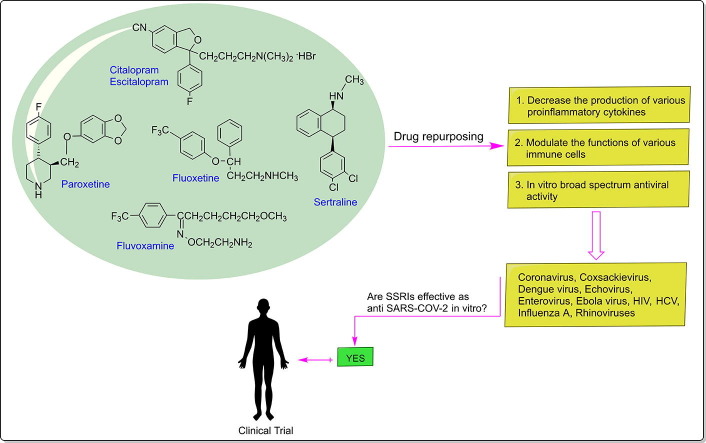
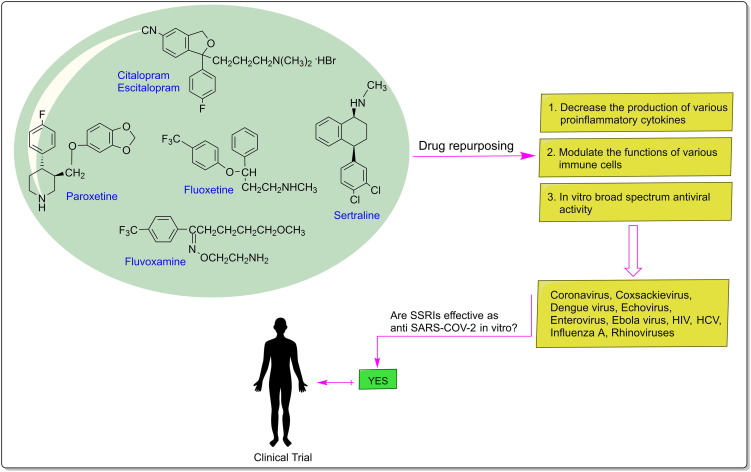
Graphical abstract
Abbreviations: ACE-2, Angiotensin converting enzyme II; ARDS, Acute respiratory distress syndrome; ALI, Acute lung injury; APOE ε4, Apolipoprotein E4; AUC, Area under the curve; Cmax, Maximum plasma concentration; COVID-2019, Coronavirus disease 2019; CS, Cytokine storm; CLpro, Chemotrypsin-like protease; CYP, Cytochrome P450; CV-B3, Coxsackievirus B3; CCR5, C-C chemokine receptor type 5; DENV, Dengue virus; EV, Enteroviruses; EBOV, Ebola virus; 5-HT, 5-Hydroxytryptamine; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus; IL-1β, Interleukin-1beta; IFN-γ, Interferon-gamma; NK, Natural Killer; NF-κB, Nuclear factor kappa B; PK, Pharmacokinetic; PD, Pharmacodynamic; Panc-1, Pancreatic cells; PLpro, Papain-like protease; RdRp, RNA-dependent RNA polymerase; SERT, Serotonin reuptake transporter; S1R, Sigma-1 receptor; SSRIs, Selective serotonin reuptake inhibitors; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SS, Serotonin syndrome; TNF-α, Tumor necrosis factor-alpha; TGF-β1, Transforming growth factor beta 1; VD, Volume of distribution
Keywords: COVID-19, SARS-CoV-2, Drug repurposing, Antidepressant drugs, Selective serotonin reuptake inhibitors (SSRIs), Anti-inflammatory
Abstract
The current 2019 novel coronavirus disease (COVID-19), an emerging infectious disease, is undoubtedly the most challenging pandemic in the 21st century. A total of 92,977,768 confirmed cases of COVID-19 and 1,991,289 deaths were reported globally up to January 14, 2021. COVID-19 also affects people’s mental health and quality of life. At present, there is no effective therapeutic strategy for the management of this disease. Therefore, in the absence of a specific vaccine or curative treatment, it is an urgent need to identify safe, effective and globally available drugs for reducing COVID-19 morbidity and fatalities. In this review, we focus on selective serotonin reuptake inhibitors (SSRIs: a class of antidepressant drugs with widespread availability and an optimal tolerability profile) that can potentially be repurposed for COVID-19 and are currently being tested in clinical trials. We also summarize the existing literature on what is known about the link between serotonin (5-HT) and the immune system. From the evidence reviewed here, we propose fluoxetine as an adjuvant therapeutic agent for COVID-19 based on its known immunomodulatory, anti-inflammatory and antiviral properties. Fluoxetine may potentially reduce pro-inflammatory chemokine/cytokines levels (such as CCL-2, IL-6, and TNF-α) in COVID-19 patients. Furthermore, fluoxetine may help to attenuate neurological complications of COVID-19.
