Abstract
Background
Cognitive impairment is an age-dependent chronic disorder that exponentially worsens with age; however, its treatment is mostly symptomatic. Moxibustion is widely accepted in East Asia as a treatment for cognitive impairment. This systematic review aimed to verify the efficacy and underlying mechanism of moxibustion in treating cognitive impairment.
Methods
Sixteen trials involving 324 animals obtained from MEDLINE (PubMed), EMBASE, the Cochrane library, the Chinese National Knowledge Infrastructure, Wan-Fang, Cqvip, the Korean Studies Information Service System, and the Oriental Medicine Advanced Searching Integrated System met the inclusion criteria. We extracted the results of behavioral tests and immunohistochemical biomarkers from the included articles and evaluated the risk of bias and reporting quality.
Results
The moxibustion group showed significantly decreased escape latency, increased crossing times, and prolonged dwelling times in the Morris water maze test. There was a significantly enhanced latency period and reduced error time in the step-down test and nerve behavior score. The effects of moxibustion were found to be mediated by suppression of oxidative stress and apoptosis, modulation of inflammation and Aβ genesis activation of vascular endothelial growth factor, and adjustment of metabolites in the tricarboxylic acid cycle and fatty acid metabolism.
Conclusion
Our results demonstrated the therapeutic efficacy of moxibustion on cognitive impairment and suggested the putative mechanism. However, considering the small number of included studies, high bias risk, low reporting quality, and the limitations of animal experimentation, our results need to be confirmed by more detailed studies.
Keywords: Animal experimentation, Cognitive impairment, Dementia, Moxibustion, Systematic review
1. Introduction
Cognitive impairment is an age-dependent chronic disorder1; globally, the number of patients is expected to reach 82 million in 2030 and 152 million in 2050.2 Alzheimer disease (AD) is the most common type of cognitive impairment,3 accounting for 60–80% of all cases,2,4 which is characterized by the accumulation of Aβ peptides, senile plaques, and intracellular neurofibrillary tangles (NFT), related to neuronal damage and premature neuronal death.4 Vascular dementia (VD) is the second most common type of cognitive impairment, which is driven by complex factors reducing cerebral blood flow and causing oxidative stress, resulting in cerebral ischemia.5
Despite the growing need for proper management of cognitive impairment in aging societies, the available therapeutic options are still mostly symptomatic, providing short-term benefit for specific condition, with a risk of adverse drug reactions (ADRs).6
Moxibustion is a widely used therapy in Eastern Asia for more than 2500 years.7,8 Moxibustion entails stimulating acupoints on the body by burning herb leaves directly (attaching moxa cones to the skin) or indirectly (interposing a substance such as ginger between the moxa cones and the skin) to transmit heat stimulation8,9 and induce pharmacological action via herbal components.10,11
In addition to its wide range of use for pain relief12,13 and inflammation control,14,15 moxibustion has been recognized as a suitable treatment for cognitive impairment, with several recent studies indicating its efficacy in patients with dementia.16,17
Acupuncture, another representative traditional Chinese medicine treatment, has been reported to be effective in cognitive enhancement, with supporting evidence from several systematic reviews (SRs),18,19,20 suggesting that its potential mechanism involves suppression of oxidative stress and neuroinflammation and modulation of glucose metabolism5 and neuronal signaling pathways.21
However, compared with acupuncture studies, the therapeutic efficacy of moxibustion for cognitive impairment has not been fully validated. Although a previous study has reported the action of moxibustion in preventing cognitive impairment,22 information on the overall therapeutic effect of moxibustion was limited, as it was focused on prevention. This SR aimed to evaluate the efficacy of moxibustion in treating cognitive impairment through a meta-analysis of animal studies. We also examined the underlying mechanisms and the quality of the supporting evidence.
2. Methods
2.1. Study search and selection
We used search terms consisting of variants of “cognitive impairment” for the target disease, “moxibustion” for the intervention, and “animal study.” The following databases were searched: MEDLINE (PubMed), EMBASE, and the Cochrane library (English language); the Chinese National Knowledge Infrastructure, Wan-Fang, and Cqvip Database (Chinese language); the Korean Studies Information Service System and Oriental Medicine Advanced Searching Integrated System (Korean language). We searched studies published from the time of inception of each database to January 2019.
2.2. Inclusion and exclusion criteria
We included animal studies that conducted randomized controlled trials on moxibustion as an intervention for cognitive disorders. We did not discriminate between different types of cognitive impairment including AD and VD. The search terms we used for describing cognitive impairment are provided in Supplementary material. All rodent models developed for any type of cognitive impairment were included.
Although we included all types of moxibustion, we excluded studies that used combined treatments. Furthermore, in the control group, we only included studies that used cognitively impaired animals without any interventions. Regarding outcome measurements, we included results from behavioral tests, immunohistochemistry, and electron microscopic analyses.
Two reviewers (SMA and SC) independently selected and evaluated the studies and subsequently discussed for confirmation. Conflicts were resolved by consulting a third reviewer (UMJ) to reach a consensus. We used the eligible studies to extract data in a standardized form suitable for animal study design as follows: study design, research institute, methodological details, procedure compatibility (performing of randomization and blinding), and therapeutic characteristics of moxibustion. Regarding measurement outcomes, we defined the primary outcomes as behavioral test results and the secondary outcomes as immunohistochemical results. For studies where data was only graphically presented, we attempted to contact the corresponding author; if unsuccessful, we extracted the data by scaling the graph using the GetData graph digitizer version 2.26.0.20 (copyright 2001–2013; S. Fedorov). Details of the included studies are shown in Table 1.
Table 1.
Characteristics of the included studies in animal model and treatment.
