Abstract
Decades of research on the human papillomavirus oncogenes, E6 and E7, have given us huge amounts of data on their expression, functions and structures. We know much about the very many cellular proteins and pathways that they influence in one way or another. However, much of this information is quite discrete, referring to one activity examined under one condition. It is now time to join the dots to try to understand a larger picture: how, where and when do all these interactions occur... and why? Examining these questions will also show how many of the yet obscure cellular processes work together for cellular and tissue homeostasis in health and disease.
Keywords: HPV, E6, E7
1. Introduction
Papillomavirus-induced cancers are addicted to the expression of the major viral oncogenes, E6 and E7, whose combined effect is required for the development and maintenance of the transformed phenotype (reviewed in Ref. [1]). Abrogation of E6 and E7 expression in tumours, or in cell-lines derived from tumours, results in growth arrest and the rapid death of the tumour cell by apoptosis or senescence, making E6 and E7 ideal potential targets for therapeutic intervention in HPV-induced cancers.
Although E6 and E7 have been the subjects of intense research over the past few decades, there are still some surprising gaps in our understanding of how they work, both to facilitate the normal life-cycle of the virus and in their contribution to malignancy. Interactome analyses have shown that they potentially interact with a very wide range of cellular proteins and affect a wide range of cellular processes [[2], [3], [4], [5]]. In some cases the biochemistry is precisely known, but the biological significance is still unclear: in other cases the biochemistry underlying an obvious biological effect is still unknown.
2. HPV life-cycle, cell cycle dysregulation and cell transformation
The papillomavirus life-cycle depends on the differentiation of the infected epithelium. The virus infects cells in the basal layer of stratified epithelium, probably through microtraumas and establishes a stable infection with genome replication occurring in tandem with cellular replication, potentially during the wound healing of the microtrauma [6]. Upon reaching confluence the cells are subject to contact inhibition and may enter terminal differentiation [7]: the differentiating infected daughter cell leaves the basement membrane and enters the mid-epithelial layers, where it would normally exit the cell cycle and cease DNA replication. To prevent this, and to facilitate HPV DNA amplification, E6 and E7 combine their activities to create a pseudo-S phase. This occurs, at least partly, through the LXCXE motif of E7 binding to the pRb tumour suppressor and, in the case of high-risk virus, inducing pRb degradation. This releases E2F from the E2F/pRb repressor complex, allowing transcriptional activation of E2F-responsive promoters and stimulating the transition of the cell from G0 to G1, and then into S-phase [[8], [9], [10]]. E7 also binds to the other pRb-related pocket proteins, p107 and p130 [11]. The consequences include increased cellular (and hence viral) DNA replication and increased symmetrical cell division, expanding the number of HPV-infected cells. The LXCXE motif is highly conserved between different HPV types and thus a major challenge in E7 studies is to distinguish between the multiple functions of the LXCXE motif (reviewed in Ref. [12]) - which are the results of its effects on the pocket proteins, which are the results of other interactions and which are a combination of both?
The amplification of the viral genome at this stage appears to require a functional viral E1 protein and also the downregulation of the Notch signalling pathway [6]. Cutaneous HPVs from the beta, delta and mu groups interact with and inhibit the Notch transactivator MAML1 [13,14], while the alphapapillomaviruses appear to downregulate Notch signalling through degradation of p53. High-risk types do this in an E6AP-dependent manner, while low risk types probably do it independently of E6AP [6,15], although some evidence suggests that E6AP might be involved [16]. A consequence of unscheduled DNA replication in normal differentiating epithelium is the triggering of p53-dependent apoptosis [17]. However, since the alpha type HPV E6s can target p53 for degradation this cellular response is circumvented, thereby promoting cell survival and completion of the viral life cycle. It is not yet clear how the beta, delta and mu HPV types overcome the p53 response, but different types of epithelia may have diverse means of responding to this particular stress, since p53 is expressed at higher levels in cervical epithelium than in dermal epithelium [18]; further investigation is needed to clarify how dermal epithelium responds. Clearly, however, blocking apoptosis and promoting proliferation by the high-risk HPV types also has the potential to cause cellular damage that could lead to the development of malignancy.
Besides the region of E7 that binds pRb and the associated pocket protein family members, many other regions, including a Casein kinase 2 (CKII) phosphorylation site and regions in the extreme N-terminus, together with large stretches of the C-terminus, are also necessary for an effective viral life cycle and for the ability to bring about cell immortalisation and cancer. While many of the biochemical factors that contribute to these biological features are known, we still have major gaps in our understanding of how their combined activities facilitate the viral life cycle and, occasionally, result in transformation.
A further important factor that determines the outcome of an infection is the permissiveness of the infected cell type for the completion of the viral life cycle. It is becoming clearer, at least in the case of cervical infections, that ectocervical cells are permissive and can support a relatively straightforward viral life cycle and resolution of the infection, whereas cells of the transformation zone or endocervix are non-permissive, leading to abortive or persistent infection and a higher risk of carcinogenesis. In addition, the originally infected cell type also appears to determine the type of cancer that ensues (reviewed in Ref. [19]). E6 and E7 proteins from diverse HPV types interact differently with the signalling pathways of the cell: these in turn are subject to changing control signals through the various stages of epithelial differentiation. It is clear that much is yet unknown or unclear.
Several cellular targets are defined as tumour suppressors or oncogenes. For example, E7 binds UBR4/p600, a protein essential for membrane morphogenesis and cell survival [20], which is thought to be required for cell transformation [21]. UBR4/p600 indeed remains an enigma. It is a critical interacting partner of all E7 proteins analysed, including high- and low-risk, as well as Rhesus PV E7 [3,21,22]. In all cases, the interaction requires an intact E7 N-terminus, and hence a simple HA tagging of E7 will destroy the interaction and block E7 transformation activity. Whether this loss of transforming activity is the consequence of said tagging is unknown, but it seems highly likely, which is itself intriguing, as low-risk E7 also interacts with p600. This raises a couple of important points. First, as is the case with E6, great care must be taken with how these viral proteins are tagged - E6 on the N-terminus and E7 on the C-terminus; even so, whether these tagged proteins are truly wild-type remains to be determined. Secondly, and most importantly, which p600 function is so critical in the viral life cycle? and what is the function that appears to be so fundamental for transforming ability? It seems likely that these two may well not be the same. The p600 protein is very large and its biology is still only poorly understood; it was identified as a pRb interactor in the nuclear matrix, and in the cytoplasm it may contribute to membrane morphology through interaction with clathrin at the leading edge of the plasma membrane; it appears to have a role in Ca2+ signalling through calmodulin and to protect against apoptosis [20]. It has recently been shown that its UBR activity is required for cell surface protein homeostasis and cell adhesion [23]. Using E7 as a tool, we have a unique opportunity to further understand this intriguing target protein.
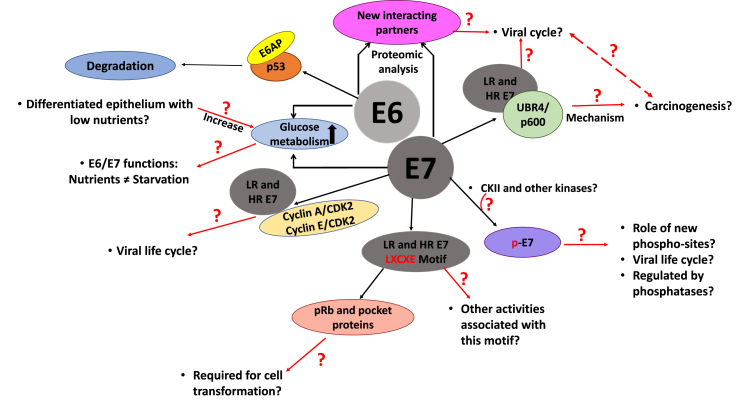
The accelerated proliferation promoted by E7 increases the accumulation of genetic aberrations, and E7 has been suggested to generate genome instability [24,25]: its interaction with gamma-tubulin, a crucial centrosome regulator, can induce centrosome duplication [26]; in addition, abnormal centriole multiplication correlates with the stabilization of Polo-like kinase 4 and the high levels of Cdk2 activity in E7 expressing cells [27,28]. The genomic alterations induced by E7 might contribute to the acquisition of further cellular mutations, contributing to cancer progression. It has been suggested that E7's stimulation of cellular DNA damage [[29], [30], [31]] ensures the availability of replicative building blocks and enzymes close to sites of viral DNA replication, and this appears to be a common strategy amongst DNA tumour viruses [32,33]. This, however, can also lead to the formation of aberrant mitoses, through E6's impairment of dsDNA break repair [34]. In Fig. 1 we summarise some of the remaining questions in cell transformation.
Fig. 1.
Schematic representation of HPV E6/E7-mediated cellular transformation, indicating the key missing links in the contexts of both viral life-cycle and HPV-mediated tumorigenesis.
3. Apoptosis
The response of the normal cell to unscheduled DNA replication is to induce either growth arrest or apoptosis, largely through the activation of p53 [17]. E6 from high-risk HPVs, in complex with the E3 ubiquitin-protein ligase, E6AP, binds to p53 and targets it for degradation via the proteasome. Interestingly, not all the p53 in the cell is degraded [35] and it is possible that E6/E6AP only targets cellular pools of p53 destined to activate the transcription of downstream pro-apoptotic factors, such as Bax, Fas, PUMAβ, Apaf-1 and PIG [36], which are all part of the intrinsic apoptotic pathway.
These cellular pools may well be differentiated by the diverse and extensive modifications that p53 undergoes in response to many cellular stresses (reviewed in Ref. [37]) and this may alter its ‘visibility’ to the E6/E6AP complex. For example, it has long been known that CKII phosphorylation of its C-terminus increases p53 DNA binding activity [38]; while, in response to DNA damage or unscheduled DNA replication, such as that induced by activation of E2F, the DDR kinases ATM and Chk1/2 phosphorylate p53 at S15 and S20, respectively, thus blocking p53's interaction with mdm2 and increasing p53 stability [39,40]. Further stress induces further phosphorylation, allowing the transactivation of proapototic factors, but not of growth suppressing factors [41,42], and it could well be these modified forms of p53 that are specifically targeted, although this has not been conclusively shown. Interestingly, p53 is also acetylated to selectively induce proapototic (PUMA and Bax), rather than growth arrest-related (p21) genes [43,44], the agent in this case being TIP60, another E6 degradatory target [45]. Furthermore CBP/p300, again, targets of E6 and E7 [46,47], also acetylate p53 to promote its transcriptional activity [48]; interestingly this may also block ubiquitination of the same residues, increasing p53 stability [49,50], all of which is abrogated by E6 and E7 targeting of CBP/p300.
In addition to p53, E6 targets a number of other cellular proteins to prevent the induction of apoptosis: the proapoptotic Bak protein is targeted for degradation by E6 proteins from high and low risk mucosal HPVs, using the ubiquitin-ligase activity of E6AP [51,52], and also by cutaneous HPV E6 [53], using the HERC1 ubiquitin ligase [54]. Bak levels are also reduced in laryngeal cells expressing HPV16, while levels of the antiapoptotic Bcl2 - an inhibitory partner of Bak and Bax - are increased, and Bax mRNA levels are also reduced, suggesting that this antiapoptotic activity can also contribute to oropharyngeal cancers [55,56].
Other apoptosis-related targets of E6 have also been identified: MAGI-1 is one of the most susceptible degradation targets of PBM-containing E6 proteins, (i.e. those from high-risk mucosal HPV types −16, −18 and −31) [[57], [58], [59]], and the release of the MAGI-1c isoform from tight junctions (TJ), its Fas-dependent cleavage, and subsequent trafficking to the nucleus is thought to be one of the earliest effector feedback signals in apoptosis [60,61], and it is specifically targeted by HPV-16 and -18 E6s [57,59,62]. Indeed, the exogenous expression in HeLa cells of a MAGI-1 mutant resistant to E6 targeting can induce apoptosis in those highly transformed cells [63].
4. Maintenance of the cancer phenotype
Once the cervical cells become malignant, both E6 and E7 are required to maintain the phenotype and promote cell survival [64,65], but it is still not clear which, of all the pathways targeted by the two oncoproteins, are essential in doing this. However, the CKII phospho-acceptor site in HPV-18 E7 does seem to be required [66]. CKII phosphorylation of E7 is needed for the induction of cell transformation [67], and increases E7's affinity for several targets [[68], [69], [70]]; cervical cancer cells with gene-edited abolition of E7's CKII recognition site are severely compromised in proliferation in low-nutrient conditions and in invasion ability [66]. These data suggest the potential of therapeutically targeting this CKII phospho-acceptor site, and, CKII inhibitors appear promising in clinical trials [71,72]. Furthermore, variants of E7 that have an additional phospho-acceptor site are also linked with increased transforming potential [73]. A major question that remains, however, is whether these variants have a more efficient viral life cycle (i.e. they produce more progeny virions per infectious cycle), or they have a more permissive host range and thus each virion has a greater chance of initiating a successful replication cycle. In addition, whilst there are known to be major changes in E7 phosphorylation through the different phases of the cell cycle, there is very little information on how the phosphorylation may change during the viral life cycle or in different phases of malignant progression.
Other phosphorylation sites in E7 have also been reported: in HPV-16 the S71 residue is phosphorylated, mainly in S-phase by an as yet unidentified kinase [74]; while the T5 and T7 residues are phosphorylated by DYRK1A [75], increasing the steady-state levels of E7. Since HPV E7 phosphorylation appears to play a fundamental role in E7 functions and stability, it is important to elucidate whether other kinases are involved during HPV infection and malignancy, how they are regulated, and which pathways and cellular targets are implicated. This information could help to design novel therapies based on kinase inhibitors [76,77]. An unexplored area of E7 phospho-regulation is the role of phosphatases: E7 interacts with cellular phosphatases PTPN14, PTPN21 and PP2A [[78], [79], [80]], but whether these phosphatases or others can regulate E7 phosphorylation is unknown. Interestingly, PP2A dysregulation has been linked to the tumorigenic activity of several small tumour viruses, suggesting combination therapy of PP2A activators with other inhibitors as a strategy for cancer therapy [81].
In addition to disrupting pRb/E2F, HPV-16 E7 interacts with E2F1 to enhance E2F1-mediated transcription, promoting the transition into S-phase independently of pRb, and this appears to correlate with carcinogenic potential [82]. Further, E7 promotes cell cycle progression by regulating cyclin dependent kinases (CDKs), binding directly to cyclin A/CDK2 and indirectly to cyclin E/CDK2 complexes [83,84]; however, the biological function of these interactions is not well understood. High-risk, but not low-risk, HPV E7 can block the cell cycle arrest induced by the p21 and p27 cyclin-dependent kinase inhibitors [85,86], and the region of E7 required to bypass these growth arrest signals correlates with its transforming activity [87], suggesting that the two processes may be related.
Much is known about HPV E7 biochemistry, but the biological roles of many of these associations are still unclear [3,5,[88], [89], [90]]. However, it is almost certain that the function of some HPV E7 targets is context-dependent: when basal and suprabasal cells are proliferating, E7 may modulate certain targets, different from those modulated when cells are differentiating. Studies to investigate the targeting of different substrates in the specific environments of differentiation and cancer progression are long overdue. Indeed, most studies are performed either at the very beginning (in monolayers) or the very end (in cancer cell lines or animal tumours) of the process of transformation and tumour progression. Even raft cultures, lasting around 14 days, are only short-term models for an infectious cycle that may take 6 months to complete.