1. Introduction
The novel coronavirus disease 2019 (COVID-19), a systemic infection potentially targeting multiple organs and functions, is currently leading to a global pandemic. The mortality rate of COVID-19 appears to be in the range of 3.4–5.5%, which is significantly higher than that for seasonal flu caused by the influenza virus (1%) [1]. At the time of writing the article (14 January 2021), humanity is struggling with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, which has already infected more than 90.9 million people and caused over 1.9 million deaths worldwide [2]. This rapid and unprecedented pandemic has caused serious psychological problems, such as anxiety (panic attacks and post-traumatic stress) and depression [3]. Structurally, SARS-CoV-2, an enveloped positive-sense single stranded RNA (+ssRNA) virus belonging to the β genus of the Coronaviridae family, has four main structural proteins including the small envelope (E) glycoprotein, membrane (M) protein, nucleocapsid (N) protein, and spike (S) glycoprotein, and also three accessory proteins include: papain-like protease (PLpro) and 3Chemotrypsin-like protease (3CLpro, also known as the main protease-Mpro), which are responsible for cleavage of viral polypeptide into functional units; and RNA-dependent RNA polymerase (RdRp), which is critical for viral replication and transcription [4], [5]. The virus penetrates the host cell via the binding of its S-protein with the angiotensin converting enzyme II (ACE-2) receptor, which is found in virtually all human organs in varying degrees [6]. Thus, it is suggested that the disruption of the interaction between ACE-2 and SARS-CoV-S protein is a potential therapeutic target for treating COVID-19. In general, S protein, PLpro, 3CLpro, RdRp and ACE-2 are the most attractive targets for the development of new drugs against COVID-19 [7]. The clinical features of COVID-19 are varied, ranging from an asymptomatic or mild symptom state (common cold-type) to acute respiratory distress syndrome (ARDS), sepsis and septic shock, multiple organ dysfunction, and, finally, death. In addition, the SARS-CoV-2 infection mediates hyper-inflammation and dysregulated immunity leading to CS. The management of the disease includes preventive and therapeutic strategies and the treatment of CS. CS is an exaggerated or aberrant host immune response to viral infection, which is considered to be one of the main causes of ARDS in COVID-19. ARDS is a potentially life-threatening condition leading to severe pulmonary edema, respiratory failure and arterial hypoxemia refractory to oxygen therapy [8], [9]. The severity of COVID-19 depends largely on the immunity and the release of inflammatory mediators including cytokines and chemokines such as interleukin (IL)-2, IL-6, IL-7, IL-1β, tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP1; also known as CCL2 [CC-chemokine ligand 2]), macrophage inflammatory protein-1 alpha (MIP-1α; also known as CCL3), ferritin, C-reactive protein (CRP), and D-dimers [10]. For example, Del Valle et al. [11] found that high serum IL-6 and TNF-α levels at presentation were strong predictors of disease severity and survival. They also proposed that serum IL-6 and TNF-α levels should be considered in the management and treatment of patients with COVID-19 to stratify prospective clinical trials, guide resource allocation and inform therapeutic options. COVID-19 crisis poses a serious threat to global public health and an effective and affordable therapeutic strategy could provide a key means of overcoming this crisis [12]. Unfortunately, till now, no effective vaccine or drug for the prevention (prophylaxis) or treatment of this contagious disease has been established. Though remdesivir, a broad spectrum anti-viral drug, an RdRp inhibitor, advanced into human clinical trials to treat COVID-19, it is still not available for most of the patients [13]. Due to the urgency of the situation, drug repurposing (i.e. testing the efficacy of existing drugs used previously to treat other diseases), as a faster and cheaper pathway, is a basic goal in order to identify possible effective therapeutic options. In this regard, multiple off-label and investigational drugs have gained significant attention due to positive preclinical or clinical data [14]. Recently, Attademo et al. [15] reported that alterations of both the serotonin and dopamine synthetic pathways might be involved in the pathophysiology of COVID-19 infection. The possible involvement of these neurotransmitters is suggested by a significant link between ACE-2 and DOPA decarboxylase (a major enzyme of both the dopamine and the serotonin synthetic pathways that catalyzes the biosynthesis of dopamine from L-3,4-dihydroxyphenylalanine and serotonin from L-5-hydroxytryptophan). The same group interestingly argues that a SARS-CoV-2-induced defective expression of ACE-2 might be paralleled by a DOPA decarboxylase dysfunction, with consequent potentially altered neurotransmitters’ levels in COVID-19 patients. However, further experimental research works are needed to evaluate this hypothesis. Also, there may be a possibility that 5-HT levels are altered in COVID-19 patients because of mental stress. In the current study, we aim to provide a to-the-point review of current literature regarding efficacy of selective serotonin reuptake inhibitors (SSRIs) as a therapeutic option for COVID-19.
