Abstract
Objective
To compare the real-world effectiveness and costs of eribulin to those of capecitabine in patients with metastatic breast cancer (MBC) pretreated with anthracyclines and taxanes.
Methods
This study extracted data from the Health and Welfare Database in Taiwan to identify MBC patients, and then eribulin and capecitabine users were matched at a 1:1 ratio by age, residential region, Charlson Comorbidity Index score, and molecular subtype of BC cell. The overall survival (OS) and time-to-treatment discontinuation (TTD) curves were plotted using the Kaplan–Meier method. Healthcare utilization and costs between the two groups were compared.
Results
A total of 24,550 MBC patients were identified, and 298 patients were enrolled in each group after matching. The median OS was 11.8 months for eribulin (95%CI: 11.5–13.5 months) and 15.2 months for capecitabine (95%CI: 15.3–17.9 months; HR = 1.7, p < 0.0001). The median TTD was 4.0 months for eribulin and 6.6 months for capecitabine (HR = 1.6; p < 0.0001). No significant difference was found between the two groups in patients with >4 prior chemotherapy agents (OS: HR 1.1, 95%CI 0.8–1.5; TTD: HR 1.2, 95%CI 0.9–1.7). The total healthcare costs per patient during the treatment period were NT$580,523.8 for eribulin versus NT$497,223.8 for capecitabine (p < 0.0001), and total medication costs were NT$438,335.8 and NT$348,438.4 (p < 0.0001), respectively.
Conclusion
Although eribulin showed an attenuated effect in the real-world setting in Taiwan, it may serve as an alternative for capecitabine in a heavy pretreated population. The total healthcare and medication costs were found to be higher with eribulin treatment.
Keywords: Real-world study, Claim database analysis, Metastatic breast cancer, Eribulin
Highlights
-
•
This study provides a better understanding of the effectiveness of eribulin in refractory MBC patients.
-
•
Despite the potential biases, our study results suggest that eribulin has shorter OS and TTD when compared to capecitabine.
-
•
The difference no longer exists when the drugs are both initiated as sixth- or later-lines of chemotherapy.
-
•
The rate of G-CSF use and the total medical and medication costs were found to be higher with eribulin treatment.
1. Introduction
Breast cancer (BC) is the most frequently diagnosed cancer, and the second most common cause of cancer-related death [1]. Despite considerable progress and advances over the years, metastatic breast cancer (MBC) remains treatable but incurable, with a 5-year survival rate greater than 25% [2,3]. There is no standard of care for patients pretreated with and resistant to anthracyclines and taxanes. Eribulin, capecitabine, and vinorelbine are the alternatives after patients become refractory to anthracyclines and taxanes. Additional options include gemcitabine, platinum agents, and liposomal anthracyclines [4,5].
Eribulin (Eribulin mesylate, Halaven®, E7389), a nontaxane microtubule dynamics inhibitor of antineoplastic drugs [6], has been proven to be an effective new chemotherapeutic agent based on the pivotal phase III randomized study EMBRACE [7]. Although another phase III trial, Study 301, failed to demonstrate the superiority of eribulin over capecitabine in terms of overall survival (OS) or progression-free survival (PFS) [8], a post hoc analysis reported a significant OS benefit for eribulin among MBC patients with human epidermal growth factor receptor 2 (HER2) negative tumors [9]. Since the U.S. Food and Drug Administration’s (FDA) approval of eribulin in November 2010, real-world evidence of eribulin has been investigated in various countries and among different ethnicities [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. However, there has been a paucity of real-world studies that included comparative agents in their study design. In addition to effectiveness analysis, cost study is also crucial, particularly for chemotherapeutic agents as they can lead to surging drug costs and toxicity, factors that increase overall costs of treatment and hinder the use of innovative medication [21]. At present, there is little published data regarding the costs associated with eribulin in treating MBC patients; an exception is a cost analysis in France that assessed the one-year health care costs of a monocentric MBC cohort [14]. The total per-patient (PP) costs were estimated at €18,694, and it was found that eribulin and its associated administration costs contributed to 79% of PP costs.
In December 2014, Taiwan FDA approved eribulin for the treatment of refractory MBC patients who have previously received an anthracycline and a taxane. A real-world study of eribulin in Taiwan appeared to show a 1-year survival rate similar to that in the EMBRACE trial [17]. However, it was not conducted nationwide, no comparative agent was included, and OS was not examined. Also, there has been no study assessing the cost of eribulin treatment in Taiwan. Since capecitabine has been commonly used as a salvage or maintenance therapy for MBC after anthracyclines or taxanes in Taiwan, and it has been used as a comparison to eribulin in previous studies, this study aimed to compare the clinical outcomes and healthcare costs of eribulin to those of capecitabine in MBC patients and to provide more insight into real-world clinical practice in Taiwan.
