Abstract
BACKGROUND:
Reduced-intensity conditioning (RIC) regimens are frequently used for allogeneic hematopoietic cell transplantation (allo-HCT) in diffuse large B-cell lymphoma (DLBCL). However, the RIC regimen with the best risk/benefit profile for allo-HCT in DLBCL is not known. This is particularly important, as patients with DLBCL undergoing allo-HCT in the future would be enriched for those whose lymphoma has failed chimeric antigen receptor T-cell (CAR-T) therapy or other novel immunotherapies, with potentially more advanced disease and suboptimal performance scores. Using the CIBMTR database, we report the outcomes of the three most commonly used allo-HCT RIC regimens in DLBCL.
METHODS:
562 adult DLBCL patients in the CIBMTR registry undergoing allo-HCT using matched related or unrelated donors, between 2008–2016 were included in the analysis. Patients received one of the three RIC regimens: fludarabine/i.v. busulfan (~6·4mg/kg) (Flu/Bu), fludarabine/melphalan (140mg/m2) (Flu/Mel140) or BCNU/etoposide/cytarabine/melphalan (BEAM).
FINDINGS:
The study cohort was divided into three groups: Flu/Bu (n=151), Flu/Mel140 (n=296) and BEAM (n=115). Relative to Flu/Bu, the Flu/Mel140 (HR=2.33, 95%CI=1.42–3.82; p=0.001) and BEAM (HR=2.54, 95%CI=1.34–4.80; p=0.004) regimens were associated with a higher non-relapse mortality (NRM) risk. Although the risk of relapse with Flu/Mel140 was lower compared to Flu/Bu (HR=0.70, 95%CI=0.52–0.95; p=0.02), this did not translate in an improvement in progression-free (HR=1.04) or overall survival (HR=1.30). There was a significantly higher risk of grade 3–4 acute graft-versus-host disease with BEAM (HR=2.19, 95%CI=1.10–4.35; p=0.03) compared to Flu/Bu. In the chemosensitive subset, multivariate analysis showed a significantly higher mortality risk with Flu/Mel140 (HR=1.48, 95%CI=1.07–2.04, p=0.02) relative to Flu/Bu conditioning.
CONCLUSIONS:
In the largest analysis comparing the impact of various RIC conditioning regimens on the survival of DLBCL patients undergoing allo-HCT, our results suggest that Flu/Bu is a better RIC choice in less fit or heavily pretreated patients due to lowest NRM risk.
Keywords: Reduced-intensity conditioning, diffuse large B-cell lymphoma, allogeneic hematopoietic cell transplant, survival
INTRODUCTION
Reduced-intensity conditioning (RIC) regimens account for the vast majority of allogeneic hematopoietic cell transplants (allo-HCT) performed for lymphomas in the U.S (1–8). Although the RIC regimens are generally associated with lower non-relapse mortality (NRM) rates relative to myeloablative conditioning (MAC) regimens, disease relapse remains the most common cause of treatment failure in lymphoma patients undergoing allo-HCT (9–12). However, even among regimens generally considered to be “reduced-intensity,” there is a spectrum of dose-intensities varying from regimens at the lower end of intensity spectrum (relying predominately on alloreactive immunological effects to eradicate disease) to higher-intensity options (that depend on both cytoreductive and immunological mechanisms to control disease). A recent Center for International Blood & Marrow Transplant Research (CIBMTR) analysis compared commonly used RIC regimens in non-Hodgkin lymphomas (NHL) (13). The study compared fludarabine/I.V. busulfan (~6.4mg/kg) (Flu/Bu), fludarabine/melphalan (140mg/m2) (Flu/Mel140), fludarabine/cyclophosphamide (Flu/Cy) and Flu/Cy+2Gy total body irradiation (Flu/Cy/2GyTBI) and found that Flu/Mel140 was associated with high NRM and inferior overall survival (OS) relative to the other RIC regimens (13). However, the study was not limited to DLBCL and included all NHL histologies. More importantly, BEAM (BCNU/etoposide/cytarabine/melphalan [BEAM]) a commonly used regimen in DLBCL, was not included in that analysis. In the future, DLBCL patients undergoing allo-HCT will be enriched for patients who have progressed despite chimeric antigen receptor T-cell (CAR-T) therapy or other novel immunotherapies, with potentially more advanced disease, compromised organ function, and suboptimal performance status. Defining a RIC platform with the best risk/benefit ratio is thus increasingly important.
We report here a registry analysis, comparing the outcomes of the three most commonly used RIC regimens in DLBCL patients undergoing allo-HCT in the United States, using the observational database of CIBMTR.
METHODS
Data source
The CIBMTR is a working group of more than 380 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin (MCW). Participating centers are required to report all transplantations consecutively and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The MCW and National Marrow Donor Program, Institutional Review Boards approved this study.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED-data includes disease type, age, gender, pre-HCT disease stage and chemotherapy-responsiveness, date of diagnosis, graft type, conditioning regimen, post-transplant disease progression and survival, development of new malignancy, and cause of death. All CIBMTR centers contribute TED-data. More detailed disease and pre- and post-transplant clinical information are collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED- and CRF-level data are collected pre-transplant, 100-days, and six months post-HCT and annually thereafter or until death. Data for the current analysis were retrieved from CIBMTR (TED and CRF) report forms.
Patients
Included in this analysis are adult (≥18 years) DLBCL patients undergoing their first RIC allo-HCT between 2008 and 2016. Eligible donors included either HLA-identical sibling donors or unrelated donors (URD) matched at the allele-level at HLA-A, HLA-B, HLA-C, and HLA-DRB1. Graft source was limited to mobilized peripheral blood stem cells. Graft-versus-host disease (GVHD) prophylaxis was limited to calcineurin inhibitor (CNI)-based approaches. The study cohort was divided into three most commonly used RIC regimens (Flu/Bu, Flu/Mel140, and BEAM) for DLBCL in the United States. Patients in the Flu/Bu cohort received a uniform intravenous busulfan dose of ~6.4mg/m2. The Flu/Mel140 cohort was limited to a melphalan dose of 140mg/m2. Allo-HCT recipients could have received in vivo T-cell depletion with antithymocyte globulin (ATG) or alemtuzumab. Patients receiving ex vivo graft manipulation were excluded. Patients receiving bone marrow grafts (n=28) were excluded due to small numbers.
Definitions and Study Endpoints
The intensity of allo-HCT conditioning regimens was determined using the existing consensus criteria (14). Disease response at the time of HCT was assessed using the International Working Group criteria in use during the era of this analysis (15).