2. Role of serotonin (5-HT) in the immune system
Serotonin, 5-Hydroxytryptamine (5-HT) or 3-(2-Aminoethyl)-1H-indol-5-ol, is often known as the happy hormone in the body; low levels of 5-HT have been noted in patients with depression [16]. It is also a precursor to melatonin in the pineal gland. Furthermore, 5-HT, a neurotransmitter (chemical messenger) in the central nervous system, plays a pivotal role in peripheral tissues, including the immune system. Blood platelets carry peripheral serotonin (close to 95% of total serotonin in the body) to various tissues and represent the major source of 5-HT for immune cells [17]. 5-HT receptors (7 classes: 5-HT1 to 5-HT7) are expressed in various human and rodent immune cells including monocytes/macrophages, dendritic cells, neutrophils, mast cells, eosinophils, B cells and T cells [18], [19]. Thus, 5-HT and 5-HT-modulating agents may have a direct effect on both innate and adaptive immune function [20]. Indeed, 5-HT is involved in modulation of pro-inflammatory cytokine/chemokine production, induction of anti-inflammatory cytokine production, activation of Natural Killer cells (or NK cells), migration and recruitment of immune cells, activation of human monocytes and prevention of monocyte apoptosis, and protection of cells against the detriment of oxidative stress [21], [22], [23], [24]. Physiologic concentrations of 5-HT reduces phagocytosis of murine macrophages [25], [26] and the production of TNF-α and interferon-gamma (IFN-γ) by human blood leucocytes [27], [28]. 5HT can also modulate human dendritic cells function by increasing the release of the cytokine IL-10, a potent cytokine with reputable anti-inflammatory properties [29]. IL-10 also reduces the levels of TNF-α and IL-6 [30]. IL-6 levels increase significantly in the early stage of inflammation, which provides evidence for rapid diagnosis of early SARS-CoV-2 infection in the clinic [31]. In human alveolar macrophages, serotonin inhibits IL-12 and TNF-α release, but it increases IL-10 production via 5-HT2 receptors [32]. Cadirci et al. [33] investigated the effects of 5-HT7 agonist (AS-19) and antagonists (SB269970) in a study on inflammation with sepsis, and showed that 5-HT7 agonist treatment decreased plasma IL-1β and IL-6 and also lung nuclear factor kappa B (NF-κB) levels. Inhibition of NF-κB activity can reduce the cell infiltration, and decrease the secretion of pro-inflammatory cytokines, thus protect the lung tissue from damage [34]. In addition, according to recent studies, 5-HT is able to inhibit lipopolysaccharide-induced inflammatory responses (IL-1β, IL-6, IL-12p40, TNF-α, and chemokine CXCL8/IL-8 release) by human monocytes and peripheral blood mononuclear [35], [36], [37]. In 2017, Ayaz et al. [38] demonstrated anti-inflammatory effects of 5-HT7 agonist administration in the in vivo and in vitro lipopolysaccharide-induced inflammation model. In 2019, Mota et al. [39] investigated the potential role of 5-HT in the brain during systemic lipopolysaccharide-induced severe septic like inflammation. They found that systemic inflammation reduced 5-HT levels in the hypothalamus favor an increased pro-inflammatory status both centrally and peripherally that converge to hypotension and hypothermia. Moreover, the authors reported that exogenously administered 5-HT is able to prevent lipopolysaccharide-induced hypotension and significantly reduce systemic TNF-α, IL-1β and IL-6 using the most well accepted experimental model of systemic inflammation [40]. Therefore, according to these studies, pharmacological regulation of the serotonergic system may modulate immune function.
