Abstract
Objective
Biomarkers are needed to characterize heterogeneity within populations at risk for type 1 diabetes. The ratio of proinsulin to C-peptide (PI:C ratio), has been proposed as a biomarker of beta cell dysfunction and is associated with progression to type 1 diabetes. However, relationships between PI:C ratios and autoantibody type and number have not been examined. We sought to characterize PI:C ratios in multiple islet autoantibody positive, single autoantibody positive and autoantibody negative relatives of individuals with type 1 diabetes.
Methods
We measured PI:C ratios and autoantibodies with both electrochemiluminescence (ECL) assays (ECL-IAA, ECL-GADA and ECL-IA2A) and radiobinding (RBA) assays (mIAA, GADA, IA2A and ZnT8A) in 98 relatives of individuals with type 1 diabetes followed in the TrialNet Pathway to Prevention Study at the Barbara Davis Center for a mean of 7.4 ± 4.1 years. Of these subjects, eight progressed to T1D, 31 were multiple autoantibody (Ab) positive, 37 were single Ab positive and 22 were Ab negative (by RBA).
Results
In cross-sectional analyses, there were no significant differences in PI:C ratios between type 1 diabetes and/or multiple Ab positive subjects (4.16 ± 4.06) compared to single Ab positive subjects (4.08 ± 4.34) and negative Ab subjects (3.72 ± 3.78) (p = 0.92) overall or after adjusting for age, sex and BMI. Higher PI:C ratios were associated with mIAA titers (p = 0.03) and showed an association with ECL-IA2A titers (p = 0.09), but not with ECL-IAA, GADA, ECL-GADA, IA2A nor ZnT8A titers. In mixed-effects longitudinal models, the trajectories of PI:C ratio over time were significantly different between the Ab negative and multiple Ab positive/type 1 diabetes groups, after adjusting for sex, age, and BMI (p = 0.04).
Conclusions
PI:C ratio trajectories increase over time in subjects who have multiple Ab or develop type 1 diabetes and may be a helpful biomarker to further characterize and stratify risk of progression to type 1 diabetes over time.
Highlights
-
•
Higher PI:C ratios are associated with mIAA titers.
-
•
Trajectories of PI:C increase overtime in multiple antibody positive subjects.
-
•
PI:C could be a useful biomarker to trend in those at risk for type 1 diabetes.
1. Introduction
Type 1 diabetes is classically thought to be an autoimmune disease due to infiltration of CD8+ T cells directed against insulin producing beta cells. It is characterized by the development of islet autoantibodies that can be present for years before onset of clinical type 1 diabetes [1]. While the presence of autoantibodies identifies individuals at risk of developing type 1 diabetes [[2], [3], [4]], progression to clinical diabetes is variable and is influenced by different factors such as type of autoantibody, titer and age at seroconversion [5]. Post-mortem studies in patients with type 1 diabetes have shown that the degree of immune cell infiltration in islets, and the composition of insulitis, can be variable among patients [6]. Histologically distinct endotypes of islet infiltration and proinsulin levels correlate with age at diagnosis [7]. There are four major islet autoantigens associated with type 1 diabetes. Insulin autoantibodies (mIAA) are typically the first autoantibody to be detected in young children [8] and correlate with age of diabetes onset [9]. Glutamic acid decarboxylase-65 autoantibodies (GADA) are antibodies against GABA-ergic neurons and pancreatic beta cells and are often the first autoantibody detected in older children [10,11]. Tyrosine phosphatase islet antigen-2 autoantibodies (IA-2A) are a major autoantigen in type 1 diabetes and an enzymatically inactive member of the tyrosine phosphatase family that regulates insulin secretion [12]. Zinc transporter 8 autoantibodies (ZnT8A) [4] can be seen in 60–80% of patients with new onset type 1 diabetes. Both IA-2A and ZnT8A tend to appear as a secondary autoantibody and IA2A has been shown to be associated with progression to diabetes [13,14].
Relatives of individuals are at an increased risk of developing type 1 diabetes [15] and are eligible for autoantibody screening through the TrialNet Pathway to Prevention study. TrialNet is an international consortium of investigators focused on studying the etiology of type 1 diabetes and preventing or reversing progression of disease [16,17]. Subjects are screened by radiobinding assays (RBA) for type 1 diabetes related autoantibodies. Recently, there has been emerging work in further refining these autoantibodies assays to improve prediction for progression to type 1 diabetes. Electrochemiluminescence (ECL) assays have been shown to be more disease specific and sensitive compared to RBA [[18], [19], [20]].