Long-term, organotypic mixed culture systems that include dermal fibroblasts and immune-modulating cells, would reflect more accurately the conditions in which the viral life-cycle occurs. In addition, the importance of the stroma and extracellular matrix in HPV infection and cancer development have long been understood [[91], [92], [93], [94], [95], [96]], but it is only now becoming technically possible to include them in model systems [[97], [98], [99], [100]]. It is also important that models are developed to reflect the tissues of origin of various different HPV-induced diseases [101]; the growth, differentiation characteristics, and tissue architecture of the ectocervix differ from those from the endocervix, or anus, and those of the tonsil are even more markedly different [19,102]. The mouse models for HPV-induced carcinogenesis (reviewed in Refs. [[103], [104], [105], [106], [107]]), in which HPV-16 E6 and E7 are stably expressed in specific tissues, have provided a wealth of information about the relative roles of E6 and E7 in carcinogenesis, however they provide little information about the virus life cycle. Considering animal models for HPV infection and carcinogenesis, the major problem is the species-specificity of papillomaviruses. Although some natural infection models, such as the canine oral papillomavirus model [108] and the murine papillomavirus 1 tail wart model [109], have given some interesting insights into low-risk mucosal [110,111], and cutaneous [112] HPV infections, respectively, they do not appear to be effective models for high-risk mucosal HPV life-cycle or carcinogenesis [113]. Recently, certain rodent PV models have shown promise [114,115], as have certain genetically modified mouse models [116], but animal models that accurately reflect high-risk mucosal HPV life cycles, and carcinogenesis in humans, are still lacking.
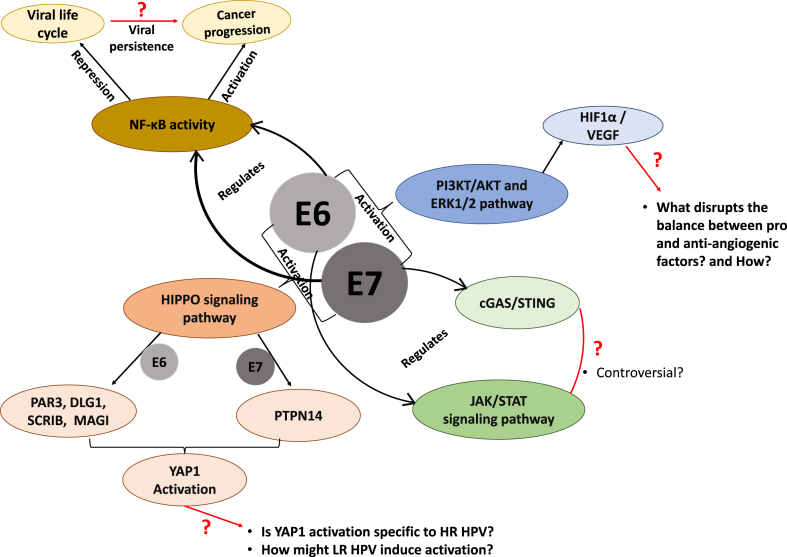
Recent interactome analyses including other HPV types have enormously increased the number of potential binding partners of E6 and E7 [[2], [3], [4], [5]], while the number of known and potential post-translational modifications of both viral and cellular proteins has similarly increased (reviewed in Ref. [70]). In addition to stimulating the degradation of p53 and pRb, E6 and E7 drive cellular proliferation through other pathways, including the HIPPO signalling pathway [117]: E6 through targeting PAR3, DLG1, Scrib and MAGI proteins [62,[118], [119], [120]], and E7 through PTPN14 [121], all of which increases YAP1 activation. E6 and E7 manipulate JAK/STAT signalling through a number of routes (reviewed in Ref. [122] and summarised in Fig. 5), and E7 has been reported to inhibit the interferon response through downregulation of the cGAS/STING response [123], although this is not yet clear and different HPV types may employ different means to do this [[124], [125], [126]]. E7 also recruits the TRIM21 ubiquitin ligase to degrade the IFI16 inflammasome, thereby contributing to the evasion of immune surveillance [127]. The contribution of insulin-activated transcription has recently come to the fore; stimulation of the insulin receptor leads to transactivation of a number of genes via recruitment of p300 (a target of E7 - [47]) and TIP60 (an E6 target - [45]) leading to chromatin modification and upregulated transcription of genes involved in glucose metabolism [128]. The role of nutrients in the tumour and cancer environment is very relevant: for example, mutation of the E7 CKII site has no deleterious effect in a nutrient-rich environment, and the effects are only seen when cancer cells are starved of nutrient [66]. In addition, HPV-18 E6's PBM-dependent binding to Sorting Nexin 27 (SNX27) modulates SNX27's interaction with its cargoes, including the GLUT1 glucose transporter, and leads to increased cellular glucose uptake [129]. So, it seems that E6 and E7 functions may have become fine-tuned to work in less than optimal conditions. This, of course makes sense, considering that viral genome amplification and progeny virion production (both energy-dependent processes) take place in the upper epithelial layers where nutrients diffusing from the basement membrane are scarce. Whether an undersupply of nutrients might alter cell morphology and functions in ways that affect the completion of the viral life-cycle is still unknown, and how the low-risk HPVs are able to overcome this restrictive environment is less well understood. Indeed, some early studies looked at the effects of nutrient levels with reference to E7 interactions [[130], [131], [132]], but the many reported interactions and biological effects thereof have not been pursued in conditions of nutrient deficiency or hypoxia.
Fig. 5.
An indication of the many cell signalling pathways manipulated by E6 and E7: many questions remain about the potentially different effects of their manipulations at different stages of the HPV life-cycle and in HPV-induced carcinogenesis.
5. Epigenetic regulation and microRNAs
It is becoming clear that epigenetic modifications are an important factor for the development of HPV-associated cancers (reviewed in Refs. [[133], [134], [135]]) and during the life cycle of the virus [136]. Like other tumour virus oncoproteins E1A and SV40 LT [[137], [138], [139]], E6 and E7 regulate the activity of histone acetyltransferases (HATs), p300 and CBP [46,47,140,141], reducing E2F- and p53-driven transcription and lowering pRb levels. E7 also decreases the activity of the HATs pCAF [142], and SRC1 [143]. Thus p300/CBP may have tumour-suppressor-like activity, although, this function seems to be context-dependent, since in tumour cells, inhibitors of p300 or p300/CBP reduce E6/E7 transcription, leading to p53 accumulation and apoptosis [144,145].
High-risk E7 interacts indirectly with class I histone deacetylases (HDACs) [146,147] to promote transcription and cell growth in differentiating epithelial cells [148]. Interestingly, mutation of the HDAC-binding domain of HPV-31 E7 reduces the episomal maintenance of the viral genome in undifferentiated cells [147], while HDAC1 and HDAC2 expression are increased in invasive carcinoma and cervical dysplasia [149], suggesting that the transcriptional regulation function of E7-HDACs may be relevant during carcinogenesis.
Dysregulation of DNA methylation is also associated with carcinogenesis [150], and several DNA tumour viruses target the methylation machinery, altering cellular and viral gene expression to override cell-cycle arrest [151]. HPV16 E7 binds and stimulates the DNA methyltransferase activity of DNMT1 and may also activate its transcription through the pRb/E2F pathway [133,152]. Like other viral oncogenes, E7 represses E-cadherin through DNMT1 activity [153,154], possibly as a means of immune evasion, preventing the migration of Langerhans cells into infected epithelium [153]. HPV E7 also induces expression of the histone lysine demethylases (KDM) KDM6A and KDM6B, leading to a global reduction of the repressive mark H3K27me3 [155,156], and a consequent increase in the levels of the cervical carcinoma biomarker p16INK4A. Paradoxically, increased p16INK4A causes G1 arrest and senescence via pRb activation [157]; however, since E7 targets pRb, this p16INK4A upregulation in HPV-infected cells cannot block cell proliferation. However, cervical cell lines seem to be dependent on the expression of KDM6A, KMD6B and p16INK4A [156,158], suggesting the possible use of KDM inhibitors to treat cervical cancer.
Recently attention has been directed at the contribution of microRNAs (miRs) to the HPV life-cycle and HPV-induced carcinogenesis. A wide range of miRs have been found to have altered expression in the presence of HPV E6/E7, affecting a wide range of centrally important cellular signalling pathways [159,160], including those involved in control of cell growth [[161], [162], [163], [164]] and apoptosis [165,166].
It is very clear how many of these modifications and miRNAs in combination, and expressed over a long period, might drive malignancy, but it is not so clear how they contribute to the virus life-cycle in the short term - which leads to the question - do some of these players have other functions that contribute to a successful life-cycle? and if so, what are they?
6. HPV E6 and E7: DNA damage response and genetic instability
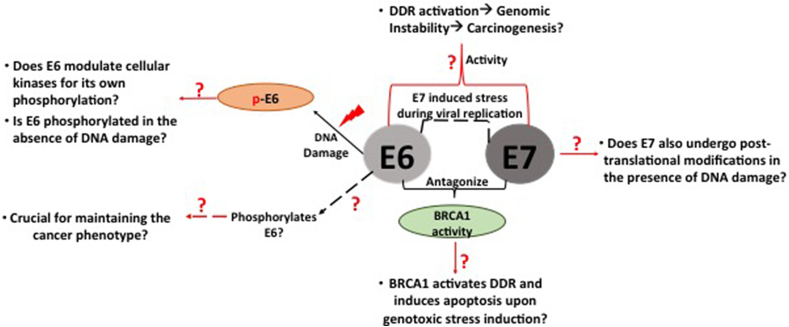
The DNA damage response (DDR) pathways safeguards the integrity of cellular genomes. Therefore, any defects or misregulation in the DDR leads to accumulation of mutations in the cells, causing genetic instability that can contribute to cancer development. HPV activates the DDR to facilitate efficient viral replication [31,167,168], and, indeed, so do many other DNA tumour viruses [169]: in HPV infection these are regulated by E6 and E7 to coordinate productive infection, but errors in doing this risk the development of malignancy. Fig. 2 illustrates some of the remaining questions related to E6 and E7's response to, and manipulation of, the DDR.
Fig. 2.
Schematic diagram representing the missing links in our knowledge of the E6 and E7 response to DNA damage, leading to genomic instability and thence to HPV-mediated carcinogenesis.
HPV E7 and E6 hijack and activate the DDR pathways by targeting pRB and p53, the two main tumour suppressors of the cells involved in controlling a plethora of DDR responsive genes. E7 targeting the pocket proteins leads to uncontrolled cell proliferation and activation of DDR responsive pathways, which in turn leads to sustained p53 activation, and the activation of cell cycle checkpoint regulators and apoptotic proteins.
The partial inactivation of DNA repair pathways and cell-cycle checkpoints by high-risk E6/E7 results in HPVs' dependence on the remaining DDR responsive pathways for their survival after DNA damage induction. Several proteins from the ATM and ATR pathways, including pCHK2, pCHK1, FANCD2 and BRCA1, which are essential for maintaining cellular genomic stability, are upregulated in HPV-positive cervical cancers and in precursor lesions, and they are crucial for completing viral DNA replication and the life-cycle [170].
E6 modulates the activity of DDR kinases, CHK1 and CHK2 through PKA, to phosphorylate itself by perturbing the transcriptional trans-activation of p53-responsive genes [171]. This may suggest how E6, utilizing the cell cycle regulatory kinases, presumably to enhance the virus life-cycle, can, in turn, enhance the survival of HPV-transformed cancer cells. However, the underlying molecular mechanism is unknown, and its elucidation could be crucial in determining the possible role of phosphorylated E6 in maintaining the integrity of the cancer cell. So far, we know that E6 is phosphorylated by exogenous DNA-damaging agents, but the question arises: is E6 phosphorylated without inducing the DDR in cervical cancer cells? - if it does, then in response to what, and in what scenario, does E6 use the same kinases that it uses under DNA damage conditions? If phosphorylation of E6 is dependent on DNA damage, then does DNA damage caused by the E7 oncoprotein, in the initial setting of viral propagation, induce E6 phosphorylation? What are the other biological consequences of this phosphorylated form of E6 in cancer progression? It will also be interesting to explore the question of whether E7 undergoes post-translational modifications, as E6 does, upon inducing DNA damage in cervical cancer.
All these observations support the premise that the survival of HPV-transformed cells depends on the preservation of a basal level of DNA repair activity, mediated by cellular machinery, to maintain a minimal genomic stability. The fact that HPV-positive cancers display no obvious mutations in the DNA damage checkpoint-responsive genes makes them potential therapeutic targets. In fact, HPV-positive cervical and head-and-neck cancers are recognised as being more radio-sensitive than HPV-negative lesions, but resistance to DNA damaging therapy still exists, as evidenced by poor survival outcomes in advanced HPV-positive cervical cancer. Therefore, therapeutic modulation of the DDR is an attractive strategy to increase the treatment response.
Increased radio-resistance in cervical cancer cells was associated with amplified activity of DNA-PKcs, the key NHEJ (non-homologous end-joining) protein [172], while DNA-PKcs inhibition correlates with enhanced chemo- and radio-sensitivity of cervical cell lines [173,174], and selective inhibitors of DNA-PKcs [175] enhance tumour cell death when used in combination with DNA damage-inducing chemotherapeutic agents and ionizing radiation [176]. However, the biochemical basis of this sensitivity is not yet clear and clinical studies are lacking. A phase I clinical trial of a DNA-PK inhibitor in combination with radiotherapy is ongoing in advanced solid tumours (Identifier number: NCT02516813), but not in HPV-positive cervical cancer, where this data suggests it might be effective.
Similarly, ATM inhibitors used with dsDNA break-stimulating agents appear promising in xenograft mouse tumour models [[177], [178], [179]] and one, AZD0156, is in phase I trial (Identifier no. NCT02588105). Observations have shown that p53-deficient cells are sensitive to ATR inhibition, as there is an increased dependence on ATR-mediated checkpoint control after the loss of p53 [180,181], and it is possible this might apply to cells with abrogated p53 due to HPV infection. Several phase I trials of ATR and ATM inhibitors are underway, however none specifically in cervical cancer. An inhibitor of Chk1 (a downstream kinase of ATR) has also been developed and a phase I trial in squamous cell cancers suggests it has low toxicity [182]. Thus, the strategic combination of CKII inhibition with DDR regulators, either within themselves, or with chemotherapy and radiotherapy, may offer therapeutic potential for HPV-mediated carcinogenesis. However, we need to be aware of possible long-term adverse effects of such treatments, and hence, an improved knowledge of the mechanism of action of these inhibitors, both biochemical and clinical, is needed.
7. Angiogenesis
Angiogenesis is required for invasive tumour growth and metastasis and plays an important role in the development and progression of cancer. HPV oncoproteins activate the angiogenic switch, promoting vessel formation: HPV16 E6 and E7 oncoproteins upregulate pro-angiogenic HIF-1α and VEGF, and reduce expression of anti-angiogenic prolyl 4-hydroxylase PHD2 in cervical carcinoma cells, together correlating with increased angiogenesis [44,[183], [184], [185]]. Blocking PI3K/Akt and ERK1/2 signalling pathways leads to inhibition of HPV-16 E6 and E7-induced HIF-1α protein accumulation, and VEGF protein secretion [44], summarised in Fig. 5. However, the precise molecular mechanism by which HPV oncoproteins disrupt the balance between pro- and anti-angiogenic factors remain unclear. E6 upregulates the VEGF promoter by binding to a region of four SP-1 sites in the VEGF proximal promoter region [186], while E7 exerts its upregulatory effect on VEGF expression through hTERT and telomerase activity [187], although whether their stimulation of VEGF expression is direct or indirect is not yet clear. This tightly controlled upregulation of VEGF indicates its importance for the HPV lifecycle, but it is obviously also important in the later stages of HPV-induced cancer and is a potential target for therapeutic approaches. Evidence suggests that the inhibition of VEGF in viral oncogenesis and viral diseases could be a promising therapeutic approach [188,189] to treating cervical intraepithelial neoplasia 3 (CIN 3) [190], or, in combination with radiotherapy, carcinoma in situ [191]. Furthermore, anti-VEGF monoclonal antibody treatment in combination with chemotherapy and radiation treatment apparently delayed metastatic progression of a number of different solid tumours [[192], [193], [194], [195]].