3. Role of 5-HT in the viral infection
The role of 5-HT in the immune response in specific viral infections has been reported. Human immunodeficiency virus (HIV) infection is a primary model for the study of 5-HT during infection [18]. 5-HT controls HIV replication in lymphocytes [41] and modulates NK cell activation in HIV-infected patients [42], [43]. NK cells are lymphocytes of the innate immune system that are important for early and effective immune reactions against infections. Also, 5-HT decreases HIV infection in human macrophages by down-regulating the expression of C-C chemokine receptor type 5 (CCR5), an essential co-receptor for HIV entry, and reduces proviral DNA synthesis (51%), probably through serotonin 5-HT1A receptor [44]. Furthermore, the infectivity of reovirus and chikungunya were found to be reduced in the presence of a specific 5-HT1B/5-HT1D receptor agonist [45], [46]. Peripheral 5-HT is also able to modulate several mechanisms of different viral infections through its receptors. For example, in 2018, Szabo et al. [47] demonstrated that activation of 5-HT2B receptor-subtype in human monocyte-derived dendritic cells suppressed the production of pro-inflammatory cytokines and chemokines (TNF-α, IL-6, IL-8, IP-10 and IL-12) during a viral stimulus. A recent study by Anderson et al. [48] revealed that viral infections with subsequent cytokine storm may contribute to suppressed 5-HT and melatonin availability. As mentioned earlier, 5-HT is a biosynthetic precursor of melatonin. It is worth noting that melatonin, as an anti-oxidative and anti-inflammatory agent, counters acute lung injury (ALI)/ARDS induced by viral and bacterial infections [49]. Wang et al. [50] evaluated the relationship between different infectious agents and depression. According to authors, there are statistically significant associations between depression and infection with Borna disease virus, herpes simplex virus-1, varicella zoster virus, Epstein-Barr virus, and Chlamydia trachomatis. It has also been reported that viral infections can trigger brain endothelial and epithelial cells to produce cytokines that impair neuronal firing in the hippocampus, leading to depressive-like symptoms [51]. Summarizing the clinical symptoms reported in SARS virus infection, there is thus the possibility that SARS virus infection affected mood by altering the 5-HT system [52]. Therefore, 5-HTR-targeting drugs could be considered as a potential approach in therapies being developed for treating anxiety and depression induced by the COVID-19 infection.
4. Selective serotonin reuptake inhibitors (SSRIs)
SSRIs are the most widely prescribed class of antidepressants and are often used as first choice medication for depression and numerous other anxiety disorders (e.g., panic disorder and obsessive–compulsive disorder) due to their efficacy, safety, and tolerability. SSRIs are generally better tolerated than most other types of antidepressants. The FDA-approved SSRIs include citalopram (more commonly called Celexa), escitalopram (Lexapro), fluoxetine (Prozac), fluvoxamine (Luvox), paroxetine (Paxil), and sertraline (Zoloft) [53]. These drugs have significantly fewer side effects compared to other types of antidepressants due to having fewer effects on adrenergic, histaminic, and cholinergic receptors. Furthermore, SSRIs have wide toxic indexes (ingestion of up to 30 times the daily dose typically produces minor or no symptoms), similar antidepressant efficacy, and similar side effect profiles. They differ, however, in their pharmacokinetic (PK) properties, which may explain their different potential for PK drug-drug interactions. SSRIs are well absorbed in the gastrointestinal tract after oral intake, and peak plasma concentrations are usually reached within 1–8 h. Sertraline, citalopram, and escitalopram exhibit linear PKs in that a change in dose leads to a proportional change in drug concentration. In contrast, paroxetine, fluvoxamine and fluoxetine have nonlinear PKs [54]. SSRIs are lipophilic compounds (logP = 2.89–5.1) and therefore exhibit a large volume of distribution (VD) (up to 45 L/kg). Values of VD greater than the total volume of body water (approximately 42 L) show that SSRIs are highly distributed into tissues. All of the SSRIs (except for fluvoxamine [77%] and escitalopram [55%]) are highly protein-bound (94–98%). Of the SSRI half-lives, fluoxetine (1–4 days), citalopram (35 h), escitalopram (27–32 h) and sertraline (26 h) have a long one with fluoxetine having the longest, paroxetine (21 h) has an intermediate one and fluvoxamine (15.6 h) has the shortest. Although the SSRIs are eliminated by hepatic biotransformation involving the cytochrome P450 (CYP) isoenzymes, they and some of their metabolites can indeed inhibit the CYP isoenzymes (see Table 1 for further details) [55]. Table 1 illustrates the relationship between SSRIs and CYP enzymes. On the other hand, SSRIs exhibit antidepressant action by blocking the serotonin reuptake transporter (SERT) at the presynaptic neuron. By blocking SERT, an increased amount of 5-HT remains in the serotonergic synaptic cleft and can stimulate postsynaptic receptors for a more extended period [56]. In addition, several studies have revealed the immunomodulatory, anti-inflammatory and antiviral properties of SSRIs. The findings of these studies are summarized in the sections below.