2. Methods
2.1. Data source
Data were extracted from the Health and Welfare Data (HWD) Science Center, Ministry of Health and Welfare, a large data repository site that preserves, manages, and analyzes health data. This study examined health data in the National Health Insurance Research Database (NHIRD), the National Cancer Registry (NCRD), and the Cause of Death Registry Database, which were all included in the HWD. The databases can be interlinked with each other using keys and encrypted personal identification numbers. The NHIRD comprises the claims data of national health insurance beneficiaries, which encompass 99.6% of the 23 million residents in Taiwan. Patient-level information such as demographic characteristics, diagnoses, medical assessments, procedures and treatments, and costs of treatments were recorded in the databases. The accuracy of NHIRD data input is ensured by a peer-review committee with a disciplinary system. The NCRD accounts for the registration of 90% of all cancers in Taiwan and preserves clinical data associated with all newly diagnosed malignant neoplasms, including date of initial diagnosis, primary cancer site, American Joint Committee on Cancer (AJCC) staging, and clinical and pathological tumor, node, metastasis staging. Data is recorded and standardized into a report by trained cancer registrars and passes through a computerized logic check to conserve fidelity and quality. The Cause of Death Registry Database preserves all causes of death (encoded by ICD-10) in Taiwan by collecting death certificates transferred from the household registration system. Patient survival and the date of expiration in the study population were confirmed by examining this database. These three national health insurance-related databases collect patients’ information until they die. As such, no patients were lost to follow up.
2.2. Study design and patients
This retrospective observational real-world study was approved by the Taipei Medical University-Joint Institutional Review Board (approval number: N201709052). Patients were enrolled from January 1, 2015 to December 31, 2016 (i.e., enrollment period), then followed until any cause of death occurred or December 31, 2017, the date of administrative censoring (i.e., follow-up period). The inclusion criteria were females aged 20 years or older, diagnosed with MBC, and who had received at least one anthracycline and one taxane. During the screening process, MBC patients were identified in both the NHIRD and the NCRD. In the NHIRD, MBC patients were selected by a diagnosis of BC (ICD-9-CM: code 174. XX or ICD-10-CM: C.50. xxx) in either an inpatient or outpatient claim along with a prescription record of chemotherapy such as vinorelbine, capecitabine, gemcitabine, and eribulin. In the NCRD, however, newly diagnosed patients were identified with a histologically or cytologically confirmed diagnosis of BC (ICD-O-3: C50. xxx) at AJCC stage IV. The MBC patients identified in the two databases between 2015 and 2016 were combined into the preliminary study cohort for further selection.
Eribulin and capecitabine users were identified based on the chemotherapeutic agent that the patient used after the treatment failure of both anthracyclines and taxanes. The index date was defined as the first date with a prescription of eribulin or capecitabine during the enrollment period. Data were traced back to January 1, 2010 and patients who were not taking eribulin or capecitabine as a single agent and those with prior use of the comparative chemotherapy agent were excluded.
2.3. Outcome measures
The primary outcome of this study is OS, defined as the time from the index date to any cause of death or last follow-up. The secondary outcomes were: (1) time to treatment discontinuation (TTD); (2) rate of use of granulocyte-colony stimulating factor (G-CSF); and (3) healthcare utilization and costs. TTD was defined as the time from the index date until the date of treatment discontinuation for any reason, including progressive disease, treatment toxicity, and death. TTD was also referred to as the treatment period in this study. The rate of G-CSF use during the treatment period was measured to elicit the rate of severe neutropenia. Healthcare utilization under assessment included the number of outpatient oncology visits, inpatient admissions, and emergency room (ER) visits during the treatment period, while the calculation of healthcare costs included costs incurred for inpatient services, outpatient services, ER visits, and medications during the treatment period.
2.4. Statistical analysis and matching
Patients’ characteristics were summarized using descriptive statistics (means or median and standard deviations [SD]) and compared between groups using the Student’s t-test for continuous data while categorical variables were summarized by frequencies and compared between groups using the Chi-square test. The OS and TTD curves were plotted using the Kaplan–Meier method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model. Healthcare costs and utilization were summarized using descriptive statistics and measured per-patient (PP) and per-patient-per-month (PPPM) during the treatment period. Healthcare utilization and various types of costs between the groups were compared using the Mann-Whitney U test. In the NHIRD, medical expenditures data were coded in point values due to the implementation of the global budget system. For ease of calculation, the points were converted to monetary values by assigning NT $1 for each point.
To control for confounding factors and minimize differences between eribulin and capecitabine users, patients in the two groups were matched at a 1:1 ratio based on age within ± one year, Charlson Comorbidity Index (CCI) score [22] within ± one point, residential area, and molecular subtype of BC cell. The matched patients (i.e., the eribulin group and the capecitabine group) were included in the subsequent analysis of this study.
Subgroup analysis was performed to investigate the outcomes of interest between eribulin and capecitabine while controlling for the line of chemotherapy treatment. First, the number of previous chemotherapy agents was calculated for identified eribulin and capecitabine users, and the two groups were matched at a 1:1 ratio based on age within ± one year, CCI score within ± one point, residential area, molecular subtype of BC cell, and early or late therapeutic line depending on whether the patients had received ≤4 previous chemotherapies or more than four (i.e., fifth- or earlier-line vs. sixth- or later-line). The variables used for matching were selected according to the EMBRACE trial and previous real-world studies [7,14,17,23]. The matching of groups was done using a macro that performs a greedy match algorithm with SAS® software, as defined elsewhere [24].
3. Results
3.1. Patients’ characteristics
From Jan 1, 2015 to Dec 31, 2016, 24,550 MBC patients were identified in the NHIRD and the NCRD. Among them, 567 patients used eribulin after treatment failure with anthracyclines and taxanes, and 1674 patients used capecitabine. After excluding patients with prior or concurrent use of the comparative chemotherapy agent and 1:1 matching, 298 patients were assigned to the eribulin and capecitabine groups, respectively.