| Study | Animal model |
Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Age (months) | Weight (g) | Sample size |
Target Disease | Acupoints | Moxa diameter (mm) | Duration (min) | Treatment period | ||
| Moxa (M, F) | Control (M, F) | |||||||||
| Du 26 | Wistar rat | 12 | 500 ± 20 | (10,0) | (10,0) | AD | GV20, BL23 | 6 | 15 | 14 times for 14 days |
| Liu 33 | ApoE(−/−) mice | 2 | 27 ∼ 29 | (11,0) | (11,0) | AD | CV8 | 20∼30 | 20 | 72 times for 78 days |
| Wang 27 | SD rat | 15 | 350 ∼450 | (10,0) | (10,0) | AD | GV20, BL23, ST36 | NR | 5 | 18 times for 21 days |
| Wang 28 | SD rat | 15 | 350 ∼450 | (10,0) | (10,0) | AD | GV20, BL23, ST36 | 8 | 5 | 18 times for 21 days |
| Wang 29 | SD rat | 15 | 350 ∼450 | (10,0) | (10,0) | AD | GV20, BL23, ST36 | NR | 5 | 18 times for 21 days |
| Jiang 31 | SD rat | 2 | 300 ± 30 | (11,0) | (10,0) | AD | GV20, GV4, GV1, CV4 | 10 | 3 or 7* | 10 times for 30 days |
| Wang 34 | Wistar rat | NR | 300 ± 30 | (12,0) | (12,0) | VD | GV20, GV24, GV14 | NR | 20 | 30 times for 31 days |
| Wang 37 | Wistar rat | 10 | 300 ± 30 | (12,0) | (12,0) | VD | GV20, GV24, GV14 | 2∼3 | 20 | 30 times for 31 days |
| Zhu 30 | SD rat | NR | NR | (12,0) | (12,0) | AD | GV20, GV16, GV14 | 20 | 20 | 15 times for 15 days |
| Wang 35 | SD rat | NR | 250 ± 10 | (7.0) | (7,0) | VD | GV20, GV24, GV14 | 5 | 20 | 30 times for 30 days |
| Weilan38 | Wistar rat | NR | 220 ± 20 | (7,0) | (7,0) | VD | GV20, GV16, ST36, GB20 | NR | 3 Zhuang | 24 times for 27 days |
| Luo 40 | Wistar rat | NR | 280 ± 20 | (10,0) | (10,0) | VD | CV6, CV12, CV17, SP10, TE5, ST36 | 5 | 3 or 4 Zhuang | 24 times for 27 days |
| Zhang 36 | Wistar rat | 10 | 250 ± 50 | (8,0) | (8,0) | VD | GV20, GV24, GV14 | 15 | 20 | 30 times for 31 days |
| Xiao 7 | SD rat | 2 | 350 ∼400 | (10,0) | (10,0) | AD | KI6, TE5, GB41, PC6, SP4, SI3, BL62, LU7 | NR | 9 Zhuang | 35 times for 35 days |
| Yu 32 | APP/PS1mice | 6 | NR | (12.0) | (12,0) | AD | CV4 | 20 | 15 | 48 times for 55 days |
| Zhu 39 | SD rat | NR | 200 ∼250 | (10,0) | (10,0) | VD | GV14, GV4, CV4 | NR | 15 | 24 times for 27 days |
Abbreviations: SDS, prague-Dawley; ApoE−/−, apolipoprotein E-deficient; APP/PS1, Amyloid precursor protein/presenilin-1 transgenic; M, male; F, female; AD, Alzheimer’s disease; DB, database; VD, vascular dementia; NR, not reported; NA, not applicable.
Jiang31 treated all participants using moxibustion for 3 min except GV20 which received moxibustion for 7 min.
2.3. Study assessment and analysis
We evaluated the overall potential bias in the included studies based on the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) study quality checklist,23 as suggested by CAMARADES24 for reporting animal data from experimental studies. The reporting quality of each study was evaluated according to the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines checklist.25
In the behavioral test, decreased escape latency in the Morris water maze (MWM) test suggests restored cognitive function, which is indicated by faster learning, maintaining a better score on average, and achieving a superior final score; meanwhile, increased cross and dwelling times from the target probe test suggest prolonged memory. Assessment of the step-down test results and neuronal behavior scores indicated a beneficial effect in extending the latency period and shortening error times.
The results of behavioral tests obtained by means and standard errors were synthesized, and meta-analyses were conducted using a random effect model, the RevMan version 5.3 (released on June 13, 2014, Cochrane Collaboration).
3. Results
3.1. Characteristics of included studies
3.1.1. Study screening
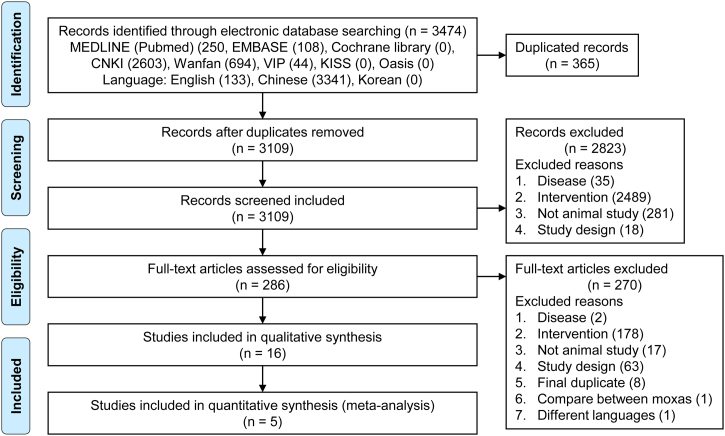
Based on our electronic search criteria, we retrieved 3474 articles. After removing duplicate studies, the titles and abstracts of 3109 studies were screened and 296 articles were selected for in-depth screening of the full text. Finally, 16 original articles met our inclusion criteria, of which five could be quantitatively synthesized. Among the 16 articles, two were dissertations not published in peer-review journals. A diagram of the study selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram for selecting related studies.
3.1.2. Fundamental study characteristics: animals
The 16 original research studies included in our analysis used a total of 324 rats and mice (age: 2–15 months; weight: 200–520 g [rats], 27–29 g [mice]). Nine studies established an AD model; among them, four studies injected Aβ (Aβ1–4226 or Aβ25–3527, 28, 29), three studies injected chemical toxin (d-galactose7,30 or streptozotocin31) into the hippocampus, and two studies established transgenic mice (amyloid precursor protein [APP]/presenilin 1 [PS1]), double-transgenic mice,32 or apolipoprotein E-deficient (ApoE−/−) mice.33 Seven studies established VD models by performing four-vessel occlusion,34, 35, 36 bilateral carotid artery occlusion,37, 38, 39 or autologous blood injection.40 The characteristics of the included studies are shown in Table 1.
3.1.3. Fundamental study characteristics: moxibustion
The most frequently used acupoint was GV20 (11 studies), followed by GV14 (6 studies), ST36 (5 studies), then GV24 and BL23 (4 studies; Supplementary material). The extra acupoints selected for treatment are described in Supplementary material. Indirect moxibustion was used in most of the studies, except for two studies where direct moxibustion was performed.38,40 Among those that used indirect moxibustion, eight burned the moxibustion about 2–3 cm above the surface of the acupoints, four placed a moxibustion cone on the skin and burned it, and two stimulated the acupoints by dropping ashes of thread soaked in herb medicine on the acupoints. The total treatment period ranged from 2 to 11 weeks, and the most frequently set time was 4 weeks (9 studies).
3.2. Quality assessment of the included studies
3.2.1. Risk of bias
Based on the checklist for evaluating the risk of bias, on average about 5.3 criteria were satisfied by the included studies (Table 2). All studies reported the implementation of randomization and possible conflicts of interest. However, no study reported the blinding process of allocation and only one study performed the blinded assessment of outcome.
Table 2.
Risk of bias in the included studies evaluated by the CAMARADES’ study quality checklist.