The primary endpoint was OS; death from any cause was considered an event, and surviving patients were censored at last follow up. Secondary endpoints included cumulative incidence of acute GVHD, chronic GVHD, NRM, progression/relapse, progression-free survival (PFS), and time to neutrophil and platelet recovery. NRM was defined as death without evidence of lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a complete remission; NRM was considered a competing risk. For PFS, a patient was considered a treatment failure at the time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. Acute GVHD and chronic GVHD were graded using established clinical criteria (16, 17). Neutrophil recovery was defined as the first of 3 successive days with an absolute neutrophil count 500/μL after post-transplant nadir. Platelet recovery was considered to have occurred on the first of 3 consecutive days with a platelet count of 20,000/μL or higher in the absence of platelet transfusion for 7 consecutive days. For GVHD and hematopoietic recovery, death without the event was considered a competing risk.
Statistical Analysis
The study compared three RIC/NMA conditioning platforms: Flu/Bu vs Flu/Mel140 vs BEAM cohorts. Associations among patient-, disease-, and transplant-related variables and outcomes of interest were evaluated using Cox proportional hazards regression for PFS, and OS, the proportional cause-specific hazards model for chronic GVHD, relapse, NRM, and logistic regression for acute GVHD. The forward stepwise selection was used to identify covariates that influenced outcomes. Covariates with a P<0.05 were considered significant. The proportional hazards assumption for Cox regression and the cause-specific hazards model was tested by evaluating time-varying effects for each risk factor and each outcome. Variables that violated the proportional hazards assumption were added using the piecewise proportional hazards models. Interactions between the main effect and significant covariates were examined. Center effect was tested using the score test for relapse, NRM, PFS, and OS and the generalized linear mixed model for acute GVHD (18). The variables considered in multivariate analysis are shown in (Table S1). Adjusted PFS and OS, and adjusted cumulative incidence rates for relapse and NRM were created based on the final model (19, 20). The cumulative incidence was calculated for hematopoietic recovery and GVHD. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics
The overall patient population (N=562) was divided into three cohorts; 151 received Flu/Bu, 296 received Flu/Mel140, and 115 received BEAM. The baseline patient-, disease-, and transplantation-related characteristics are shown in Table 1. There were no significant differences between the three cohorts in terms of patient’s gender, HCT-comorbidity index (HCT-CI), donor type, donor/recipient sex match, donor/recipient CMV match, and prior median lines of chemotherapy before allo-HCT. Significantly more patients in the Flu/Bu (45%) cohort were ≥ 60 years of age compared to Flu/Mel140 (33%) or BEAM cohorts (20%; P=0.004). There were significantly more patients in the Flu/Bu and Flu/Mel140 cohorts who had a Karnofsky performance score (KPS) of ≥ 90, chemosensitive disease at allo-HCT, and a history of prior autologous HCT (auto-HCT) relative to the BEAM cohort (Table 1). ATG/alemtuzumab use in conditioning was more frequently used in Flu/Bu (35%) and BEAM (38%) cohorts, while rituximab with conditioning was predominantly used in the BEAM (49%) cohort. Patients in the BEAM cohort had a significantly shorter time from diagnosis to transplant compared with Flu/Bu and Flu/Mel140 groups. Among total transplants reported each year, the proportion of patients receiving Flu/Mel140 increased from 2012 onward (Table 1).
Table 1.
Baseline characteristics of DLBCL patients receiving RIC/NMA conditioning regimens followed by allo-HCT between 2008–2016
| Flu/Bu N (%) | Flu/Mel 140 N (%) | BEAM N (%) | p-value | |
|---|---|---|---|---|
| Number of patients | 151 | 296 | 115 | |
| Median patient age, years (range) | 59 (25–72) | 56 (22–73) | 53 (22–69) | 0.004 |
| ≥60 | 68 (45) | 99 (33) | 23 (20) | |
| Male sex | 87 (58) | 190 (64) | 74 (64) | 0.36 |
| Karnofsky performance score ≥ 90 | 83 (55) | 175 (59) | 45 (39) | <0.001 |
| Missing | 1 (1) | 6 (2) | 10 (9) | |
| HCT-CI | 0.06 | |||
| 0 | 34 (23) | 98 (33) | 41 (36) | |
| 1–2 | 51 (34) | 72 (24) | 35 (30) | |
| ≥3 | 64 (42) | 118 (40) | 35 (30) | |
| Missing | 2 (1) | 8 (3) | 4 (4) | |
| Patient race | 0.04 | |||
| Caucasian | 142 (94) | 257 (87) | 98 (85) | |
| Other | 9 (6) | 39 (13) | 17 (15) | |
| Remission at HCT | <0.001 | |||
| CR | 68 (45) | 126 (43) | 37 (32) | |
| PR | 60 (40) | 124 (42) | 36 (31) | |
| Resistant | 16 (11) | 42 (14) | 41 (36) | |
| Untreated/Unknown | 4 (3) | 3 (1) | 0 | |
| Not reported | 3 (2) | 1 (0.3) | 1 (1) | |
| History of prior auto-HCT | 83 (55) | 133 (45) | 11 (10) | <0.001 |
| Donor type | 0.25 | |||
| Matched related donor | 70 (46) | 153 (52) | 65 (57) | |
| Matched unrelated donor | 81 (54) | 143 (48) | 50 (43) | |
| Median time from diagnosis to HCT, months (range) | 34 (3–340) | 27 (<l-386) | 14 (2–258) | <0.001 |
| GVHD prophylaxis | <0.001 | |||
| CNI + MMF+−other (s) | 40 (26) | 39 (13) | 34 (30) | |
| CNI + MTX+−other (s) | 96 (64) | 176 (60) | 62 (54) | |
| CNI + other (s) | 15 (10) | 81 (27) | 19 (16) | |
| ATG/alemtuzumab use in conditioning | 53 (35) | 71 (24) | 44 (38) | 0.005 |
| Rituximab use in conditioning | 2 (1) | 32 (11) | 56 (49) | <0.001 |
| Donor/recipient sex match | 0.29 | |||
| F-M | 25 (17) | 69 (23) | 20 (17) | |
| Other combinations | 126 (83) | 227 (77) | 94 (82) | |
| Missing | 0 | 0 | 1 (1) | |
| Donor/recipient CMV match | 0.93 | |||
| Donor-/recipient+ | 42 (28) | 79 (27) | 33 (29) | |
| Others | 108 (71) | 212 (71) | 81 (70) | |
| Not reported | 1 (1) | 5 (2) | 1 (1) | |
| Year of transplant | 0.03 | |||
| 2008 | 12 (7.9) | 12 (4.1) | 12 (10.4) | |
| 2009 | 14 (9.3) | 29 (9.8) | 14 (12.2) | |
| 2010 | 8 (5.3) | 33 (11.1) | 11 (9.6) | |
| 2011 | 14 (9.3) | 23 (7.8) | 13 (11.3) | |
| 2012 | 22 (14.6) | 31 (10.5) | 11 (9.6) | |
| 2013 | 29 (19.2) | 36 (12.2) | 18 (15.7) | |
| 2014 | 24 (15.9) | 40 (13.5) | 10 (8.7) | |
| 2015 | 13 (8.6) | 45 (15.2) | 17 (14.8) | |
| 2016 | 15 (9.9) | 47 (15.9) | 9 (7.8) | |
| Median number of lines of chemotherapy (range) | 2 (1–5) | 3 (1–5) | 3 (1–5) | 0.37 |
| Median follow-up of survivors (range), months | 48 (4–100) | 37 (2–101) | 60 (3–96) |
Abbreviations: DLBCL- Diffuse large B-cell lymphoma; RIC/NMA- Reduced Intensity Conditioning/Non-myeloablative; allo-HCT- Allogeneic hematopoietic cell transplantation; Flu/Bu- Fludarabine/Busulfan; Flu/Mel- Fludarabine/Melphalan; BEAM- BCNU/etoposide/cytarabine/melphalan; HCT-CI- Hematopoietic Cell Transplantation Specific Comorbidity Index; auto-HCT- Autologous Hematopoietic cell transplantation; GVHD- Graft versus Host Disease; CNI- Calcineurin inhibitor; MMF- Mycophenolate mofetil; MTX- methotrexate; ATG- Anti-Thymocyte Globulin.
