adults with moderate to severe active SLE [27]. Sifalimumab treatment also reduced the IGS not only in SLE [28], but also in dermatomyositis and polymyositis patients [29]. Nevertheless, its development was stopped for repurposing to anifrolumab. Rontalizumab, another humanised anti–IFN–α mAb, did not meet its primary and secondary endpoints in a 24-week phase II clinical trial in SLE, mainly owing to low responses in patients presenting a high IGS [30].
Collectively, these results confirm the interest of targeting the type I IFN pathway in SLE and other autoimmune diseases, in which there is an ongoing need for fine-tuned therapies. In this context, we now report the extensive characterization of S95021, a potent IFN-α-neutralizing mAb originally derived by limit-dilution cloning from memory B cells of APS1/APECED patients [31] and considered as a promising candidate therapy in selected autoimmune diseases.
2. Methods
Procedures concerning gene design and synthesis, mAbs expression, purification and characterization, binding kinetics by SPR and anti IFN-α SIMOA assay are available in online supplementary files.
2.1. Luciferase immunoprecipation system (LIPS) assay
LIPS experiments were conducted in order to assess the IFN-α-binding potency of S95021. Coding sequences of human IFN-α subtypes without signal peptides were cloned into modified pPK-CMV-F4 fusion vector (PromoCell GmbH, Heidelberg, Germany) downstream of NanoLuc luciferase (Promega, USA), that was cloned into the plasmid instead of Firefly luciferase. HEK 293 cells were transfected with the constructs and secreted NanoLuc-antigen fusion proteins were collected with the supernatants after 48 h. Luciferase immunoprecipitation system (LIPS) assay was adapted from Ref. [32] and performed in 96-well MultiScreen filter HTS plates (Millipore) at room temperature in 50 mM Tris, pH 7.5 buffer containing 100 mM NaCl, 5 mM MgCl2 and 1% Triton X-100. Serial dilutions of S95021, sifalimumab or rontalizumab were captured onto Protein G Agarose beads (25 μL of 4% suspension, Exalpha Biologicals) and incubated for 1 h with NanoLuc-conjugated IFNs 106 LU per well. After washing, Nano-Glo® Luciferase Assay Reagent was added (Promega, USA) and luminescence intensity measured with Victor X plate reader (Perkin Elmer Life Sciences). Effective concentration 50 (EC50) values were calculated according to the dose-response curves using Prism 8 (GraphPad Software, San Diego, CA).
2.2. Cell-based assay of human IFN-α neutralization in HEK-blueTM cells
The IFN-neutralizing capacity of S95021 and benchmark antibodies was tested with the help of reporter HEK-BlueTM IFN-α/β cells (InvivoGen) that express alkaline phosphatase (AP) under the inducible ISG54 promoter after ISGF binding to the IFN-stimulated response elements (ISRE) in the promoter as previously reported [33]. The cells were grown in DMEM (Naxo), containing 10% heat inactivated FBS and supplemented with 30 μg/mL blasticidin (InvivoGen) and 100 μg/mL Zeocin (InvivoGen). IFN-α2a was purchased from Miltenyi Biotech (Bergisch Gladbach, Germany) and all other subtypes were from PBL Assay Science and used at the following final concentrations: IFN-α2a 200 U/ml, IFN-α1 50 U/ml, IFN-α4, IFN-α5, IFN-α7 and IFN-α21 25 U/ml, IFN-α10 and IFN-α17 12.5 U/ml, IFN-α8 6.25 U/ml, IFN-α6 3 U/ml, IFN-α16 1.5 U/ml and IFN-α14 0.6 U/ml. Cells were stimulated with optimized concentrations of type I IFNs that were preincubated for 2 h with serial dilutions of monoclonal antibodies. QUANTI-Blue TM (InvivoGen) colorimetric enzyme assay was used to determine AP in the cell culture supernatants after 21 h of incubation. OD was measured at 620 nm with a Multiscan MCC/340 (Labsystems) ELISA reader. IC50 values were calculated from the dose-response curves.
2.3. PBMC stimulation experiments
All IFN-α subtypes, IFN-β or IFN-γ were purchased from PBL Assay Science (Piscataway, NJ, USA). IFN-α or plasmas obtained from SLE patients were preincubated with serial dilutions of S95021, sifalimumab or rontalizumab for 1 h at 37 °C and 5% CO2 and added to PBMC from healthy donors for 15 min at 37 °C to measure STAT1 phosphorylation, or overnight to assess the IFN signature by RT-qPCR. STAT1 phosphorylation was assessed with the Beckman Coulter PerFix EXPOSE kit. Flow cytometry experiments were performed on Cytoflex LX (Beckman) and data analyzed using Flowjo software. For RT-qPCR analyses, IFN-α16, IFN-β or IFN-γ were used at 1000 IU/mL and plasmas at a 1:2 dilution. The gene expression of 18 interferon-stimulated genes and 2 housekeeping genes (Supplementary Table 1) was quantified. The median fold change of the 18 selected genes compared to the median of the combined data of healthy controls was used to calculate an IFN score for each patient.
2.4. STAT1 phosphorylation assay
For the STAT1 phosphorylation assay, cells were fixed for 10 min at room temperature using Beckman Coulter PerFix EXPOSE Fixation Buffer and permeabilized for 5 min at 37 °C using Beckman Coulter PerFix EXPOSE permeabilizing Buffer. Cells were stained with BV421-anti-STAT1 pY701 (clone 4a, BD Biosciences) and the cell surface markers FITC-CD3 (Biolegend), PE-CD56, APC-CD14 A700-CD19 (Beckman) for 1 h at room temperature protected from light. Flow cytometry analyses were performed on Cytoflex LX (Beckman). Results were analyzed using Flowjo software.
2.5. RT-qPCR analyses: IFN signature
Total RNA was extracted using the RNeasy Mini Kit (Qiagen), with the optional on-column DNAse I digestion. Upon extraction, total RNA samples were quantified on Nanodrop 2000 (ThermoFisher Scientific™) and analyzed on 2100 Bioanalyzer (Agilent) using RNA 6000 pico chips to determine RNA quality (RIN).