However, a clearer understanding of whether these anti-angiogenic agents normalize or disrupt the vascular structure will permit their optimal clinical use. Most clinical trials are designed to measure changes in tumour size and may not shed light on changes in their vascular biology. It would also be interesting to determine if these drugs have any effects on the functions of HPV E6/E7 oncoproteins per se, since these drugs target their downstream effector proteins.
8. Evading the immune response
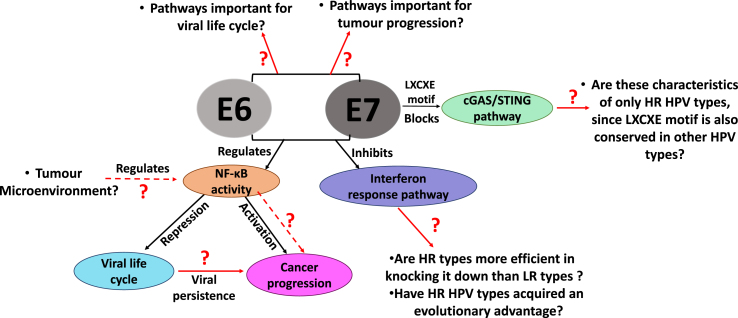
Most virus infections trigger a variety of immune responses that must be suppressed to allow the virus to replicate and generate viral progeny. Therefore, viruses have evolved many strategies to manipulate and circumvent the immune system by targeting different cellular pathways that would lead to its clearance from the host cell [196], see also Fig. 5. As part of the innate immune response, interferon release is an early line of defence to fight viral infection, and papillomaviruses have developed ways to disrupt this by targeting several genes involved in its activation [197]. Some of the remaining questions about how E6 and E7 do this are illustrated in Fig. 3.
Fig. 3.
Schematic diagram representing the unanswered questions about how E6 and E7 contribute to evasion of the host cell immune responses to enable the viral life-cycle, but also contributing to virus-induced carcinogenesis.
HPV-16 E6 was reported to interact with Interferon response factor-3 (IRF-3), a transcriptional regulator of type I IFN (IFN-α/β). This interaction may attenuate IRF3 transcriptional activity, reducing the induction of IFN β, although this has not been confirmed [198]. In addition, HPV-18 E6 acts more strongly than HPV-11 E6 to prevent the activation of Tyk-2 kinase, thus reducing its activation of STAT1 and STAT2 and preventing the activation of INF-α stimulated JAK-STAT signalling [199]. Over-expression of high-risk HPV-16 E6 in cervical keratinocytes leads to reduced production of IFN-α and-β mRNAs and diminished intracellular expression of IFN-α, while re-localising STAT1 from the nucleus, thus preventing its transactivation of interferon-responsive genes [200].
The cGAS/STING pathway also activates type I interferon signalling and may induce cell death through apoptosis, pyroptosis and necroptosis [201]. During the initial stages of HPV infection, the virus uses the cellular vesicular trafficking system for the HPV genome to gain entry to the nucleus, and it is possible that this also allows it to evade GAS/STING surveillance, at that stage of the life cycle, at least [202]. However, HPV-18 E7 was reported to bind and inhibit the STING protein [123], via the LXCXE motif, thereby reducing the production of proinflammatory cytokines.
E7 also recruits the TRIM21 ubiquitin ligase to degrade IFI16, a viral DNA sensor, thus inhibiting inflammasome-induced pyroptosis [127]. Another DNA-detection protein, Toll-like receptor 9 (TLR9), is also suppressed in keratinocytes expressing E6/E7 [203], although in this case the downregulation seems to be at the transcriptional level [204,205]. Interestingly, there are differences between high-risk HPV types in their downregulation of TLR9: HPV-16 reduces the levels of TLR9 more efficiently than HPV-18 [203], while the low-risk HPV-6 does not affect TLR9 transcription, suggesting that HPV's ability to subvert this aspect of the immune response might correlate with its carcinogenic potential, with a more immunosuppressive microenvironment in mucosal epithelia favouring a persistent infection and progression of the cancerous phenotype.
Thus, interferon responses are targeted by HPV at multiple points, highlighting the importance of interferon signalling in antiviral immune responses and how HPV counteracts that response. It is interesting to note that the E6 and E7 proteins of the high-risk viruses – but not the low-risk viruses – can selectively inhibit the interferon response. This raises the interesting question of what makes high-risk HPV types more efficient in counteracting immune surveillance. Are they more predisposed to evolve this ability owing to differential selective pressure to avoid their clearance, which allows them to accumulate genetic mutations giving additional opportunities to evade and target the immune response pathways, further correlating with their increased oncogenicity? Do these genetic alterations in the high-risk viruses coincide with regions of the proteins that are important for immune function?
Finally, it is worth remembering that all viruses, whether HPV or others, such as EBV MCPyV and HTLV-1, that induce cancer in the host must persist for many years. Thus, whilst overcoming the immune response is an essential weapon in a virus's armoury, needed to complete the life cycle, it is also a critical part of the ability to cause cancer. A major unanswered question is, undoubtedly: which immune pathways are critical only for productive viral infection, and which others play a fundamental role in HPV-driven tumorigenesis?
HPV E6 and E7 oncoproteins are also known to downregulate the NF-кB pathway [[206], [207], [208], [209]], which plays a crucial role in eliciting immune responses by stimulating the expression of the genes involved in the production of cytokines, cytokines receptors and antigen presentation [200]. NF-кB activates the interleukin-8 (IL-8) promotor, a chemoattractant for T lymphocytes and neutrophils, and HPV E6 and E7 can repress IL-8 mRNA in keratinocytes [210]. In the case of E7, binding to pCAF protein is required for IL-8 downregulation, and cervical cells expressing wild type HPV-16 E7 have reduced NF-кB activity [289], whilst cells expressing an E7 mutant in pRb binding do not [208]. Various HPV E7 mutants show a reduced ability to prevent NF-кB transactivation, suggesting that multiple regions of E7 are required. Controversially, the NF-кB pathway is highly activated in cervical cancers and some studies suggest that E6/E7 oncoproteins promote this activation [200,289,212]. It is therefore possible that NF-кB signalling pathways play an inhibitory role during HPV infection, or in the initial stages of the viral infection, however, once the epithelial cells are transformed, NF-кB activation might promote tumorigenesis. In agreement with this hypothesis, there appears to be a significant association between NF-кB activation, tumour progression and poor prognosis in cervical cancer [213], and studies in other carcinomas have shown that NF-кB is dispensable during early premalignant phases, but is essential for subsequent tumour promotion [214]. Interestingly, both high-and low-risk E7 proteins can counteract imiquimod-induced NF-кB activation, preventing IL-6 production [209], suggesting again that E7's inactivation of this transcription factor may be more related to the HPV life cycle than to malignancy.
Activation of NF-kB changes the tumour microenvironment, favouring the progression of inflammatory associated cancers. Therefore, it is likely that the HPV mediated activation of NF-kB in cervical cancer and HSILs is likely to be partially in response to the signals or chemokines released by the tumour microenvironment [214]. Hence, it will be very interesting to determine whether the tumour microenvironment plays any role in HPV-induced regulation of NF-kB activity and the progression to carcinogenesis. Taken together, NFκB modulation seems to first facilitate viral persistence and immune evasion, and later to drive tumour progression, but because of the many conflicting results and the complexity of its signalling networks, great caution is required in interpreting the role of NFκB in HPV-induced carcinogenesis, and further work is still required, particularly at different stages of tumour development.
9. PDZ-binding motifs and cellular polarity
The cancer-associated HPV types characteristically have a PDZ binding motif (PBM) at the extreme C-terminus of E6, through which they target a number of cellular proteins containing PDZ domains - 80–90 amino acid domains that form binding pockets involved in multiple protein-protein interactions. Deletion of the HPV-16 E6 PBM prevents deregulation of the cell cycle, cell immortalisation, and the formation of cancer in transgenic mice [[215], [216], [217], [218]], at least in part through its enhancement of the nuclear localisation of YAP1 [219]. However, the PBM is also required for a number of activities that prevent the cellular changes that lead to malignancy - lack of the PBM in the context of the whole HPV-18 genome leads to an increase in the frequency of abnormal mitoses [220] - and for the episomal maintenance of the viral genome [[221], [222], [223]].
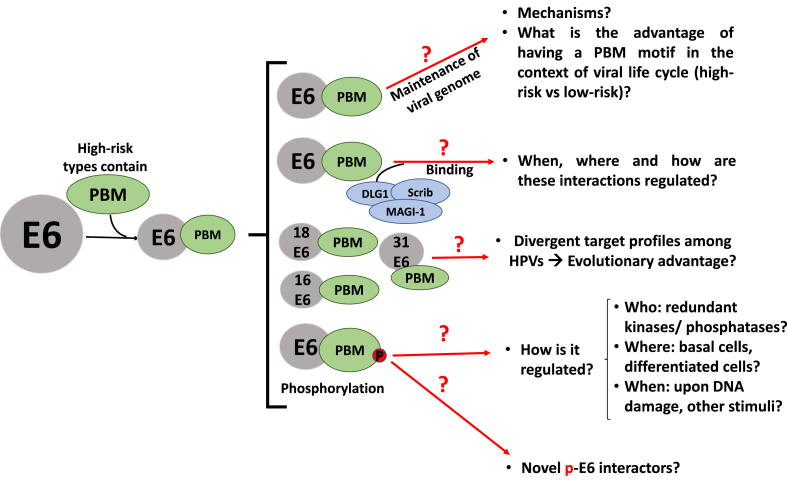
The most well-known PDZ domain-containing targets of the high-risk E6 PBM are proteins involved in cellular polarity, including core components of the polarity control pathways - DLG1, hScrib, PATJ, PAR3, as well as the tight junction protein MAGI-1 (mentioned above), all of which are targeted for ubiquitin-mediated degradation at the proteasome, thus disrupting cellular polarity control (reviewed in Refs. [154,224,225]) However, the diverse sequences of the E6 PBMs from different HPV types leads to their quite distinct target selectivity profiles [226,227], -illustrated in Table 1, with DLG1 being almost the only target consistently bound by all E6 PBMs, suggesting that it may have been the original PDZ target in the adaptation to replication in the muco-cutaneous evolutionary niche [228,229]. Interestingly, stronger binding to Scrib by the E6 PBM appears to correlate with stronger cancer association of the HPV type [227,230], as does the degree of flexibility of binding, exemplified by the number of polarity proteins targeted. It is also interesting to note that while E6 induces Scrib degradation, it has also been shown to cooperate with Scrib in maintaining active mTORC signalling in HeLa cells [231], thus confirming yet again that many of the E6 interactions are context-dependent. However, a key element appears to be the multifunctionality of HPV-16 and HPV-18 E6s targeting multiple different PDZ-containing proteins and pathways. It is intriguing that the most prominent high-risk types - HPV-16 and -18 - are also the most multifunctional; does this multifunctionality associate with increased fitness of the virus? Certainly, targeting multiple pathways would seem to increase cancer-causing ability. However, a major question remains as to which PDZ interactions are relevant at different stages of the life-cycle and at different stages of malignancy (Fig. 4). Indeed, in low-grade lesions Scrib and DLG1 are very highly expressed and mislocalised [232], which could be indicative of an oncogenic function, which later is no longer needed and ultimately both proteins are absent from late-stage cancers. Finally, how the targeting of PDZ proteins cooperates with other biochemical targets still remains to be defined.
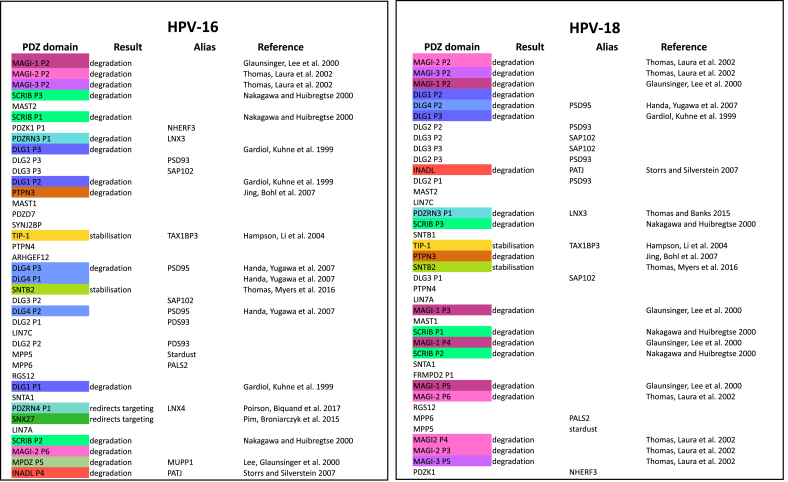
Table 1.
HPV-16 and HPV-18 E6s bind the same target proteins with different affinities and through different PDZ domains.
It has been shown in a number of studies that the E6 oncoproteins of different HPV types differ in their binding affinities for their various cellular target proteins [4,227,284]. This was demonstrated particularly clearly by Ref. [4]; who measured the binding affinities of HPV-16 and HPV-18 E6 for a huge array of different PDZ domains and in Table 1 we show the most strongly-bound PDZ domains of the two E6s in order, starting with the highest affinity, and with the previously confirmed interacting proteins defined in different colours.
It is immediately clear that the order of the most-favoured binding partners differs between the two E6s, although the second PDZ domains of the MAGI protein family members are confirmed as the highest binders of both [62,63,285], while SCRIB and DLG1 are, respectively, the second-favoured proteins of HPV-16 and HPV-18, as would be expected from previous results [226] and that HPV-16, but not HPV-18, binds to PDZRN4 [5].
Interestingly, however, the specific PDZ domain of a given protein also seems to differ: DLG1 binding was initially determined using HPV-18 and PDZ2 was defined as the binding domain, however HPV-16 appears to favour PDZ3; given the sequence differences between the two PBMs (16E6 is -SSRTRRETQL; 18E6 is -RLQRRRETQV) this may reflect the binding capacity of each PDZ domain for either a Leu (16E6) or Val (18E6) as the final residue, and there may well be a contribution from the upstream serines to 16E6 binding [229,286]. Thus, although the high-risk E6 proteins target a common set of PDZ-containing cellular proteins, it is clear that they may not all target them through the same PDZ domain and thus may compete with the binding of different cellular PDZ-binding proteins, with potentially differing secondary downstream effects within the infected cell.
It is also clear from this list that although many of these cellular proteins have been previously defined as E6 targets [[5], [59], [118], [119], [227], [287], [288], [290], [291], [292], [294], [295]], nearly half of the PDZ domains bound in this assay are from proteins that have not been so defined, and it might well be worth examining more closely their potential as targets, under various cellular conditions such as differentiation, hypoxia, or low nutrients.
Fig. 4.
Some crucial questions that need to be answered to understand the role of HR HPV E6 PBM in causing carcinogenesis.
Although E6 induces degradation of the AJ proteins DLG1 and Scrib, and the tight junction MAGI-1, it also stabilises the ZO-2 TJ protein to increase cell proliferation [227,233], while E6 and E7 together downregulate the AJ protein E-cadherin through cdc6 induction to increase proliferative signalling.
The E6 PBM is regulated by phosphorylation: upon phosphorylation the binding affinity switches completely from PDZ domains to members of the 14-3-3 family [[234], [235], [236]], although the kinases involved differ between different HPV types [171,236,237]. Interestingly, this PDZ to 14-3-3 phospho-switch has also been demonstrated in a number of cellular protein PBMs [238], suggesting that this may be a common method of specificity control, and also that there are contexts in which PDZ protein-dependent and 14-3-3 protein-dependent signalling are inversely linked in the cell. Indeed, 14-3-3 remains an enigma and it is still unclear whether specific isoforms are more relevant than others. There are also major open questions about whether the phosphorylation of E6 might also confer the ability to interact with other cellular targets.