Hematopoietic recovery
The cumulative incidence of neutrophil recovery at day 28 was 99% (95%CI=96–100) in the Flu/Bu group compared to 97% (95%CI=95–99) and 94% (95%CI=89–98) in the Flu/Mel140 and BEAM cohorts respectively (p=0.17; Table 2). The cumulative incidence of platelet recovery at day 100 was significantly higher in Flu/Bu group [99% (95%CI=97–100)] compared to Flu/Mel140 [89% (95%CI=85–93)] and BEAM groups [89% (95%CI=82–94)] (p<0.001; Table 2).
Table 2.
Engraftment, GVHD and adjusted transplant outcomes
| Flu/Bu (N = 151) | Flu/Mel 140 (N = 296) | BEAM (N = 115) | |||||
|---|---|---|---|---|---|---|---|
| Outcomes | N | Prob (95% Cl) | N | Prob (95% Cl) | N | Prob (95% Cl) | P Value |
| Neutrophil recovery | 148 | 293 | 115 | ||||
| 28-days | 99 (96–100)% | 97 (95–99)% | 94 (89–98)% | 0.17 | |||
| Platelet recovery | 145 | 288 | 114 | ||||
| 100-days | 99 (97–100)% | 89 (85–93)% | 89 (82–94)% | <0.001 | |||
| Grade II-IV acute GVHD | 143 | 284 | 110 | ||||
| 6 months | 45 (37–53)% | 38 (33–44)% | 44 (35–53)% | 0.38 | |||
| Grade III-IV acute GVHD | 143 | 283 | 110 | ||||
| 6 months | 13 (8–19)% | 13 (9–17)% | 22 (15–30)% | 0.10 | |||
| Chronic GVHD | 141 | 270 | 107 | ||||
| 1-year | 38 (30–46)% | 42 (36–48)% | 31 (23–40)% | 0.16 | |||
| Adjusted NRM | 151 | 295 | 114 | ||||
| 1-year | 10 (5–14)% | 20 (16–25)% | 19 (11–26)% | 0.002 | |||
| 4-year | 16 (10–23)% | 29 (23–34)% | 25 (17–34)% | 0.02 | |||
| Adjusted Progression/relapse | 151 | 295 | 114 | ||||
| 1-year | 49 (41–56)% | 33 (28–39)% | 39 (31–48)% | 0.007 | |||
| 4-year | 54 (46–62)% | 40 (35–46)% | 42 (33–51)% | 0.02 | |||
| Adjusted PFS | 151 | 295 | 114 | ||||
| 1-year | 44 (37–52)% | 46 (41–52)% | 42 (34–51)% | 0.75 | |||
| 4-year | 33 (26–41)% | 33 (27–38)% | 34 (25–43)% | 0.95 | |||
| Adjusted OS | 151 | 296 | 115 | ||||
| 1-year | 68 (61–75)% | 63 (58–69)% | 53 (44–63)% | 0.07 | |||
| 4-year | 50 (41–58)% | 42 (36–48)% | 41 (31–51)% | 0.31 | |||
Abbreviations: N- Number; Prob- Probability; CI- Confidence Interval; GVHD- Graft versus Host Disease; Flu/Bu- Fludarabine/Busulfan; Flu/Mel- Fludarabine/Melphalan; BEAM- BCNU/etoposide/cytarabine/melphalan; NRM- Non-Relapse Mortality; PFS- Progression Free Survival; OS- Overall Survival
Acute and chronic GVHD
The cumulative incidence of grade II-IV acute GVHD at day 180 (Table 2) was 45% (95%CI=37–53) for Flu/Bu, 38% (95%CI=33–44) for Flu/Mel140 and 44% (95%CI=35–53) for BEAM (p=0.38). The corresponding rates of grades III-IV acute GVHD in similar order, were 13% (95%CI=8–19), 13% (95%CI=9–17) and 22% (95%CI=15–30), respectively, (p=0.10). After adjusting for GVHD prophylaxis and donor type, the multivariate analysis (Table 3) showed a significantly higher risk of grade III-IV acute GVHD with BEAM conditioning (HR=2.2, 95%CI=1.10–4.35, p=0.03) relative to the Flu/Bu cohort (Table 3).
Table 3.