Total RNA (40 ng/20 μL) were reversed transcribed into cDNA using random primers, dNTPS, RNAse inhibitor and multiScribe RT enzyme from the High Capacity cDNA Reverse Transcription kit (Applied biosystems) with the following conditions: 25 °C for 10 min, 37 °C for 2 h, 85 °C for 5 min. Considering a retro-transcription efficiency of 100% and a final volume of 20 μL, cDNA solution concentration has been regarded as equal to 2 ng/μL. Specific target amplification of 1.25 μL cDNA was carried out with 1.25 μL of pool of the TaqMan™ Gene Expression Assays (0.2X, Life Technologies) and 2.5 μL TaqMan Preamp Master Mix 2X (Life Technologies) at 95 °C for 10 min, followed by 15 PCR cycles at 95 °C for 15 s and 60 °C for 4 min. The pre-amplification product was diluted 1:5 in TE buffer 10 mM Tris, 1 mM EDTA, pH 8.0 Solution (Teknova). Real-time PCR was conducted on Fluidigm 96.96 Dynamic Arrays using the BioMark™ HD system according to the manufacturer’s protocol. 2.25 μL of cDNA were combined to 2.5 μL TaqMan Universal PCR Master Mix (Life technologies, 4,324,020) and 20X GE Sample loading Reagent (Fluidigm, 85,000,735) before being loaded into a 96.96 Dynamic Array. In the same way, 2.5 μL of TaqMan assays and Assay loading Reagent 2X (Fluidigm, 85,000,736) were combined before being loaded into a 96.96 Dynamic Array. Ct values were calculated using the BioMark Real-Time PCR Analysis software (Fluidigm) with the linear derivative baseline subtraction method and an user calculated threshold (set at 0.01). Quantifiable expression is defined by Ct values of 24 or lower, and undetectable expressions by Ct values over 40. The Ct values were normalized to endogenous controls by subtracting the average of its PPIA and B2M expression levels for each sample. Selection of the most stable reference genes for normalization was evaluated using geNorm. The list of genes and assay ID is reported in Supplementary Table 1.
2.6. Human samples and patients characteristics
PBMCs and plasmas from SLE and pSS patients were purchased from Tebu-bio. All patients were Caucasian females. Their characteristics are reported in Supplementary Table 2. Plasma IFN-α levels were measured using a Simoa assay as previously described [31].
2.7. Statistical analyses
Statistical analyses were performed using the GraphPad Prism 8 software.
3. Results
3.1. S95021 mAb characterization
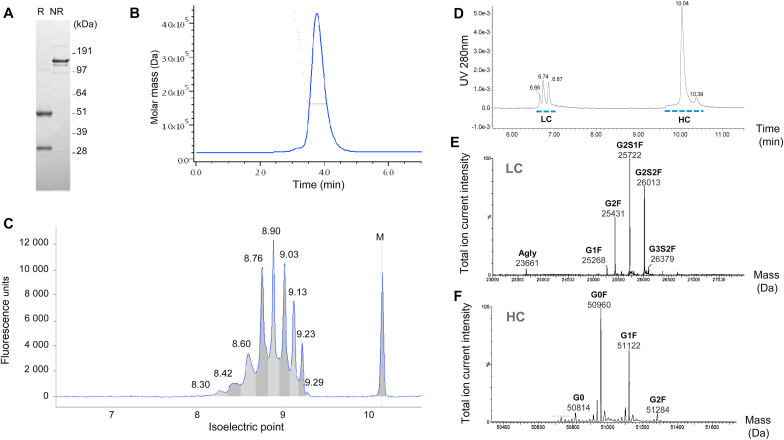
Heavy chain (HC) and Light chain (LC), and the intact monoclonal antibody (mAb) were evidenced by SDS-PAGE under reducing and non-reducing conditions, respectively (Fig. 1A). The antibody resolved as a symmetrical peak with a molar mass at ~150 kDa, matching with the expected mass of human IgG1, with > 98% homogeneity as estimated by SEC-UV-MALS (Fig. 1B). Other minor species (~2%) consisting of dimeric mAb and higher-order oligomers, were also detected by SEC-UV-MALS. cIEF detected nine charge variants with pIs ranging from 8.30 to 9.29, with the most abundant species estimated at 8.76 and 8.90 (Fig. 1C).
Fig. 1.
Characterization of S95021. (A) SDS-PAGE of purified S95021 mAb under reducing (R) and non-reducing (NR) conditions. The position of the standard mass markers is depicted on the right side of the gel. (B) SEC-UV-MALS chromatogram of S95021. Continuous line: UV chromatogram at 280 nm; dotted line: MALS-based mass estimation obtained using ASTRA (Y axis). (C) cIEF electropherogram of S95021. Measured isoelectric points are indicated above peaks (10.17 pI marker is indicated as M). (D-F) LC-MS mass measurement and N-glycan identification of S95021. (D) RP-UHPLC-UV chromatogram of S95021 following reduction. Peaks corresponding to LC and HC are depicted with dotted lines. (E) Deconvoluted MS spectrum of LC glycoforms with main glycoforms indicated. (F) Deconvoluted MS spectrum of HC glycoforms. The relative content of N-glycans is reported in Supplementary Table 3.
Following deglycosylation, the intact mass measured by LC-MS was 146,349.8 Da, in agreement with the theoretical mass of 146,348.9 Da (mass accuracy < 7 ppm). The masses of LC and HC were determined following reduction of the non-deglycosylated mAb, evidencing 3 and 2 major peaks corresponding to LC and HC glycoforms, respectively (Fig. 1D). The first peak contained LC decorated with non-sialylated complex-type N-glycans, whereas mono- and di-sialylated motifs were evidenced within the second and third peaks, respectively. LC was almost fully N-glycosylated at position N28 as evidenced from MS/MS data. The LC N-glycans were mostly mono- and disialylated with 40% of bigalactosyl, monosialylated core-fucosylated biantennary complex-type N-glycans (G2S1F) and 30% of disialylated species (G2S2F) (Fig. 1E and Supplementary Table 3). This N-glycosylation accounts for most of the heterogeneity observed by cIEF. HC MS analysis confirmed Fc N-glycosylation consisting of G0, G0F, G1F, G2F as expected for mAbs produced in CHO cells, with appropriate mass accuracy below 20 ppm (Fig. 1F and Supplementary Table 3).