Given this heterogeneity within disease, additional biomarkers are needed to further characterize type 1 diabetes for personalized treatments and prevention trials. Markers of beta cell dysfunction in type 1 diabetes have been explored as a way to further characterize and predict those who may go on to develop type 1 diabetes. Proinsulin to C-peptide (PI:C) ratios have emerged as a promising biomarker to characterize progression to type 1 diabetes [21]. Elevations in PI:C ratios preceded the diagnosis of type 1 diabetes especially in younger children [22] and aberrant proinsulin processing correlates with islet infiltration [7]. However, PI:C ratio trajectories over time as well as relationships between PI:C ratios and autoantibody titers have not been examined.
We sought to characterize PI:C ratios in multiple islet autoantibody positive, single autoantibody positive and autoantibody negative relatives of individuals with type 1 diabetes in a cohort of patients from the TrialNet Pathway to Prevention Study followed at the Barbara Davis Center for Diabetes.
2. Research design and methods
2.1. Study participants
Nondiabetic relatives of patients with type 1 diabetes were recruited to the TrialNet Pathway to Prevention Study (ClinicalTrials.gov identifier: NCT00097292), as previously described [16]. All TrialNet subjects followed in the Pathway to Prevention study at the Barbara Davis Center for Diabetes with available ECL and proinsulin/C-peptide data were included in this study (N = 98). All study participants gave informed consent, and the study was approved by the local Ethics Committee. In the TrialNet Pathway to Prevention Study, subjects who are initially autoantibody negative are annually retested for islet autoantibodies until age 18, while autoantibody positive subjects are monitored every 6–12 months with RBA testing, HbA1c and OGTTs. In this study, participants were classified as autoantibody negative, single antibody positive and multiple antibody positive according to their RBA status longitudinally over time. ECL assays are not routinely measured in the TrialNet Pathway to Prevention Study. For this study, all participants had ECL assays measured at baseline as we included all TrialNet subjects followed in the Pathway to Prevention study at the Barbara Davis Center for Diabetes with available ECL and proinsulin/C-peptide data (N = 98). Since ECL assays are not measured longitudinally, we analyzed antibody titers for both RBA and ECL at baseline. Participants were classified as autoantibody negative (N = 22), single antibody positive if they were confirmed single antibody positive (N = 37) and multiple antibody positive if they were ever multiple antibody positive (N = 31); 8 subjects progressed to type 1 diabetes. Participants who were single antibody positive but not confirmed on repeat testing, i.e. transient single antibody positive, were included in the autoantibody negative group. Individuals with single confirmed autoantibody positive or multiple islet autoantibodies were offered baseline assessment of oral glucose tolerance test (OGTT), and were monitored with autoantibody testing, HbA1c and OGTT at 6- or 12-month intervals depending on estimated risk [16]. In the TrialNet Pathway to Prevention Study, siblings who are initially autoantibody negative are annually retested for islet autoantibodies until age 18, while autoantibody positive subjects are monitored every 6–12 months. Type 1 diabetes was diagnosed according to the TrialNet definitions of development of diabetes which include the American Diabetes Association criteria [23] as well as the following criteria if subjects are not unequivocally symptomatic: two diabetic OGTTs, not on the same day; diabetic OGTT and FPG ≥ 126 mg/dl, not on the same day; diabetic OGTT and HbA1c ≥ 6.5% (these may be on the same day); FPG ≥ 126 mg/dl and HbA1c ≥ 6.5% (these may be on the same day). Participants were followed for a mean ± SD of 7.4 ± 4.1 years.
2.2. Biochemical testing
All participants were screened for GADA, IA-2A, mIAA and ZnT8A measured by radioimmunoassay (RBA) in the TrialNet Core Laboratory at the Barbara Davis Center for Childhood Diabetes, Aurora, Colorado, as previously described [2,3]. In the 2020 Islet Autoantibody Standardization Program Workshop, sensitivities and specificities for the RBA were 62% and 99% respectively for mIAA, 78% and 99% respectively for GADA, 72% and 100% respectively for IA-2A, and 74% and 100% respectively for ZnT8A. Electrochemiluminescence assays (ECL) were performed for ECL-IAA, ECL-GADA, and ECL-IA2A [19,24]. In the 2020 Islet Autoantibody Standardization Program Workshop, sensitivities and specificities for ECL were 66% and 99% respectively for IAA, 78% and 100% respectively for GADA, and 72% and 100% respectively for IA-2A.