Multivariable analysis of DLBCL patients receiving RIC conditioning regimens
| N | OR | OR Lower CI | OR Upper CI | p-value | |
|---|---|---|---|---|---|
| Grade 3–4 acute GVHD | |||||
| Conditioning regimen | |||||
| Flu/Bu | 143 | 1 | 0.061 | ||
| Flu/ Mel 140 | 283 | 1.26 | 0.68 | 2.35 | 0.47 |
| BEAM | 110 | 2.19 | 1.10 | 4.35 | 0.03 |
| Grade 3–4 acute GHVD adjusted for significant covariates: GVHD prophylaxis and donor type. | |||||
| Chronic GVHD | |||||
| Conditioning regimen | |||||
| Flu/Bu | 143 | 1 | 0.401 | ||
| Flu/ Mel 140 | 279 | 1.09 | 0.80 | 1.47 | 0.59 |
| BEAM | 108 | 0.84 | 0.56 | 1.27 | 0.41 |
| Chronic GVHD adjusted for significant covariates: GVHD prophylaxis and ATG/alemtuzumab use in conditioning. | |||||
| Non-relapse mortality | |||||
| Conditioning regimen | |||||
| Flu/Bu | 151 | 1 | 0.0021 | ||
| Flu/ Mel 140 | 296 | 2.33 | 1.42 | 3.82 | 0.001 |
| BEAM | 115 | 2.54 | 1.34 | 4.80 | 0.004 |
| NRM adjusted for significant covariates: age, HCT-CI, remission status at HCT, prior auto-HCT, and GVHD prophylaxis | |||||
| Progression/relapse | |||||
| Conditioning regimen | |||||
| Flu/Bu | 151 | 1 | 0.071 | ||
| Flu/ Mel 140 | 296 | 0.70 | 0.52 | 0.95 | 0.02 |
| BEAM | 115 | 0.81 | 0.56 | 1.19 | 0.29 |
| Progression/relapse adjusted for significant covariates: age and remission at HCT. | |||||
| Progression free survival | |||||
| Conditioning regimen | |||||
| Flu/Bu | 151 | 1 | 0.921 | ||
| Flu/ Mel 140 | 296 | 1.04 | 0.81 | 1.35 | 0.75 |
| BEAM | 115 | 1.07 | 0.78 | 1.45 | 0.69 |
| Progression free survival adjusted for significant covariates: remission at HCT and GVHD prophylaxis. | |||||
| Overall survival | |||||
| Conditioning regimen | |||||
| Flu/Bu | 151 | 1 | 0.111 | ||
| Flu/ Mel 140 | 296 | 1.30 | 0.98 | 1.73 | 0.07 |
| BEAM | 115 | 1.44 | 0.99 | 2.10 | 0.05 |
| Overall survival adjusted for significant covariates: KPS, remission status at HCT, and prior auto-HCT. | |||||
Abbreviations: DLBCL- Diffuse large B-cell lymphoma; RIC- Reduced Intensity Conditioning; allo-HCT- Allogeneic hematopoietic cell transplantation; Flu/Bu- Fludarabine/Busulfan; Flu/Mel- Fludarabine/Melphalan; BEAM- BCNU/etoposide/cytarabine/melphalan; HCT-CI- Hematopoietic Cell Transplantation Specific Comorbidity Index; auto-HCT- Autologous Hematopoietic cell transplantation; GVHD- Graft versus Host Disease; CNI- Calcineurin inhibitor; MMF- Mycophenolate mofetil; MTX- methotrexate; ATG- Anti-Thymocyte Globulin.
Overall P values show whether the main effect was significant based on the Wald test in the final model. The other P values are from pairwise comparisons between two conditioning regimens. All pairwise comparisons were from the Wald test.
Please see Table S1 in the supplementary appendix for the co-variates included in the multivariable analysis.
The cumulative incidence of chronic GVHD at 1-year (Table 2) was 38% (95%CI=30–46) for Flu/Bu, 42% (95%CI=36–48) for Flu/Mel140 and 31% (95%CI=23–40) for BEAM (p=0.16). After adjusting for GVHD prophylaxis and ATG/alemtuzumab use in conditioning, the multivariate analysis (Table 3) showed no significant difference in the risk of chronic GVHD in patients who received Flu/Mel140 (HR=1·09) or BEAM (HR=0·84) relative to Flu/Bu (Figure 1a).
Figure 1:
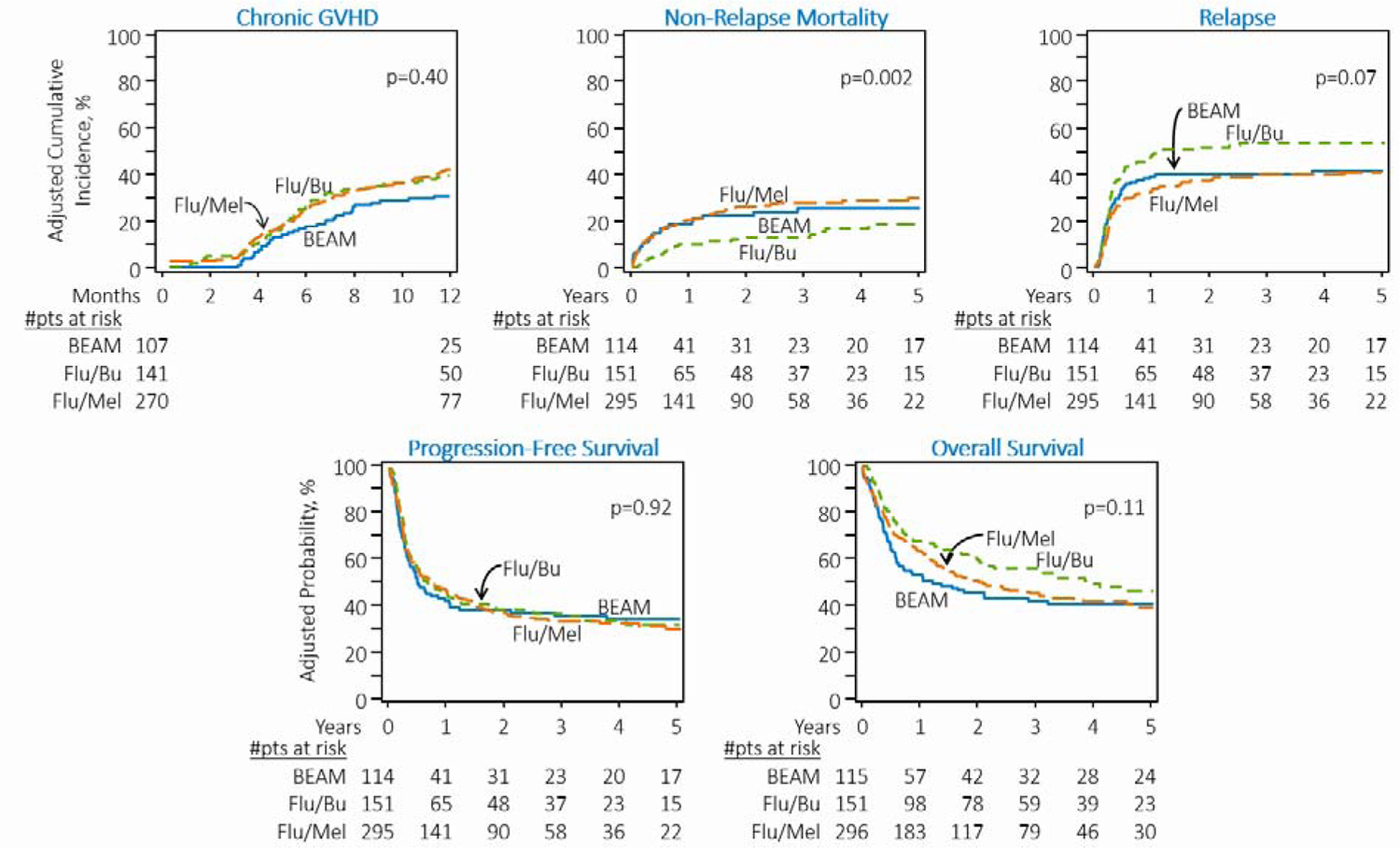
Adjusted transplantation outcomes of DLBCL patients receiving RIC regimens. A) Cumulative incidence of chronic graft-versus-host-disease. B) Cumulative incidence of non-relapse mortality. C) Cumulative incidence of lymphoma relapse/progression. D) Progression-free survival. E) Overall survival
NRM and relapse
The 4-year adjusted cumulative incidence of NRM for Flu/Bu, Flu/Mel140, and BEAM cohorts was 16%, 29%, and 25%, respectively (p=0.02, Table 2). After adjusting for age, HCT-CI, history of prior autologous HCT, remission status at allo-HCT and GVHD prophylaxis in multivariate analysis, Flu/Mel140 (HR=2.33, 95%CI=1.42–3.82, p=0.001) and BEAM (HR=2.54, 95%CI=1.34–4.80, p=0.004) conditioning regimens were associated with a significantly higher risk of NRM when compared to Flu/Bu (Table 3, Figure 1b).