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | Total count of Y | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Du 26 | Y | Y | Y | N | N | Nn | Y | N | Y | Y | 6 |
| Liu 33 | Y | Y | Y | N | Y | Nn | Y | N | N | Y | 6 |
| Wang 27 | Y | Y | Y | N | N | Nn | Y | N | N | Y | 5 |
| Wang 28 | Y | Y | Y | N | N | Nk | Y | N | Y | Y | 6 |
| Wang 29 | Y | Y | Y | N | N | Nk | Y | N | Y | Y | 6 |
| Jiang31 | Y | N | Y | N | N | Nk | Y | N | N | Y | 4 |
| Wang 34 | Y | N | Y | N | N | Nk | Y | N | N | Y | 4 |
| Wang 37 | Y | Y | Y | N | N | Nk | Y | N | Y | Y | 6 |
| Zhu 30 | Y | Y | Y | N | N | Nn | Y | N | N | Y | 6 |
| Wang 35 | Y | Y | Y | N | N | Nn | Y | N | Y | Y | 6 |
| Weilan38 | Y | Y | Y | N | N | Nk | Y | N | Y | Y | 6 |
| Luo 40 | Y | Y | Y | N | N | Nk | Y | N | N | Y | 5 |
| Zhang 36 | Y | Y | Y | N | N | Nk | Y | N | N | Y | 5 |
| Xiao 7 | Y | Y | Y | N | N | Nn | Y | N | N | Y | 5 |
| Yu 32 | N | Y | Y | N | N | Nn | Y | N | N | Y | 4 |
| Zhu 39 | N | Y | Y | N | N | Nk | Y | N | Y | Y | 5 |
| Total count of Y | 14 | 14 | 16 | 0 | 1 | 0 | 16 | 0 | 7 | 16 |
(1) Publication in peer-reviewed journal (2) Statement of control of temperature (3) Randomization of treatment or control (4) Allocation concealment (5) Blinded assessment of outcome (6) Avoidance of anesthetics with marked intrinsic properties (7) Use of a suitable animal model (8) Sample size calculation (9) Statement of compliance with regulatory requirements (10) Statement regarding possible conflict of interest.
Y, Yes; N, No; Nn, Not necessary; Nk, Not known.
3.2.2. Reporting quality
Regarding the ARRIVE guidelines (Table 3), all studies fully presented four items, including the title, objectives, sample size per group, and funding. Regarding the experimental procedure, although most studies detailed “how it was conducted,” only four studies mentioned “why it was conducted.” Although all studies reported the sample size, the method and reference of sample calculation were not reported, and the assessment regarding the statistical approach was not properly disclosed.
Table 3.
Reporting quality assessment of the treatment studies based upon the ARRIVE guidelines.
| Study | ARRIVE Guideline |
|||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Introduction |
Methods |
Results |
Discussion |
|||||||||||||||||||||||||||||||||||
| Title | Abstract | Back ground |
Objec tives | Ethical statement | Study design |
Experimental procedure |
Experimental animals |
Housing/ husbandry |
Sample size |
Allocating animals to experimental groups |
Experimental outcomes | Statistical methods |
Baseline data | Numbers analyzed |
Outcomes and estimation | Adverse events |
Interpretation/scientific implications |
Generalizability / Translation | Funding | |||||||||||||||||||
| a | b | a | b | c | a | b | c | d | a | b | a | b | c | a | b | c | a | b | a | b | c | a | b | a | b | a | b | c | ||||||||||
| Du 26 | F | P | F | F | F | F | F | P | P | F | P | P | N | F | P | F | F | N | F | N | NA | P | N | F | P | F | N | P | F | NA | F | N | N | F | F | P | F | F |
| Liu 33 | F | P | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | N | F | N | NA | F | N | F | F | F | N | P | F | P | P | N | N | F | F | N | F | F |
| Wang 27 | F | P | P | P | F | N | F | F | P | F | P | F | N | F | F | P | P | N | F | N | NA | P | N | F | P | F | N | F | F | NA | F | N | N | F | N | N | P | F |
| Wang 28 | F | P | P | P | F | N | F | F | P | F | F | F | N | F | F | N | N | N | F | N | NA | P | N | F | P | F | N | P | F | NA | F | N | N | F | N | N | P | F |
| Wang 29 | F | P | P | P | F | F | F | P | P | F | F | F | F | F | F | P | P | N | F | N | NA | P | N | F | F | F | N | F | F | F | F | N | N | F | N | N | P | F |
| Jiang31 | F | P | P | P | F | F | F | F | P | F | F | F | F | F | F | F | P | P | F | N | NA | P | N | F | F | F | N | N | F | F | F | N | N | F | N | N | P | F |
| Wang 34 | F | P | F | P | F | N | F | P | F | F | F | F | P | F | F | F | P | P | F | N | NA | F | N | F | F | F | N | N | F | F | F | N | N | P | N | N | P | F |
| Wang 37 | F | P | F | P | F | N | F | F | F | F | F | F | N | F | F | F | P | P | F | N | NA | F | N | F | F | F | N | N | F | F | F | N | N | P | N | N | N | F |
| Zhu 30 | F | P | F | P | F | F | F | P | F | F | F | F | N | P | F | P | P | N | F | N | NA | F | N | F | F | F | N | P | F | F | F | N | N | F | N | N | N | F |
| Wang 35 | F | P | F | P | F | N | F | P | F | F | F | F | N | F | F | F | N | N | F | F | NA | F | N | F | NA | F | N | P | F | F | F | N | N | F | N | N | N | F |
| Weilan38 | F | P | F | P | F | F | F | P | F | F | F | F | N | F | F | F | F | P | F | N | NA | F | N | F | F | F | N | P | F | F | F | N | N | F | N | N | P | F |
| Luo 40 | F | P | F | P | F | N | F | F | F | F | F | F | N | F | F | F | F | P | F | N | NA | F | N | F | F | F | N | F | F | F | F | N | N | F | P | N | P | F |
| Zhang 36 | F | P | F | P | F | N | F | F | F | F | F | F | N | F | F | F | P | P | F | N | NA | F | N | F | F | F | N | F | F | F | F | N | N | F | P | N | P | F |
| Xiao 7 | F | P | F | P | F | N | F | P | F | F | F | F | N | F | F | P | F | P | F | N | NA | F | N | F | F | F | N | P | F | F | F | N | N | F | F | N | P | F |
| Yu 32 | F | F | F | P | F | N | F | F | F | F | F | F | N | F | F | P | F | N | F | N | NA | F | N | F | F | F | N | P | F | NA | F | N | N | F | F | N | P | F |
| Zhu 39 | F | F | F | P | F | F | F | F | F | F | F | F | N | F | F | P | F | N | F | N | NA | F | N | F | F | F | F | F | F | F | F | N | N | F | F | N | P | F |
Abbreviations: ARRIVE, Animal Research: Reporting In Vivo Experiments; F, fully reported; P, partially reported; N, not reported; NA, not applicable.
3.2.3. Data acquisition for analysis
Regarding the analysis of the behavioral test results, we excluded studies that reported duplicated data29 or omitted detailed values33 from the outcome analysis. Furthermore, studies reporting graph-shaped results33,35,41,42 were scaled by the GetData graph digitizer program, and the means and standard deviations were extracted.
3.3. Outcome analysis of the included studies
3.3.1. Results of primary outcome: behavioral experiments
Among the studies that performed behavioral tests, those with duplicated29 and insufficient data33 were excluded, whereas results presented in the form of graphs33,35,41,42 were converted to numerical values. Consequently, we obtained results from 11 behavioral tests and finally included five studies that performed the MWM test and two that performed the step-down and nerve behavioral tests for analysis.