We measured pro-insulin level using an RBA (HPI-15 K; Millipore) [22]. This assay detects 100% human intact proinsulin, 95% human Des (31,32) proinsulin, and <0.1% human Des (64,65) proinsulin. Cross-reactivity for both human C-peptide and human insulin are <0.1% and has been extensively validated [25]. C-peptide values were measure using the Tosoh two-site immunoenzymometric assay (Tosoh Bioscience).
2.3. Statistical analysis
Statistical analyses were performed using PRISM (GraphPad Software, Inc., La Jolla, CA) and SAS version 9.4 (SAS Institute, Cary, NC, USA). ECL and RBA autoantibody titers were converted to SD units away from threshold (z-scores) for analyses. Interestingly, of the 8 subjects who progressed to type 1 diabetes, 5 were single confirmed autoantibody positive and 3 were multiple autoantibody positive prior to diagnosis. Due to the small number of individuals diagnosed with type 1 diabetes in this cohort, multiple autoantibody positive subjects and those who developed type 1 diabetes were combined in this analysis. In cross-sectional analyses, linear models were used to compare PI:C ratio by autoantibody status. PI:C ratios were analyzed over time using mixed effects longitudinal models while adjusting for age, sex and BMI. Of note, age and BMI were positively correlated (r2 = 0.51, p < 0.0001). Pearson’s correlation analyses were used to relate the RBA and ECL antibody titers to PI:C ratio. Results were considered statistically significant with p-value < 0.05.
3. Results
Subjects were classified as either autoantibody negative (n = 22), single confirmed autoantibody positive (n = 37) or multiple autoantibody positive/diagnosed with type 1 diabetes (n = 39) based on RBA testing. Baseline characteristics are shown in Table 1. The multiple autoantibody and type 1 diabetes cohort were younger (22.6 ± 13.0 years) than the single autoantibody positive subjects (31.4 ± 13.1 years) and autoantibody negative subjects (28.1 ± 14.6 years) (p = 0.02). There was no statistically significant difference for sex or ethnicity between the populations. Those with multiple autoantibodies and/or type 1 diabetes had a higher hemoglobin A1c (5.3 ± 0.4%) compared to the autoantibody negative and single autoantibody positive subjects (5.0 ± 0.4%) (p = 0.007). The presence of the high-risk haplotype DR4 was more common in those with multiple autoantibodies and/or type 1 diabetes (26/39, 67%) compared to the single autoantibody positive subjects (21/37, 57%) and the autoantibody negative subjects (6/22, 29%) (p = 0.02). There was no statistically significant difference in the presence of the high-risk HLA haplotype DR3 between the groups.
Table 1.
Characteristics of subjects.
| All (n = 98) | Negative Ab (n = 22) | Single Ab Positive (n = 37) |
Multiple Positive and type 1 diabetes (n = 39) |
P Value | |
|---|---|---|---|---|---|
| Age at screening (years) | 27.2 ± 13.8 | 28.1 ± 14.6 | 31.4 ± 13.1 | 22.6 ± 13 | 0.02 |
| Sex (Female) | 56 (57%) | 9 (41%) | 26 (70%) | 21 (54%) | 0.08 |
| Race NHW Hispanic Other |
80 (82%) 16 (16%) 2 (2%) |
20 (91%) 2 (9%) 0 (0)% |
30 (81%) 7 (19%) 0 (0%) |
30 (77%) 7 (18%) 2 (5%) |
0.49 |
| BMI (kg/m2) | 25.3 ± 6.0 | 26 ± 6.1 | 26.7 ± 6.1 | 23.6 ± 5.7 | 0.10 |
| DR3 Present | 35 (36%) | 5 (24%) | 15 (41%) | 15 (38%) | 0.41 |
| DR4 Present | 53 (55%) | 6 (29%) | 21 (57%) | 26 (67%) | 0.02 |
| DR 3/4 | 13 (13%) | 1 (5%) | 5 (14%) | 7 (18%) | 0.39 |
| HbA1c % | 5.1 ± 0.4 | 5.0 ± 0.4 | 5.0 ± 0.4 | 5.3 ± 0.4 | 0.007 |
| Follow up time (years) | 7.4 ± 4.1 | 9.5 ± 3.7 | 6.2 ± 4.0 | 7.3 ± 4.0 | 0.01 |
Ab: islet autoantibody.