The adjusted cumulative incidence of relapse/progression at 4-years for Flu/Bu, Flu/Mel140 and BEAM cohorts was 54%, 40%, and 42%, respectively (p=0.02, Table 2). After adjusting for age and remission status at allo-HCT, the multivariate analysis showed that the Flu/Mel140 cohort had a significantly lower risk of relapse/progression compared with the Flu/Bu cohort (HR=0.70, 95%CI=0.52–0.95, p=0.02) (Table 3, Figure 1c). Relative to Flu/Bu, the BEAM cohort was not associated with a significantly lower risk of relapse/progression (Table 3).
Progression-free survival
The 4-year adjusted PFS for the Flu/Bu, Flu/Mel140, and BEAM cohorts was 33%, 33%, and 34%, (p=0.95, Table 2). After adjusting for remission status at allo-HCT and GVHD prophylaxis, the multivariate analysis did not show a significantly improved PFS with Flu/Mel140 (HR=1.04) or BEAM (HR=1.07) relative to Flu/Bu conditioning (overall p=0.92; Table 3, Figure 1d).
Overall survival
The 4-year adjusted OS for the Flu/Bu, Flu/Mel140 and BEAM cohorts was 50%, 42%, and 41%, (p=0.31, Table 2). However, after adjusting for KPS, remission status at allo-HCT and history of prior autologous HCT, multivariate analysis did not show a significantly higher mortality risk with Flu/Mel140 (HR=1.30) or BEAM (HR=1.44) relative to Flu/Bu conditioning (overall p=0.11; Table 3, Figure 1e).
There was no center effect noted for NRM (p=0.80), relapse/progression (p=0.20), PFS (p=0.52) or OS (p=0.54).
Outcomes of chemosensitive patients at allo-HCT
The proportion of patients in complete or partial remission at the time of allo-HCT was higher in the Flu/Bu (85%) and Flu/Mel140 (85%) cohorts compared with the BEAM cohort (63%; p<0.01). We thus performed a subgroup analysis restricted to patients with chemosensitive disease. In the chemosensitive subset, after adjusting for significant covariates in the multivariate analysis, Flu/Mel140 (HR=2.19, 95%CI=1.26–3.81, p=0.005) and BEAM (HR=2.27, 95%CI=1.07–4.83, p=0.03) conditioning regimens remained associated with a significantly higher risk of NRM versus Flu/Bu (Table 4, Figure 2a). In line with the overall analysis (Table 3), the Flu/Mel140 cohort had a significantly lower risk of relapse/progression compared with Flu/Bu cohort (HR=0.69, 95%CI=0.49–0.96, p=0.03) (Table 4, Figure 1b). Relative to Flu/Bu, the BEAM cohort was not associated with a significantly lower risk of relapse/progression. Neither the Flu/Mel140 (HR=1.02) nor BEAM (HR=1.02) cohort was associated with significantly improved PFS relative to the Flu/Bu cohort after adjusting for the significant covariates (overall p=0.99; Table 4, Figure 1c). After adjusting for KPS, HCT-CI, remission status at allo-HCT, and history of prior autologous HCT, multivariate analysis showed a significantly higher mortality risk with Flu/Mel140 (HR=1.48, 95%CI=1.07–2.04, p=0.02) relative to Flu/Bu conditioning. The mortality risk was not significantly different with BEAM (HR=1.56, 95%CI=0.98–2.46, p=0.059) compared to Flu/Bu conditioning (Table 4, Figure 1d). The adjusted NRM, relapse/progression, PFS, and OS outcomes are provided in Table S2.
Table 4.
Multivariable analysis of chemosensitive DLBCL patients receiving RIC conditioning regimens
| N | OR | OR Lower CI | OR Upper CI | p-value | |
|---|---|---|---|---|---|
| Non-relapse mortality | |||||
| Conditioning regimen | |||||
| Flu/Bu | 128 | 1 | 0.021 | ||
| Flu/ Mel 140 | 250 | 2.19 | 1.26 | 3.81 | 0.005 |
| BEAM | 73 | 2.27 | 1.07 | 4.83 | 0.03 |
| NRM adjusted for significant covariates: age, HCT-CI, remission status at HCT, prior auto-HCT, and GVHD prophylaxis. | |||||
| Progression/relapse | |||||
| Conditioning regimen | |||||
| Flu/Bu | 128 | 1 | 0.091 | ||
| Flu/ Mel 140 | 250 | 0.69 | 0.49 | 0.96 | 0.03 |
| BEAM | 73 | 0.81 | 0.52 | 1.27 | 0.36 |
| Progression/relapse adjusted for significant covariates: age and remission at HCT. | |||||
| Progression free survival | |||||
| Conditioning regimen | |||||
| Flu/Bu | 128 | 1 | 0.991 | ||
| Flu/ Mel 140 | 250 | 1.02 | 0.77 | 1.36 | 0.87 |
| BEAM | 73 | 1.02 | 0.70 | 1.48 | 0.93 |
| Progression free survival adjusted for significant covariates: remission at HCT and GVHD prophylaxis. | |||||
| Overall survival | |||||
| Conditioning regimen | |||||
| Flu/Bu | 128 | 1 | 0.041 | ||
| Flu/ Mel 140 | 250 | 1.48 | 1.07 | 2.04 | 0.02 |
| BEAM | 73 | 1.56 | 0.98 | 2.46 | 0.059 |
| Overall survival adjusted for significant covariates: KPS, HCT-CI, remission status at HCT, and prior auto-HCT. | |||||
Abbreviations: DLBCL- Diffuse large B-cell lymphoma; RIC- Reduced Intensity Conditioning; allo-HCT- Allogeneic hematopoietic cell transplantation; Flu/Bu- Fludarabine/Busulfan; Flu/Mel- Fludarabine/Melphalan; BEAM- BCNU/etoposide/cytarabine/melphalan; HCT-CI- Hematopoietic Cell Transplantation Specific Comorbidity Index; auto-HCT- Autologous Hematopoietic cell transplantation; GVHD- Graft versus Host Disease
Overall P values show whether the main effect was significant based on the Wald test in the final model. The other P values are from pairwise comparisons between two conditioning regimens. All pairwise comparisons were from the Wald test.