Table 1.
SSRI drugs and CYP enzymes.
| SSRIs | Major elimination pathway(s) | Other elimination pathway(s) | Inhibitory effect on CYP isoenzyme(s) |
|---|---|---|---|
| Citalopram | CYP2C19 | CYP2D6, CYP3A4 | CYP2D6 (weak) |
| Escitalopram | CYP2C19 | CYP2D6, CYP3A4 | CYP2D6 (weak) |
| Fluoxetine | CYP2D6 | CYP2C9, CYP2C19, CYP3A4 | CYP2D6 (strong), CYP2C9 (moderate), CYP2C19, (weak to moderate), CYP3A4 (weak to moderate), CYP1A2 (weak) |
| Fluvoxamine | CYP1A2, CYP2D6 | – | CYP2D6 (weak), CYP1A2 (strong), CYP2C19 (strong), CYP2C9 (moderate), CYP3A4 (moderate) |
| Paroxetine | CYP2D6 | CYP3A4 | CYP2D6 (strong), CYP1A2 (weak), CYP2C9 (weak), CYP2C19 (weak), CYP3A4 (weak) |
| Sertraline | CYP2B6 | CYP2C9, CYP2C19, CYPC2D6, CYP3A4 | CYP2D6 (weak to moderate), CYP1A2 (weak), CYP2C9 (weak), CYP2C19 (weak), CYP3A4 (weak) |
5. SSRIs and immune system
SSRIs have been shown to alter several aspects of immune cell functioning. For example, Frank et al. [57] demonstrated that in vitro exposure of mononuclear cells to fluoxetine and paroxetine directly increase NK-cell activity. Several authors also found significant increases in NK cells counts or activity following SSRI treatment of depressed individuals [58], [59], [60]. Moreover, Evans et al. [42] and Benton et al. [61] found that the administration of citalopram to HIV-seropositive women exerted a number of immunomodulatory effects, including enhanced NK cell innate immunity, decreased HIV replication in latently infected T-cell and macrophage cell lines, and inhibited acute HIV infection of macrophages. Therefore, it could be told that SSRIs may have an adjuvant medication role in immune restitution of patients infected with HIV. The studies by Pellegrino et al. [62], [63] showed that in vivo administration of fluoxetine to rats similarly decreased lymphocyte proliferation when induced by mitogens ex vivo. Moreover, Canan et al. [64] reported that escitalopram treatment may have a lymphocyte proliferative effect. According to the authors, the possible treatment of depression with escitalopram must be carried out with caution, in patients with immunological disturbances. In another study, Chang et al. [65] suggested that fluoxetine has a protective role against cell death in concentrations between 100 pM and 1 μM and a dose-dependent effect on the proliferation of neural stem cells. Hernandez et al. [66] also achieved a significant increase in B-cell numbers and NK proliferation following long-term (52-week) SSRI treatment. In addition, the ex-vivo immunomodulatory effect of SSRIs on human T cells was elucidated by Taler et al. [67]. The authors found that a higher concentration of paroxetine and sertraline (IC50 = 10 µM) was associated with inhibition of T-cell proliferation and reduced secretion of TNF-α. Thus, according to the above-mentioned studies, it seems that SSRIs can modulate the functions of various immune cells. On the other hand, SSRIs have anti-inflammatory effects and they achieve this effect through the decrease of pro-inflammatory cytokine production and increase of anti-inflammatory cytokines. In 2011, a meta-analysis of twenty-two studies by Hannestad et al. [68] demonstrated that SSRI treatment may decrease levels of IL-1β, IL-6 and possibly TNF-α. Kubera et al. [28], [37] and Maes et al. [69] found that sertraline and fluoxetine significantly reduced IFN-γ and increased IL-10 production. Hence, both SSRIs significantly decreased the IFN-γ/IL-10 production ratio. Tuglu et al. [70] found a significant decrease of TNF-α plasma levels after 6 weeks of SSRI treatment. Sluzewska et al. [71] also found a decrease of elevated IL-6 levels in depressed patients after 8 weeks of fluoxetine. Furthermore, Sharma et al. [72] described that sertraline in combination with oseltamivir (an antiviral neuraminidase inhibitor) increased survival, reduced mortality, and reduced pulmonary