BMI: body mass index.
NHW: Non-Hispanic White.
P values by ANOVA, chi-square test or Fisher’s exact test were used to compare negative ab, single ab and multiple positive/type 1 diabetes cohorts.
There was no statistical difference in cross sectional PI:C ratios analyses by autoantibody status, both overall or after adjusting for age, sex and BMI. Subjects with negative autoantibodies had a PI:C ratio of 3.72 ± 3.78, those with single autoantibodies had a PI:C ratio of 4.08 ± 4.34 and those with multiple autoantibodies and/or type 1 diabetes had a PI:C ratio of 4.16 ± 4.06 (p = 0.92).
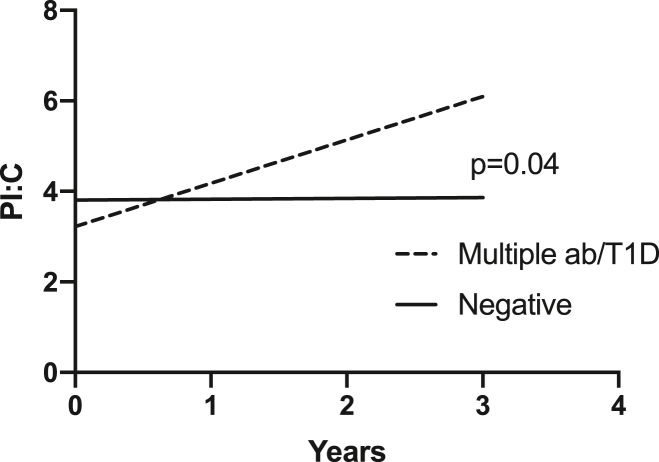
Mixed-effects longitudinal models were adjusted for sex, age and BMI. The trajectories of PI:C ratio over time were significantly different between the multiple autoantibody positive and/or type 1 diabetes subjects compared to the subjects who were autoantibody negative (p = 0.04) (Fig. 1).
Fig. 1.
Trajectories of PI:C ratio over time.
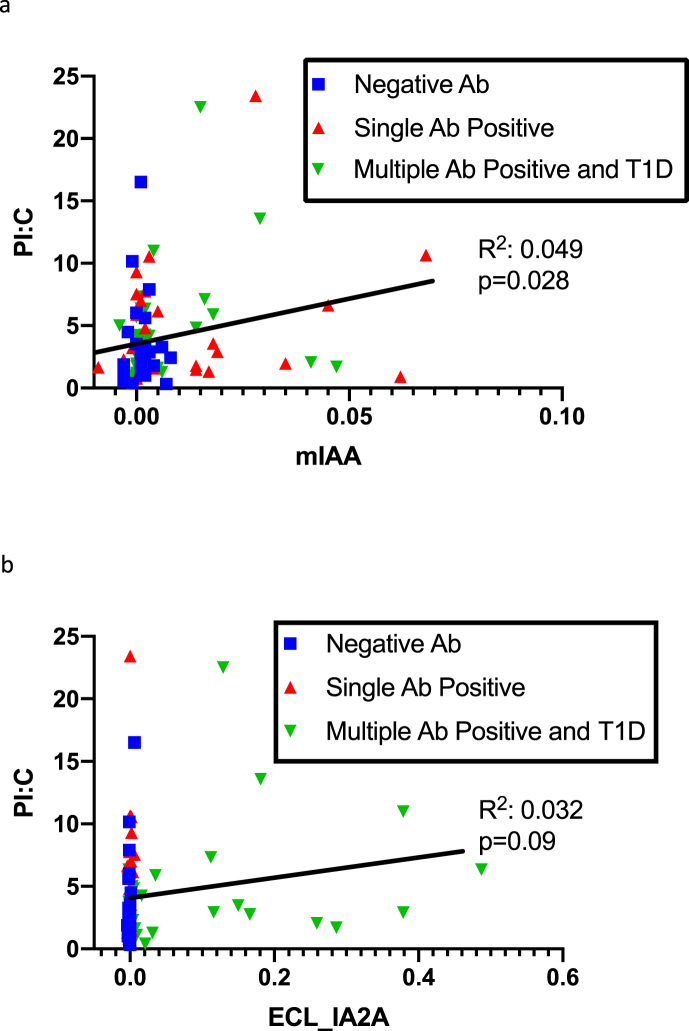
Pearson’s correlation analyses were used to relate the titers of autoantibodies with PI:C ratios (Table 2). Higher PI:C ratios were correlated with higher mIAA titers (R2 = 0.049, p = 0.028) and showed a borderline association with ECL-IA2A titers (R2 = 0.032, p = 0.09) (Fig. 2). All other autoantibody titers by RBA or ECL did not correlate with PI:C ratio levels.