10. Interactions with ubiquitin ligases
E6 and E7 co-opt cellular E3 ubiquitin-protein ligases to label their cellular target proteins (reviewed in Refs. [5,239,240]). The best known of these interactions are the E6/E6AP interaction with p53 [241] and the E7/CUL2 interaction with pRb [242], but a number of other partnerships have been identified. Determining the relevance of these interactions is complicated by the fact that E6 requires E6AP for its stability [243], and thus the presence of E6AP is necessary for the development of cancers in transgenic mice [215]. It is clear that in several cases more than one ligase can be used, for example p53, MAGI-1 and Scrib can be degraded in E6AP-dependent and -independent manners [58,119,[244], [245], [246], [247], [248], [249]]. It would be interesting to know if the cellular pools of the target proteins are the same in each case, since it has been clearly shown by proteasome inhibition of HPV-positive and HPV-negative tumour cell lines that E6 can discriminate between different pools of its target proteins [250], with high levels of nuclear DLG1 being rescued in inhibitor-treated HPV-positive cells only. In addition, E6 preferentially targets the MAGI-1c isoform [57], which is the only isoform with a nuclear localisation sequence [251].
Interestingly, E6 uses the ligase EDD to induce the degradation of TIP-60 [252], but also enhances EDD's activity in inducing the degradation of E6AP itself [253], which in turn leads to a reduction in E6 levels. This type of negative feedback is also seen when E6 induces the degradation of Scrib [119], while Scrib enhances the expression of E6 [231]. This suggests that these interactions may be involved in maintaining the correct levels of the HPV oncoproteins to further the virus life-cycle while not pushing the cell dangerously close to immortalisation, in which case the life-cycle would cease.
11. Proliferative and differentiation-inducing signalling
HPV E6 targets NOTCH, a signalling pathway that has both tumour suppressor and oncogenic outcomes depending on context [254,255], and which is important in keratinocyte differentiation [256,257]. NOTCH signalling is repressed by E6 proteins from non-alpha types, through binding to MAML-1 transcriptional coactivators [14,16], repressing MAML-1's enhancement of NOTCH signalling and thus delaying keratinocyte differentiation [13,258]. MAML-1 binding occurs through a motif similar to that used by alpha-HPV E6 proteins to bind E6AP [259,260]; alpha-HPV E6s have not been shown to bind MAML-1 in this way, and this may be a defining difference between mucosal and cutaneous HPVs, reflecting potential differences in keratinocyte differentiation-inducing signalling in the respective host cell types. Nonetheless high-risk alpha HPVs do target NOTCH signalling: NOTCH downregulation is required to maintain E6/E7 expression in cervical cancer cell lines [261,262] and loss of NOTCH function stimulates HPV-16-driven head and neck carcinogenesis [263].
E6AP is required for E6 to induce degradation of NHERF1 [264,265], thus relieving NHERF1 inhibition of canonical Wnt/β-catenin signalling and allowing nuclear accumulation of β-catenin [266]. However, since E6AP alone can enhance β-catenin nuclear accumulation [267], the role of the E6-E6AP interaction is not yet entirely clear, and different HPV types may vary in their methods of achieving the same effects. It has been suggested that the E6 PBM is also required [268], but since low-risk HPV E6 also appears to induce NHERF1 degradation [269], this may not be necessary. NHERF1 targeting by both high- and low-risk E6s suggests that, like the similarly targeted Bak [52], the downstream effects of the interaction are essential for the virus life cycle. However, E6's targeting of the related NHERF2 [270], leading to upregulated p27 and Cyclin D, is PBM-dependent and may enhance tumorigenic potential. This suggests the potential danger for the high-risk viruses in targeting multiple proliferative signalling pathways at the same time.
12. Telomerase activation
A key feature of E6-induced malignancy appears to be activation of Telomerase [271,272], and indeed it is a feature of the majority, if not all, human cancers (reviewed in Ref. [273]). HPV E6s activate telomerase through pathways that are still controversial: with various studies suggesting or refuting the requirements for cooperation with myc [[274], [275], [276], [277], [278]], NFX1 [279,280], or E6AP [[280], [281],[211], [282], [283]]. It is entirely possible that, as with other HPV oncogene activities, different virus types employ different mechanisms to achieve the same end. In addition, it is not clear to what extent the potential involvement of E6AP may be related to E6AP's stabilising effect upon E6 [243], rather to than its ubiquitin ligase activity. By whatever means the activation is effected, a major question is why this interaction arose in the first place? Clearly in its classical function, the activation of telomerase is irrelevant for the viral life-cycle, but can play a role in tumorigenesis. This brings us to the wider question of multifunctional proteins, and whether the activities targeted by E6 and E7 to enhance the viral life-cycle differ from those that play a role in tumour development, and the latter are unfortunate by-products or mistakes.
13. Conclusion
It is clear that identification of a potential interaction between an HPV oncoprotein and a cellular target is only the start; the context-dependence must be clarified. Determining whether the interaction is place-specific: i.e. where in the cell, or where in the epithelium: whether it is time specific: i.e what phase of the cell cycle, or when in the life cycle. The target's context is also vitally important - certain HPV targets can have both tumour suppressive and oncogenic characteristics, e.g. DLG1, Scrib, MAGI-1, p53. So not only is it necessary to determine what is targeted, but also, when, where, how...and why.
In the case of many target proteins, for example Telomerase, or PDZ proteins, it may well be that we don't yet understand all their biological effects and, as a corollary, such unknown functions may be what the virus is aiming to target or manipulate. Hence, the great power of virology lies in using uniquely evolved viral interactions to unpick the mechanisms regulating cellular homeostasis in life and death.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We would like to thank Om Basukala for very useful discussions on the manuscript, we are also grateful to Giannino Del Sal. AV and OT-C are recipients of ICGEB Arturo Falaschi Fellowships. LB is a recipient of Grant no. IG2019 - ID. 23572 from the Associazione Italiana per la Ricerca sul Cancro.
References
- 1.Hoppe-Seyler K., Bossler F., Braun J.A., Herrmann A.L., Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26(2):158–168. doi: 10.1016/j.tim.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Rozenblatt-Rosen O., Deo R.C., Padi M., Adelmant G., Calderwood M.A., Rolland T., Grace M., Dricot A., Askenazi M., Tavares M., Pevzner S.J., Abderazzaq F., Byrdsong D., Carvunis A.R., Chen A.A., Cheng J., Correll M., Duarte M., Fan C., Feltkamp M.C., Ficarro S.B., Franchi R., Garg B.K., Gulbahce N., Hao T., Holthaus A.M., James R., Korkhin A., Litovchick L., Mar J.C., Pak T.R., Rabello S., Rubio R., Shen Y., Singh S., Spangle J.M., Tasan M., Wanamaker S., Webber J.T., Roecklein-Canfield J., Johannsen E., Barabasi A.L., Beroukhim R., Kieff E., Cusick M.E., Hill D.E., Munger K., Marto J.A., Quackenbush J., Roth F.P., DeCaprio J.A., Vidal M. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature. 2012;487(7408):491–495. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White E.A., Sowa M.E., Tan M.J.A., Jeudy S., Hayes S.D., Santha S., Münger K., Harper J.W., Howley P.M. Systematic identification of interactions between host cell proteins and E7 oncoproteins from diverse human papillomaviruses. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(5):E260–E267. doi: 10.1073/pnas.1116776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincentelli R., Luck K., Poirson J., Polanowska J., Abdat J., Blemont M., Turchetto J., Iv F., Ricquier K., Straub M.L., Forster A., Cassonnet P., Borg J.P., Jacob Y., Masson M., Nomine Y., Reboul J., Wolff N., Charbonnier S., Trave G. Quantifying domain-ligand affinities and specificities by high-throughput holdup assay. Nat. Methods. 2015;12(8):787–793. doi: 10.1038/nmeth.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirson J., Biquand E., Straub M.L., Cassonnet P., Nominé Y., Jones L., van der Werf S., Travé G., Zanier K., Jacob Y. Mapping the interactome of HPV E6 and E7 oncoproteins with the ubiquitin‐proteasome system. FEBS J. 2017;284(19):3171–3201. doi: 10.1111/febs.14193. [DOI] [PubMed] [Google Scholar]
- 6.Murakami I., Egawa N., Griffin H., Yin W., Kranjec C., Nakahara T., Kiyono T., Doorbar J. Roles for E1-independent replication and E6-mediated p53 degradation during low-risk and high-risk human papillomavirus genome maintenance. PLoS Pathog. 2019;15(5) doi: 10.1371/journal.ppat.1007755. e1007755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White E.A. Manipulation of epithelial differentiation by HPV oncoproteins. Viruses. 2019;11(4) doi: 10.3390/v11040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyson N., Howley P.M., Munger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 9.Munger K., Werness B.A., Dyson N., Phelps W.C., Harlow E., Howley P.M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies R., Hicks R., Crook T., Morris J., Vousden K. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J. Virol. 1993;67(5):2521–2528. doi: 10.1128/jvi.67.5.2521-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu T., Ferril S., Snider A., Barbosa M. In-vivo analysis of hpv e7 protein association with prb, p107 and p130. Int. J. Oncol. 1995;6(1):167–174. doi: 10.3892/ijo.6.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Palopoli N., Gonzalez Foutel N.S., Gibson T.J., Chemes L.B. Short linear motif core and flanking regions modulate retinoblastoma protein binding affinity and specificity. Protein Eng. Des. Sel. 2018;31(3):69–77. doi: 10.1093/protein/gzx068. [DOI] [PubMed] [Google Scholar]
- 13.Meyers J.M., Uberoi A., Grace M., Lambert P.F., Munger K. Cutaneous HPV8 and MmuPV1 E6 proteins target the NOTCH and TGF-beta tumor suppressors to inhibit differentiation and sustain keratinocyte proliferation. PLoS Pathog. 2017;13(1) doi: 10.1371/journal.ppat.1006171. e1006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers J.M., Grace M., Uberoi A., Lambert P.F., Munger K. Inhibition of TGF-beta and NOTCH signaling by cutaneous papillomaviruses. Front. Microbiol. 2018;9:389. doi: 10.3389/fmicb.2018.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storey A., Thomas M., Kalita A., Harwood C., Gardiol D., Mantovani F., Breuer J., Leigh I.M., Matlashewski G., Banks L. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393(6682):229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 16.Brimer N., Drews C.M., Vande Pol S.B. Association of papillomavirus E6 proteins with either MAML1 or E6AP clusters E6 proteins by structure, function, and evolutionary relatedness. PLoS Pathog. 2017;13(12) doi: 10.1371/journal.ppat.1006781. e1006781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Ko L.J., Jayaraman L., Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10(19):2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 18.Kranjec C., Holleywood C., Libert D., Griffin H., Mahmood R., Isaacson E., Doorbar J. Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch. J. Pathol. 2017;242(4):448–462. doi: 10.1002/path.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doorbar J., Griffin H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019;7:176–179. doi: 10.1016/j.pvr.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakatani Y., Konishi H., Vassilev A., Kurooka H., Ishiguro K., Sawada J., Ikura T., Korsmeyer S.J., Qin J., Herlitz A.M. p600, a unique protein required for membrane morphogenesis and cell survival. Proc. Natl. Acad. Sci. U. S. A. 2005;102(42):15093–15098. doi: 10.1073/pnas.0507458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh K.W., DeMasi J., Ogawa H., Nakatani Y., Howley P.M., Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc. Natl. Acad. Sci. U. S. A. 2005;102(32):11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMasi J., Huh K.W., Nakatani Y., Munger K., Howley P.M. Bovine papillomavirus E7 transformation function correlates with cellular p600 protein binding. Proc. Natl. Acad. Sci. U. S. A. 2005;102(32):11486–11491. doi: 10.1073/pnas.0505322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.T., Lee Y.J., Tasaki T., Hwang J., Kang M.J., Yi E.C., Kim B.Y., Kwon Y.T. The N-recognin UBR4 of the N-end rule pathway is required for neurogenesis and homeostasis of cell surface proteins. PloS One. 2018;13(8) doi: 10.1371/journal.pone.0202260. e0202260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duensing S., Lee L.Y., Duensing A., Basile J., Piboonniyom S., Gonzalez S., Crum C.P., Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. U. S. A. 2000;97(18):10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akagi K., Li J., Broutian T.R., Padilla-Nash H., Xiao W., Jiang B., Rocco J.W., Teknos T.N., Kumar B., Wangsa D., He D., Ried T., Symer D.E., Gillison M.L. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014;24(2):185–199. doi: 10.1101/gr.164806.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen C.L., Eichwald C., Nibert M.L., Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J. Virol. 2007;81(24):13533–13543. doi: 10.1128/JVI.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duensing A., Liu Y., Tseng M., Malumbres M., Barbacid M., Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25(20):2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korzeniewski N., Treat B., Duensing S. The HPV-16 E7 oncoprotein induces centriole multiplication through deregulation of Polo-like kinase 4 expression. Mol. Canc. 2011;10:61. doi: 10.1186/1476-4598-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y., Munger K. Human papillomavirus type 16 E7 oncoprotein engages but does not abrogate the mitotic spindle assembly checkpoint. Virology. 2012;432(1):120–126. doi: 10.1016/j.virol.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordignon V., Di Domenico E.G., Trento E., D'Agosto G., Cavallo I., Pontone M., Pimpinelli F., Mariani L., Ensoli F. How human papillomavirus replication and immune evasion strategies take advantage of the host DNA damage repair machinery. Viruses. 2017;9(12):390. doi: 10.3390/v9120390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bristol M.L., Das D., Morgan I.M. Why human papillomaviruses activate the DNA damage response (DDR) and how cellular and viral replication persists in the presence of DDR signaling. Viruses. 2017;9(10) doi: 10.3390/v9100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luftig M.A. Viruses and the DNA damage response: activation and antagonism. Ann. Rev. Virol. 2014;1(1):605–625. doi: 10.1146/annurev-virology-031413-085548. [DOI] [PubMed] [Google Scholar]
- 33.Gaglia M.M., Munger K. More than just oncogenes: mechanisms of tumorigenesis by human viruses. Curr. Opinion Virol. 2018;32:48–59. doi: 10.1016/j.coviro.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin K.H., Ahn J.H., Kang M.K., Lim P.K., Yip F.K., Baluda M.A., Park N.H. HPV-16 E6 oncoprotein impairs the fidelity of DNA end-joining via p53-dependent and -independent pathways. Int. J. Oncol. 2006;28(1):209–215. [PubMed] [Google Scholar]
- 35.Mantovani F., Banks L. Inhibition of E6 induced degradation of p53 is not sufficient for stabilization of p53 protein in cervical tumour derived cell lines. Oncogene. 1999;18(22):3309–3315. doi: 10.1038/sj.onc.1202688. [DOI] [PubMed] [Google Scholar]