Figure 2:
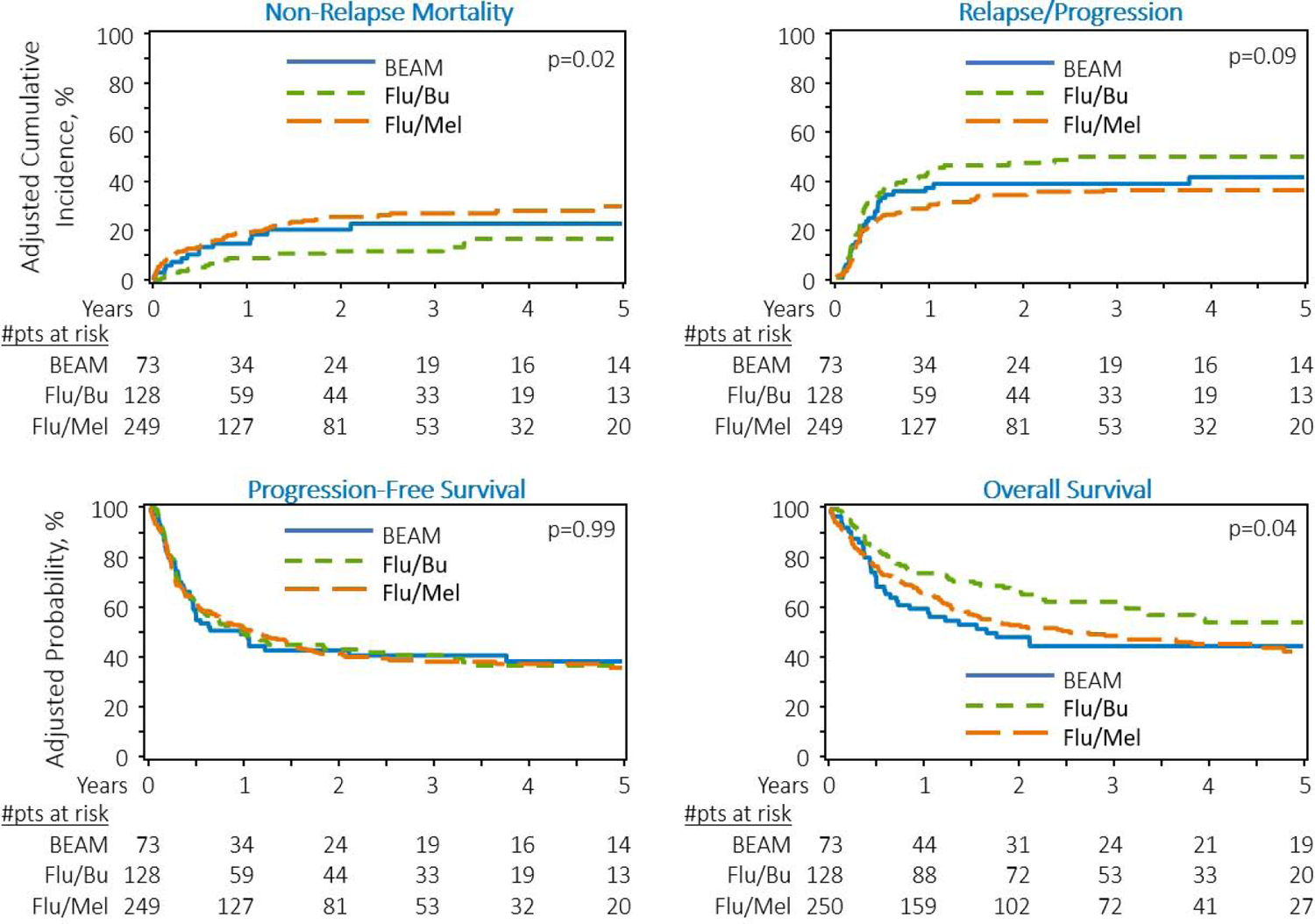
Adjusted transplantation outcomes of chemosensitive DLBCL patients receiving RIC regimens. A) Cumulative incidence of non-relapse mortality. B) Cumulative incidence of lymphoma relapse/progression. C) Progression-free survival. D) Overall survival
Outcomes of chemoresistant patients at allo-HCT
The proportion of patients with chemoresistant disease at the time of allo-HCT was higher in the BEAM cohort (36%) compared with Flu/Bu (11%) and Flu/Mel140 (14%) cohorts (p<0.001). In the chemoresistant subset, after adjusting for significant covariates in the multivariate analysis, BEAM (HR=9.65, 95%CI=1.93–48.21, p=0.006) conditioning regimen was associated with a significantly higher risk of NRM relative to Flu/Bu (Table S3). The Flu/Mel140 (HR=0.61) and BEAM (HR=0.59) cohorts were not associated with a significantly lower risk of relapse/progression relative to Flu/Bu cohort (overall p=0.41; Table S3). Finally, on multivariate analysis, after adjusting for significant covariates, neither of the RIC regimens (Flu/Mel 140 or BEAM) were associated with an improvement in PFS (overall p=0.80) or OS (overall p=0.35) compared with Flu/Bu cohort (Table S3).
Causes of death
The most common cause of death in all the cohorts was recurrent/progressive lymphoma; 57% (n = 72) with Flu/Bu, 41% (n=158) with Flu/Mel140, and 51% (n=34) with BEAM. Infectious complications and GVHD were other common causes of death (Table 5).
Table 5.