LC and HC primary sequences were assessed by LC-MS/MS with complementary peptide maps, confirming the expected amino acid sequences with 98% and 100% sequence coverage, for the HC and LC, respectively (Supplementary Fig. 2). Besides N-glycosylation, other typical modifications included asparagine deamidation and methionine oxidation.
3.2. Several criteria evidence binding and neutralization of all IFN-α subtypes by S95021
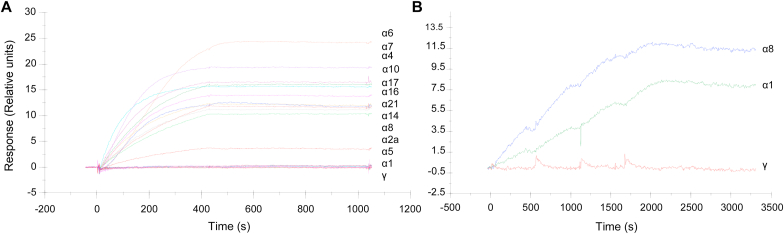
The binding of S95021 to the twelve human IFN-α subtypes was analyzed by SPR (Fig. 2A). Beforehand, the active concentration of each IFN was determined by calibration-free-concentration-analysis to have a better estimation of affinity parameters. All IFN-α subtypes bound strongly to S95021. Nevertheless, IFN-α1 showed slower association rate without reaching the equilibrium. KD could be measured for IFN-α1 (1.10−11 M) and IFN-α8 (6.10−12 M) in a multidose assay, owing to a faster dissociation rate than other subtypes (Fig. 2B). Association and dissociation constants for IFN-α1 and IFN-α8 are reported in Supplementary Table 4. In an independent experiment, the measured KD for IFNα-2b was 5.4.10−12 M. The affinity for other IFN-α subtypes could not be determined as kinetics parameters were outside the limit of Biacore instrument, strongly suggesting subpicomolar KD values. S95021 did not interact with other type I IFNs nor with IFN-γ.
Fig. 2.
Binding analysis by SPR of IFN-α subtypes flowed over captured S95021. (A) Binding in single dose assay of the 12 IFN-α subtypes at 250 pM over captured S95021. (B) Binding analysis of IFN-α1 and IFN-α8 flowed over captured S95021. IFN-α1, IFN-α8 and the negative control IFN-γ were injected in single cycle kinetics binding cycles, as 2-fold dilution series with top at 750 nM.
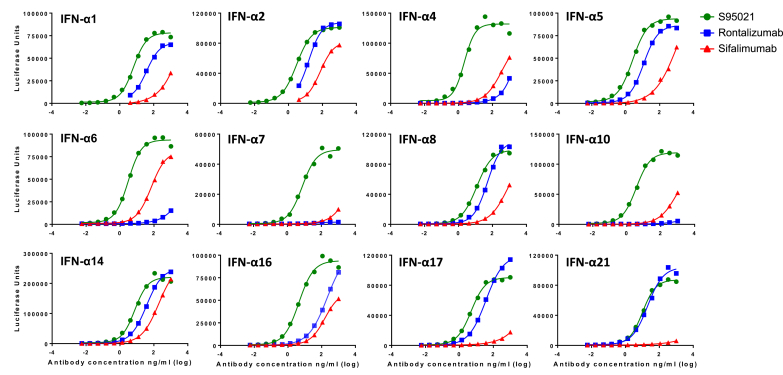
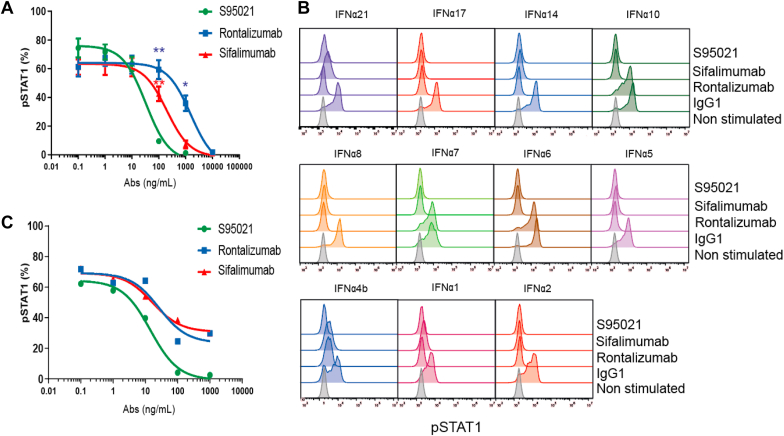
The capacity of S95021 to bind all IFN-α subtypes was further evaluated by LIPS assay and compared to the binding capacity of rontalizumab and sifalimumab (Fig. 3 and Supplementary Table 5). S95021 uniformly bound all IFN-α subtypes with much better efficacy, with favorable EC50 ratios ranging from 2 (for IFN-α21) to 48 (for IFN-α16) when compared with rontalizumab, and from 22 (for IFN-α2) to 437 (for IFN-α5) when compared to sifalimumab.
Fig. 3.
Binding activity of S95021, rontalizumab and sifalimumab to IFN-α subtypes using luciferase based immunoprecipitation system (LIPS). LIPS assay was carried out with NanoLuc-IFN fusion proteins and serial dilutions of monoclonal antibodies. Dose-response curves used for effective concentration 50 (EC50) calculation are depicted for each IFN-α subtype. EC50 values are reported in Supplementary Table 5.