- 36.Giampieri S., Garcia-Escudero R., Green J., Storey A. Human papillomavirus type 77 E6 protein selectively inhibits p53-dependent transcription of proapoptotic genes following UV-B irradiation. Oncogene. 2004;23(34):5864–5870. doi: 10.1038/sj.onc.1207711. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Tavana O., Gu W. p53 modifications: exquisite decorations of the powerful guardian. J. Mol. Cell Biol. 2019;11(7):564–577. doi: 10.1093/jmcb/mjz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hupp T.R., Meek D.W., Midgley C.A., Lane D.P. Regulation of the specific DNA binding function of p53. Cell. 1992;71(5):875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 39.Shieh S.Y., Ikeda M., Taya Y., Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91(3):325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 40.Shieh S.Y., Ahn J., Tamai K., Taya Y., Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 41.Olsson A., Manzl C., Strasser A., Villunger A. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 2007;14(9):1561–1575. doi: 10.1038/sj.cdd.4402196. [DOI] [PubMed] [Google Scholar]
- 42.Taira N., Nihira K., Yamaguchi T., Miki Y., Yoshida K. DYRK2 is targeted to the nucleus and controls p53 via Ser46 phosphorylation in the apoptotic response to DNA damage. Mol. Cell. 2007;25(5):725–738. doi: 10.1016/j.molcel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Sykes S.M., Mellert H.S., Holbert M.A., Li K., Marmorstein R., Lane W.S., McMahon S.B. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell. 2006;24(6):841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang X., Zhang Q., Nishitani J., Brown J., Shi S., Le A.D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin. Canc. Res. 2007;13(9):2568–2576. doi: 10.1158/1078-0432.CCR-06-2704. [DOI] [PubMed] [Google Scholar]
- 45.Jha S., Vande Pol S., Banerjee N.S., Dutta A.B., Chow L.T., Dutta A. Destabilization of TIP60 by human papillomavirus E6 results in attenuation of TIP60-dependent transcriptional regulation and apoptotic pathway. Mol. Cell. 2010;38(5):700–711. doi: 10.1016/j.molcel.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel D., Huang S.M., Baglia L.A., McCance D.J. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18(18):5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernat A., Avvakumov N., Mymryk J.S., Banks L. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene. 2003;22(39):7871. doi: 10.1038/sj.onc.1206896. [DOI] [PubMed] [Google Scholar]
- 48.Gu W., Shi X.L., Roeder R.G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387(6635):819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 49.Gu W., Roeder R.G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 50.Reed S.M., Quelle D.E. p53 acetylation: regulation and consequences. Cancers. 2014;7(1):30–69. doi: 10.3390/cancers7010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas M., Banks L. Inhibition of bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17(23):2943–2954. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- 52.Thomas M., Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J. Gen. Virol. 1999;80(Pt 6):1513–1517. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 53.Jackson S., Harwood C., Thomas M., Banks L., Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14(23):3065–3073. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holloway A., Simmonds M., Azad A., Fox J.L., Storey A. Resistance to UV-induced apoptosis by beta-HPV5 E6 involves targeting of activated BAK for proteolysis by recruitment of the HERC1 ubiquitin ligase. Int. J. Canc. 2015;136(12):2831–2843. doi: 10.1002/ijc.29350. [DOI] [PubMed] [Google Scholar]
- 55.Du J., Chen G., Vlantis A., Chan P., Tsang R., Van Hasselt C. Resistance to apoptosis of HPV 16-infected laryngeal cancer cells is associated with decreased Bak and increased Bcl-2 expression. Canc. Lett. 2004;205(1):81–88. doi: 10.1016/j.canlet.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 56.Magal S.S., Jackman A., Ish-Shalom S., Botzer L.E., Gonen P., Schlegel R., Sherman L. Downregulation of Bax mRNA expression and protein stability by the E6 protein of human papillomavirus 16. J. Gen. Virol. 2005;86(Pt 3):611–621. doi: 10.1099/vir.0.80453-0. [DOI] [PubMed] [Google Scholar]
- 57.Glaunsinger B.A., Lee S.S., Thomas M., Banks L., Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19(46):5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pim D., Thomas M., Javier R., Gardiol D., Banks L. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene. 2000;19(6):719–725. doi: 10.1038/sj.onc.1203374. [DOI] [PubMed] [Google Scholar]
- 59.Thomas M., Glaunsinger B., Pim D., Javier R., Banks L. HPV E6 and MAGUK protein interactions: determination of the molecular basis for specific protein recognition and degradation. Oncogene. 2001;20(39):5431–5439. doi: 10.1038/sj.onc.1204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gregorc U., Ivanova S., Thomas M., Guccione E., Glaunsinger B., Javier R., Turk V., Banks L., Turk B. Cleavage of MAGI-1, a tight junction PDZ protein, by caspases is an important step for cell-cell detachment in apoptosis. Apoptosis. 2007;12(2):343–354. doi: 10.1007/s10495-006-0579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanova S., Repnik U., Banks L., Turk V., Turk B. Cellular localization of MAGI-1 caspase cleavage products and their role in apoptosis. Biol. Chem. 2007;388(11):1195–1198. doi: 10.1515/BC.2007.141. [DOI] [PubMed] [Google Scholar]
- 62.Thomas M., Laura R., Hepner K., Guccione E., Sawyers C., Lasky L., Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21(33):5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- 63.Kranjec C., Massimi P., Banks L. Restoration of MAGI-1 expression in human papillomavirus-positive tumor cells induces cell growth arrest and apoptosis. J. Virol. 2014;88(13):7155–7169. doi: 10.1128/JVI.03247-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Butz K., Ristriani T., Hengstermann A., Denk C., Scheffner M., Hoppe-Seyler F. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene. 2003;22(38):5938–5945. doi: 10.1038/sj.onc.1206894. [DOI] [PubMed] [Google Scholar]
- 65.Yoshinouchi M., Yamada T., Kizaki M., Fen J., Koseki T., Ikeda Y., Nishihara T., Yamato K. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol. Ther. 2003;8(5):762–768. doi: 10.1016/j.ymthe.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Basukala O., Mittal S., Massimi P., Bestagno M., Banks L. The HPV-18 E7 CKII phospho acceptor site is required for maintaining the transformed phenotype of cervical tumour-derived cells. PLoS Pathog. 2019;15(5) doi: 10.1371/journal.ppat.1007769. e1007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Firzlaff J.M., Luscher B., Eisenman R.N. Negative charge at the casein kinase II phosphorylation site is important for transformation but not for Rb protein binding by the E7 protein of human papillomavirus type 16. Proc. Natl. Acad. Sci. U. S. A. 1991;88(12):5187–5191. doi: 10.1073/pnas.88.12.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Massimi P., Pim D., Storey A., Banks L. HPV-16 E7 and adenovirus E1a complex formation with TATA box binding protein is enhanced by casein kinase II phosphorylation. Oncogene. 1996;12(11):2325–2330. [PubMed] [Google Scholar]
- 69.Rey O., Lee S., Baluda M.A., Swee J., Ackerson B., Chiu R., Park N.H. The E7 oncoprotein of human papillomavirus type 16 interacts with F-actin in vitro and in vivo. Virology. 2000;268(2):372–381. doi: 10.1006/viro.1999.0175. [DOI] [PubMed] [Google Scholar]
- 70.Basukala O., Sarabia-Vega V., Banks L. Human papillomavirus oncoproteins and post-translational modifications: generating multifunctional hubs for overriding cellular homeostasis. Biol. Chem. 2020;401(5):585–599. doi: 10.1515/hsz-2019-0408. [DOI] [PubMed] [Google Scholar]
- 71.Solares A.M., Santana A., Baladron I., Valenzuela C., Gonzalez C.A., Diaz A., Castillo D., Ramos T., Gomez R., Alonso D.F., Herrera L., Sigman H., Perea S.E., Acevedo B.E., Lopez-Saura P. Safety and preliminary efficacy data of a novel casein kinase 2 (CK2) peptide inhibitor administered intralesionally at four dose levels in patients with cervical malignancies. BMC Canc. 2009;9:146. doi: 10.1186/1471-2407-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perea S.E., Baladron I., Valenzuela C., Perera Y. CIGB-300: a peptide-based drug that impairs the Protein Kinase CK2-mediated phosphorylation. Semin. Oncol. 2018;45(1–2):58–67. doi: 10.1053/j.seminoncol.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Zine El Abidine A., Tomaic V., Bel Haj Rhouma R., Massimi P., Guizani I., Boubaker S., Ennaifer E., Banks L. A naturally occurring variant of HPV-16 E7 exerts increased transforming activity through acquisition of an additional phospho-acceptor site. Virology. 2017;500:218–225. doi: 10.1016/j.virol.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 74.Massimi P., Banks L. Differential phosphorylation of the HPV-16 E7 oncoprotein during the cell cycle. Virology. 2000;276(2):388–394. doi: 10.1006/viro.2000.0514. [DOI] [PubMed] [Google Scholar]
- 75.Liang Y.J., Chang H.S., Wang C.Y., Yu W.C. DYRK1A stabilizes HPV16E7 oncoprotein through phosphorylation of the threonine 5 and threonine 7 residues. Int. J. Biochem. Cell Biol. 2008;40(11):2431–2441. doi: 10.1016/j.biocel.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Gross S., Rahal R., Stransky N., Lengauer C., Hoeflich K.P. Targeting cancer with kinase inhibitors. J. Clin. Invest. 2015;125(5):1780–1789. doi: 10.1172/JCI76094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han M., Sun D. Rational creation and systematic analysis of cervical cancer kinase-inhibitor binding profile. J. Comput. Aided Mol. Des. 2019;33(7):689–698. doi: 10.1007/s10822-019-00211-1. [DOI] [PubMed] [Google Scholar]
- 78.Pim D., Massimi P., Dilworth S.M., Banks L. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene. 2005;24(53):7830–7838. doi: 10.1038/sj.onc.1208935. [DOI] [PubMed] [Google Scholar]
- 79.Szalmas A., Tomaic V., Basukala O., Massimi P., Mittal S., Konya J., Banks L. The PTPN14 tumor suppressor is a degradation target of human papillomavirus E7. J. Virol. 2017;91(7) doi: 10.1128/JVI.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yun H.Y., Kim M.W., Lee H.S., Kim W., Shin J.H., Kim H., Shin H.C., Park H., Oh B.H., Kim W.K., Bae K.H., Lee S.C., Lee E.W., Ku B., Kim S.J. Structural basis for recognition of the tumor suppressor protein PTPN14 by the oncoprotein E7 of human papillomavirus. PLoS Biol. 2019;17(7) doi: 10.1371/journal.pbio.3000367. e3000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eichhorn P.J., Creyghton M.P., Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta. 2009;1795(1):1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Hwang S.G., Lee D., Kim J., Seo T., Choe J. Human papillomavirus type 16 E7 binds to E2F1 and activates E2F1-driven transcription in a retinoblastoma protein-independent manner. J. Biol. Chem. 2002;277(4):2923–2930. doi: 10.1074/jbc.M109113200. [DOI] [PubMed] [Google Scholar]
- 83.Tommasino M., Adamczewski J.P., Carlotti F., Barth C.F., Manetti R., Contorni M., Cavalieri F., Hunt T., Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8(1):195–202. [PubMed] [Google Scholar]
- 84.McIntyre M.C., Ruesch M.N., Laimins L.A. Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology. 1996;215(1):73–82. doi: 10.1006/viro.1996.0008. [DOI] [PubMed] [Google Scholar]
- 85.Zerfass-Thome K., Zwerschke W., Mannhardt B., Tindle R., Botz J.W., Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13(11):2323–2330. [PubMed] [Google Scholar]
- 86.Jones D.L., Alani R.M., Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11(16):2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Demers G.W., Espling E., Harry J.B., Etscheid B.G., Galloway D.A. Abrogation of growth arrest signals by human papillomavirus type 16 E7 is mediated by sequences required for transformation. J. Virol. 1996;70(10):6862–6869. doi: 10.1128/jvi.70.10.6862-6869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee K.A., Shim J.H., Kho C.W., Park S.G., Park B.C., Kim J.W., Lim J.S., Choe Y.K., Paik S.G., Yoon D.Y. Protein profiling and identification of modulators regulated by the E7 oncogene in the C33A cell line by proteomics and genomics. Proteomics. 2004;4(3):839–848. doi: 10.1002/pmic.200300626. [DOI] [PubMed] [Google Scholar]
- 89.Lee K.A., Kang J.W., Shim J.H., Kho C.W., Park S.G., Lee H.G., Paik S.G., Lim J.S., Yoon D.Y. Protein profiling and identification of modulators regulated by human papillomavirus 16 E7 oncogene in HaCaT keratinocytes by proteomics. Gynecol. Oncol. 2005;99(1):142–152. doi: 10.1016/j.ygyno.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 90.Roman A., Munger K. The papillomavirus E7 proteins. Virology. 2013;445(1–2):138–168. doi: 10.1016/j.virol.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bissell M.J., Radisky D. Putting tumours in context. Nat. Rev. Canc. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pickard A., Cichon A.C., Barry A., Kieran D., Patel D., Hamilton P., Salto-Tellez M., James J., McCance D.J. Inactivation of Rb in stromal fibroblasts promotes epithelial cell invasion. EMBO J. 2012;31(14):3092–3103. doi: 10.1038/emboj.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maeda N., Maenaka K. The roles of matricellular proteins in oncogenic virus-induced cancers and their potential utilities as therapeutic targets. Int. J. Mol. Sci. 2017;18(10):2198. doi: 10.3390/ijms18102198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spurgeon M.E., Lambert P.F. Human papillomavirus and the stroma: bidirectional crosstalk during the virus life cycle and carcinogenesis. Viruses. 2017;9(8) doi: 10.3390/v9080219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poltavets V., Kochetkova M., Pitson S.M., Samuel M.S. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 2018;8:431. doi: 10.3389/fonc.2018.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villa P.L., Jackson R., Eade S., Escott N., Zehbe I. Isolation of biopsy-derived, human cervical keratinocytes propagated as monolayer and organoid cultures. Sci. Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-36150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roberts S., Evans D., Mehanna H., Parish J.L. Modelling human papillomavirus biology in oropharyngeal keratinocytes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019;374(1773) doi: 10.1098/rstb.2018.0289. 20180289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Gregorio V., Urciuolo F., Netti P.A., Imparato G. In vitro organotypic systems to model tumor microenvironment in human papillomavirus (HPV)-Related cancers. Cancers. 2020;12(5) doi: 10.3390/cancers12051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jackson R., Maarsingh J.D., Herbst-Kralovetz M.M., Van Doorslaer K. 3D oral and cervical tissue models for studying papillomavirus host-pathogen interactions. Curr. Protoc. Microbiol. 2020;59(1):e129. doi: 10.1002/cpmc.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rijal G., Li W. Native-mimicking in vitro microenvironment: an elusive and seductive future for tumor modeling and tissue engineering. J. Biol. Eng. 2018;12:20. doi: 10.1186/s13036-018-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng H., Hillpot E., Mondal S., Khurana K.K., Woodworth C.D. HPV16-Immortalized cells from human transformation zone and endocervix are more dysplastic than ectocervical cells in organotypic culture. Sci. Rep. 2018;8(1):15402. doi: 10.1038/s41598-018-33865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lambert P.F. Transgenic mouse models of tumor virus action. Ann. Rev. Virol. 2016;3(1):473–489. doi: 10.1146/annurev-virology-100114-054908. [DOI] [PubMed] [Google Scholar]