inflammation in mice infected with a lethal dose of influenza A H1N1 virus. According to the authors, sertraline had no significant effect on virus replication in vitro and in vivo, but significantly reduced lung inflammation. Obuchowicz et al. [73] demonstrated that imipramine and fluoxetine suppressed lipopolysaccharide-induced activation NF-κB and the production of TNF-α, IL-1β and IL-10 even at a very low concentration. Shenoy et al. [74] also showed that citalopram completely suppressed anti-CD3 triggered IL-2 production, severely reduced IL-4 and partially suppressed IL-17 production. Tucker et al [75] found that blood levels of IL-1β significantly reduced in patients with posttraumatic stress disorder after treatment with citalopram and sertraline. In another study, Roumestan et al. [76] described that fluoxetine decreased TNF-α expression as well as the activity of NF-κB and activator protein-1, in septic shock and allergic asthma animal models. Besides, SSRIs may modulate the inflammatory response not only by direct serotonergic mechanisms. For example, in 2019, Rosen et al. [77] identify the endoplasmic reticulum-resident protein Sigma-1 receptor (S1R) as an essential inhibitor of cytokine production. The authors reported that the S1R ligand fluvoxamine can enhance survival in mouse models of inflammation and sepsis and can inhibit the inflammatory response in human peripheral blood cells. Other studies have also demonstrated that SSRIs exert anti-inflammatory effects on microglia, the principal cells within the CNS that regulate and respond to inflammatory factors [78], [79], [80]. For example, fluoxetine significantly reduced TNF-α, IL-6 and NO production in lipopolysaccharide-stimulated microglial cells [78]. In 2017, Shi et al. [81] found that the presence of apolipoprotein E (APOE ε4) allele has been associated with increased pro-inflammatory cytokines (such as TNF-α, IL-6) and microglial activation. It is well-known that APOE ε4 allele is a major genetic risk factor for Alzheimer's disease (AD) [82]. Studies have also shown that the APOE ε4 allele may lead to AD pathology through an altered inflammatory state [83]. Interestingly, Wang et al. [84] provided evidence that APOE ε4 could lead to increased SARS-CoV-2 susceptibility in both neurons and astrocytes. However, additional studies are needed to clarify an association between APOE ε4, inflammation, and COVID-19 infection. On the other hand, SSRIs increase circulating transforming growth factor beta 1 (TGF-β1: a potent anti-inflammatory cytokine) in depressed patients [85]. A recent study by Torrisi et al. [86] showed that a long-term (24 days) treatment with fluoxetine or vortioxetine (both at the dose of 10 mg/kg) in mice can revert both β-amyloid-induced depressive-like behavior and memory impairment by increasing the release of TGF-β1. TGF-β1 is also a key regulator of pulmonary fibrosis as well as other fibrotic diseases of various organs. Accordingly, Xiong et al [87] suggested that increased expression of TGF-β in COVID-19 patients might be the cause of pulmonary fibrosis. Interestingly, the work of Marques-Deak et al. [88] demonstrated that SSRI administration increases pro-inflammatory cytokines levels. Frick et al. [89] also described that the treatment with fluoxetine for 4 weeks increased T cell proliferation and Th1-like cytokines (IFN-γ and TNF-α) production. In another study, Hernández et al. [90] showed an increment in IL-2 and IL-1β after 52 weeks of SSRI treatment. Recently, Keaton et al. [91] reported a unique immunobiological profile linked to increased suicide risk. Accordingly, Amitai et al. [92] found that an increase in IL-6 levels during 8 weeks of fluoxetine treatment is a risk factor for the emergence of SSRI-associated suicidality. In general, despite some conflicting data, it appears that SSRI drugs are able to modulate the immune response.