3.2.2. Morris water maze test
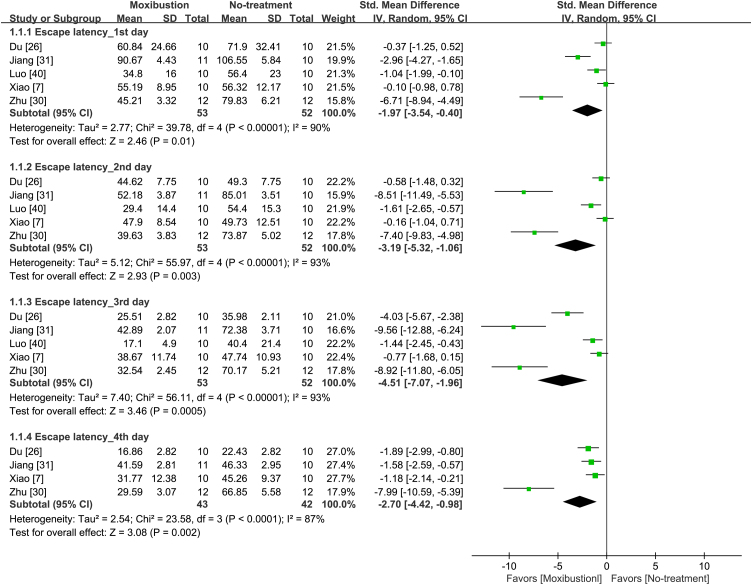
Among the nine studies that performed MWM tests, five26,30,31,36,40 that reported the individual results for MWM tests conducted for 4 days were included in the meta-analysis. Regarding escape latencies, the moxibustion group showed a significantly decreased escape latency compared to the control group after analysis of results measured from days 1 to 4 (Fig. 2). Additionally, from the 1st to the 3rd day, there was a decreasing tendency of the SMD of escape time (1st day: SMD: −1.97; 95% CI: −3.54, −0.40; p = 0.01, I2 = 90%; 2nd day: SMD: −3.19; 95% CI: −5.32, −1.06; p < 0.001, I2 = 93%; 3rd day: SMD: −4.51; 95% CI: −7.07, −1.96; p < 0.001, I2 = 93%). The greatest difference in the SMD between the moxibustion and control group was observed on the 3rd day.
Fig. 2.
Forest plot of escape latency in the Morris water maze test from the 1st to 4th day.
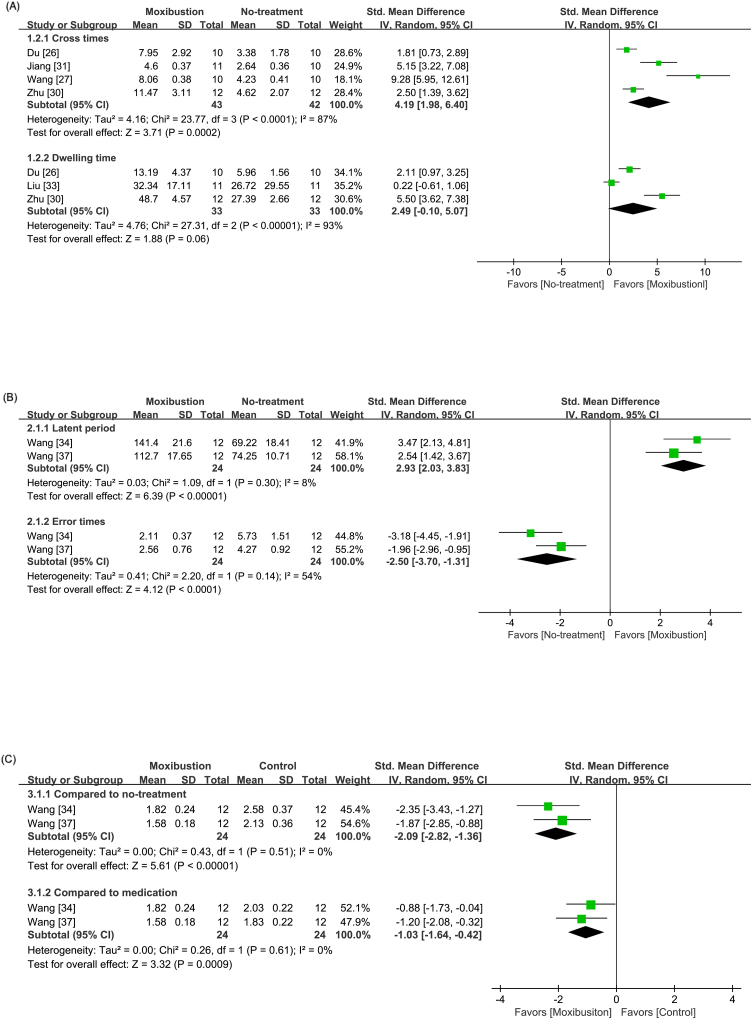
Regarding platform crossing times, the moxibustion group presented significantly increased crossing times compared to the control group (SMD: 4.19; 95% CI: 1.98, 6.40; p < 0.001; Fig. 3A). The dwelling time was reported in four different formats as follows: time spent in the quadrant,43,44,45 percentage of time dwelling,30,33,41,42 percentage of total distance traveled within the quadrant,46 and picture tracking of the animal’s motion.27,31 Meta-analysis indicated a significantly increased dwelling time in the quadrant in the moxibustion group compared with that in the control group (SMD: 2.49; 95% CI: −0.10, 5.07; p < 0.001); however, the heterogeneity remained high (I2 = 93%). Three studies,27,31,46 albeit not included in the meta-analysis, showed significantly decreased distance within the quadrant where animals in the moxibustion group traveled.
Fig. 3.
Forest plot of (A) cross times and dwelling time in the Morris water maze test (B) latency period and error times in the step-down test. (C) nerve behavior score.
3.2.3. Step-down test
Among the three studies that performed step-down tests, two studies34,37 reporting same methods were meta-analyzed and showed significantly extended latent periods (SMD: 2.93; 95% CI: 2.03, 3.83; p < 0.001; Fig. 3B (A)) and shortened error times (SMD: −2.50; 95% CI: −3.70, −1.31; p < 0.001; Fig. 3B (B)) in the moxibustion group.
In another study,33 the moxibustion group showed significantly prolonged latency and decreased error counts in both training and retention stages.
3.2.4. Nerve behavior score
Two of the studies34,37 that conducted step-down tests also determined nerve behavior scores. The results were scored by a five-point scale in reference to a previous study47 Consequently, we found significantly decreased nerve behavior scores in the moxibustion group compared with the control group (SMD: −2.09; 95% CI: −2.82, −1.36; p < 0.001; Fig. 3C).
3.2.5. Results of the secondary outcome: putative immunohistochemical biomarkers
Fourteen of the included studies investigated various immunohistochemical biomarkers for pathogenic characteristics (Table 4 and Fig. 4).
Table 4.