Patients’ baseline characteristics were comparable between the two treatment groups (Table 1). Most of the patients were ages 51–60 years, with a mean of 54.3 years in both groups. Half of the patients were from the northern district (51.7%), approximately 85% of the patients had a CCI score of eight or greater, more than half of the patients were with hormone receptor expression (87.6%), and most of the patients had received at least four chemotherapy (CT) agents.
Table 1.
Baseline characteristics of study patients after matching.
| Characteristic | Eribulin (n = 298) |
Capecitabine (n = 298) |
||
|---|---|---|---|---|
| n | % | N | % | |
| Age | ||||
| 20–30 | 0 | 0.0 | 0 | 0.0 |
| 31–40 | 12 | 4.0 | 11 | 3.7 |
| 41–50 | 82 | 27.5 | 85 | 28.5 |
| 51–60 | 129 | 43.3 | 135 | 45.3 |
| 61–70 | 71 | 23.8 | 64 | 21.5 |
| 71–80 | 4 | 1.3 | 3 | 1.0 |
| >=81 | 0 | 0.0 | 0 | 0.0 |
| Mean (SD) [Median], years | 54.3 (7.9) [55] | 54.3 (7.9) [55] | ||
| P value |
0.97 |
|||
| Geographical region | ||||
| North | 154 | 51.7 | 154 | 51.7 |
| South | 93 | 31.2 | 93 | 31.2 |
| Central | 51 | 17.1 | 51 | 17.1 |
| East | 0 | 0 | 0 | 0 |
| P value |
1 |
|||
| CCI score | ||||
| 2 | 22 | 7.4 | 28 | 9.4 |
| 3 | 20 | 6.7 | 15 | 5.0 |
| 4 | 4 | 1.3 | 3 | 1.0 |
| 8 | 124 | 41.6 | 129 | 43.3 |
| 9 | 80 | 26.8 | 79 | 26.5 |
| 10 | 33 | 11.1 | 37 | 12.4 |
| >=11 | 15 | 5.0 | 7 | 2.3 |
| Mean (SD) [Median], points | 7.8 (2.4) [8] | 7.7 (2.4) [8] | ||
| P value |
0.68 |
|||
| Cancer cell type (phenotype) | ||||
| ER/PR positive | 261 | 87.6 | 261 | 87.6 |
| HER2 positive | 90 | 30.2 | 90 | 30.2 |
| ER/PR and HER2 positive | 74 | 24.8 | 74 | 24.8 |
| ER/PR and HER2 negative | 21 | 7.0 | 21 | 7.0 |
| P value | 1 | |||
| Number of previous CT agents | ||||
| 2 | 3 | 1.0 | 5 | 1.7 |
| 3 | 54 | 18.1 | 102 | 34.2 |
| 4 | 67 | 22.5 | 87 | 29.2 |
| 5 | 78 | 26.2 | 55 | 18.5 |
| 6 | 52 | 17.4 | 25 | 8.4 |
| 7 | 36 | 12.1 | 20 | 6.7 |
| >=8 | 8 | 2.7 | 4 | 1.3 |
| Mean (SD) [Median] | 4.9 (1.4) [5] | 4.2 (1.3) [4] | ||
| P value | <0.0001 | |||
| History of chemotherapy agents | ||||
| Doxorubicin/liposomal doxorubicin | 135 | 45.3 | 110 | 36.9 |
| Epirubicin | 189 | 63.4 | 204 | 68.5 |
| Docetaxel | 243 | 81.5 | 249 | 83.6 |
| Paclitaxel | 190 | 63.8 | 129 | 43.3 |
| Cyclophosphamide | 258 | 86.6 | 279 | 93.6 |
| Cisplatin | 99 | 33.2 | 71 | 23.8 |
| Carboplatin | 16 | 5.4 | 5 | 1.7 |
| Gemcitabine | 146 | 49.0 | 83 | 27.9 |
| Vinorelbine | 164 | 55.0 | 115 | 38.6 |
| Methotrexate | 15 | 5.0 | 17 | 5.7 |
| P value | 0.29 | |||
CCI Charlson Comorbidity Index, ER Estrogen receptor, HER2 Human epidermal receptor 2, PR Progesterone receptor, SD Standard deviation.
3.2. Effectiveness and the use of G-CSF
Until the date of administrative censoring (i.e. 31st December 2017), there were 214 (71.8%) deaths in the eribulin group and 157 (52.7%) deaths in the capecitabine group. The 1-year survival rate was 49.0% (deaths = 152) in the eribulin group and 65.1% (deaths = 104) in the capecitabine group. Median OS was 11.8 months (95%CI, 11.5–13.5 months) for eribulin compared to 15.2 months (95%CI, 15.3–17.9 months) for capecitabine, resulting in an HR of 1.7 (95%CI, 1.4 to 2.1; p < 0.0001). The Kaplan-Meier graph of OS is presented in Fig. 1A.
Fig. 1.
Kaplan-Meier graph of (A) OS and (B) TTD.