Table 2.
Correlation of PI:C ratios with Autoantibody Titers.
| Correlation | P-Value | |
|---|---|---|
| GADA | 0.17 | 0.10 |
| IA2A | 0.11 | 0.26 |
| mIAA | 0.22 | 0.03 |
| ZnT8A | 0.003 | 1.0 |
| ECL_GADA | 0.03 | 0.80 |
| ECL_IA2A | 0.18 | 0.09 |
| ECL_IAA | 0.04 | 0.70 |
ECL: electrochemiluminescence.
Fig. 2.
(a)Correlation of mIAA to PI:C. (b): Correlation of ECL- IA2A to PI:C.
4. Conclusions/discussion
In this study of 98 relatives of individuals with type 1 diabetes followed longitudinally over time for a mean of 7.4 years, PI:C ratio trajectories increase in subjects who have multiple autoantibodies and/or develop type 1 diabetes compared to those who are autoantibody negative. In addition, this is the first study to show that higher PI:C ratios were associated with mIAA and ECL-IA2A titers. PI:C ratios and especially their trajectories over time might be a helpful biomarker to further stratify risk of progression to type 1 diabetes and select potential subjects for prevention trials.
Type 1 diabetes is a complex, multifactorial disease and current data suggests the concept of disease endotypes rather than considering type 1 diabetes as a single disease [26]. Distinct diabetes endotypes correlate with age at diagnosis [27], autoantibody type [28], genetic risk [29] and potential environmental exposures [30]. Further characterization of endotypes will help guide intervention and prevention trials as well as personalized clinical management. While diagnosis of type 1 diabetes has clear clinical criteria once patients present with symptoms and hyperglycemia, the pre-clinical period is variable. Even in genetically at-risk relatives there is a considerable amount of heterogeneity in those who progress to type 1 diabetes and in the rate of progression to clinical disease. Consistent with the findings in this study, higher mIAA and IA-2A titers were associated with rate of progression to diabetes in antibody positive subjects followed in The Environmental Determinants of Diabetes in the Young study, after adjusted for first-degree relative status, number of autoantibodies, age at first persistent confirmed autoantibodies, and HLA genotypes [31]. This may be clinically relevant to further understand those at the greatest risk for progressing to type 1 diabetes and to characterize individuals who may benefit from intervention and/or prevention studies. Further characterizing and stratifying risk of progression is needed and PI:C ratios and ECL assays are emerging biomarkers that may aid in our understanding of beta cell dysfunction and progression to clinical diabetes.
Beta cell stress can be evident when insulin demand exceeds the beta cells’ capacity to secrete insulin. However, timing of beta cell decline within the first year of diagnosis is highly variable [32]. In this setting of progressive beta cell death, processing of proinsulin is disrupted and can be a marker of cell dysfunction. Abnormalities in processing insulin can be seen in those with longstanding diabetes [33]. Cross-sectional elevated PI:C ratios were associated with progression to type 1 diabetes in autoantibody positive relatives 1 year before diagnosis [22] and PI:C ratios can be significantly elevated at diagnosis of type 1 diabetes and persist into the honeymoon period [34,35]. Markers of beta cell stress such as PI:C ratios trajectories over time could be used to further characterize heterogeneity within disease and offer potential personalized clinical management of patients at early stages of type 1 diabetes.