Cause of death
| Flu/Bu | Flu/Mel 140 | BEAM | |
| Number of patients | 72 | 158 | 67 |
| Primary disease | 41 (57) | 65 (41) | 34 (51) |
| Infection | 6 (8) | 16 (10) | 5 (8) |
| GVHD | 4 (6) | 8 (5) | 8 (12) |
| IpN | 1 (1) | 1 (1) | 0 |
| Organ failure | 1 (1) | 12 (8) | 3 (5) |
| Second malignancy | 0 | 1 (1) | 1 (1) |
| Hemorrhage | 0 | 1 (1) | 1 (1) |
| Vascular | 0 | 1 (1) | 0 |
| Other HCT related cause, NOS | 13 (18) | 30 (19) | 9 (13) |
| Not reported | 6 (8) | 23 (15) | 6 (9) |
Abbreviations: Flu/Bu- Fludarabine/Busulfan; Flu/Mel- Fludarabine/Melphalan; BEAM- BCNU/etoposide/cytarabine/melphalan; GVHD- Graft versus Host Disease; IPn, Idiopathic pneumonia syndrome; HCT- Hematopoietic Cell Transplantation
DISCUSSION
Prospective randomized controlled studies comparing outcomes among various RIC regimens in DLBCL have not been performed. Here, we report the largest registry analysis of DLBCL patients undergoing RIC allo-HCT with either Flu/Bu, Flu/Mel140 or BEAM conditioning, and make several important observations. First, the probability of platelet recovery was lower following more intense RIC (i.e. Flu/Mel140 and BEAM) relative to Flu/Bu. Second, BEAM conditioning was associated with a significantly higher risk of grade 3–4 acute GVHD. Third, BEAM and Flu/Mel140 were associated with a significantly higher NRM risk relative to Flu/Bu conditioning. Fourth, the risk of relapse was significantly lower following Flu/Mel140 conditioning but did not translate into survival benefit due to higher NRM. Lastly, none of these regimens provided a PFS or OS benefit in the overall study population, but in chemosensitive patients, Flu/Bu was associated with significantly reduced mortality risk.
Unlike myeloid disorders, where the benefit of conditioning intensity in younger and fit patients is well established (21, 22), in lymphomas, the benefit for higher intensity regimens remains debatable. Whether MAC offers an advantage over RIC options in NHL patients undergoing allo-HCT has been examined. A CIBMTR analysis for patients with chemosensitive DLBCL showed no difference in 5-year PFS and OS between MAC and RIC/NMA regimens (5, 11). Similarly, even in patients with chemorefractory DLBCL, the intensity of conditioning regimens (MAC vs. RIC) does not appear to impact survival outcomes (5, 12, 23). Although BEAM is considered as a MAC regimen by the European Society for Blood and Marrow Transplantation (EBMT), it is categorized as RIC by CIBMTR based on the consensus criteria (14). While prior CIBMTR studies have reported the outcomes of MAC vs RIC/NMA, there are limited comparative data published on the relative efficacy and toxicity of the individual RIC regimens commonly used in DLBCL. A multicenter retrospective study (n=136) compared outcomes of two RIC regimens (Flu/Mel with Flu/Bu) in lymphomas and showed that Flu/Bu was associated with a significantly lower risk of acute GVHD and NRM and improved OS relative to Flu/Mel (24). However, the study was limited by a small sample size of aggressive NHLs (n=65) and did not include BEAM as a conditioning regimen. A recent CIBMTR study reported the outcome of four RIC/NMA regimens: Flu/Bu, Flu/Mel140, Flu/Cy, and Flu/Cy/2GyTBI, in NHL patients (13). In that study, relative to Flu/Bu, Flu/Mel140 was associated with a significantly higher risk of NRM translating to inferior OS (HR=1.34, 95%CI=1.13–1.59; p<0.001) (13). However, that study included all NHL histologies (B-cell NHL including aggressive and indolent NHLs and T-cell NHL) and did not include BEAM conditioning, a commonly used RIC regimen in DLBCL. In the current study, we compared the three commonly used RIC regimens (Flu/Bu, Flu/Mel140 and BEAM) in DLBCL and found that Flu/Mel140 and BEAM were associated with a significantly higher risk of NRM compared to Flu/Bu. In line with the previous study, the risk of relapse was significantly lower with Flu/Mel140; however, this did not translate into survival benefit in the current study.
A recent single-center retrospective study (n=70) evaluated the outcomes of R-BEAM (n=47) versus other RIC regimens (n=23; Flu/Bu/TBI +/− rituximab and Flu/Mel/TBI +/− rituximab) in DLBCL patients (25). In the study, the cumulative incidence of grade 3–4 acute GVHD was significantly higher in the R-BEAM group relative to the RIC cohort, however, there was no difference in the cumulative incidence of relapse, NRM, or survival outcomes between the two cohorts. Relative to R-BEAM, the other regimens in the RIC cohort had a similar 3-year risk of relapse (25.5% versus 17.4%), NRM (39.7% versus 39.1%), and OS (34.4% versus 43.5%). One of the major limitations of the study was the small sample size that precluded robust multivariable modeling. Another important drawback was the imbalance between the groups, especially the RIC cohort that was predominantly comprised of the Flu/Bu/TBI +/− rituximab. Of note, the addition of TBI to the RIC regimens in the study likely increased the toxicity (8, 26).
Historically, DLBCL was the most common indication for allo-HCT in NHL, but with newly available options, these numbers are decreasing. The advent of CD19 CAR T-cell therapy has revolutionized the treatment of relapsed/refractory DLBCL patients with good response rates (27–29). Bispecific antibodies have also shown promising activity in multiply relapsed DLBCL patients including those with do not respond or progress following CAR-T cell therapy (30). Thus, in the future, patients undergoing allo-HCT will be enriched for those who are potentially heavily pretreated. Hence, defining a RIC platform with the best risk/benefit ratio is increasingly important in current practice. Our results suggest that Flu/Bu is a better RIC platform in less fit or heavily pretreated patients due to the lowest NRM risk, and reduced risk of mortality (particularly in patients with chemosensitive disease).
We would like to acknowledge that the consensus criteria allows a wide array of regimens to be classified as RIC, with some at the lower spectrum of intensity (e.g. Flu/Bu and associated lower NRM risk), while others are at the high end of intensity spectrum (e.g. Flu/Mel140 and BEAM) with much higher NRM risk, in elderly DLBLC patients. Any observational study comparing different interventions is subject to the preferences of the treating centers/physicians owing to the complex criteria for selection of the conditioning regimen. However, we did not notice any center effect, in this analysis. We acknowledge that the patients in the BEAM cohort were probably enriched for those who were not candidates for auto-HCT, likely due to chemoresistance. To address this bias, we did a subgroup analysis of the three RIC regimens and evaluated the outcomes of the chemosensitive patients at allo-HCT. The results were generally in line with the main analysis except for significantly higher risk of mortality with Flu/Mel140. When restricting the analysis to patients with chemoresistant at allo-HCT, the BEAM cohort had a significantly higher risk for NRM relative to Flu/Bu, however, there was no survival (PFS or OS) benefit seen with any of the RIC regimens in this analysis. These results are in line with a previously published CIBMTR study that showed no survival benefit with higher conditioning regimen intensity in chemorefractory patients at allo-HCT (12). While it has been previously shown that the addition of rituximab to RIC regimens improved PFS (1), we cannot examine the effect of rituximab at conditioning in our study as only a few patients received rituximab at conditioning (n=90). More importantly, it was predominantly used with BEAM conditioning (n=56) thereby precluding additional analysis. The reason for more frequent use of rituximab with BEAM conditioning (compared with Flu/Bu and Flu/Mel140) in our study is not clear. While this is likely a reflection of center policies, it is plausible that the high percentage of chemoresistant patients in the BEAM cohort could have been the driving factor behind the addition of rituximab to BEAM. Lastly, as alemtuzumab-BEAM was shown to be toxic by other groups, we tried to evaluate the toxicity associated with this. However, only 13 patients in the current study received alemtuzumab-BEAM precluding any additional evaluation.