Median TTD was 4.0 months (95%CI 5.5–6.9) in the eribulin group and 6.6 months (95%CI 9.0–11.1) in the capecitabine group (HR 1.6; 95%CI 1.3–1.9; p < 0.0001). Until the date of administrative censoring, there were 280 and 261 (94.0% and 87.6%, respectively) treatment discontinuations in the eribulin and capecitabine groups, respectively, with a median TTD of 3.8 months and 4.7 months, respectively. The Kaplan-Meier graph of TTD is plotted in Fig. 1B.
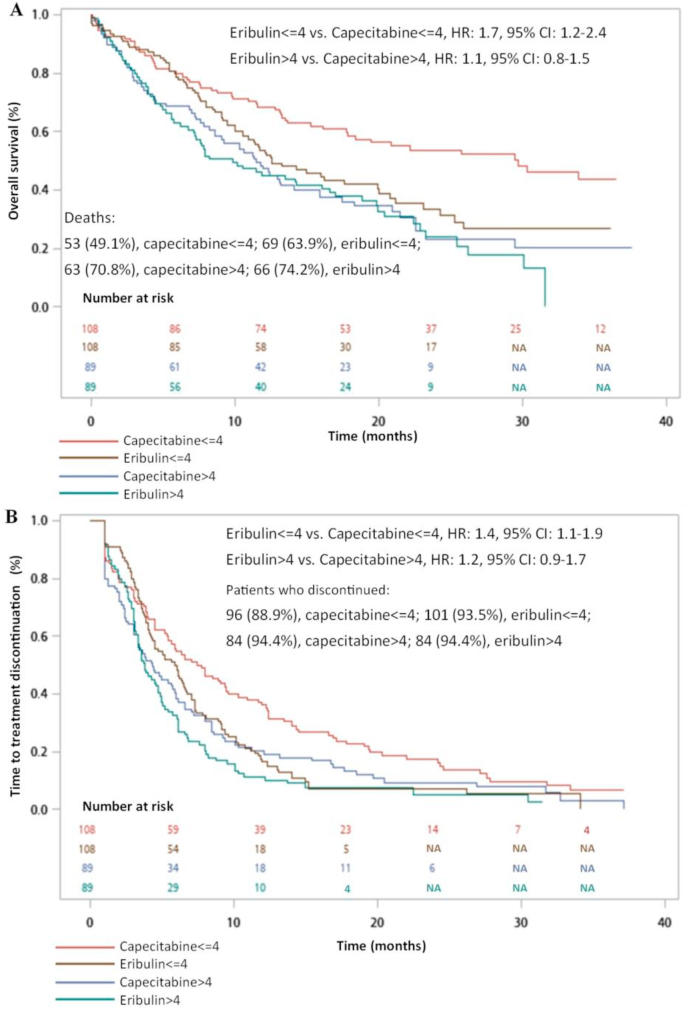
Subgroup analysis (Fig. 2) demonstrated that when capecitabine and eribulin were initiated in patients with >4 prior chemotherapy agents (i.e., the study treatment was at least a sixth- or later-line treatment), there was no significant difference between the two groups’ median OS or TTD (OS: HR 1.1, 95%CI 0.8–1.5; TTD: HR 1.2, 95%CI 0.9–1.7). However, capecitabine performed better than eribulin when each was initiated as a fifth-line or earlier chemotherapy treatment (OS: HR 1.7, 95%CI 1.2–2.4; TTD: HR 1.4, 95%CI 1.1–1.9).
Fig. 2.
Subgroup analysis of (A) OS and (B) TTD by the number of previous chemotherapy agents.
Among patients receiving eribulin, 135 (45.3%) received G-CSF during the treatment period. In comparison, 41 (13.8%) patients taking capecitabine received with the same. A univariate logistic regression analysis suggested the odds ratio to be 5.2 (95%CI 3.5–7.8, p < 0.0001).
3.3. Healthcare utilization and costs
Healthcare utilization and costs PP as well as PPPM during the treatment period were calculated and are summarized in Table 2, Table 3, respectively. As shown in Table 2, although patients receiving eribulin treatment tended to visit an outpatient oncologist less frequently (p = 0.049), the mean PP medical cost of outpatient oncology visits was higher than that for patients receiving capecitabine (NT$405,654.1 vs. NT$371,177.7; p = 0.003). As for the emergency room and hospitalization costs, no difference was found between the two groups. The mean total PP medical costs for eribulin and capecitabine were NT$ 580,523.8 and NT$ 497,223.8, respectively (p < 0.0001). Outpatient costs contributed to approximately 70% of the total medical costs of both eribulin and capecitabine while drug costs contributed to a greater proportion of total medical costs for eribulin than for capecitabine (60.4% vs. 15.3%).
Table 2.
Healthcare utilization and costs per-patient (eribulin vs. capecitabine).