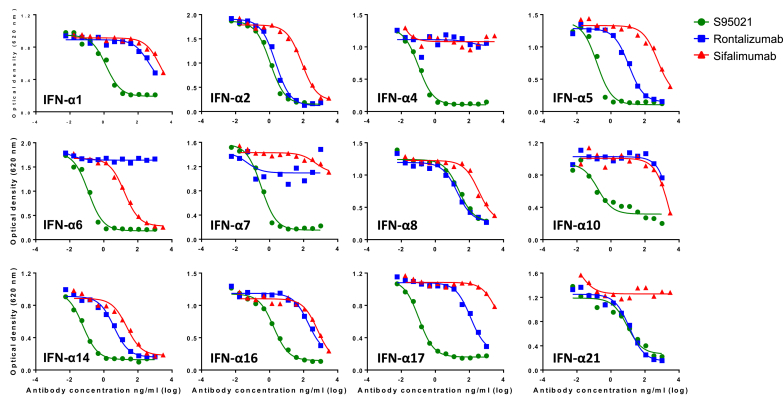
This binding capacity was further assessed in HEK-Blue IFN-α/β cells for which stimulation with human IFN-α subtypes results in activation of the JAK/STAT/ISGF3 pathway. Similarly, S95021 potently inhibited the activity of all IFN-α subtypes to a much higher extent than rontalizumab or sifalimumab (Fig. 4 and Supplementary Table 6). Of note, several IFN-α subtypes were inhibited poorly or not at all by the latter antibodies.
Fig. 4.
Neutralization of the effect of IFN-α subtypes by S95021, rontalizumab and sifalimumab in reporter HEK-BlueTM IFN-α/β cells. IFN-α subtypes were incubated with serial dilutions of monoclonal antibodies and tested for bioactivity on reporter cells. Dose-response curves used for inhibitory concentration 50 (IC50) calculation are depicted for each IFN-α subtype. IC50 values are reported in Supplementary Table 6.
3.3. S95021 Inhibits STAT1 phosphorylation better than benchmark mAbs
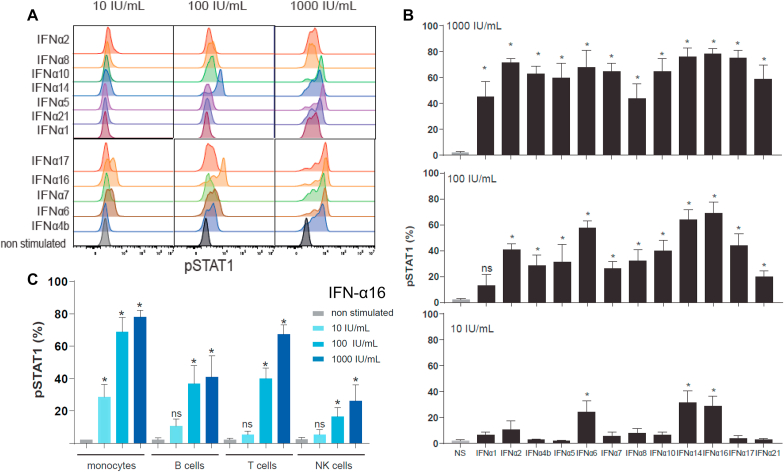
We then analyzed the activity of each IFN-α subtype on STAT1 phosphorylation, an early marker of IFNAR activation, in human healthy PBMCs gated on CD14+ monocytes (Fig. 5A and B). IFN-α6, -14 and -16 were the most potent subtypes with P-STAT1 induction starting from 10 IU/mL, followed by IFN-α -17, -2, -10, -8, -5, -4b -7 and -21 from 100 IU/mL, and IFN-α1 only at 1000 IU/mL.
Fig. 5.
IFN-α subtypes induce P-STAT1 at different levels in immune cell populations. (A) Representative FACS plots showing the dose-dependent induction of STAT1 phosphorylation by IFN-α subtypes in human healthy PBMCs gated in CD14+ monocytes. (B) Percentage of P-STAT1 positive cells in human heathy PBMCs gated in CD14+ monocytes after stimulation with IFN-α subtypes. (C) Percentage of P-STAT1 positive cells in human heathy PBMCs (gated in CD14+ monocytes, CD20+ B cells, CD3+ T cells and CD16+CD56+ NK cells) after stimulation with IFN-α16. Values in (B) and (C) are means and SEM from experiments completed with 4 individual donors. Significance was calculated using Mann-Whitney test with ∗p < 0.05; ns, not significant.
IFN-α16 was selected as a prototypical subtype to determine the response of different immune cell populations to IFN-α stimulation (Fig. 5C). Monocytes showed the highest level of P-STAT1 induction and were more responsive at lower IFN-α concentration than B, T, and NK immune cells, consistent with their higher cell surface IFNAR expression [34].
The neutralizing potency of S95021 on STAT1 phosphorylation was then studied in PBMCs from human healthy individuals stimulated by each IFN-α subtype (Fig. 6A and B). IFN-α16-induced P-STAT1 was strongly and dose-dependently neutralized by S95021 with a mean IC50 value of 19.5 ng/mL (Fig. 6A). By comparison, mean IC50 values for sifalimumab and rontalizumab were 180 and 1660 ng/mL, respectively. To compare the relative potency of S95021, sifalimumab and rontalizumab on STAT1 phosphorylation induced by other IFN-α subtypes (Fig. 6B), the concentration of each mAb was fixed at the highest neutralization value observed in the IFN-α16 experiment, i.e. 1 μg/mL for S95021 and 10 μg/mL for sifalimumab and rontalizumab. At 1 μg/mL, S95021 fully neutralized STAT1 phosphorylation induced by all IFN-α subtypes. At a 10-fold higher concentration, rontalizumab failed to efficiently neutralize IFN-α6, -7, -10 and -4b, and sifalimumab was less potent in neutralizing IFN-α -21, -5, -4b and -1. Neither S95021 nor the benchmark mAbs affected IFN-γ or IFN-β-induced P-STAT1 (Supplementary Fig. 3).
Fig. 6.
S95021 is more potent than benchmark antibodies in neutralizing P-STAT1 after IFN-α stimulation in control healthy PBMCs. P-STAT1 analysis was assessed in human healthy PBMCs gated in CD14+ monocytes. (A) Dose-dependent neutralization of STAT1 phosphorylation by S95021, rontalizumab or sifalimumab after IFN-α16 stimulation. (B) Relative intrinsic neutralization potency of S95021 (1 μg/mL), sifalimumab (10 μg/mL) or rontalizumab (10 μg/mL) on STAT1 phosphorylation induced by IFN-α subtypes. (C) Dose-dependent neutralization of STAT1 phosphorylation by S95021, rontalizumab or sifalimumab after incubation with plasma from an individual SLE donor. Values in (A) and (B) are means and SEM from experiments completed with 5 and 3 individual donors, respectively. Significance was calculated using Bonferroni-Holms adjustment on group after a two-way Anova compared with the condition S95021. ∗∗p < 0.01 and ∗p < 0.05.