Our analysis is the largest comparative study evaluating outcomes of commonly used RIC regimens in DLBCL patients. Although Flu/Mel140 was associated with a significantly lower risk of relapse, this did not translate into superior survival due to significantly higher NRM. Similarly, BEAM was associated with a significantly higher risk of NRM. Although all the regimens evaluated provided comparable survival outcomes in the overall patient population, Flu/Bu provided superior survival relative to Flu/Mel140 in chemosensitive patients. In the absence of prospective randomized studies in the field, our results suggest that Flu/Bu is a better RIC platform in less fit or heavily pretreated patients due to the lowest NRM risk.
Supplementary Material
HIGHLIGHTS.
Reduced-intensity conditioning regimen with the best risk/benefit profile for allo-HCT in DLBCL is not known. This is important, as patients with DLBCL undergoing allo-HCT in the future would be enriched for those whose lymphoma has failed chimeric antigen receptor T-cell therapy or other novel immunotherapies, with potentially more advanced disease and suboptimal performance scores.
Our results suggest that Flu/Bu is a better RIC choice in less fit or heavily pretreated patients due to the lowest NRM risk.
ACKNOWLEDGMENTS
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors do not have any relevant competing interests pertaining to the manuscript
References:
- 1.Epperla N, Ahn KW, Ahmed S, Jagasia M, DiGilio A, Devine SM, et al. Rituximab-containing reduced-intensity conditioning improves progression-free survival following allogeneic transplantation in B cell non-Hodgkin lymphoma. J Hematol Oncol. 2017;10(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klyuchnikov E, Bacher U, Kroger NM, Hari PN, Ahn KW, Carreras J, et al. Reduced-Intensity Allografting as First Transplantation Approach in Relapsed/Refractory Grades One and Two Follicular Lymphoma Provides Improved Outcomes in Long-Term Survivors. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(12):2091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh N, Karmali R, Rocha V, Ahn KW, DiGilio A, Hari PN, et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. J Clin Oncol. 2016;34(26):3141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenske TS, Ahn KW, Graff TM, DiGilio A, Bashir Q, Kamble RT, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016;174(2):235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah NN, Ahn KW, Litovich C, Fenske TS, Ahmed S, Battiwalla M, et al. Outcomes of Medicare-age eligible NHL patients receiving RIC allogeneic transplantation: a CIBMTR analysis. Blood Adv. 2018;2(8):933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epperla N, Ahn KW, Armand P, Jaglowski S, Ahmed S, Kenkre VP, et al. Fludarabine and Busulfan versus Fludarabine, Cyclophosphamide, and Rituximab as Reduced-Intensity Conditioning for Allogeneic Transplantation in Follicular Lymphoma. Biol Blood Marrow Transplant. 2018;24(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamadani M, Khanal M, Ahn KW, Litovich C, Chow VA, Eghtedar A, et al. Higher Total Body Irradiation Dose Intensity in Fludarabine/TBI-Based Reduced-Intensity Conditioning Regimen Is Associated with Inferior Survival in Non-Hodgkin Lymphoma Patients Undergoing Allogeneic Transplantation. Biol Blood Marrow Transplant. 2020;26(6):1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avivi I, Canals C, Vernant JP, Wulf G, Nagler A, Hermine O, et al. Matched unrelated donor allogeneic transplantation provides comparable long-term outcome to HLA-identical sibling transplantation in relapsed diffuse large B-cell lymphoma. Bone Marrow Transplant. 2014;49(5):671–8. [DOI] [PubMed] [Google Scholar]
- 10.Dreger P, Sureda A, Ahn KW, Eapen M, Litovich C, Finel H, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3(3):360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacher U, Klyuchnikov E, Le-Rademacher J, Carreras J, Armand P, Bishop MR, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120(20):4256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamadani M, Saber W, Ahn KW, Carreras J, Cairo MS, Fenske TS, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant. 2013;19(5):746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh N, Ahmed S, Ahn KW, Khanal M, Litovich C, Aljurf M, et al. Association of Reduced-Intensity Conditioning Regimens With Overall Survival Among Patients With Non-Hodgkin Lymphoma Undergoing Allogeneic Transplant. JAMA Oncology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(5):579–86. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 17.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. The American journal of medicine. 1980;69(2):204–17. [DOI] [PubMed] [Google Scholar]
- 18.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145–56; discussion 57–9. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. J Clin Oncol. 2020;38(12):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Kampen RJ, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29(10):1342–8. [DOI] [PubMed] [Google Scholar]
- 24.Kekre N, Marquez-Malaver FJ, Cabrero M, Pinana J, Esquirol A, Soiffer RJ, et al. Fludarabine/Busulfan versus Fludarabine/Melphalan Conditioning in Patients Undergoing Reduced-Intensity Conditioning Hematopoietic Stem Cell Transplantation for Lymphoma. Biol Blood Marrow Transplant. 2016;22(10):1808–15. [DOI] [PubMed] [Google Scholar]
- 25.Modi D, Kim S, Surapaneni M, Ayash L, Alavi A, Ratanatharathorn V, et al. R-BEAM versus Reduced-Intensity Conditioning Regimens in Patients Undergoing Allogeneic Stem Cell Transplantation for Relapsed Refractory Diffuse Large B Cell Lymphoma. Biol Blood Marrow Transplant. 2020;26(4):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong S, Le-Rademacher J, Artz A, McCarthy PL, Logan BR, Pasquini MC. Comparison of non-myeloablative conditioning regimens for lymphoproliferative disorders. Bone Marrow Transplant. 2015;50(3):367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 29.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang ML, Arnason JE, et al. Pivotal Safety and Efficacy Results from Transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed/Refractory (R/R) Large B Cell Lymphomas. Blood. 2019;134(Supplement_1):241–. [Google Scholar]
- 30.Schuster SJ, Bartlett NL, Assouline S, Yoon S-S, Bosch F, Sehn LH, et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood. 2019;134(Supplement_1):6–.31273004 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