| Eribulin |
Capecitabine |
p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | Mean | 95%CI | N | Median | Mean | 95%CI | ||
| Healthcare utilization | |||||||||
| Outpatient oncology visits (n) | 278 | 17.0 | 28.4 | 24.8–32.0 | 281 | 23.0 | 38.8 | 33.6–43.9 | 0.049 |
| Emergency room visits (n) | 85 | 1.0 | 2.0 | 1.6–2.4 | 73 | 1.0 | 2.3 | 1.8–2.8 | 0.68 |
| Hospitalizations (n) | 160 | 2.0 | 4.3 | 3.3–5.3 | 157 | 2.0 | 3.6 | 2.8–4.4 | 0.40 |
| Days of hospitalization (n) | 160 | 13.0 | 32.8 | 23.0–42.6 | 157 | 14.0 | 36.8 | 10.6–63.0 | 0.64 |
| Medication cost | |||||||||
| Cost of eribulin/capecitabine | 298 | 254,300.0 | 350,458.6 | 312,554.0–388,363.2 | 298 | 46,144.0 | 76,247.7 | 66,776.6–85,718.8 | <0.0001 |
| Cost of G-CSF | 298 | 0.0 | 16,899.6 | 12,897.0–20,902.3 | 298 | 0.0 | 2313.8 | 1217.3–3410.3 | <0.0001 |
| Total medication cost | 298 | 305,335.0 | 438,335.8 | 388,488.1–488,183.5 | 298 | 154,731.0 | 348,438.4 | 294,184.5–402,692.3 | <0.0001 |
| Medical cost | |||||||||
| Cost of outpatient oncology visits | 298 | 262,319.0 | 405,654.1 | 353,578.1–457,730.1 | 298 | 181,910.5 | 371,177.7 | 313,071.4–429,284.0 | 0.003 |
| Cost of emergency room visits | 298 | 0.0 | 2880.2 | 2055.9–3704.5 | 298 | 0.0 | 2865.3 | 1490.8–4239.9 | 0.18 |
| Cost of hospitalizations | 298 | 25,524.5 | 171,989.5 | 125,334.7–218,644.3 | 298 | 11,733.5 | 123,180.8 | 95,876.9–150,484.7 | 0.45 |
| Total medical cost | 298 | 367,653.0 | 580,523.8 | 514,217.1–646,830.4 | 298 | 282,622.5 | 497,223.8 | 431,400.1–563,047.6 | <0.0001 |
G-CSF granulocyte-colony stimulating factor.
Table 3.
Healthcare utilization and costs per-patient-per-month (eribulin vs. capecitabine).
| Eribulin |
Capecitabine |
p value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | Mean | 95%CI | N | Median | Mean | 95%CI | ||
| Healthcare utilization | |||||||||
| Outpatient oncology visits (n) | 278 | 5.1 | 5.6 | 5.2–5.9 | 281 | 4.0 | 4.7 | 4.3–5.1 | <0.0001 |
| Emergency room visits (n) | 85 | 0.4 | 0.7 | 0.5–0.9 | 73 | 0.2 | 0.7 | 0.3–1.0 | 0.002 |
| Hospitalizations (n) | 160 | 0.9 | 1.5 | 1.1–2.0 | 157 | 0.4 | 1.2 | 0.8–1.6 | 0.0006 |
| Days of hospitalization (n) | 160 | 5.2 | 11.6 | 9.1–14.0 | 157 | 2.1 | 14.8 | 7.5–22.1 | 0.01 |
| Medication cost | |||||||||
| Cost of eribulin/capecitabine | 298 | 75,493.8 | 88,414.2 | 80,803.2–96,025.3 | 298 | 9471.2 | 12,137.8 | 10,273.6–14,002.1 | <0.0001 |
| Cost of G-CSF | 298 | 0.0 | 5293.9 | 3515.5–7072.3 | 298 | 0.0 | 974.0 | 100.1–1848.0 | <0.0001 |
| Total medication cost | 298 | 89,062.4 | 107,366.5 | 98,030.6–116,702.4 | 298 | 34,673.4 | 47,438.6 | 41,168.5–53,708.7 | <0.0001 |
| Medical cost | |||||||||
| Cost of outpatient oncology visits | 298 | 87,487.4 | 88,442.7 | 81,706.4–95,179.0 | 298 | 35,212.1 | 42,246.9 | 38,072.7–46,421.0 | <0.0001 |
| Cost of emergency room visits | 298 | 0.0 | 967.1 | 593.8–1340.5 | 298 | 0.0 | 913.2 | 232.0–1594.5 | 0.09 |
| Cost of hospitalizations | 298 | 3425.2 | 59,574.5 | 45,350.4–73,798.7 | 298 | 748.5 | 60,361.6 | 23,910.6–96,812.5 | 0.10 |
| Total medical cost | 298 | 114,335.0 | 148,984.3 | 134,563.9–163,404.8 | 298 | 51,706.4 | 103,521.6 | 65,821.9–141,221.4 | <0.0001 |
G-CSF granulocyte-colony stimulating factor.
As presented in Table 3, the mean PPPM medical cost of oncology visits for eribulin was NT$88,442.7, which doubled that of capecitabine (NT$42,246.9). Similar to PP costs, the PPPM emergency room costs were comparable between the two groups, albeit eribulin patients’ utilization was higher. The mean PPPM total medical cost of eribulin was NT$ 148,984.3 (95%CI, NT$134,563.9-NT$163,404.8), with around 60% of it resulting from eribulin’s drug cost and the cost of outpatient oncology visits. In contrast, the mean PPPM total medical cost of capecitabine (NT$ 103,521.6; 95%CI, NT$ 65,821.9-NT$141,221.4) was mainly driven by hospitalizations (58.3%).
Overall, compared to capecitabine, eribulin had both higher total medical costs and higher total medication costs PP and PPPM.
4. Discussion
This is the first real-world study investigating the effectiveness and cost of eribulin in Taiwan. Also, to the best of our knowledge, it is the first observational study that included capecitabine as a comparator to eribulin.