- 104.Lambert P.F., Pan H., Pitot H.C., Liem A., Jackson M., Griep A.E. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1993;90(12):5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brake T., Lambert P.F. Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc. Natl. Acad. Sci. U. S. A. 2005;102(7):2490–2495. doi: 10.1073/pnas.0409883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strati K., Lambert P.F. Role of Rb-dependent and Rb-independent functions of papillomavirus E7 oncogene in head and neck cancer. Canc. Res. 2007;67(24):11585–11593. doi: 10.1158/0008-5472.CAN-07-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stelzer M.K., Pitot H.C., Liem A., Schweizer J., Mahoney C., Lambert P.F. A mouse model for human anal cancer. Canc. Prev. Res. 2010;3(12):1534–1541. doi: 10.1158/1940-6207.CAPR-10-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicholls P.K., Klaunberg B.A., Moore R.A., Santos E.B., Parry N.R., Gough G.W., Stanley M.A. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection. Virology. 1999;265(2):365–374. doi: 10.1006/viro.1999.0060. [DOI] [PubMed] [Google Scholar]
- 109.Handisurya A., Day P.M., Thompson C.D., Buck C.B., Pang Y.Y., Lowy D.R., Schiller J.T. Characterization of Mus musculus papillomavirus 1 infection in situ reveals an unusual pattern of late gene expression and capsid protein localization. J. Virol. 2013;87(24):13214–13225. doi: 10.1128/JVI.02162-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jahan-Parwar B., Chhetri D.K., Hart S., Bhuta S., Berke G.S. Development of a canine model for recurrent respiratory papillomatosis. Ann. Otol. Rhinol. Laryngol. 2003;112(12):1011–1013. doi: 10.1177/000348940311201203. [DOI] [PubMed] [Google Scholar]
- 111.Doorbar J. The papillomavirus life cycle. J. Clin. Virol. 2005;32(Suppl 1):S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Stubenrauch F., Straub E., Klein K., Kramer D., Iftner T., Wong M. Expression OF E8∧ E2 IS required for wart formation BY mouse papillomavirus 1 IN VIVO. J. Virol. 2021 doi: 10.1128/JVI.01930-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Porcellato I., Brachelente C., Guelfi G., Reginato A., Sforna M., Bongiovanni L., Mechelli L. A retrospective investigation on canine papillomavirus 1 (CPV1) in oral oncogenesis reveals dogs are not a suitable animal model for high-risk HPV-induced oral cancer. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0112833. e112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uberoi A., Lambert P.F. Rodent papillomaviruses. Viruses. 2017;9(12) doi: 10.3390/v9120362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wei T., Buehler D., Ward-Shaw E., Lambert P.F. An infection-based murine model for papillomavirus-associated head and neck cancer. mBio. 2020;11(3) doi: 10.1128/mBio.00908-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scagnolari C., Cannella F., Pierangeli A., Pilgrim R.M., Antonelli G., Rowley D., Wong M., Best S., Xing D., Roden R.B. Insights into the role of innate immunity in cervicovaginal papillomavirus infection from studies using gene-deficient mice. J. Virol. 2020;94(12) doi: 10.1128/JVI.00087-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishio M., To Y., Maehama T., Aono Y., Otani J., Hikasa H., Kitagawa A., Mimori K., Sasaki T., Nishina H., Toyokuni S., Lydon J.P., Nakao K., Wah Mak T., Kiyono T., Katabuchi H., Tashiro H., Suzuki A. Endogenous YAP1 activation drives immediate onset of cervical carcinoma in situ in mice. Canc. Sci. 2020;111(10):3576–3587. doi: 10.1111/cas.14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gardiol D., Kuhne C., Glaunsinger B., Lee S.S., Javier R., Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18(40):5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- 119.Nakagawa S., Huibregtse J.M. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell Biol. 2000;20(21):8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Facciuto F., Bugnon Valdano M., Marziali F., Massimi P., Banks L., Cavatorta A.L., Gardiol D. Human papillomavirus (HPV)-18 E6 oncoprotein interferes with the epithelial cell polarity Par3 protein. Mol. Oncol. 2014;8(3):533–543. doi: 10.1016/j.molonc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.White E.A., Munger K., Howley P.M. High-risk human papillomavirus E7 proteins target PTPN14 for degradation. mBio. 2016;7(5) doi: 10.1128/mBio.01530-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morgan E.L., Macdonald A. Manipulation of JAK/STAT signalling by high-risk HPVs: potential therapeutic targets for HPV-associated malignancies. Viruses. 2020;12(9) doi: 10.3390/v12090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lau L., Gray E.E., Brunette R.L., Stetson D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350(6260):568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 124.Shaikh M.H., Bortnik V., McMillan N.A., Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019;132:162–165. doi: 10.1016/j.micpath.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Bortnik V., Wu M., Julcher B., Salinas A., Nikolic I., Simpson K.J., McMillan N.A., Idris A. Loss of HPV type 16 E7 restores cGAS-STING responses in human papilloma virus-positive oropharyngeal squamous cell carcinomas cells. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 126.Luo X., Donnelly C.R., Gong W., Heath B.R., Hao Y., Donnelly L.A., Moghbeli T., Tan Y.S., Lin X., Bellile E. HPV16 drives cancer immune escape via NLRX1-mediated degradation of STING. J. Clin. Invest. 2020;130(4) doi: 10.1172/JCI129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song Y., Wu X., Xu Y., Zhu J., Li J., Zou Z., Chen L., Zhang B., Hua C., Rui H., Zheng Q., Zhou Q., Wang Q., Cheng H. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int. J. Biol. Sci. 2020;16(15):2924–2937. doi: 10.7150/ijbs.50074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lai Y., He Z., Zhang A., Yan Z., Zhang X., Hu S., Wang N., He H. Tip60 and p300 function antagonistically in the epigenetic regulation of HPV18 E6/E7 genes in cervical cancer HeLa cells. Genes Genom. 2020;42(6):691–698. doi: 10.1007/s13258-020-00938-4. [DOI] [PubMed] [Google Scholar]
- 129.Ganti K., Massimi P., Manzo-Merino J., Tomaic V., Pim D., Playford M.P., Lizano M., Roberts S., Kranjec C., Doorbar J., Banks L. Interaction of the human papillomavirus E6 oncoprotein with sorting nexin 27 modulates endocytic cargo transport pathways. PLoS Pathog. 2016;12(9) doi: 10.1371/journal.ppat.1005854. e1005854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galloway D.A., McDougall J.K. The disruption of cell cycle checkpoints by papillomavirus oncoproteins contributes to anogenital neoplasia. Semin. Canc. Biol. 1996;7(6):309–315. doi: 10.1006/scbi.1996.0040. [DOI] [PubMed] [Google Scholar]
- 131.Zwerschke W., Mazurek S., Massimi P., Banks L., Eigenbrodt E., Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc. Natl. Acad. Sci. U. S. A. 1999;96(4):1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mazurek S., Zwerschke W., Jansen-Durr P., Eigenbrodt E. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem. J. 2001;356(Pt 1):247–256. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Durzynska J., Lesniewicz K., Poreba E. Human papillomaviruses in epigenetic regulations. Mutat. Res. Rev. Mutat. Res. 2017;772:36–50. doi: 10.1016/j.mrrev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 134.Soto D., Song C., McLaughlin-Drubin M.E. Epigenetic alterations in human papillomavirus-associated cancers. Viruses. 2017;9(9) doi: 10.3390/v9090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mac M., Moody C.A. Epigenetic regulation of the human papillomavirus life cycle. Pathogens. 2020;9(6) doi: 10.3390/pathogens9060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burley M., Roberts S., Parish J.L. Epigenetic regulation of human papillomavirus transcription in the productive virus life cycle. Semin. Immunopathol. 2020;42(2):159–171. doi: 10.1007/s00281-019-00773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goodman R.H., Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–1577. [PubMed] [Google Scholar]
- 138.Chan H.M., La Thangue N.B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 2001;114(13):2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 139.Ferrari R., Berk A.J., Kurdistani S.K. Viral manipulation of the host epigenome for oncogenic transformation. Nat. Rev. Genet. 2009;10(5):290–294. doi: 10.1038/nrg2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zimmermann H., Degenkolbe R., Bernard H.U., O'Connor M.J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 1999;73(8):6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jansma A.L., Martinez-Yamout M.A., Liao R., Sun P., Dyson H.J., Wright P.E. The high-risk HPV16 E7 oncoprotein mediates interaction between the transcriptional coactivator CBP and the retinoblastoma protein pRb. J. Mol. Biol. 2014;426(24):4030–4048. doi: 10.1016/j.jmb.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Avvakumov N., Torchia J., Mymryk J.S. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene. 2003;22(25):3833–3841. doi: 10.1038/sj.onc.1206562. [DOI] [PubMed] [Google Scholar]
- 143.Baldwin A., Huh K.W., Munger K. Human papillomavirus E7 oncoprotein dysregulates steroid receptor coactivator 1 localization and function. J. Virol. 2006;80(13):6669–6677. doi: 10.1128/JVI.02497-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He H., Lai Y., Hao Y., Liu Y., Zhang Z., Liu X., Guo C., Zhang M., Zhou H., Wang N., Luo X.G., Huo L., Ma W., Zhang T.C. Selective p300 inhibitor C646 inhibited HPV E6-E7 genes, altered glucose metabolism and induced apoptosis in cervical cancer cells. Eur. J. Pharmacol. 2017;812:206–215. doi: 10.1016/j.ejphar.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 145.Kessler U., Cattori V., Labelle M., Hentsch B. Oral small molecule modulators of p300/CBP to reprogram cancer cells and reactivate the p53 pathway in HPV-associated carcinomas. J. Clin. Oncol. 2018;36(15_suppl) e14581-e14581. [Google Scholar]
- 146.Brehm A., Nielsen S.J., Miska E.A., McCance D.J., Reid J.L., Bannister A.J., Kouzarides T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J. 1999;18(9):2449–2458. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Longworth M.S., Laimins L.A. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J. Virol. 2004;78(7):3533–3541. doi: 10.1128/JVI.78.7.3533-3541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Longworth M.S., Wilson R., Laimins L.A. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 2005;24(10):1821–1830. doi: 10.1038/sj.emboj.7600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang B.H., Laban M., Leung C.H., Lee L., Lee C.K., Salto-Tellez M., Raju G.C., Hooi S.C. Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ. 2005;12(4):395–404. doi: 10.1038/sj.cdd.4401567. [DOI] [PubMed] [Google Scholar]
- 150.Locke W.J., Guanzon D., Ma C., Liew Y.J., Duesing K.R., Fung K.Y.C., Ross J.P. DNA methylation cancer biomarkers: translation to the clinic. Front. Genet. 2019;10:1150. doi: 10.3389/fgene.2019.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Burgers W.A., Blanchon L., Pradhan S., de Launoit Y., Kouzarides T., Fuks F. Viral oncoproteins target the DNA methyltransferases. Oncogene. 2007;26(11):1650–1655. doi: 10.1038/sj.onc.1209950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McCabe M.T., Davis J.N., Day M.L. Regulation of DNA methyltransferase 1 by the pRb/E2F1 pathway. Canc. Res. 2005;65(9):3624–3632. doi: 10.1158/0008-5472.CAN-04-2158. [DOI] [PubMed] [Google Scholar]
- 153.Laurson J., Khan S., Chung R., Cross K., Raj K. Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein. Carcinogenesis. 2010;31(5):918–926. doi: 10.1093/carcin/bgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Banks L., Pim D., Thomas M. Human tumour viruses and the deregulation of cell polarity in cancer. Nat. Rev. Canc. 2012;12(12):877–886. doi: 10.1038/nrc3400. [DOI] [PubMed] [Google Scholar]
- 155.Hyland P.L., McDade S.S., McCloskey R., Dickson G.J., Arthur K., McCance D.J., Patel D. Evidence for alteration of EZH2, BMI1, and KDM6A and epigenetic reprogramming in human papillomavirus type 16 E6/E7-expressing keratinocytes. J. Virol. 2011;85(21):10999–11006. doi: 10.1128/JVI.00160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McLaughlin-Drubin M.E., Crum C.P., Munger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl. Acad. Sci. U. S. A. 2011;108(5):2130–2135. doi: 10.1073/pnas.1009933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Agger K., Cloos P.A., Rudkjaer L., Williams K., Andersen G., Christensen J., Helin K. The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes Dev. 2009;23(10):1171–1176. doi: 10.1101/gad.510809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McLaughlin-Drubin M.E., Park D., Munger K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc. Natl. Acad. Sci. U. S. A. 2013;110(40):16175–16180. doi: 10.1073/pnas.1310432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harden M.E., Munger K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology. 2017;508:63–69. doi: 10.1016/j.virol.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Babion I., Miok V., Jaspers A., Huseinovic A., Steenbergen R.D.M., van Wieringen W.N., Wilting S.M. Identification of deregulated pathways, key regulators, and novel miRNA-mRNA interactions in HPV-mediated transformation. Cancers. 2020;12(3) doi: 10.3390/cancers12030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zheng Z.M., Wang X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim. Biophys. Acta. 2011;1809(11–12):668–677. doi: 10.1016/j.bbagrm.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zang B., Huang G., Wang X., Zheng S. HPV-16 E6 promotes cell growth of esophageal cancer via downregulation of miR-125b and activation of Wnt/beta-catenin signaling pathway. Int. J. Clin. Exp. Pathol. 2015;8(10):13687–13694. [PMC free article] [PubMed] [Google Scholar]
- 163.Fujii T., Shimada K., Asano A., Tatsumi Y., Yamaguchi N., Yamazaki M., Konishi N. MicroRNA-331-3p suppresses cervical cancer cell proliferation and E6/E7 expression by targeting NRP2. Int. J. Mol. Sci. 2016;17(8) doi: 10.3390/ijms17081351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu F., Zhang S., Zhao Z., Mao X., Huang J., Wu Z., Zheng L., Wang Q. MicroRNA-27b up-regulated by human papillomavirus 16 E7 promotes proliferation and suppresses apoptosis by targeting polo-like kinase2 in cervical cancer. Oncotarget. 2016;7(15):19666–19679. doi: 10.18632/oncotarget.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu W., Feng L., Li P., Wang Y., Du Y., Chen X., Wu S., Zhao G., Lou W. Effects of HPV-16 infection on hypopharyngeal squamous cell carcinoma and FaDu cells. Oncol. Rep. 2016;35(1):99–106. doi: 10.3892/or.2015.4340. [DOI] [PubMed] [Google Scholar]
- 166.Mou Z., Xu X., Dong M., Xu J. MicroRNA-148b acts as a tumor suppressor in cervical cancer by inducing G1/S-phase cell cycle arrest and apoptosis in a caspase-3-dependent manner. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res.: Int. Med. J. Exp. Clin. Res. 2016;22:2809. doi: 10.12659/MSM.896862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fradet-Turcotte A., Bergeron-Labrecque F., Moody C.A., Lehoux M., Laimins L.A., Archambault J. Nuclear accumulation of the papillomavirus E1 helicase blocks S-phase progression and triggers an ATM-dependent DNA damage response. J. Virol. 2011;85(17):8996–9012. doi: 10.1128/JVI.00542-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Anacker D.C., Moody C.A. Modulation of the DNA damage response during the life cycle of human papillomaviruses. Virus Res. 2017;231:41–49. doi: 10.1016/j.virusres.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pancholi N.J., Price A.M., Weitzman M.D. Take your PIKK: tumour viruses and DNA damage response pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0269. 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moody C.A., Laimins L.A. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 2009;5(10) doi: 10.1371/journal.ppat.1000605. e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thatte J., Massimi P., Thomas M., Boon S.S., Banks L. The human papillomavirus E6 PDZ binding motif links DNA damage response signaling to E6 inhibition of p53 transcriptional activity. J. Virol. 2018;92(16) doi: 10.1128/JVI.00465-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beskow C., Skikuniene J., Holgersson Å., Nilsson B., Lewensohn R., Kanter L., Viktorsson K. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br. J. Canc. 2009;101(5):816–821. doi: 10.1038/sj.bjc.6605201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tian X., Chen G., Xing H., Weng D., Guo Y., Ma D. The relationship between the down-regulation of DNA-PKcs or Ku70 and the chemosensitization in human cervical carcinoma cell line HeLa. Oncol. Rep. 2007;18(4):927–932. [PubMed] [Google Scholar]