6. SSRIs and viral infections
There are some studies showing possible antiviral effects of SSRIs. For example, Kristiansen et al [93] demonstrated that paroxetine and femoxetine reduced p24 antigen levels in an in vitro HIV inhibition cell culture system. According to the authors, these compounds could be used in combination with other anti-retroviral drugs in HIV-1 infected patients with AIDS-related dementia. Greeson et al. [94] suggested that citalopram treatment inhibits HIV cell entry and replication, through downregulating CD4 expression and chemokine receptor expression (CCR5, CXCR4), and may reduce susceptibility of immune cells to HIV infection and decrease inflammation. Letendre et al. [95] also reported that SSRIs (citalopram and sertraline) may reduce HIV replication in cerebrospinal fluid and improve neuropsychological performance. In another study, Johansen et al. [96] identified 171 different anti-Ebola virus (EBOV) compounds in a high-throughput screen. Two drugs, sertraline and bepridil, inhibited EBOV cell entry in vitro and in vivo. These drugs offer potential for repurposing for EBOV disease, either as single agents or in combinations. Benton et al. [61] showed that citalopram significantly downregulated the reverse transcriptase response in both the acute and chronic infection models. Zuo et al. [97] screened more than 1100 compounds to identify potentially novel compounds with antiviral efficacy against enteroviruses (EV). The authors found that fluoxetine and its metabolite norfluoxetine inhibited the replication of Coxsackievirus B3 (CV-B3) in HeLa cells. Subsequently, Ulferts et al. [98] demonstrated that fluoxetine inhibited the replication of CV-B3, EV-D68, EV-D70, Echovirus-1, Echovirus-9 and Echovirus-11 in vitro in a human system. Alidjinou et al. [99] also demonstrated that fluoxetine can inhibit the replication of CV-B4 in human pancreatic cells (Panc-1 cell line). According to the authors, fluoxetine cleared the virus from Panc-1 cell cultures chronically infected with CV-B4. In 2019, a report from Bauer et al. [100] showed that only the S-enantiomer of fluoxetine inhibits CV-B3 and also EV-D68. They observed that the S-enantiomer of fluoxetine also exerts antiviral activity against rhinoviruses. Recently, the same group synthesized a new series of fluoxetine analogues and evaluated them for their antiviral activity. They demonstrated that these analogues inhibited CV-B3 and EV-D68 replication, but not EV-A71 or representatives of the EV-C species (poliovirus and CV-A24). According to the authors, the structural features of the trifluoro-phenoxy moiety and the amino moiety are essential for the antiviral activity whereas the 3-phenyl moiety seems dispensable [101]. Fluoxetine was also shown to inhibit dengue virus (DENV) and hepatitis C virus (HCV), two members of the Flaviviridae family [102], [103]. For example, Young et al [103] demonstrated that fluoxetine inhibited HCV infection and blocked the production of reactive oxygen species and lipid accumulation in Huh7.5 cells. The authors suggested that adding fluoxetine intervention to the current IFN-α regimen might improve the efficacy of anti-HCV treatment in chronic hepatitis C patients. Interestingly, Gofshteyn et al. [104] reported that off-label use of fluoxetine treated an immunocompromised child with chronic enterovirus encephalitis. According to the authors, fluoxetine can be useful to fight EV-induced diseases in humans. In summary, the studies included here indicate that SSRI, especially fluoxetine, treatment may be beneficial for many viral infections.
7. Repurposing SSRIs for treatment of COVID-19
As noted above, there are several plausible mechanisms through which SSRIs may exert a beneficial effect in this novel infectious disease (Fig. 1 ). Schloer et al. [105] found that fluoxetine treatment inhibited SARS-CoV-2 infection in human bronchial epithelial cell line (Calu-3) and Vero E6 cells, with an EC50 value below 1 μM. Also, they reported that the application of 10 μM fluoxetine severely reduced viral titres up to 99%. The study by Zimniak et al. [106] also supports antiviral effects of fluoxetine on SARS-CoV-2, although this effect was not observed for other SSRIs, including escitalopram and paroxetine. So the antiviral effect is not related to the 5-HT reuptake receptor. The authors suggested that treatment with fluoxetine decreased viral protein expression, indicating that the drug works upstream of gene expression. In this screening, fluoxetine significantly suppressed SARS-CoV-2 replication at a concentration of 0.8 μg/mL, and the EC50 was determined with 0.38 μg/mL. In another study, treatment with fluoxetine-remdesivir combination was found to inhibited the production of infectious SARS-CoV-2 particles (greater than90%) in the polarized Calu-3 cell culture model and displayed synergistic effects in commonly used reference models for drug interaction [107]. According to the authors, fluoxetine-remdesivir