Patient characteristics in this study moderately mirror those in previous studies. The average age of our study sample was 54 years old, which is the same as that in the EMBRACE trial and Study 301, and the proportion of study patients with estrogen receptor positive or HER2 positive receptor was also comparable [7,8]. However, compared to those two clinical trials, patients in the present study were receiving MBC treatment for a worse baseline health condition, as indicated by higher CCI scores and a greater number of previous lines of chemotherapy. These characteristics were instead similar to those in the eribulin real-world study by Hurtaud et al. where study patients were closer to routine clinical practice, with 19.5% of patients having received more than six previous chemotherapy regimens [14].
Compared to Study 301, the only clinical trial to compare eribulin and capecitabine, a shorter OS of eribulin was observed in the present study (15.9 months vs. 11.8 months). The discrepancy may be explained by the differences between clinical trials and real-world studies and also by the fact that 99.3% of patients in Study 301 had received no more than two previous CT regimens for advanced disease. With regards to HR status, among the HR-population in the Study 301, although the OS and PFS of the Eribulin group were longer than those of the capecitabine group, the difference did not reach statistical significance (p = 0.05 and 0.82, respectively) [25]. Moreover, in two other clinical trials (eribulin vs. physician choice in one; eribulin vs. vinorelbine in the other), the efficacy of eribulin in patients with positive hormone receptor is similar to that in patients with negative hormone receptor status [26,27]. In a further analysis, we explored the potential effect of HR and HER2 status using multiple regression analysis, but no statistical significance was observed. Although there is no real-world study comparing eribulin and capecitabine to serve as a reference, the median OS of eribulin observed in this study is slightly longer than those of other real-world studies involving eribulin in Denmark, Italy, and Japan [11,12,15]. Also, the 1-year survival of patients on eribulin observed in the present study mirrors the result published by Hurtaud et al. in France (42%) [14]. Therefore, even though the effectiveness of eribulin was not as good as that of capecitabine, the performance of eribulin in this study is comparable to and even better than that reported in other real-world studies. Moreover, in the subgroup analysis, we discovered that eribulin exhibited the same benefits as capecitabine when both were administered in chemotherapy lines greater than five. Our findings provided a better understanding of the effectiveness of eribulin since few published studies have inspected the impact of the line of treatment.
The rate of G-CSF use in this study indicates that the rate of severe neutropenia in eribulin users observed in Taiwan was higher than those in Europe and the U.S [7,8,10,12]. It has been suggested that severe neutropenia could be more pronounced in east Asian populations, which is particularly reflected in the use of eribulin as a late-line treatment [28]. Indeed, an incidence rate of 57.4% for grade 4 neutropenia was observed in eribulin users in South Korea, and up to two-thirds of eribulin users had neutropenia in Japan [16,18].
The cost items included in this analysis were comprehensive and consisted of all healthcare products and services reimbursed by the NHI. Although the estimated treatment costs cannot be compared with other studies directly due to different health care policies and systems among countries and variations in study methods, previous cost and cost-effectiveness studies conducted in the US, the UK, and the Southeast Netherlands also concluded that eribulin was expensive and not cost-effective when compared to other chemotherapies [23,29,30].
At an advanced stage of MBC, whether to continue with chemotherapy is a tough decision, as the benefits are debatable [31]. Since November 2010 when eribulin received approval from the US FDA, studies have been conducted to examine its real-world effectiveness and costs. However, no study has compared the benefit of eribulin with another chemotherapy agent. Moreover, although capecitabine is effective and commonly prescribed, its adverse events of hand-foot syndrome occurred in 50–60% of its users [32], and some of the cases were extreme, leaving physicians with no choice but to discontinue the treatment. In this study, eribulin was found to show comparable benefits to capecitabine in patients who had received more than four chemotherapy agents; therefore, eribulin may serve as an alternative for patients who have been heavily pretreated and for whom capecitabine is contraindicated. The costs analysis in this study discovered that the high treatment cost of eribulin was mainly driven by the high drug acquisition cost. Although the price of eribulin has dropped 9.7% since being approved by the Taiwan FDA, additional price negotiation is warranted. Moreover, as eribulin was found to be less effective and costlier than its comparator, further comprehensive health technology assessments of eribulin should be performed using real-world data. To further minimize selection bias and the imbalance in the line of chemotherapy between eribulin and capecitabine, future research aiming to evaluate eribulin as used in various lines of chemotherapy is warranted. In addition, the best sequential use of eribulin after the failure of anthracyclines and taxanes also needs to be examined.
The major limitation to this study resulted from the use of the HWD, which makes the study subject to potential data coding errors and missing data. Also, we were unable to know whether the assigned treatment was actually administered. Nevertheless, considering the high drug cost and the administration setting (i.e., in hospitals), the chance of not being administered is quite low. In addition, the absence of information about lab tests and biomedical images in the study databases prevented us from evaluating PFS. Although TTD was designed as a surrogate for PFS to demonstrate the effectiveness of comparators, care should be taken when comparing our TTD to PFS reported in other studies. Similarly, a G-CSF prescription was used as a proxy for the occurrence of severe neutropenia, which may have overestimated the rate of adverse events since prophylactic treatment may have also been included. Also, only reimbursed medications were recorded in the HWD so patients’ treatment history may not be complete, particularly for late-line therapies. Moreover, although great efforts were made to alleviate potential confounding, the effects of selection bias and unequal disease burden/spread may still have existed. Finally, the follow-up period was limited by data availability (the last date of available data in the HWD was Dec 31, 2017).