- 174.Fuhrman C.B., Kilgore J., LaCoursiere Y.D., Lee C.M., Milash B.A., Soisson A.P., Zempolich K.A. Radiosensitization of cervical cancer cells via double-strand DNA break repair inhibition. Gynecol. Oncol. 2008;110(1):93–98. doi: 10.1016/j.ygyno.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 175.Davidson D., Amrein L., Panasci L., Aloyz R. Small molecules, inhibitors of DNA-PK, targeting DNA repair, and beyond. Front. Pharmacol. 2013;4:5. doi: 10.3389/fphar.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dong J., Ren Y., Zhang T., Wang Z., Ling C.C., Li G.C., He F., Wang C., Wen B. Inactivation of DNA-PK by knockdown DNA-PKcs or NU7441 impairs non-homologous end-joining of radiation-induced double strand break repair. Oncol. Rep. 2018;39(3):912–920. doi: 10.3892/or.2018.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Golding S.E., Rosenberg E., Valerie N., Hussaini I., Frigerio M., Cockcroft X.F., Chong W.Y., Hummersone M., Rigoreau L., Menear K.A., O'Connor M.J., Povirk L.F., van Meter T., Valerie K. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol. Canc. Therapeut. 2009;8(10):2894–2902. doi: 10.1158/1535-7163.MCT-09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Batey M.A., Zhao Y., Kyle S., Richardson C., Slade A., Martin N.M., Lau A., Newell D.R., Curtin N.J. Preclinical evaluation of a novel ATM inhibitor, KU59403, in vitro and in vivo in p53 functional and dysfunctional models of human cancer. Mol. Canc. Therapeut. 2013;12(6):959–967. doi: 10.1158/1535-7163.MCT-12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Biddlestone-Thorpe L., Sajjad M., Rosenberg E., Beckta J.M., Valerie N.C., Tokarz M., Adams B.R., Wagner A.F., Khalil A., Gilfor D., Golding S.E., Deb S., Temesi D.G., Lau A., O'Connor M.J., Choe K.S., Parada L.F., Lim S.K., Mukhopadhyay N.D., Valerie K. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin. Canc. Res. 2013;19(12):3189–3200. doi: 10.1158/1078-0432.CCR-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Charrier J.D., Durrant S.J., Golec J.M., Kay D.P., Knegtel R.M., MacCormick S., Mortimore M., O'Donnell M.E., Pinder J.L., Reaper P.M., Rutherford A.P., Wang P.S., Young S.C., Pollard J.R. Discovery of potent and selective inhibitors of ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase as potential anticancer agents. J. Med. Chem. 2011;54(7):2320–2330. doi: 10.1021/jm101488z. [DOI] [PubMed] [Google Scholar]
- 181.Reaper P.M., Griffiths M.R., Long J.M., Charrier J.D., Maccormick S., Charlton P.A., Golec J.M., Pollard J.R. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat. Chem. Biol. 2011;7(7):428–430. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- 182.Hong D.S., Moore K., Patel M., Grant S.C., Burris H.A., 3rd, William W.N., Jr., Jones S., Meric-Bernstam F., Infante J., Golden L., Zhang W., Martinez R., Wijayawardana S., Beckmann R., Lin A.B., Eng C., Bendell J. Evaluation of prexasertib, a checkpoint kinase 1 inhibitor, in a phase ib study of patients with squamous cell carcinoma. Clin. Canc. Res. 2018;24(14):3263–3272. doi: 10.1158/1078-0432.CCR-17-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Toussaint-Smith E., Donner D.B., Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23(17):2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- 184.Durczak M., Jagodzinski P.P. Apicidin upregulates PHD2 prolyl hydroxylase gene expression in cervical cancer cells. Anti Canc. Drugs. 2010;21(6):619–624. doi: 10.1097/cad.0b013e328339848b. [DOI] [PubMed] [Google Scholar]
- 185.Li G., He L., Zhang E., Shi J., Zhang Q., Le A.D., Zhou K., Tang X. Overexpression of human papillomavirus (HPV) type 16 oncoproteins promotes angiogenesis via enhancing HIF-1alpha and VEGF expression in non-small cell lung cancer cells. Canc. Lett. 2011;311(2):160–170. doi: 10.1016/j.canlet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 186.Lopez-Ocejo O., Viloria-Petit A., Bequet-Romero M., Mukhopadhyay D., Rak J., Kerbel R.S. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19(40):4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 187.Li F., Cui J. Human telomerase reverse transcriptase regulates vascular endothelial growth factor expression via human papillomavirus oncogene E7 in HPV-18-positive cervical cancer cells. Med. Oncol. 2015;32(7):199. doi: 10.1007/s12032-015-0649-0. [DOI] [PubMed] [Google Scholar]
- 188.Hamden K.E., Whitman A.G., Ford P.W., Shelton J.G., McCubrey J.A., Akula S.M. Raf and VEGF: emerging therapeutic targets in Kaposi's sarcoma-associated herpesvirus infection and angiogenesis in hematopoietic and nonhematopoietic tumors. Leukemia. 2005;19(1):18–26. doi: 10.1038/sj.leu.2403532. [DOI] [PubMed] [Google Scholar]
- 189.Tsang J., Lee V.H., Kwong D.L. Novel therapy for nasopharyngeal carcinoma--where are we. Oral Oncol. 2014;50(9):798–801. doi: 10.1016/j.oraloncology.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 190.Rader J.S., Sill M.W., Beumer J.H., Lankes H.A., Benbrook D.M., Garcia F., Trimble C., Tate Thigpen J., Lieberman R., Zuna R.E., Leath C.A., 3rd, Spirtos N.M., Byron J., Thaker P.H., Lele S., Alberts D. A stratified randomized double-blind phase II trial of celecoxib for treating patients with cervical intraepithelial neoplasia: the potential predictive value of VEGF serum levels: an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;145(2):291–297. doi: 10.1016/j.ygyno.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Argiris A., Bauman J.E., Ohr J., Gooding W.E., Heron D.E., Duvvuri U., Kubicek G.J., Posluszny D.M., Vassilakopoulou M., Kim S., Grandis J.R., Johnson J.T., Gibson M.K., Clump D.A., Flaherty J.T., Chiosea S.I., Branstetter B., Ferris R.L. Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer. Ann. Oncol. 2016;27(8):1594–1600. doi: 10.1093/annonc/mdw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Willett C.G., Duda D.G., di Tomaso E., Boucher Y., Ancukiewicz M., Sahani D.V., Lahdenranta J., Chung D.C., Fischman A.J., Lauwers G.Y., Shellito P., Czito B.G., Wong T.Z., Paulson E., Poleski M., Vujaskovic Z., Bentley R., Chen H.X., Clark J.W., Jain R.K. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 2009;27(18):3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Crane C.H., Eng C., Feig B.W., Das P., Skibber J.M., Chang G.J., Wolff R.A., Krishnan S., Hamilton S., Janjan N.A., Maru D.M., Ellis L.M., Rodriguez-Bigas M.A. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(3):824–830. doi: 10.1016/j.ijrobp.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 194.Willett C.G., Duda D.G., Ancukiewicz M., Shah M., Czito B.G., Bentley R., Poleski M., Fujita H., Lauwers G.Y., Carroll M., Tyler D., Mantyh C., Shellito P., Chung D.C., Clark J.W., Jain R.K. A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer. Oncol. 2010;15(8):845–851. doi: 10.1634/theoncologist.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee N.Y., Zhang Q., Pfister D.G., Kim J., Garden A.S., Mechalakos J., Hu K., Le Q.T., Colevas A.D., Glisson B.S., Chan A.T., Ang K.K. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13(2):172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bussey K.A., Brinkmann M.M. Strategies for immune evasion by human tumor viruses. Curr. Opin. Virol. 2018;32:30–39. doi: 10.1016/j.coviro.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 197.Alcami A., Koszinowski U.H. Viral mechanisms of immune evasion. Trends Microbiol. 2000;8(9):410–418. doi: 10.1016/S0966-842X(00)01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ronco L.V., Karpova A.Y., Vidal M., Howley P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12(13):2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li S., Labrecque S., Gauzzi M.C., Cuddihy A.R., Wong A.H., Pellegrini S., Matlashewski G.J., Koromilas A.E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18(42):5727–5737. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- 200.Nees M., Geoghegan J.M., Hyman T., Frank S., Miller L., Woodworth C.D. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J. Virol. 2001;75(9):4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Motwani M., Pesiridis S., Fitzgerald K.A. DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet. 2019;20(11):657–674. doi: 10.1038/s41576-019-0151-1. [DOI] [PubMed] [Google Scholar]
- 202.Uhlorn B.L., Jackson R., Li S., Bratton S.M., Van Doorslaer K., Campos S.K. Vesicular trafficking permits evasion of cGAS/STING surveillance during initial human papillomavirus infection. PLoS Pathog. 2020;16(11) doi: 10.1371/journal.ppat.1009028. e1009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hasan U.A., Bates E., Takeshita F., Biliato A., Accardi R., Bouvard V., Mansour M., Vincent I., Gissmann L., Iftner T., Sideri M., Stubenrauch F., Tommasino M. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007;178(5):3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 204.Hasan U.A., Zannetti C., Parroche P., Goutagny N., Malfroy M., Roblot G., Carreira C., Hussain I., Muller M., Taylor-Papadimitriou J., Picard D., Sylla B.S., Trinchieri G., Medzhitov R., Tommasino M. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J. Exp. Med. 2013;210(7):1369–1387. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pacini L., Savini C., Ghittoni R., Saidj D., Lamartine J., Hasan U.A., Accardi R., Tommasino M. Downregulation of toll-like receptor 9 expression by beta human papillomavirus 38 and implications for cell cycle control. J. Virol. 2015;89(22):11396–11405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Spitkovsky D., Hehner S.P., Hofmann T.G., Moller A., Schmitz M.L. The human papillomavirus oncoprotein E7 attenuates NF-kappa B activation by targeting the Ikappa B kinase complex. J. Biol. Chem. 2002;277(28):25576–25582. doi: 10.1074/jbc.M201884200. [DOI] [PubMed] [Google Scholar]
- 207.Havard L., Rahmouni S., Boniver J., Delvenne P. High levels of p105 (NFKB1) and p100 (NFKB2) proteins in HPV16-transformed keratinocytes: role of E6 and E7 oncoproteins. Virology. 2005;331(2):357–366. doi: 10.1016/j.virol.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 208.Vandermark E.R., Deluca K.A., Gardner C.R., Marker D.F., Schreiner C.N., Strickland D.A., Wilton K.M., Mondal S., Woodworth C.D. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology. 2012;425(1):53–60. doi: 10.1016/j.virol.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Richards K.H., Wasson C.W., Watherston O., Doble R., Eric Blair G., Wittmann M., Macdonald A. The human papillomavirus (HPV) E7 protein antagonises an Imiquimod-induced inflammatory pathway in primary human keratinocytes. Sci. Rep. 2015;5 doi: 10.1038/srep12922. 12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang S.M., McCance D.J. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J. Virol. 2002;76(17):8710–8721. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.James M.A., Lee J.H., Klingelhutz A.J. HPV16‐E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int. J. Canc. 2006;119(8):1878–1885. doi: 10.1002/ijc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.da Costa R.M.G., Bastos M.M., Medeiros R., Oliveira P.A. The NFκB signaling pathway in papillomavirus-induced lesions: friend or foe? Anticancer Res. 2016;36(5):2073–2083. [PubMed] [Google Scholar]
- 213.Li J., Jia H., Xie L., Wang X., Wang X., He H., Lin Y., Hu L. Association of constitutive nuclear factor-kappaB activation with aggressive aspects and poor prognosis in cervical cancer. Int. J. Gynecol. Canc. 2009;19(8):1421–1426. doi: 10.1111/IGC.0b013e3181b70445. [DOI] [PubMed] [Google Scholar]
- 214.Pikarsky E., Porat R.M., Stein I., Abramovitch R., Amit S., Kasem S., Gutkovich-Pyest E., Urieli-Shoval S., Galun E., Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. 7007. [DOI] [PubMed] [Google Scholar]
- 215.Shai A., Brake T., Somoza C., Lambert P.F. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Canc. Res. 2007;67(4):1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Spanos W.C., Geiger J., Anderson M.E., Harris G.F., Bossler A.D., Smith R.B., Klingelhutz A.J., Lee J.H. Deletion of the PDZ motif of HPV16 E6 preventing immortalization and anchorage-independent growth in human tonsil epithelial cells. Head Neck. 2008;30(2):139–147. doi: 10.1002/hed.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shai A., Pitot H.C., Lambert P.F. E6-associated protein is required for human papillomavirus type 16 E6 to cause cervical cancer in mice. Canc. Res. 2010;70(12):5064–5073. doi: 10.1158/0008-5472.CAN-09-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cortes-Malagon E.M., Palacios-Reyes C., Romero-Cordoba S., Mendoza-Villanueva D., Escobar-Herrera J., Verdejo-Torres O., Contreras R.G., Fernadez-Tilapa G., Moreno-Eutimio M.A., Moreno J., Hidalgo-Miranda A., Gariglio P., Bonilla-Delgado J. The PDZ-binding motif of HPV16-E6 oncoprotein modulates the keratinization and stemness transcriptional profile in vivo. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/7868645. 7868645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Webb Strickland S., Brimer N., Lyons C., Vande Pol S.B. Human Papillomavirus E6 interaction with cellular PDZ domain proteins modulates YAP nuclear localization. Virology. 2018;516:127–138. doi: 10.1016/j.virol.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marsh E.K., Delury C.P., Davies N.J., Weston C.J., Miah M.A., Banks L., Parish J.L., Higgs M.R., Roberts S. Mitotic control of human papillomavirus genome-containing cells is regulated by the function of the PDZ-binding motif of the E6 oncoprotein. Oncotarget. 2017;8(12):19491. doi: 10.18632/oncotarget.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee C., Laimins L.A. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J. Virol. 2004;78(22):12366–12377. doi: 10.1128/JVI.78.22.12366-12377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nicolaides L., Davy C., Raj K., Kranjec C., Banks L., Doorbar J. Stabilization of HPV16 E6 protein by PDZ proteins, and potential implications for genome maintenance. Virology. 2011;414(2):137–145. doi: 10.1016/j.virol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 223.Delury C.P., Marsh E.K., James C.D., Boon S.S., Banks L., Knight G.L., Roberts S. The role of protein kinase A regulation of the E6 PDZ-binding domain during the differentiation-dependent life cycle of human papillomavirus type 18. J. Virol. 2013;87(17):9463–9472. doi: 10.1128/JVI.01234-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ganti K., Broniarczyk J., Manoubi W., Massimi P., Mittal S., Pim D., Szalmas A., Thatte J., Thomas M., Tomaic V., Banks L. The human papillomavirus E6 PDZ binding motif: from life cycle to malignancy. Viruses. 2015;7(7):3530–3551. doi: 10.3390/v7072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thomas M., Banks L. Upsetting the balance: when viruses manipulate cell polarity control. J. Mol. Biol. 2018;430(19):3481–3503. doi: 10.1016/j.jmb.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Thomas M., Massimi P., Navarro C., Borg J.P., Banks L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene. 2005;24(41):6222–6230. doi: 10.1038/sj.onc.1208757. [DOI] [PubMed] [Google Scholar]
- 227.Thomas M., Myers M.P., Massimi P., Guarnaccia C., Banks L. Analysis of multiple HPV E6 PDZ interactions defines type-specific PDZ fingerprints that predict oncogenic potential. PLoS Pathog. 2016;12(8) doi: 10.1371/journal.ppat.1005766. e1005766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Van Doorslaer K., DeSalle R., Einstein M.H., Burk R.D. Degradation of human PDZ-proteins by human alphapapillomaviruses represents an evolutionary adaptation to a novel cellular niche. PLoS Pathog. 2015;11(6) doi: 10.1371/journal.ppat.1004980. e1004980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sarabia-Vega V., Banks L. Acquisition of a phospho-acceptor site enhances HPV E6 PDZ-binding motif functional promiscuity. J. Gen. Virol. 2020;101(9):954–962. doi: 10.1099/jgv.0.001236. [DOI] [PubMed] [Google Scholar]