To further study the efficacy of S95021 in a pathological context, we examined whether addition of S95021 to the plasma of a SLE patient containing high IFN-α levels (20,000 fg/mL) would also translate into P-STAT1 inhibition. The SLE plasma by itself induced 67.5% of P-STAT1 in human healthy PBMCs. S95021 neutralized P-STAT1 in a dose-dependent manner with nearly maximal efficacy at 100 ng/mL, whereas approximately 30% of cells remained P-STAT1 positive when incubated with sifalimumab or rontalizumab at 1 μg/mL (Fig. 6C), likely reflecting the failure of these two antibodies to neutralize all IFN-α subtypes.
3.4. Inhibition of type I IFN gene expression by S95021 in comparison with benchmark mAbs
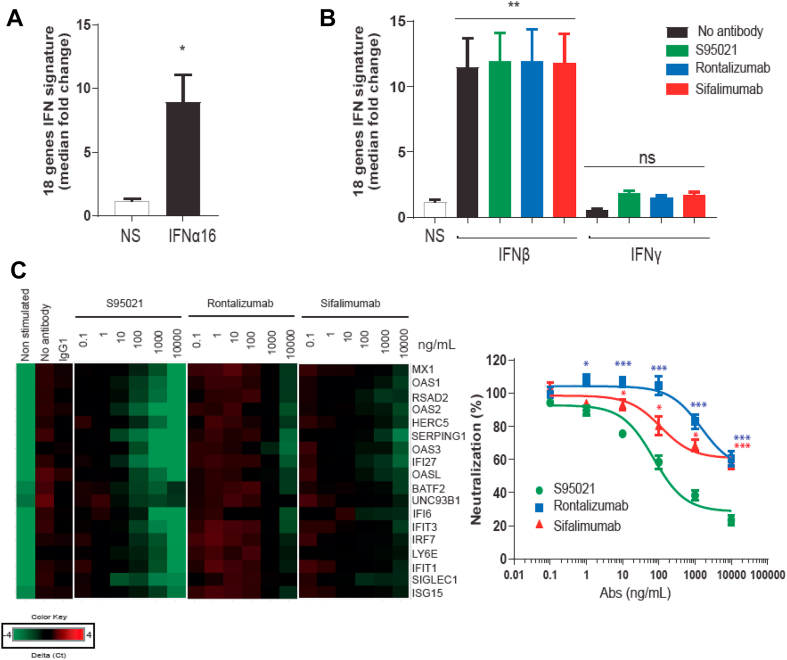
PBMCs from healthy control donors were stimulated with IFN-α16, IFN-β or IFN-γ in order to identify the most relevant genes modulated by type I IFN from among a broad selection reported in the literature. Final gene candidates were selected when expression in PBMCs was both stimulated after exposure to SLE/pSS patient plasma and inhibited by S95021 in a dose-dependent manner. The magnitude of expression of the selected genes was then determined in PBMCs from SLE/pSS patients in comparison with healthy donors (Supplementary Fig. 4A). The median fold change of the 18 selected genes (listed in Supplementary Table 1) was used to calculate an IFN score. In the tested samples from 19 SLE and 14 pSS patients, this score showed a good correlation (r = 0.76 and r = 0.65, respectively) with the plasma IFN-α levels measured by SIMOA (Supplementary Fig. 4B).
The effect of IFN-α16 on the IGS in control PBMCs is presented in Fig. 7A. The basal IGS was stimulated by IFN-α16 by a mean of 7.8-fold. As expected, the IFN score was increased by IFN-β but not IFN-γ (Fig. 7B). None of the mAbs was able to neutralize the IFN-β-induced IGS, confirming their specificity towards IFN-α. By contrast, S95021 suppressed the IFN-α16-induced IGS in a dose-dependent manner by an average of 77.2% at 10 μg/mL compared to control conditions (Fig. 7C). At this concentration, sifalimumab or rontalizumab decreased the IGS by about 40%, a level that was attained with S95021 from 0.1 μg/mL.
Fig. 7.
S95021 is more potent than benchmark antibodies in neutralizing the type I IFN gene signature induced by IFN-α16 in control healthy PBMCs. (A) Induction of the 18 IFN gene signature by 1000 IU/mL IFN-α16. (B) Effect of S95021, rontalizumab or sifalimumab (each mAb at 10 μg/mL) on the 18 IFN gene signature after stimulation of PBMCs with 1000 IU/mL IFN-β or IFN-γ compared with untreated condition. (C) Heatmap showing the differential expression and dose-dependent neutralization of individual genes of the 18 IFN gene signature by S95021, rontalizumab or sifalimumab after stimulation with 1000 IU/mL IFN-α16 (left panel). The percentage of neutralization of the IFN gene signature in comparison with the untreated condition is shown on the right panel. Values in (A, B and C) are means and SEM from experiments completed with 5 individual donors. Significance was calculated using (A, B) Mann-Whitney test and (C) Bonferroni-Holms adjustment on group after a two-way Anova compared with the condition S95021. ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05 (C) ns; not significant.