5. Conclusions
This population-based real-world study provides a better understanding of the effectiveness of eribulin in refractory MBC patients who had failed to respond to anthracyclines and taxanes in Taiwan. Despite the potential biases, our study results suggest that eribulin has shorter OS and TTD when compared to capecitabine; however, that difference no longer exists when the drugs are both initiated as sixth- or later-lines of chemotherapy. Therefore, eribulin may serve as an alternative for those heavily pretreated patients and those for whom capecitabine is contraindicated. In addition, the rate of G-CSF use and the total medical and medication costs were found to be higher with eribulin treatment.
Funding
This study was funded by a research grant provided by Taipei Medical University (TMU104-AE1-B16).
Ethical approval
The study was approved by Taipei Medical University- Joint Institutional Review Board (Approval number: N201709052).
Declaration of competing interest
All author declare that they have no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA: Canc J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Dawood S., Broglio K., Ensor J., Hortobagyi G., Giordano S.H. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundquist M., Brudin L., Tejler G. Improved survival in metastatic breast cancer 1985-2016. Breast. 2017;31:46–50. doi: 10.1016/j.breast.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., André F., Harbeck N., Aguilar Lopez B., Barrios C.H., Bergh J. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)††These guidelines were developed by the European school of oncology (ESO) and the European society for medical oncology (ESMO) Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano S.H., Elias A.D., Gradishar W.J. NCCN guidelines updates: breast cancer. J Natl Compr Canc Netw : J Natl Compr Canc Netw. 2018;16(5s):605–610. doi: 10.6004/jnccn.2018.0043. [DOI] [PubMed] [Google Scholar]
- 6.Jordan M.A., Kamath K., Manna T., Okouneva T., Miller H.P., Davis C., Littlefield B.A., Wilson L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Canc Therapeut. 2005;4(7):1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 7.Cortes J., O’Shaughnessy J., Loesch D., Blum J.L., Vahdat L.T., Petrakova K., Chollet P., Manikas A., Dieras V., Delozier T. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman P.A., Awada A., Twelves C., Yelle L., Perez E.A., Velikova G., Olivo M.S., He Y., Dutcus C.E., Cortes J. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol : Off J Am Soc Clin Oncol. 2015;33(6):594–601. doi: 10.1200/JCO.2013.52.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pivot X., Im S.A., Guo M., Marme F. Subgroup analysis of patients with HER2-negative metastatic breast cancer in the second-line setting from a phase 3, open-label, randomized study of eribulin mesilate versus capecitabine. Breast Canc. 2018;25(3):370–374. doi: 10.1007/s12282-017-0826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aftimos P., Polastro L., Ameye L., Jungels C., Vakili J., Paesmans M., van den Eerenbeemt J., Buttice A., Gombos A., de Valeriola D. Results of the Belgian expanded access program of eribulin in the treatment of metastatic breast cancer closely mirror those of the pivotal phase III trial. Eur J Canc (Oxford, England : 1990. 2016;60:117–124. doi: 10.1016/j.ejca.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Barni S., Livraghi L., Morritti M., Vici P., Michelotti A., Cinieri S., Fontanella C., Porcu L., Del Mastro L., Puglisi F. Eribulin in the treatment of advanced breast cancer: real-world scenario from 39 Italian centers - ESEMPiO study. Future Oncol. 2019;15(1):33–44. doi: 10.2217/fon-2018-0324. [DOI] [PubMed] [Google Scholar]
- 12.Brems-Eskildsen A.S., Kristoffersen K.B., Linnet S., Lorincz T., Langkjer S.T. Efficacy and toxicity of eribulin treatment in metastatic breast cancer patients. Acta Oncol. 2019;58(1):119–121. doi: 10.1080/0284186X.2018.1497301. [DOI] [PubMed] [Google Scholar]
- 13.Jacot W., Heudel P.E., Fraisse J., Gourgou S., Guiu S., Dalenc F., Pistilli B., Campone M., Levy C., Debled M. Real-life activity of eribulin mesylate among metastatic breast cancer patients in the multicenter national observational ESME program. Int J Canc. 2019;145(12):3359–3369. doi: 10.1002/ijc.32402. [DOI] [PubMed] [Google Scholar]