Outcomes evaluated in the included studies.
| Study | Outcome | Signal pathway |
|---|---|---|
| Behavioral test | ||
| Du 26 | 1. Morris water maze a) Escape latency b) Crossing times c) Dwelling time | Apoptosis rates ↓ |
| Liu 33 | 1. Morris water maze c) Dwelling time | GFAP ↓, Aβ ↓ |
| Wang 27 | 1. Morris water maze a) Escape latency b) Crossing times | Bcl-2 ↑, Bax ↓, Caspase-3 ↓ |
| Wang 28 | None | Aβ ↓ |
| Wang 29 | Duplicated with Wang 27 | Morphologic change |
| Jiang 31 | 1. Morris water maze b) Crossing times | IL-1β ↓, IL-2 ↑, Aβ ↓ |
| Wang 34 | 2. Step-down test a) Latent period b) Error times 3. Nerve behavior score | VEGF ↑, flt-1 ↑, flk-1mRNA ↑ |
| Wang 37 | 2. Step-down test a) Latent period b) Error times 3. Nerve behavior score | VEGF ↑, flt-1 ↑, bFGF ↑, bFGF-r ↑ |
| Zhu 30 | 1. Morris water maze a) Escape latency b) Crossing times c) Dwelling time | PS1 ↓, BACE-1 ↓, serum IL-6 ↓, Aβ ↓ |
| Wang 35 | None | morphologic change |
| Weilan 38 | NA | Bax ↓, Bcl-2 ↑, C-fos ↓ |
| Luo 40 | 1. Morris water maze a) Escape latency b) Crossing times | NA |
| Zhang 36 | NA | Vascular endothelial cell proliferation↑, migration↑ |
| Xiao 7 | 1. Morris water maze a) Escape latency | Bax ↓, Bcl-2 ↑, SOD2 ↑, MDA ↓, GSH-Px ↑ ChAT ↑, AChE ↓ |
| Yu 32 | NA | Metabolites of TCA ↑ Metabolites of fatty acid ↓ Mono / polyunsaturated fatty acids ↑ |
| Zhu 39 | 1. Morris water maze a) Escape latency | NF-κB (NF-κBp65, NFκBp50) ↓, TNF-α ↓, IL-1β ↓, morphologic change |
Abbreviations: TCA, tricarboxylic acid; FA, fatty acid; P-p38 MAPK, phospho-p38 mitogen-activated protein kinase; Aβ, β-amyloid; GFAP, glial fibrillary acidic protein; IL, interleukin; VEGF, vascular endothelial growth factor; Flt-1, fms-like tyrosine kinase 1; bFGF, basic fibroblast growth factor; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; SOD, superoxide dismutase; MDA, malondialdehyde; BACE-1, β-site APP cleaving enzyme 1; PS1, presenilin-1; APP, Amyloid precursor protein; GSH-Px, glutathione peroxidase; AChE, acetylcholinesterase; ChAT, choline acetyltransferase; TNF-α, tumor necrosis factor-α; NF- kB, nuclear factor-kappa.
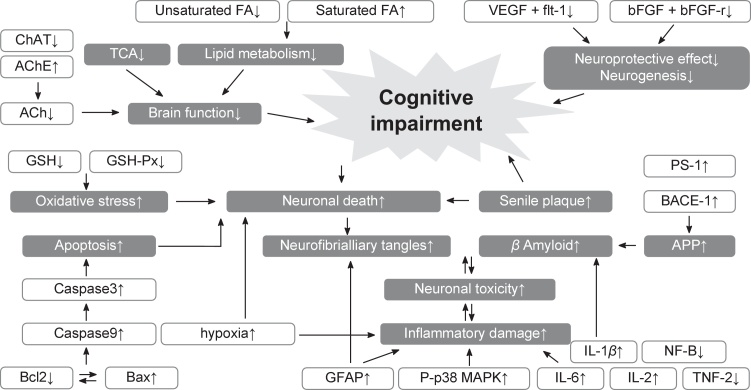
Fig. 4.
Putative mechanisms underlying cognitive impairment. The mechanisms identified in the figure represent those that could be modulated by moxibustion based on the reviewed studies.
Liu et al33 reported decreased levels of glial fibrillary acidic protein (GFAP), whereas three studies30,31,39 verified attenuated inflammatory damage after moxibustion treatment. Three studies7,27,38 demonstrated apoptosis-related factors that were controlled after moxibustion treatment; they also reported the upregulation of B-cell lymphoma 2 (Bcl-2) and downregulation of Bcl-2–associated X protein (Bax). The morphological changes of neuronal cells in the control group were compared with those of the moxibustion group,29,30 and recovery of morphological characteristics of neuronal cells was observed in the moxibustion group. Four studies28,30,31,33,41 investigated Aβ genesis and found it to be significantly restrained in the moxibustion group, with Zhu et al30 reporting decreased levels of PS1 and β-site APP cleaving enzyme 1 (BACE-1) in the moxibustion group, indicating the efficacy of moxibustion in regulating Aβ genesis.
Two studies34,37 found that moxibustion improved VEGF levels and related factors. Furthermore, Zhang et al36 postulated that proliferation and migration of vascular endothelial cells occurred in the moxibustion group. Yu32 also investigated attenuated metabolites of the TCA cycle and fatty acid metabolism after moxibustion.
4. Discussion
4.1. Summary of the main findings
The outcomes of this SR verified the efficacy of moxibustion in the treatment of cognitive impairment. Moxibustion treatment significantly improved the results of the behavioral test, the primary outcomes; it also showed efficacy against neurodegenerative disorders as shown through the outcomes of the immunohistochemical biomarkers, the secondary outcomes. These effects include attenuated acetylcholine (ACh) deficit and mitochondrial oxidative stress, attenuated inflammation and APP secretion, enhanced vascular endothelial growth factor (VEGF) activity and proliferation of vascular endothelial cells, and modulation of metabolites of the tricarboxylic acid (TCA) cycle and fatty acid metabolism.
4.2. Overall completeness and applicability of the evidence
4.2.1. Suppressing ACh deficits and mitochondrial oxidative stress
The accumulation of Aβ1–42 has been reported to ACh deficit48 related oxidative stress,49 which leads to metabolic malfunctions in the brain.50,51 Liu et al33 reported the upregulation of choline acetyltransferase (ChAT) and GSH-Px and downregulation of AChE in the moxibustion group, suggesting that moxibustion could treat cognition loss by attenuating ACh deficits and mitochondrial oxidative stress.
4.2.2. Controlling APP secretase and Aβ genesis
Since Aβ is generated from β-secretase, including BACE-1 or γ-secretase composed of PS1 or PS2,52,53 inhibiting γ- or β-secretase has been targeted for AD treatment.54 Zhu et al30 reported the downregulation of PS1 and BACE-1 after moxibustion treatment, indicating the effect of moxibustion in preventing Aβ genesis and other five studies also demonstrated reduced Aβ formation 26,28,30,33,55 by moxibustion treatment.
4.2.3. Attenuating apoptosis
Accumulated Aβ in AD and ischemia in VD are considered to induce massive neuronal apoptosis.56,57 In response to apoptotic signals, overexpressed Bax promotes cell death by antagonizing the Bcl-2 complex.58 Two studies7,27 indicated the apoptosis-suppressive effect of moxibustion by demonstrating modulated Bcl-2 and Bax in the moxibustion group. Further, Weilan et al38 reported the downregulation of C-fos protein and, two other studies26,29 demonstrated the mitigated apoptotic morphology of cells after moxibustion treatment.