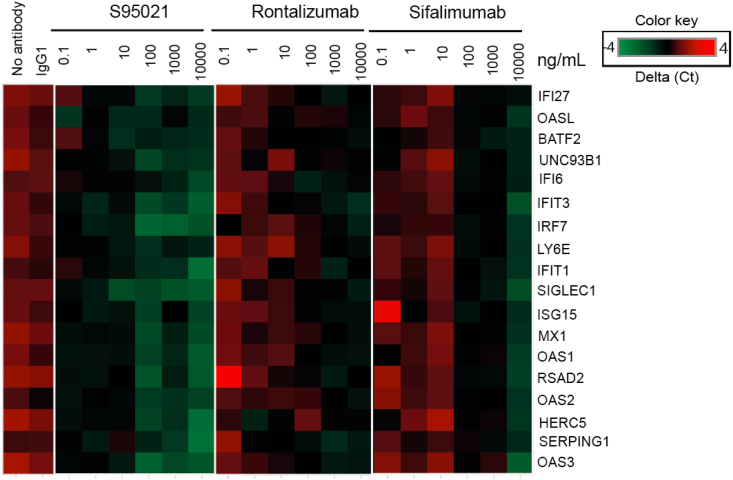
We then determined whether S95021 could reduce the IGS induced by SLE plasma in PBMCs from healthy donors. Fig. 8 shows the heatmap integrating data from 6 SLE patients. The IGS was neutralized by S95021 in a dose-dependent manner, with an impact on gene expression detectable at 0.1 ng/mL, whereas rontalizumab and sifalimumab were active only at a 1000-fold higher concentration. S95021 similarly decreased the IGS induced by plasma from pSS patients from 1 ng/mL (Supplementary Fig. 5).
Fig. 8.
S95021 is more potent than benchmark antibodies in neutralizing the type I IFN gene signature induced by plasma from SLE patients in control healthy PBMCs. Heatmap showing the differential expression and dose-dependent neutralization of individual genes of the 18 IFN gene signature by S95021, rontalizumab or sifalimumab after stimulation with plasma from SLE donors. The heatmap is representative of 6 individual SLE donors.
4. Discussion
Here we have characterized the anti–IFN–α mAb S95021 to be considered as a valid candidate therapy for a wide range of autoimmune diseases driven by IFN-α. S95021 was initially derived (under the initial name of 19D11), from APS1/APECED patients that are documented to be enriched in high autoantibody responses relative to healthy controls [31]. As a fully human antibody, S95021 can be used for therapeutic purposes without the need for further engineering usually required for antibodies produced by classical phage display or immunization. The expected benefits and high therapeutic potential of naturally occurring human antibodies have been emphasized [35]. Such antibodies predominantly belong to the IgM class, and as the first line of antibody defense, tend to have lower antigen binding affinities than IgG. As an IgG1, S95021 demonstrates excellent binding affinity, with KD reaching picomolar to sub-picomolar values towards all IFN-α subtypes. It was previously reported that 19D11 bound to conformational epitopes of IFN-α2 [31]. The high degree of similarity between IFN-α subtypes (between 87.3% and 90.5% compared to IFN-α2) and the observation that S95021 binds to all of them with quite similar affinities strongly suggest that conserved conformational epitopes are involved in the binding region.
Characterization of S95021 demonstrated the presence of four main charge variants, that are most likely manifestations of expected N-glycosylation. As for ~20% of IgGs from healthy donors, S95021 is N-glycosylated both within the Fab and Fc regions [36], bearing complex-type glycans. S95021 Fab (LC) N-glycosylation displays sialic acids that were described as potential enhancers of serum protein half-life [37]. It is commonly known that low amounts of N-glycoyl neuraminic acid (Neu5Gc) or α-galactosylated glycans could induce a risk of allergic reactions in patients. Based on LC-MS analyses, no mass difference corresponding to Neu5Gc addition were detected, whereas mass differences corresponding to non immunogenic N-acetyl neuraminic acid (Neu5Ac) were evidenced. Likewise, the known immunogenic galactose-α-1,3-galactose was not revealed by LC-MS.
S95021 displays a potent pan–IFN–α neutralizing profile, that was superior to that of two anti–IFN–α benchmark antibodies, sifalimumab and rontalizumab, that previously reached the phase 2 clinical stage. The broad neutralizing capacity of S95021 is a key property to treat SLE or pSS patients. Although the exact composition of IFN-α subtypes in plasma from such patients has not been documented, we confirmed here that each IFN-α subtype could potentially contribute to the pathophysiology by inducing P-STAT1 at different levels in immune cells, thereby strengthening arguments for using a pan IFN-α neutralizing mAb. While the significance of the multiplicity of type I IFN genes is not fully resolved, there is some evidence that individual IFN-α subtypes bind to different sites on IFNAR and with different affinities, potentially explaining differences in the extent of P-STAT1 induction [38,39].
Various type I IFN targeting therapeutic approaches have entered human trials, including IFN-α vaccines aiming at inducing endogenous anti-IFN antibodies. In a phase I/IIa study, IFN-α-kinoid immunization disrupted B-cell tolerance and generated high titers of polyclonal IFN-α-neutralizing antibodies lasting for up to 36 months without affecting T-cell tolerance [[40], [41], [42]]. Long-lasting antibodies may represent a potential issue as those cannot be “turned off” until the response wanes. Another approach involved targeting the type I IFN receptor IFNAR using the mAb, anifrolumab. By this means, and by contrast to the selective profile of S95021 towards IFN-α, anifrolumab was intended to neutralize the biological activity of all type I IFNs represented in humans, encompassing IFN-α, -β, -ε, -κ, and -ω [21]. Theoretical limitations to this approach are that the potential association of IFN-β, -ε, -κ, or -ω with SLE, pSS, dermatomyositis or interferonopathies, among others, still remains poorly documented and elusive. They are also known as important effectors in host cell protection against viruses. As such, broad inhibition of the whole type I IFN signaling might increase susceptibility to viral infections. Whereas an acceptable safety profile was observed in SLE patients receiving anifrolumab over one year, there was a higher incidence of upper respiratory tract and herpes zoster infections compared to placebo [24]. Additionally, despite sharing the same receptor, distinct type I IFNs could elicit different regulatory properties beyond control of the immune response, depending on their distribution and the tissue-specific pattern of signaling effectors and IFN-stimulated genes [43,44]. As an example, IFN-ε maintains homeostasis of the reproductive tract and is hormonally-regulated as a function of the estrus cycle [45]. Consequently, we consider that targeting IFN-α specifically would be a safe and focused option. This also takes into account that IFN-α is described as the main agent provocateur in the pathogenesis of selected autoimmune diseases, with observations that high circulating IFN-α levels predominate in these patients and its neutralization ablates the global type I IFN gene signature in activated immune cells [[18], [19], [20],22,[25], [26], [27], [28], [29],46].