- 230.Yoshimatsu Y., Nakahara T., Tanaka K., Inagawa Y., Narisawa-Saito M., Yugawa T., Ohno S.I., Fujita M., Nakagama H., Kiyono T. Roles of the PDZ-binding motif of HPV 16 E6 protein in oncogenic transformation of human cervical keratinocytes. Canc. Sci. 2017;108(7):1303–1309. doi: 10.1111/cas.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kranjec C., Tomaić V., Massimi P., Nicolaides L., Doorbar J., Banks L. The high-risk HPV E6 target scribble (hScrib) is required for HPV E6 expression in cervical tumour-derived cell lines. Papillomavirus Res. 2016;2:70–77. doi: 10.1016/j.pvr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gardiol D., Zacchi A., Petrera F., Stanta G., Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int. J. Canc. 2006;119(6):1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- 233.Hernandez-Monge J., Garay E., Raya-Sandino A., Vargas-Sierra O., Diaz-Chavez J., Popoca-Cuaya M., Lambert P.F., Gonzalez-Mariscal L., Gariglio P. Papillomavirus E6 oncoprotein up-regulates occludin and ZO-2 expression in ovariectomized mice epidermis. Exp. Cell Res. 2013;319(17):2588–2603. doi: 10.1016/j.yexcr.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 234.Kuhne C., Gardiol D., Guarnaccia C., Amenitsch H., Banks L. Differential regulation of human papillomavirus E6 by protein kinase A: conditional degradation of human discs large protein by oncogenic E6. Oncogene. 2000;19(51):5884–5891. doi: 10.1038/sj.onc.1203988. [DOI] [PubMed] [Google Scholar]
- 235.Boon S.S., Banks L. High-risk human papillomavirus E6 oncoproteins interact with 14-3-3zeta in a PDZ binding motif-dependent manner. J. Virol. 2013;87(3):1586–1595. doi: 10.1128/JVI.02074-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Boon S.S., Tomaic V., Thomas M., Roberts S., Banks L. Cancer-causing human papillomavirus E6 proteins display major differences in the phospho-regulation of their PDZ interactions. J. Virol. 2015;89(3):1579–1586. doi: 10.1128/JVI.01961-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Narayan N., Subbaiah V.K., Banks L. The high-risk HPV E6 oncoprotein preferentially targets phosphorylated nuclear forms of hDlg. Virology. 2009;387(1):1–4. doi: 10.1016/j.virol.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 238.Espejo A.B., Gao G., Black K., Gayatri S., Veland N., Kim J., Chen T., Sudol M., Walker C., Bedford M.T. PRMT5 C-terminal phosphorylation modulates a 14-3-3/PDZ interaction switch. J. Biol. Chem. 2017;292(6):2255–2265. doi: 10.1074/jbc.M116.760330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Thomas M., Banks L. Viral oncoproteins and ubiquitination: accessing a cellular toolbox for modifying protein function. FEBS J. 2017;284(19):3168–3170. doi: 10.1111/febs.14208. [DOI] [PubMed] [Google Scholar]
- 240.Dukic A., Lulic L., Thomas M., Skelin J., Bennett Saidu N.E., Grce M., Banks L., Tomaic V. HPV oncoproteins and the ubiquitin proteasome system: a signature of malignancy? Pathogens. 2020;9(2) doi: 10.3390/pathogens9020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Scheffner M., Huibregtse J.M., Vierstra R.D., Howley P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 242.Huh K., Zhou X., Hayakawa H., Cho J.Y., Libermann T.A., Jin J., Harper J.W., Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 2007;81(18):9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tomaić V., Pim D., Banks L. The stability of the human papillomavirus E6 oncoprotein is E6AP dependent. Virology. 2009;393(1):7–10. doi: 10.1016/j.virol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 244.Huibregtse J.M., Scheffner M., Howley P.M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10(13):4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Huibregtse J.M., Scheffner M., Howley P.M. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol. Cell Biol. 1993;13(8):4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Grm H.S., Banks L. Degradation of hDlg and MAGIs by human papillomavirus E6 is E6-AP-independent. J. Gen. Virol. 2004;85(10):2815–2819. doi: 10.1099/vir.0.80035-0. [DOI] [PubMed] [Google Scholar]
- 247.Massimi P., Shai A., Lambert P., Banks L. HPV E6 degradation of p53 and PDZ containing substrates in an E6AP null background. Oncogene. 2008;27(12):1800–1804. doi: 10.1038/sj.onc.1210810. [DOI] [PubMed] [Google Scholar]
- 248.Padash Barmchi M., Gilbert M., Thomas M., Banks L., Zhang B., Auld V.J. A Drosophila model of HPV E6-induced malignancy reveals essential roles for magi and the insulin receptor. PLoS Pathog. 2016;12(8) doi: 10.1371/journal.ppat.1005789. e1005789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vats A., Thatte J., Banks L. Identification of E6AP-independent degradation targets of HPV E6. J. Gen. Virol. 2019;100(12):1674–1679. doi: 10.1099/jgv.0.001331. [DOI] [PubMed] [Google Scholar]
- 250.Massimi P., Gammoh N., Thomas M., Banks L. HPV E6 specifically targets different cellular pools of its PDZ domain-containing tumour suppressor substrates for proteasome-mediated degradation. Oncogene. 2004;23(49):8033–8039. doi: 10.1038/sj.onc.1207977. [DOI] [PubMed] [Google Scholar]
- 251.Dobrosotskaya I., Guy R.K., James G.L. MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J. Biol. Chem. 1997;272(50):31589–31597. doi: 10.1074/jbc.272.50.31589. [DOI] [PubMed] [Google Scholar]
- 252.Subbaiah V.K., Zhang Y., Rajagopalan D., Abdullah L.N., Yeo-Teh N.S., Tomaic V., Banks L., Myers M.P., Chow E.K., Jha S. E3 ligase EDD1/UBR5 is utilized by the HPV E6 oncogene to destabilize tumor suppressor TIP60. Oncogene. 2016;35(16):2062–2074. doi: 10.1038/onc.2015.268. [DOI] [PubMed] [Google Scholar]
- 253.Tomaic V., Pim D., Thomas M., Massimi P., Myers M.P., Banks L. Regulation of the human papillomavirus type 18 E6/E6AP ubiquitin ligase complex by the HECT domain-containing protein EDD. J. Virol. 2011;85(7):3120–3127. doi: 10.1128/JVI.02004-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Radtke F., Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat. Rev. Canc. 2003;3(10):756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 255.Kuncharin Y., Sangphech N., Kueanjinda P., Bhattarakosol P., Palaga T. MAML1 regulates cell viability via the NF-κB pathway in cervical cancer cell lines. Exp. Cell Res. 2011;317(13):1830–1840. doi: 10.1016/j.yexcr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 256.Rangarajan A., Talora C., Okuyama R., Nicolas M., Mammucari C., Oh H., Aster J.C., Krishna S., Metzger D., Chambon P. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20(13):3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Okuyama R., Nguyen B.-C., Talora C., Ogawa E., di Vignano A.T., Lioumi M., Chiorino G., Tagami H., Woo M., Dotto G.P. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev. Cell. 2004;6(4):551–562. doi: 10.1016/s1534-5807(04)00098-x. [DOI] [PubMed] [Google Scholar]
- 258.Meyers J.M., Spangle J.M., Munger K. The human papillomavirus type 8 E6 protein interferes with NOTCH activation during keratinocyte differentiation. J. Virol. 2013;87(8):4762–4767. doi: 10.1128/JVI.02527-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Brimer N., Lyons C., Wallberg A.E., Vande Pol S.B. Cutaneous papillomavirus E6 oncoproteins associate with MAML1 to repress transactivation and NOTCH signaling. Oncogene. 2012;31(43):4639–4646. doi: 10.1038/onc.2011.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tan M.J.A., White E.A., Sowa M.E., Harper J.W., Aster J.C., Howley P.M. Cutaneous β-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(23):E1473–E1480. doi: 10.1073/pnas.1205991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Talora C., Sgroi D.C., Crum C.P., Dotto G.P. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 2002;16(17):2252–2263. doi: 10.1101/gad.988902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Talora C., Cialfi S., Segatto O., Morrone S., Kim Choi J., Frati L., Paolo Dotto G., Gulino A., Screpanti I. Constitutively active Notch1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathways. Exp. Cell Res. 2005;305(2):343–354. doi: 10.1016/j.yexcr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 263.Nyman P.E., Buehler D., Lambert P.F. Loss of function of canonical Notch signaling drives head and neck carcinogenesis. Clin. Canc. Res. 2018;24(24):6308–6318. doi: 10.1158/1078-0432.CCR-17-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lichtig H., Gilboa D.A., Jackman A., Gonen P., Levav-Cohen Y., Haupt Y., Sherman L. HPV16 E6 augments Wnt signaling in an E6AP-dependent manner. Virology. 2010;396(1):47–58. doi: 10.1016/j.virol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 265.Accardi R., Rubino R., Scalise M., Gheit T., Shahzad N., Thomas M., Banks L., Indiveri C., Sylla B.S., Cardone R.A., Reshkin S.J., Tommasino M. E6 and E7 from human papillomavirus type 16 cooperate to target the PDZ protein Na/H exchange regulatory factor 1. J. Virol. 2011;85(16):8208–8216. doi: 10.1128/JVI.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang Q., Qin Q., Song R., Zhao C., Liu H., Yang Y., Gu S., Zhou D., He J. NHERF1 inhibits beta-catenin-mediated proliferation of cervical cancer cells through suppression of alpha-actinin-4 expression. Cell Death Dis. 2018;9(6):1–14. doi: 10.1038/s41419-018-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sominsky S., Kuslansky Y., Shapiro B., Jackman A., Haupt Y., Rosin-Arbesfeld R., Sherman L. HPV16 E6 and E6AP differentially cooperate to stimulate or augment Wnt signaling. Virology. 2014;468–470:510–523. doi: 10.1016/j.virol.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 268.Bonilla-Delgado J., Bulut G., Liu X., Cortes-Malagon E.M., Schlegel R., Flores-Maldonado C., Contreras R.G., Chung S.H., Lambert P.F., Uren A., Gariglio P. The E6 oncoprotein from HPV16 enhances the canonical Wnt/beta-catenin pathway in skin epidermis in vivo. Mol. Canc. Res. 2012;10(2):250–258. doi: 10.1158/1541-7786.MCR-11-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Drews C.M., Case S., Vande Pol S.B. E6 proteins from high-risk HPV, low-risk HPV, and animal papillomaviruses activate the Wnt/beta-catenin pathway through E6AP-dependent degradation of NHERF1. PLoS Pathog. 2019;15(4) doi: 10.1371/journal.ppat.1007575. e1007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Saidu N.E.B., Filić V., Thomas M., Sarabia-Vega V., Đukić A., Miljković F., Banks L., Tomaić V. PDZ domain-containing protein NHERF-2 is a novel target of human papillomavirus 16 (HPV-16) and HPV-18. J. Virol. 2019;94(1) doi: 10.1128/JVI.00663-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Klingelhutz A.J., Foster S.A., McDougall J.K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380(6569):79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 272.Kiyono T., Foster S.A., Koop J.I., McDougall J.K., Galloway D.A., Klingelhutz A.J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396(6706):84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 273.Yuan X., Larsson C., Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene. 2019;38(34):6172–6183. doi: 10.1038/s41388-019-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gewin L., Galloway D.A. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J. Virol. 2001;75(15):7198–7201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Oh S.T., Kyo S., Laimins L.A. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 2001;75(12):5559–5566. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Veldman T., Horikawa I., Barrett J.C., Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 2001;75(9):4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Liu X., Disbrow G.L., Yuan H., Tomaic V., Schlegel R. Myc and human papillomavirus type 16 E7 genes cooperate to immortalize human keratinocytes. J. Virol. 2007;81(22):12689–12695. doi: 10.1128/JVI.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Liu X., Dakic A., Chen R., Disbrow G.L., Zhang Y., Dai Y., Schlegel R. Cell-restricted immortalization by human papillomavirus correlates with telomerase activation and engagement of the hTERT promoter by Myc. J. Virol. 2008;82(23):11568–11576. doi: 10.1128/JVI.01318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Katzenellenbogen R.A., Egelkrout E.M., Vliet-Gregg P., Gewin L.C., Gafken P.R., Galloway D.A. NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells. J. Virol. 2007;81(8):3786–3796. doi: 10.1128/JVI.02007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Xu M., Luo W., Elzi D.J., Grandori C., Galloway D.A. NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes. Mol. Cell Biol. 2008;28(15):4819–4828. doi: 10.1128/MCB.01969-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Liu X., Yuan H., Fu B., Disbrow G.L., Apolinario T., Tomaić V., Kelley M.L., Baker C.C., Huibregtse J., Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J. Biol. Chem. 2005;280(11):10807–10816. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- 282.Bedard K.M., Underbrink M.P., Howie H.L., Galloway D.A. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J. Virol. 2008;82(8):3894–3902. doi: 10.1128/JVI.01818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sekaric P., Cherry J.J., Androphy E.J. Binding of human papillomavirus type 16 E6 to E6AP is not required for activation of hTERT. J. Virol. 2008;82(1):71–76. doi: 10.1128/JVI.01776-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Muench P., Hiller T., Probst S., Florea A.-M., Stubenrauch F., Iftner T. Binding of PDZ proteins to HPV E6 proteins does neither correlate with epidemiological risk classification nor with the immortalization of foreskin keratinocytes. Virology. 2009;387(2):380–387. doi: 10.1016/j.virol.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 285.Fournane S., Charbonnier S., Chapelle A., Kieffer B., Orfanoudakis G., Trave G., Masson M., Nomine Y. Surface plasmon resonance analysis of the binding of high-risk mucosal HPV E6 oncoproteins to the PDZ1 domain of the tight junction protein MAGI-1. J. Mol. Recogn. 2011;24(4):511–523. doi: 10.1002/jmr.1056. [DOI] [PubMed] [Google Scholar]
- 286.Zhang Y., Dasgupta J., Ma R.Z., Banks L., Thomas M., Chen X.S. Structures of a human papillomavirus (HPV) E6 polypeptide bound to MAGUK proteins: mechanisms of targeting tumor suppressors by a high-risk HPV oncoprotein. J. Virol. 2007;81(7):3618–3626. doi: 10.1128/JVI.02044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hampson L., Li C., Oliver A.W., Kitchener H.C., Hampson I.N. The PDZ protein Tip-1 is a gain of function target of the HPV16 E6 oncoprotein. Int. J. Oncol. 2004;25(5):1249–1256. [PubMed] [Google Scholar]
- 288.Handa K., Yugawa T., Narisawa-Saito M., Ohno S.-I., Fujita M., Kiyono T. E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein. J. Virol. 2007;81(3):1379–1389. doi: 10.1128/JVI.01712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.James M.A., Lee J.H., Klingelhutz A.J. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 2006;80(11):5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jing M., Bohl J., Brimer N., Kinter M., Pol S.B.V. Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J. Virol. 2007;81(5):2231–2239. doi: 10.1128/JVI.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lee S.S., Glaunsinger B., Mantovani F., Banks L., Javier R.T. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J. Virol. 2000;74(20):9680–9693. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pim D., Broniarczyk J., Bergant M., Playford M.P., Banks L. A novel PDZ domain interaction mediates the binding between human papillomavirus 16 L2 and sorting nexin 27 and modulates virion trafficking. J. Virol. 2015;89(20):10145–10155. doi: 10.1128/JVI.01499-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Storrs C.H., Silverstein S.J. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J. Virol. 2007;81(8):4080–4090. doi: 10.1128/JVI.02545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thomas M., Banks L. PDZRN3/LNX3 is a novel target of human papillomavirus type 16 (HPV-16) and HPV-18 E6. J. Virol. 2015;89(2):1439–1444. doi: 10.1128/JVI.01743-14. [DOI] [PMC free article] [PubMed] [Google Scholar]