4.2.4. Regulating inflammation
In response to neuronal toxicity, activated microglia and astrocytes lead to increased levels of GFAP,59,60 which is correlated with NFTs,61,62 cytokines such as interleukin (IL) and tumor necrosis factor-α (TNF-α),63 and pro-inflammatory proteins such as nuclear factor κB (NF-κb). IL-1β is genetically correlated with a high risk of AD,64 IL-6 is elevated shortly after ischemic events, while IL-2 reduces amyloid plaque load.65
Jiang et al31 proposed that moxibustion alleviated neuroinflammation based on findings of decreased IL-1β and increased IL-2 levels, which were consistent with the findings of Zhu et al,30 Liu et al,33 and Zhu et al39 regarding attenuated pro-inflammatory factors after moxibustion treatment.30,66
4.2.6. Modulating metabolites of TCA cycle and fatty acid metabolism
Deficits in mitochondrial enzymes of the TCA are related to clinical disability in AD67; meanwhile lipid metabolism malfunction can contribute to the pathogenesis68 of brain injuries and neuropsychiatric disorders.69 Yu32 observed that moxibustion increases the levels of metabolic products of the TCA cycle and fatty acid metabolism.
4.2.7. Activating the VEGF
In response to ischemia,70 the angiogenic factor VEGF induces neuroprotective effects.71 Therefore, decreased levels of VEGF cause chronic ischemia of neurons.71 Three studies demonstrated that moxibustion treatment ameliorated ischemia-driven memory loss by improving levels of VEGF and basic fibroblast growth factor and its receptor. In addition, Zhang et al36 reported the improved proliferation and migration of endothelial cells in moxibustion-treated VD rats (Fig. 4).
4.3. Potential biases in the review process
There are several limitations inducing potential biases in the review process. First, the low reporting quality reduced the credibility of the study results. Ambiguity in the model selection and outcome assessment presents a high risk of assessment bias, and insufficient reporting of excluded animals may indicate a high risk of reporting bias. Second, insufficient between-group baseline adjustments and differences in the therapeutic protocol could potentially distort the results, causing high heterogeneity between studies. Third, the obtained results were insufficient to allow generalized conclusions regarding every possible cognitive impairment condition. The small number of included studies and different reporting formats impeded the analysis of some studies, and the included animal models do not encompass all possible cognitive impairment cases.
4.4. Comparison to previous reviews and implications
Compared to our previous SR22 investigating the efficacy of moxibustion in preventing cognitive impairment, this study is more focused on the therapeutic effects of moxibustion. In both SRs, moxibustion treatment improved the behavioral test scores, and suppression of apoptosis and inflammation appeared to be the common mechanism induced by moxibustion. In preventive research, increased activity of neurotrophins, heat shock protein, and modulation of the cell cycle were demonstrated to be mediated by moxibustion; meanwhile, this study suggests modulation of metabolites and mitochondrial oxidative stress as the therapeutic mechanism of moxibustion.
4.5. Implication for clinical trials
In previous clinical studies, moxibustion treatment groups showed increased clinical scores accompanying attenuated metabolic factors including lower blood cholesterol levels,72 suppressed oxidative stress,17 and regulated balance between plasma thromboxane B2 and 6-keto-PG1α.73 These findings have a part in common with the results of the present animal SR analysis. However, there is limited direct connection. The animal model design is inherently limited in terms of interpretation and application of human pathology. Unclear understanding of the pathology of human cognitive impairment makes it difficult to implement the results from an animal model in the treatment of humans.74
Despite the limitations, animal research has played a pivotal role in understanding the biological mechanisms of cognitive diseases and evaluating the efficacy of the drugs.75 Although designing an ideal animal study and implementing a clinical study is challenging, interpreting research using various models and improved evaluation methods might be helpful in overcoming the limitations of biased analyses.
4.6. Conclusions
This SR showed that moxibustion might be beneficial in treating cognitive impairment. Its mechanism might encompass the suppression of oxidative stress and apoptosis, modulation of inflammation and Aβ genesis, enhancement of VEGF activity, and adjustment of metabolites of the TCA cycle and fatty acid metabolism. However, there were several limitations of this review, including the small number of included studies that lacked a common study design. Furthermore, the low reporting quality induced a high risk of bias and impeded the validation of the findings. More specific and rigorous trials with large sample sizes are needed to validate the efficacy of moxibustion for cognitive impairment and thoroughly examine the underlying mechanisms.
Author contributions
Sungmin Aum: Conceptualization, Formal analysis, Investigation, Writing - original draft. Seon Choe: Formal analysis, Investigation. Mudan Cai: Writing - review & editing. Ui Min Jerng: Writing - review & editing, Supervision. Jun-Hwan Lee: Writing - review & editing, Supervision.
Conflicts of interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or in the decision to publish the results.
Funding
This work was supported in part by the Korea Institute of Oriental Medicine (Grant No.KSN1621051).
Ethical statement
This research did not involve any human or animal experiment.
Data availability
The data will be made available upon reasonable request.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.imr.2020.100680.
Contributor Information
Ui Min Jerng, Email: breeze@sangji.ac.kr.
Jun-Hwan Lee, Email: omdjun@kiom.re.kr.
Supplementary material
The following are Supplementary data to this article:
References
- 1.Anekonda T.S., Reddy P.H. Can herbs provide a new generation of drugs for treating Alzheimer’s disease? Brain Res Brain Res Rev. 2005;50:361–376. doi: 10.1016/j.brainresrev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Mental health and older adults, <http://www.who.int/mediacentre/factsheets/fs381/en/>; Published 2016.