Limitations of this study include the small sample size as PI:C ratios and ECL assays have only been measured in a subset of subjects followed in TrialNet. Strengths of this study include a well characterized cohort of relatives of individuals with type 1 diabetes followed longitudinally over a mean period of 7.4 years.
To our knowledge this is the first study to analyze PI:C ratio trajectories over time in subjects at risk for type 1 diabetes. Monitoring of PI:C ratios longitudinally may be a helpful biomarker to further characterize and stratify risk of progression to type 1 diabetes. While PI:C ratios were correlated with mIAA and ECL-IA2A titers, larger studies are needed to elucidate potential causal relationships between PI:C ratios and autoantibody titers.
Author contribution
Author Contributions: Dr. Taylor M. Triolo is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. T.M.T. researched data, wrote manuscript. L.P researched data, reviewed/edited manuscript. S.S. researched data, reviewed/edited manuscript. K.S. researched data, reviewed/edited manuscript. L.Y researched data, reviewed/edited manuscript. P.A.G. contributed to discussion, reviewed/edited manuscript. C.E-M. contributed to discussion, reviewed/edited manuscript. A.K.S. designed the study, contributed to discussion, reviewed/edited manuscript. We would like to thank the subjects and their families. The authors have no conflicts of interest to disclose.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by NIH grants R21 DK119800 and R01 DK093954, UC4 DK104166, U01DK127786 (to C.E. M) U.S. Department of Veterans Affairs Merit Award I01BX001733 (to C.E.-M.), and gifts from the Sigma Beta Sorority, the Ball Brothers Foundation, and the George and Frances Ball Foundation (to C.E.-M.). The authors acknowledge the support of the Indiana Diabetes Research Center Translation Core (P30 DK097512). T.M.T is funded by the NIDDK K12 training grant (K12DK094712). The contents of this Article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38(6):989–996. doi: 10.2337/dc15-0101. Epub 2015/05/23, PubMed PMID: 25998291. [DOI] [PubMed] [Google Scholar]
- 2.Yu L., Rewers M., Gianani R., Kawasaki E., Zhang Y., Verge C. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J. Clin. Endocrinol. Metab. 1996;81(12):4264–4267. doi: 10.1210/jcem.81.12.8954025. Epub 1996/12/01, PubMed PMID: 8954025. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacio E., Yu L., Williams A.K., Eisenbarth G.S., Bingley P.J., Marcovina S.M. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J. Clin. Endocrinol. Metab. 2010;95(7):3360–3367. doi: 10.1210/jc.2010-0293. Epub 2010/05/07, PubMed PMID: 20444913; PubMed Central PMCID: PMCPMC2928900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenzlau J.M., Juhl K., Yu L., Moua O., Sarkar S.A., Gottlieb P. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2007;104(43):17040–17045. doi: 10.1073/pnas.0705894104. Epub 2007/10/19, PubMed PMID: 17942684; PubMed Central PMCID: PMCPMC2040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler A.G., Rewers M., Simell O., Simell T., Lempainen J., Steck A. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. J. Am. Med. Assoc. 2013;309(23):2473–2479. doi: 10.1001/jama.2013.6285. Epub 2013/06/20, PubMed PMID: 23780460; PubMed Central PMCID: PMCPMC4878912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell-Thompson M., Fu A., Kaddis J.S., Wasserfall C., Schatz D.A., Pugliese A. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. doi: 10.2337/db15-0779. Epub 2015/11/20, PubMed PMID: 26581594; PubMed Central PMCID: PMCPMC4764143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leete P., Oram R.A., McDonald T.J., Shields B.M., Ziller C., Roep B.O. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia. 2020;63(6):1258–1267. doi: 10.1007/s00125-020-05115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimpimäki T., Kupila A., Hämäläinen A.M., Kukko M., Kulmala P., Savola K. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J. Clin. Endocrinol. Metab. 2001;86(10):4782–4788. doi: 10.1210/jcem.86.10.7907. Epub 2001/10/16, PubMed PMID: 11600541. [DOI] [PubMed] [Google Scholar]