A critical issue is to select patients more likely to benefit from anti–IFN–α therapies, considering diseases heterogeneity [[47], [48], [49]]. Given the difficulty in measuring circulating IFN-α levels by ELISA, the IGS has been employed in clinical trials as a readout of efficacy, and is supposed to reflect the level of involvement of IFN-α in causing the disease. However, this footprint by itself may not be appropriate as a predictive biomarker of treatment efficacy, since it is not strictly correlated to disease activity. Thus, the search for additional relevant biomarkers is critical to ensure adequate patient stratification and monitor treatment efficacy. In this context, the availability of the IFN-α SIMOA assay [46], allowing the sensitive detection of all IFN-α subtypes, should facilitate patient stratification in clinical trials with S95021, particularly by discriminating patients with significant IGS but low IFN-α levels.
We conclude that the fully human and pan–IFN–α neutralizing antibody S95021 represents a valid therapeutic option for patients with selected autoimmune diseases such as SLE and pSS. In light of its exquisite properties, it is anticipated to display efficacy in blunting IFN-α-driven inflammatory responses in patients, without affecting natural antiviral immune responses. As such, S95021 is currently being developed to enter clinical evaluation in human.
Authors statement
FD, CO, BF, HD, SB, AC, GL, KK and FDC designed the experiments. FD, HD, ALG, LH, and ED performed the experiments. All authors participated to data collection and interpretation. FD, CO, KK, EN, ACH, PM and FDC wrote and revised the manuscript and all authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The study was supported by ImmunoQure AG and grants PUT1367 and 391 PRG377 from the Estonian Research Council to Dr. Kisand and Dr. Haljasmägi. Dr. Kisand and Dr. Hayday have a patent US20190071499A1 pending to Les laboratoires Servier, and Dr. Hayday reports personal fees from ImmunoQure outside the submitted work.
Acknowledgements
The authors would like to thank Drs Pärt Peterson, Ed Stuart, and Sara Cipolat for helpful discussions. The authors gratefully acknowledge Aude Le Gall, Jérôme Dupuis, Manuel Picca, Edouard Geyer, Kevin Roger, Dominique Favale and Claire Semler-Collery for their expert technical assistance in this project. The study was supported by ImmunoQure AG and the Estonian Research Council grants PUT1367 and 391 PRG377.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2021.100093.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Crow M.K., Olferiev M., Kirou K.A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. 2019;14:369–393. doi: 10.1146/annurev-pathol-020117-043952. [DOI] [PubMed] [Google Scholar]
- 2.Novick D., Cohen B., Rubinstein M. The human interferon α/β receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 3.Barrat F.J., Crow M.K., Ivashkiv L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streuli M., Nagata S., Weissmann C. At least three human type alpha interferons: structure of alpha 2. Science. 1980;209:1343–1347. doi: 10.1126/science.6158094. [DOI] [PubMed] [Google Scholar]
- 5.Antonelli G., Scagnolari C., Moschella F., Proietti E. Twenty-five years of type I interferon-based treatment: a critical analysis of its therapeutic use. Cytokine Growth Factor Rev. 2015;26:121–131. doi: 10.1016/j.cytogfr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rönnblom L.E., Alm G.V., Oberg K.E. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann. Intern. Med. 1991;115:178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 7.Rönnblom L.E., Alm G.V., Oberg K.E. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 1990;227:207–210. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez Roman J., Castillo Palma M.J., Garcia Diaz E., Ferrer Ordínez J.A. Systemic lupus erythematosus induced by recombinant alpha interferon treatment. Med. Clin. 1994;102:198. [PubMed] [Google Scholar]
- 9.Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crow M.K., Kirou K.A., Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 12.Han G.M., Chen S.L., Shen N., Ye S., Bao C.D., Gu Y.Y. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Gene Immun. 2003;4:177–186. doi: 10.1038/sj.gene.6363966. [DOI] [PubMed] [Google Scholar]
- 13.Dall’Era M., Cardarelli P., Preston B., Witte A., Davis J.C., Jr. Type I interferon correlates with serological and clinical manifestations of SLE. Ann. Rheum. Dis. 2005;64:1692–1697. doi: 10.1136/ard.2004.033753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bengtsson A.A., Sturfelt G., Truedsson L., Blomberg J., Alm G., Vallin H. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 15.Feng X., Wu H., Grossman J.M., Hanvivadhanakul P., FitzGerald J.D., Park G.S. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J., Zhao M., Chang C., Wu H., Lu Q. Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin. Rev. Allergy Immunol. 2020;59:248–272. doi: 10.1007/s12016-020-08798-2. [DOI] [PubMed] [Google Scholar]
- 17.Crow Y.J. Type I interferonopathies: mendelian type I interferon up-regulation. Curr. Opin. Immunol. 2015;32:7–12. doi: 10.1016/j.coi.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Kirou K.A., Gkrouzman E. Anti-interferon alpha treatment in SLE. Clin. Immunol. 2013;148:303–312. doi: 10.1016/j.clim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Bodewes I.L.A., Versnel M.A. Interferon activation in primary Sjögren’s syndrome: recent insights and future perspective as novel treatment target. Expet Rev. Clin. Immunol. 2018;14:817–829. doi: 10.1080/1744666X.2018.1519396. [DOI] [PubMed] [Google Scholar]
- 20.Arshanapalli A., Shah M., Veerula V., Somani A.K. The role of type I interferons and other cytokines in dermatomyositis. Cytokine. 2015;73:319–325. doi: 10.1016/j.cyto.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Riggs J.M., Hanna R.N., Rajan B., Zerrouki K., Karnell J.L., Sagar D. Characterisation of anifrolumab, a fully human anti-interferon receptor antagonist antibody for the treatment of systemic lupus erythematosus. Lupus Sci. Med. 2018;5 doi: 10.1136/lupus-2018-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirou K.A., Lee C., George S., Louca K., Papagiannis I.G., Peterson M.G.E. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 23.Furie R.A., Morand E.F., Bruce I.N., Manzi S., Kalunian K.C., Vital E.M. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:e208–e219. doi: 10.1016/S2665-9913(19)30076-1. [DOI] [PubMed] [Google Scholar]