- 3.Yankner B.A. Mechanisms of neuronal degeneration in alzheimer’s disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 4.Abramov A.Y., Duchen M.R. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philosophical transactions of the Royal Society of London Series B. Prog Nucl Energy 6 Biol Sci. 2005;360:2309–2314. doi: 10.1098/rstb.2005.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Y., Zhu W., Wang X.R., Yang J.W., Xiao L.Y., Liu Y. Mechanisms of acupuncture on vascular dementia—A review of animal studies. Neurochem Int. 2017;107:204–210. doi: 10.1016/j.neuint.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Piersanti M., Capannolo M., Turchetti M., Serroni N., De Berardis D., Evangelista P. Increase in mortality rate in patients with dementia treated with atypical antipsychotics: A cohort study in outpatients in Central Italy. Riv Psichiatr. 2014;49:34–40. doi: 10.1708/1407.15623. [DOI] [PubMed] [Google Scholar]
- 7.Xiao M. Guangxi University of Traditional Chinese Medicine; 2018. Effect of expression and behavior of eight methods of Ling gui acupuncture moxibustion on AD on rat hippocampal Bax and Bcl-2 learning [Master degree] [Google Scholar]
- 8.Shen X., Ding G., Wei J., Zhao L., Zhou Y., Deng H. An infrared radiation study of the biophysical characteristics of traditional moxibustion. Complement Ther Med. 2006;14:213–219. doi: 10.1016/j.ctim.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Xu X., Cao L., Mwandalima C.J., Wang Z., Liu L., Sun Z. Protocol for systematic review and meta-analysis: Moxibustion for treating ankylosing spondylitis. Eur J Integr Med. 2017;12:142–146. [Google Scholar]
- 10.Kawakita K., Shinbara H., Imai K., Fukuda F., Yano T., Kuriyama K. How do acupuncture and moxibustion act? - focusing on the progress in Japanese acupuncture research. J Pharmacol Sci. 2006;100:443–459. doi: 10.1254/jphs.crj06004x. [DOI] [PubMed] [Google Scholar]
- 11.Okada K., Kawakita K. Analgesic Action of Acupuncture and Moxibustion: A Review of Unique Approaches in Japan. 2007;6:11-17. [DOI] [PMC free article] [PubMed]
- 12.Ewies A., Olah K. Moxibustion in breech version—a descriptive review. Acupunct Med. 2002;20:26–29. doi: 10.1136/aim.20.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Neri I., Airola G., Contu G., Allais G., Facchinetti F., Benedetto C. Acupuncture plus moxibustion to resolve breech presentation: A randomized controlled study. J Matern Fetal Neonatal Med. 2004;15:247–252. doi: 10.1080/14767050410001668644. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Wu X.H. Study on the influence of metformin on castration-resistant prostate cancer PC-3 cell line biological behavior by its inhibition on PLCepsilon gene-mediated Notch1/Hes and androgen receptor signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:1918–1923. [PubMed] [Google Scholar]
- 15.Tu J.F., Yang J.W., Lin L.L., Wang T.Q., Du Y.Z., Liu Z.S. Efficacy of electro-acupuncture and manual acupuncture versus sham acupuncture for knee osteoarthritis: Study protocol for a randomised controlled trial. Trials. 2019;20:79. doi: 10.1186/s13063-018-3138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H., Wang P., Yang J., Liu G. Impacts of moxibustion on vascular dementia and neuropeptide substance content in cerebral spinal fluid. Zhongguo Zhen jiu = Chinese Acupuncture & Moxibustion. 2011;31(1):19–22. [PubMed] [Google Scholar]
- 17.Ganghui L. Effects of combination of acupuncture and moxibustion with chinese drugs on lipid peroxide and antioxidase in patients of vascular dementia. World J Acupunct Moxibustion. 1998 [Google Scholar]
- 18.Shao S., Tang Y., Guo Y., Tian Z., Xiang D., Wu J. Effects of acupuncture on patients with Alzheimer’s disease: Protocol for a systematic review and meta-analysis. Medicine. 2019;98 doi: 10.1097/MD.0000000000014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon C.Y., Lee B., Suh H.W., Chung S.Y., Kim J.W. Efficacy and safety of auricular acupuncture for cognitive impairment and dementia: a systematic review. Evid Based Complement Altern Med. 2018;2018 doi: 10.1155/2018/3426078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung C.Y., Wu X., Chung V.C., Tang E.C., Wu J.C., Lau A.Y. Overview of systematic reviews with meta-analyses on acupuncture in post-stroke cognitive impairment and depression management. Integr Med Res. 2019;8:145–159. doi: 10.1016/j.imr.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung M.C., Yip K.K., Ho Y.S., Siu F.K., Li W.C., Garner B. Mechanisms underlying the effect of acupuncture on cognitive improvement: A systematic review of animal studies. J Neuroimmune Pharmacol. 2014;9:492–507. doi: 10.1007/s11481-014-9550-4. [DOI] [PubMed] [Google Scholar]
- 22.Choe S., Cai M., Jerng U.M., Lee J.H. The efficacy and underlying mechanism of moxibustion in preventing cognitive impairment: A systematic review of animal studies. Exp Neurobiol. 2018;27:1–15. doi: 10.5607/en.2018.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macleod M., O’Collins T., Howells D., Donnan G. Pooling of animal experimental data reveals influence of study design and publication Bias. Stroke; J Cereb Circ. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 24.CAMARADES . 2014. Reporting of animal data from experimental studies. Published. [Google Scholar]
- 25.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Y., Liu R., Sun G., Meng P., Song J. Pre-moxibustion and moxibustion prevent Alzheimer’s disease. Neural Regen Res. 2013;8:2811–2819. doi: 10.3969/j.issn.1673-5374.2013.30.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S., Sun G., Ma J., Li X., Wan B. Effects of moxibustion on behaviors and Bcl-2, Bax, Caspase-3 in Hippocampus of alzheimer’s disease model rats. World Sci Technol?Modern Tradit Chin Med Materia Medica. 2015;17:1243–1248. [Google Scholar]
- 28.Wang S., Ma J., Sun G., Shen F., Li X., Wan B. Effect of moxibustion on morphology of Hippocampus in alzheimer’ s disease model rats. Chin J Gerontol. 2015;36:4993–4995. [Google Scholar]
- 29.Wang S., Sun G., Ma J., Li X., Wan B. Effects of moxibustion on behaviors and hippocampal ultrastructure of alzheimer’s disease model rats. Chin J Inform TCM. 2015;22:58–61. [Google Scholar]
- 30.Zhu C., Jian J., Han W., Yang J. Effect of moxibusiton on learning-memory ability and hippocampal Amyloid β protein overexpression in Mild Cognitive Impairment rats. Acupunct Res. 2016;41:131–137. [PubMed] [Google Scholar]
- 31.Jiang M., Liang J., Wang J. Effect of Moxibustion on the Learning and Memory of Rat Models of Alzheimer’s Disease and the Expression of Hippocampal Aβ, IL-1β and IL-2. Shanghai J Acu-mox. 2016;35:870–875. [Google Scholar]
- 32.Yu M. Beijing University of Chinese Medicine; 2017. Metabolomics study of urine and plasma targets in APP/PS1 double transgenic AD mice by moxibustion and Aiwu [master’s degree] [Google Scholar]
- 33.Liu J., Zhao B., Cui Y., Huang Y., Huang C., Huang J. Effects of shenque moxibustion on behavioral changes and brain oxidative state in apolipoprotein e-deficient mice. Evid Based Complement Altern Med. 2015;2015 doi: 10.1155/2015/804804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P.Y., Jun Z., Qing P. Moxibustion head acupoints on vascular dementia rats affection of VEGF and its receptor Flt-1, Flk-1mRNA expression. J Emerg Tradit Chin Med. 2013 [Google Scholar]
- 35.Wang M., Qing P., Wu S., Zhang F., Ma Z., Zhang Y. Effect of Huayu Tongluo Moxibustion on the Ultrastructure of the Frontal of SVD Rats. J Clin Acupunct Moxibust. 2015;31:59–61. [Google Scholar]
- 36.Zhang M, Yang J, Wang P, Wang P, editors. The Effect of Stasis-eliminating and Meridian-unblocking Moxibustion on Vascular Endothelial Cell Proliferation and Migration in VD Rats. 2017 World Acupuncture Academic Conference and 2017 China Acupuncture Association Annual Meeting; 2017; Beijing China.