- 9.Steck A.K., Johnson K., Barriga K.J., Miao D., Yu L., Hutton J.C. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34(6):1397–1399. doi: 10.2337/dc10-2088. Epub 2011/05/13, PubMed PMID: 21562325; PubMed Central PMCID: PMCPMC3114355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson M.A., Kaufman D.L., Newman D., Tobin A.J., Maclaren N.K. Islet cell cytoplasmic autoantibody reactivity to glutamate decarboxylase in insulin-dependent diabetes. J. Clin. Invest. 1993;91(1):350–356. doi: 10.1172/jci116192. Epub 1993/01/01, PubMed PMID: 8423231; PubMed Central PMCID: PMCPMC330033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krischer J.P., Lynch K.F., Schatz D.A., Ilonen J., Lernmark Å., Hagopian W.A. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–987. doi: 10.1007/s00125-015-3514-y. Epub 2015/02/11, PubMed PMID: 25660258; PubMed Central PMCID: PMCPMC4393776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan M.S., Lu J., Goto Y., Notkins A.L. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13(5):505–514. doi: 10.1089/dna.1994.13.505. Epub 1994/05/01, PubMed PMID: 8024693. [DOI] [PubMed] [Google Scholar]
- 13.Verge C.F., Gianani R., Kawasaki E., Yu L., Pietropaolo M., Jackson R.A. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45(7):926–933. doi: 10.2337/diab.45.7.926. Epub 1996/07/01, PubMed PMID: 8666144. [DOI] [PubMed] [Google Scholar]
- 14.Steck A.K., Vehik K., Bonifacio E., Lernmark A., Ziegler A.G., Hagopian W.A. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: the environmental Determinants of diabetes in the young (TEDDY) Diabetes Care. 2015;38(5):808–813. doi: 10.2337/dc14-2426. Epub 2015/02/11, PubMed PMID: 25665818; PubMed Central PMCID: PMCPMC4407751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orban T., Sosenko J.M., Cuthbertson D., Krischer J.P., Skyler J.S., Jackson R. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32(12):2269–2274. doi: 10.2337/dc09-0934. Epub 2009/09/11, PubMed PMID: 19741189; PubMed Central PMCID: PMCPMC2782989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahon J.L., Sosenko J.M., Rafkin-Mervis L., Krause-Steinrauf H., Lachin J.M., Thompson C. The TrialNet natural history study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr. Diabetes. 2009;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. Epub 2008/10/01, PubMed PMID: 18823409. [DOI] [PubMed] [Google Scholar]
- 17.Skyler J.S., Greenbaum C.J., Lachin J.M., Leschek E., Rafkin-Mervis L., Savage P. Type 1 Diabetes TrialNet--an international collaborative clinical trials network. Ann. N. Y. Acad. Sci. 2008;1150:14–24. doi: 10.1196/annals.1447.054. Epub 2009/01/06, PubMed PMID: 19120262; PubMed Central PMCID: PMCPMC2918900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L., Dong F., Miao D., Fouts A.R., Wenzlau J.M., Steck A.K. Proinsulin/Insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care. 2013;36(8):2266–2270. doi: 10.2337/dc12-2245. Epub 2013/02/21, PubMed PMID: 23423694; PubMed Central PMCID: PMCPMC3714529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao D., Guyer K.M., Dong F., Jiang L., Steck A.K., Rewers M. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 2013;62(12):4174–4178. doi: 10.2337/db13-0534. Epub 2013/08/27, PubMed PMID: 23974918; PubMed Central PMCID: PMCPMC3837058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao P., Liu T., Li X., Ning L., Yin J., Han K. Highly sensitive, label-free colorimetric assay of trypsin using silver nanoparticles. Biosens. Bioelectron. 2013;49:20–24. doi: 10.1016/j.bios.2013.04.038. Epub 2013/05/28, PubMed PMID: 23708813. [DOI] [PubMed] [Google Scholar]
- 21.Loopstra-Masters R.C., Haffner S.M., Lorenzo C., Wagenknecht L.E., Hanley A.J. Proinsulin-to-C-peptide ratio versus proinsulin-to-insulin ratio in the prediction of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetologia. 2011;54(12):3047–3054. doi: 10.1007/s00125-011-2322-2. Epub 2011/10/01, PubMed PMID: 21959959. [DOI] [PubMed] [Google Scholar]