- 24.Morand E.F., Furie R., Tanaka Y., Bruce I.N., Askanase A.D., Richez C. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 2020;382:211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 25.Merrill J.T., Wallace D.J., Petri M., Kirou K.A., Yao Y., White W.I. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann. Rheum. Dis. 2011;70:1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 26.Petri M., Wallace D.J., Spindler A., Chindalore V., Kalunian K., Mysler E. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013;65:1011–1021. doi: 10.1002/art.37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamashta M., Merrill J.T., Werth V.P., Furie R., Kalunian K., Illei G.G. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016;75:1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao Y., Richman L., Higgs B.W., Morehouse C.A., de los Reyes M., Brohawn P. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- 29.Higgs B.W., Zhu W., Morehouse C., White W.I., Brohawn P., Guo X. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann. Rheum. Dis. 2014;73:256–262. doi: 10.1136/annrheumdis-2012-202794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalunian K.C., Merrill J.T., Maciuca R., McBride J.M., Townsend M.J., Wei X. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE) Ann. Rheum. Dis. 2016;75:196–202. doi: 10.1136/annrheumdis-2014-206090. [DOI] [PubMed] [Google Scholar]
- 31.Meyer S., Woodward M., Hertel C., Vlaicu P., Haque Y., Kärner J. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 2016;166:582–595. doi: 10.1016/j.cell.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burbelo P.D., Ching K.H., Mattson T.L., Light J.S., Bishop L.R., Kovacs J.A. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem. Biophys. Res. Commun. 2007;352:889–895. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 33.Breivik L., Oftedal B.E., Bøe Wolff A.S., Bratland E., Orlova E.M., Husebye E.S. A novel cell-based assay for measuring neutralizing autoantibodies against type I interferons in patients with autoimmune polyendocrine syndrome type 1. Clin. Immunol. 2014;153:220–227. doi: 10.1016/j.clim.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Pogue S.L., Preston B.T., Stalder J., Bebbington C.R., Cardarelli P.M. The receptor for Type I IFNs is highly expressed on peripheral blood B cells and monocytes and mediates a distinct profile of differentiation and activation of these cells. J. Interferon Cytokine Res. 2004;24:131139. doi: 10.1089/107999004322813372. [DOI] [PubMed] [Google Scholar]
- 35.Dimitrov J.D. Harnessing the therapeutic potential of ’rogue’ antibodies. Trends Pharmacol. Sci. 2020;41:409–417. doi: 10.1016/j.tips.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Jefferis R. Antibody therapeutics: isotype and glycoform selection. Expet Opin. Biol. Ther. 2007;7:1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 37.Bork K., Horstkorte R., Weidemann W. Increasing the sialylation of therapeutic glycoproteins: the potential of the sialic acid biosynthetic pathway. J. Pharmacol. Sci. 2009;98:3499–3508. doi: 10.1002/jps.21684. [DOI] [PubMed] [Google Scholar]
- 38.Yonehara S., Yonehara-Takahashi M., Ishii A., Nagata S. Different binding of human interferon alpha 1 and alpha 2 to common receptors on human and bovine cells: studies with recombination interferons produced in Escherichia coli. J. Biol. Chem. 1983;258:9046–9049. [PubMed] [Google Scholar]
- 39.Hu R., Gan Y., Liu J., Miller D., Zoon K.C. Evidence for multiple binding sites for several components of human lymphoblastoid interferon-α. J. Biol. Chem. 1993;268:12591–12595. [PubMed] [Google Scholar]
- 40.Houssiau F.A., Thanou A., Mazur M., Ramiterre E., Gomez Mora D.A., Misterska-Skora M. IFN-alpha kinoid in systemic lupus erythematosus: results from a phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis. 2020;79:347–355. doi: 10.1136/annrheumdis-2019-216379. [DOI] [PubMed] [Google Scholar]
- 41.Lauwerys B.R., Hachulla E., Spertini F., Lazaro E., Jorgensen C., Mariette X. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α–kinoid. Arthritis Rheum. 2013;65:447–456. doi: 10.1002/art.37785. [DOI] [PubMed] [Google Scholar]
- 42.Ducreux J., Houssiau A., Vandepapelière P., Jorgensen C., Lazaro E., Spertini F. Interferon α kinoid induces neutralizing anti-interferon α antibodies that decrease the expression of interferon-induced and B cell activation associated transcripts: analysis of extended follow-up data from the interferon α kinoid phase I/II study. Rheumatology. 2016;55:1901–1905. doi: 10.1093/rheumatology/kew262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S.F., Gong M.J., Zhao F.R., Shao J.J., Xie Y.L., Zhang Y.G. Type I interferons: distinct biological activities and current applications for viral infection. Cell. Physiol. Biochem. 2018;51:2377–2396. doi: 10.1159/000495897. [DOI] [PubMed] [Google Scholar]
- 44.Hertzog P.J., Williams B.R. Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 2013;24:217–225. doi: 10.1016/j.cytogfr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Marks Z.R.C., Campbell N., deWeerd N.A., Lim S.S., Gearing L.J., Bourke N.M. Properties and functions of the novel type I interferon epsilon. Semin. Immunol. 2019;43:101328. doi: 10.1016/j.smim.2019.101328. [DOI] [PubMed] [Google Scholar]
- 46.Rodero M.P., Decalf J., Bondet V., Hunt D., Rice G.I., Werneke S. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J. Exp. Med. 2017;214:1547–1555. doi: 10.1084/jem.20161451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tocut M., Shoenfeld Y., Zandman-Goddard G. Systemic lupus erythematosus: an expert insight into emerging therapy agents in preclinical and early clinical development. Expet Opin. Invest. Drugs. 2020;20:1151–1162. doi: 10.1080/13543784.2020.1807004. [DOI] [PubMed] [Google Scholar]
- 48.Lever E., Alves M.R., Isenberg D.A. Towards precision medicine in systemic lupus erythematosus. Pharmgenomics Pers. Med. 2020;4:39–49. doi: 10.2147/PGPM.S205079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noaiseh G., Baer A.N. Toward better outcomes in Sjogren’s syndrome: the promise of a stratified medicine approach. Best Pract. Res. Clin. Rheumatol. 2020;34:101475. doi: 10.1016/j.berh.2019.101475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.