- 37.Wang P., Tang J.Y., Yang J. Effects of moxibustion on the expressions of hippocampal VEGF, flt-1, bFGF, and bFGF-r in vascular dementia rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:97–101. [PubMed] [Google Scholar]
- 38.Weilan Q., Yan Y., Xuemei L., Wei S., Xiaohan L., Qingke M. Effect of medicated thread moxibustion on apoptosis of hippocampal neurons in rat models of chronic cerebral ischemic vascular dementia. Cell Mol Biol (Noisy-le-grand) 2018;64:107–112. [PubMed] [Google Scholar]
- 39.Zhu W., Yang K., Cai S., Wang Y., Song X. Effect of warming yang and supplementing kidney moxibustion therapy on the NF-κB signaling pathway of vascular dementia rats. World J Acupunct - Moxibustion. 2018;28:44–49. [Google Scholar]
- 40.Luo B, Guo Y, Peng M, Zhang Y, Wang Y, Qi B. The effect of “Yiqi Tiaoxue, Fuben Peiyuan” medicine moxibustion on spatial memory of rats with multiple infarct dementia. 2018;38:2718-20.
- 41.Yu J, Jia M, Gao L, Zhang, Yong S, Bao Y. Effects of grain-sized moxibustion on learning and memory ability and amyloid deposition of transgenic Alzheimer's desease mice. Acupunct Research 2014;39. [PubMed]
- 42.Bao Y., Zhang Y., Chu J. Effects of grain-sized moxibustion on expression of Aβ1-42 in prefrontal cortex and hippocampus in double-transgenic AD mice. Chinese Acupuncture & Moxibustion. 2015;35(1):59–65. [PubMed] [Google Scholar]
- 43.Liu R. Hubei university of Chinese medicine: Hubei university of Chinese medicine; 2012. The effect of preventive moxibustion on expression of 14-3-3 protein and apoptosis of hippocampal neurons in rats of Alzheimer’s disease. [Google Scholar]
- 44.Kim M.H., Seo J.Y., Han M.K., Kang H.R., Kim S.S., Kim B.R. Chronic administration of Artemisia annua L. Attenuates oxidative stress induced by D-galactose in mice. FASEB J. 2014;28 [Google Scholar]
- 45.Li J. Hubei University of Chinese Medicine: Hubei University of Chinese Medicine; 2011. The experimental study of the expression of Pre-stimulation with moxibustion on CDK5, P27kip1 in rats of Alzheimer’s disease. [Google Scholar]
- 46.Li X. Hubei University of Chinese Medicine: Hubei University of Chinese Medicine; 2012. Research on the action mechanism of p38MAPK signal pathway in treating the rats with Alzheimer disease by preventative moxibustion. [Google Scholar]
- 47.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 48.Khan M.B., Khan M.B., Khan A., Ahmed M.E., Ishrat T., Tabassum R. Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem Int. 2012;61:1081–1093. doi: 10.1016/j.neuint.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 49.Martin J.B. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 50.Ejaz Ahmed M., Khan M.M., Javed H., Vaibhav K., Khan A., Tabassum R. Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem Int. 2013;62:492–501. doi: 10.1016/j.neuint.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Neha, Sodhi R.K., Jaggi A.S., Singh N. Animal models of dementia and cognitive dysfunction. Life Sci. 2014;109:73–86. doi: 10.1016/j.lfs.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 52.Mirra S.S., Hart M.N., Terry R.D. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–144. [PubMed] [Google Scholar]
- 53.O’Brien R.J., Wong P.C. Amyloid precursor protein processing and alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heneka M.T., Carson M.J., Khoury J.E., Landreth G.E., Brosseron F., Feinstein D.L. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wever K.E., Menting T.P., Rovers M., van der Vliet J.A., Rongen G.A., Masereeuw R. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X., Wu J., Yu C., Tang Y., Liu J., Chen H. Lychee seed saponins improve cognitive function and prevent neuronal injury via inhibiting neuronal apoptosis in a rat model of alzheimer’s disease. Nutrients. 2017;9 doi: 10.3390/nu9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimohama S. Apoptosis in Alzheimer’s disease—an update. Apoptosis. 2000;5:9–16. doi: 10.1023/a:1009625323388. [DOI] [PubMed] [Google Scholar]
- 58.Oltvai Z.N., Milliman C.L., Korsmeyer S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 59.Kato S., Gondo T., Hoshii Y., Takahashi M., Yamada M., Ishihara T. Confocal observation of senile plaques in Alzheimer’s disease: Senile plaque morphology and relationship between senile plaques and astrocytes. Pathol Int. 1998;48:332–340. doi: 10.1111/j.1440-1827.1998.tb03915.x. [DOI] [PubMed] [Google Scholar]
- 60.Pekny M., Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 61.Vehmas A.K., Kawas C.H., Stewart W.F., Troncoso J.C. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24:321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 62.Kamphuis W., Middeldorp J., Kooijman L., Sluijs J.A., Kooi E.-J., Moeton M. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol Aging. 2014;35:492–510. doi: 10.1016/j.neurobiolaging.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 63.Wang W.Y., Tan M.S., Yu J.T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swardfager W., Lanctot K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Alves S., Churlaud G., Audrain M., Michaelsen-Preusse K., Fol R., Souchet B. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain. 2017;140:826–842. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H., Zhou S., Song X., Xu W., Li L., Su S. Regulating effect of moxibustion on MCP-1 and NF-κB in the colonic mucosa tissue of rats with Crohn’s disease. World J Acupunct - Moxibustion. 2016;26:33–40. [Google Scholar]
- 67.Bubber P., Haroutunian V., Fisch G., Blass J.P., Gibson G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 68.Wong M.W., Braidy N., Poljak A., Pickford R., Thambisetty M., Sachdev P.S. Dysregulation of lipids in Alzheimer’s disease and their role as potential biomarkers. Alzheimer Demen. 2017;13:810–827. doi: 10.1016/j.jalz.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Hussain G., Anwar H., Rasul A., Imran A., Qasim M., Zafar S. Lipids as biomarkers of brain disorders. Crit Rev Food Sci Nutr. 2019:1–24. doi: 10.1080/10408398.2018.1529653. [DOI] [PubMed] [Google Scholar]
- 70.Svensson B., Peters M., Konig H.G., Poppe M., Levkau B., Rothermundt M. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- 71.Storkebaum E., Carmeliet P. VEGF: A critical player in neurodegeneration. J Clin Invest. 2004;113:14–18. doi: 10.1172/JCI200420682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang P., Yang J., Liu G., Chen H., Yang F. Effects of moxibustion at head-points on levels of somatostatin and arginine vasopressin from cerebrospinal fluid in patients with vascular dementia: a randomized controlled trial. Chin J Tradit Chin Med Phar. 2010;8:636–640. doi: 10.3736/jcim20100706. [DOI] [PubMed] [Google Scholar]
- 73.Liang Y. Effect of acupuncture-moxibustion plus chinese medicinal herbs on plasma TXB2, 6-Keto-PGF1α in patients with vascular dementia. World J Acupunct Moxibustion. 1998;23(2):17–19. [Google Scholar]
- 74.Götz J., Bodea L.G., Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19:583–598. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- 75.Goodarzi P., Payab M., Alavi-Moghadam S., Larijani B., Rahim F., Bana N. Development and validation of alzheimer’s disease animal model for the purpose of regenerative medicine. Cell Tissue Bank. 2019;20:141–151. doi: 10.1007/s10561-019-09773-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon reasonable request.