- 22.Sims E.K., Chaudhry Z., Watkins R., Syed F., Blum J., Ouyang F. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016;39(9):1519–1526. doi: 10.2337/dc15-2849. Epub 2016/07/08, PubMed PMID: 27385327; PubMed Central PMCID: PMCPMC5001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes A. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. doi: 10.2337/dc17-S005. Epub 2016/12/17, PubMed PMID: 27979889. [DOI] [PubMed] [Google Scholar]
- 24.Yu L., Miao D., Scrimgeour L., Johnson K., Rewers M., Eisenbarth G.S. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 2012;61(1):179–186. doi: 10.2337/db11-0670. Epub 2011/11/30, PubMed PMID: 22124462; PubMed Central PMCID: PMCPMC3237666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims E.K., Bahnson H.T., Nyalwidhe J., Haataja L., Davis A.K., Speake C. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. 2019;42(2):258–264. doi: 10.2337/dc17-2625. Epub 2018/12/12, PubMed PMID: 30530850; PubMed Central PMCID: PMCPMC6341288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglia M., Ahmed S., Anderson M.S., Atkinson M.A., Becker D., Bingley P.J. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5–12. doi: 10.2337/dc19-0880. Epub 2019/11/23, PubMed PMID: 31753960; PubMed Central PMCID: PMCPMC6925574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leete P., Oram R.A., McDonald T.J., Shields B.M., Ziller C., Ts team. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia. 2020;63(6):1258–1267. doi: 10.1007/s00125-020-05115-6. Epub 2020/03/17, PubMed PMID: 32172310; PubMed Central PMCID: PMCPMC7228905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krischer J.P., Lynch K.F., Schatz D.A., Ilonen J., Lernmark A., Hagopian W.A. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–987. doi: 10.1007/s00125-015-3514-y. Epub 2015/02/11, PubMed PMID: 25660258; PubMed Central PMCID: PMCPMC4393776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redondo M.J., Geyer S., Steck A.K., Sharp S., Wentworth J.M., Weedon M.N. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care. 2018;41(9):1887–1894. doi: 10.2337/dc18-0087. Epub 2018/07/14, PubMed PMID: 30002199; PubMed Central PMCID: PMCPMC6105323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rewers M., Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–2348. doi: 10.1016/S0140-6736(16)30507-4. 10035, Epub 2016/06/16, PubMed PMID: 27302273; PubMed Central PMCID: PMCPMC5571740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steck A.K., Vehik K., Bonifacio E., Lernmark A., Ziegler A.G., Hagopian W.A. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: the environmental Determinants of diabetes in the young (TEDDY) Diabetes Care. 2015;38(5):808–813. doi: 10.2337/dc14-2426. Epub 2015/02/11, PubMed PMID: 25665818; PubMed Central PMCID: PMCPMC4407751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao W., Gitelman S., DiMeglio L.A., Boulware D., Greenbaum C.J. Type 1 diabetes TrialNet study G. Fall in C-peptide during first 4 Years from diagnosis of type 1 diabetes: variable relation to age, HbA1c, and insulin dose. Diabetes Care. 2016;39(10):1664–1670. doi: 10.2337/dc16-0360. Epub 2016/07/17, PubMed PMID: 27422577; PubMed Central PMCID: PMCPMC5033079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sims E.K., Syed F., Nyalwidhe J., Bahnson H.T., Haataja L., Speake C. Abnormalities in proinsulin processing in islets from individuals with longstanding T1D. Transl. Res. 2019;213:90–99. doi: 10.1016/j.trsl.2019.08.001. Epub 2019/08/24, PubMed PMID: 31442418; PubMed Central PMCID: PMCPMC6783367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snorgaard O., Hartling S.G., Binder C. Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33(1):36–42. doi: 10.1007/BF00586459. Epub 1990/01/01, PubMed PMID: 2303172. [DOI] [PubMed] [Google Scholar]
- 35.Watkins R.A., Evans-Molina C., Terrell J.K., Day K.H., Guindon L., Restrepo I.A. Proinsulin and heat shock protein 90 as biomarkers of beta-cell stress in the early period after onset of type 1 diabetes. Transl. Res. 2016;168:96–106 e1. doi: 10.1016/j.trsl.2015.08.010. Epub 2015/09/24, PubMed PMID: 26397425; PubMed Central PMCID: PMCPMC4839287. [DOI] [PMC free article] [PubMed] [Google Scholar]