- 14.Hurtaud A., Donnadieu A., Escalup L., Cottu P.H., Baffert S. Costs associated with Eribulin treatment for patients with metastatic breast cancer in a comprehensive cancer center in France. Breast. 2016;30:73–79. doi: 10.1016/j.breast.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Iizumi S., Shimoi T., Tsushita N., Bun S., Shimomura A., Noguchi E., Kodaira M., Yunokawa M., Yonemori K., Shimizu C. Efficacy and safety of eribulin in patients with locally advanced or metastatic breast cancer not meeting trial eligibility criteria: a retrospective study. BMC Canc. 2017;17(1):819. doi: 10.1186/s12885-017-3846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park Y.H., Kim T.Y., Im Y.H., Lee K.S., Park I.H., Sohn J., Lee S.H., Im S.A., Kim J.H., Kim S.H. Feasibility and efficacy of eribulin mesilate in Korean patients with metastatic breast cancer: Korean multi-center phase IV clinical study results. Canc Res Treat: Off J Kor Canc Assoc. 2017;49(2):423–429. doi: 10.4143/crt.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rau K.M., Ou-Yang F., Chao T.C., Kuo Y.L., Cheng T.F., Chao T.Y., Chen D.R., Tzeng Y.D., Wang B.W., Liu C.Y. Effect of eribulin on patients with metastatic breast cancer: multicenter retrospective observational study in Taiwan. Breast Canc Res Treat. 2018;170(3):583–591. doi: 10.1007/s10549-018-4778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakata Y., Matsuoka T., Ohashi S., Koga T., Toyoda T., Ishii M. Use of a healthcare claims database for post-marketing safety assessments of eribulin in Japan: a comparative assessment with a prospective post-marketing surveillance study. Drugs - Real World Outc. 2019;6(1):27–35. doi: 10.1007/s40801-019-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka N., Ogura K., Hattori A., Inoue H., Yukawa H., Sakaguchi S., Matsuoka A., Kodera A., Naritaka Y., Hirano A. [Treatment out come of eribulin in patients with advanced or metastatic breast cancer who resistant to anthracycline and taxane] Gan to kagaku ryoho Canc Chemother. 2017;44(12):1200–1202. [PubMed] [Google Scholar]
- 20.Tanaka T., Ueno M., Nakashima Y., Chinen S., Sato E., Masaki M., Mogi A., Sasaki H., Tamura K., Takamatsu Y. Retrospective analysis of the efficacy and safety of eribulin therapy for metastatic breast cancer in daily practice. Thorac Canc. 2017;8(5):523–529. doi: 10.1111/1759-7714.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niraula S., Amir E., Vera-Badillo F., Seruga B., Ocana A., Tannock I.F. Risk of incremental toxicities and associated costs of new anticancer drugs: a meta-analysis. J Clin Oncol : Off J Am Soc Clin Oncol. 2014;32(32):3634–3642. doi: 10.1200/JCO.2014.55.8437. [DOI] [PubMed] [Google Scholar]
- 22.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., Saunders L.D., Beck C.A., Feasby T.E., Ghali W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 23.Lopes G., Gluck S., Avancha K., Montero A.J. A cost effectiveness study of eribulin versus standard single-agent cytotoxic chemotherapy for women with previously treated metastatic breast cancer. Breast Canc Res Treat. 2013;137(1):187–193. doi: 10.1007/s10549-012-2326-8. [DOI] [PubMed] [Google Scholar]
- 24.The PSMATCH Procedure [https://documentation.sas.com/?docsetId=statug&docsetTarget=statug_psmatch_examples04.htm&docsetVersion=14.3&locale=en].
- 25.Twelves C., Awada A., Cortes J., Yelle L., Velikova G., Olivo M.S., Song J., Dutcus C.E., Kaufman P.A. Subgroup Analyses from a phase 3, open-label, randomized study of eribulin mesylate versus capecitabine in pretreated patients with advanced or metastatic breast cancer. Breast Canc Basic Clin Res. 2016;10:77–84. doi: 10.4137/BCBCR.S39615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes J., O’Shaughnessy J., Loesch D., Blum J.L., Vahdat L.T., Petrakova K., Chollet P., Manikas A., Diéras V., Delozier T. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 27.Yuan P., Hu X., Sun T., Li W., Zhang Q., Cui S., Cheng Y., Ouyang Q., Wang X., Chen Z. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: a randomised clinical trial. Eur J Canc (Oxford, England : 1990. 2019;112:57–65. doi: 10.1016/j.ejca.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Kok V.C. Eribulin in the management of advanced breast cancer: implications of current research findings. Breast Canc Basic Clin Res. 2015;9:109–115. doi: 10.4137/BCBCR.S32787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhalgh J., Bagust A., Boland A., Oyee J., Trevor N., Beale S., Dundar Y., Hockenhull J., Proudlove C., O’Reilly S. Eribulin for the treatment of advanced or metastatic breast cancer: a NICE single technology appraisal. Pharmacoeconomics. 2015;33(2):137–148. doi: 10.1007/s40273-014-0214-2. [DOI] [PubMed] [Google Scholar]
- 30.Pouwels X., Ramaekers B.L.T., Geurts S.M.E., Erdkamp F., Vriens B., Aaldering K.N.A., van de Wouw A.J., Dercksen M.W., Smilde T.J., Peters N. An economic evaluation of eribulin for advanced breast cancer treatment based on the Southeast Netherlands advanced breast cancer registry. Acta Oncol. 2020:1–8. doi: 10.1080/0284186X.2020.1775289. [DOI] [PubMed] [Google Scholar]
- 31.Bonotto M., Gerratana L., Iacono D., Minisini A.M., Rihawi K., Fasola G., Puglisi F. Treatment of metastatic breast cancer in a real-world scenario: is progression-free survival with first line predictive of benefit from second and later lines? Oncol. 2015;20(7):719–724. doi: 10.1634/theoncologist.2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degen A., Alter M., Schenck F., Satzger I., Völker B., Kapp A., Gutzmer R. The hand-foot-syndrome associated with medical tumor therapy – classification and management. JDDG J der Deutschen Dermatol Gesellschaft. 2010;8(9):652–661. doi: 10.1111/j.1610-0387.2010.07449.x. [DOI] [PubMed] [Google Scholar]