combination is promising therapeutic option to control SARS-CoV-2 infection and severe progression of COVID-19. Hoertel et al. [108] reported the first large observational study of antidepressant use in COVID-19. They performed a retrospective, multicentre cohort study examining the association between antidepressant use and the risk of intubation or death in 7345 adults hospitalized with COVID-19 at Assistance Publique-Hôpitaux de Paris, France, between 24th January and 1th April 2020. Of these, 257 patients received SSRIs, 71 received SNRIs, 59 received tricyclic antidepressants, 94 tetracyclic antidepressants, 44 received α2-antagonist antidepressants and 6885 received no antidepressant treatment. The authors concluded that treatment with SSRIs (fluoxetine and escitalopram) and SNRIs (venlafaxine) reduced the risk of intubation or death in hospitalized patients with COVID-19. However, to substantiate the robustness of the findings, further studies with larger sample sizes and longer follow-up periods are required. Therefore, prospective randomized controlled trials are recommended to be able to assess the efficacy and safety of these ‘repurposed drugs’ as adjuncts to standard COVID-19 treatment. As for fluvoxamine, a randomized, double-blind, placebo-controlled, phase-II clinical trial study is currently undergoing (NCT04342663) [109]. In the control group, the patients (n = 80) received 300 mg fluvoxamine daily (3 capsules per day) for 15 days and followed for up to 30 days. Preliminary results showed that fluvoxamine, if given early in the course of COVID-19, significantly reduced the likelihood of hospitalization. It is thought that fluvoxamine may prevent clinical deterioration in patients with mild COVID-19 by stimulating the S1R [77], which regulates pro-inflammatory cytokine production. Future studies should investigate this promising avenue of research. Another clinical trial is currently underway to study the effect of fluoxetine in reducing intubation and death in COVID-19 patients (NCT04377308) [110]. Currently, fluoxetine has already reached phase IV clinical trials with 20–60 mg of daily dose given to patients (n = 1000) that are infected with SARS-CoV-2.
Fig. 1.
Drug repurposing of SSRIs.
8. Drug-drug interactions
Drug interactions are usually categorized into two main groups, pharmacokinetic (PK) and pharmacodynamic (PD). SSRIs may inhibit specific CYP isoenzymes, causing PK interactions with other drugs that are metabolized by these enzymes; a PD interaction can also occur when SSRIs are given in combination with other serotonin-elevating agents (including tricyclic antidepressants, monoamine oxidase inhibitors, linezolid, tramadol, meperidine, cocaine, buspirone, reserpine, lithium, tryptophan, fentanyl, triptans, amphetamines, St. John’s wort, gingko biloba and S-adenosyl-methionine), which may lead to potentially severe serotonin toxicity, or serotonin syndrome (SS), a classification of potentially life-threatening symptoms [111], [112]. These symptoms can include agitation, hyperthermia, tachycardia, hallucinations, and muscle twitching. First-line management of SS includes discontinuation of the offending serotonergic agents and provision of supportive care, which can include benzodiazepines (such as diazepam and lorazepam) and cyproheptadine, a nonspecific 5-HT1A and 5-HT2A antagonist, to counteract the increased synaptic 5-HT levels [113]. Most pharmacokinetic drug interactions of SSRIs occur at metabolic level involving the CYP enzyme system [114]. SSRIs differ considerably in their potency to inhibit individual CYPs, as shown in Table 1. This may help guide selection of an appropriate compound for the individual patient. Fluoxetine, its active metabolite (norfluoxetine) and paroxetine are potent inhibitors of CYP2D6 isoenzyme and therefore can significantly increase the plasma concentrations and adverse effects of drugs that are predominantly metabolized by this isozyme [55], [115]. For example, co-administration of fluoxetine/paroxetine and β-blockers (e.g., carvedilol, metoprolol, propranolol and timolol) that are metabolized by 2D6 isoenzyme can result in increased β-blocker exposure, which can lead to drug toxicity and events such as decreased heart rate (bradycardia), hypotension and falls, especially in older persons [116], [117]. Moreover, there is evidence that fluoxetine can increase the plasma levels of the tricyclic antidepressants several fold even at the usual dosage of 20 mg/day [118]. For example, desipramine (50 mg) mean plasma concentrations were increased 4.4-fold when used with 20 mg fluoxetine for 20 days [119]. CYP2D6 is also known to be involved in the metabolism of opioid analgesics (e.g., tramadol and codeine), class I antiarrhythmic drugs, first-generation H1-blockers, and antipsychotic drugs (e.g., haloperidol, risperidone, clozapine, and thioridazine) [120], [121]. Therefore, concomitant use of fluoxetine with any of these drugs poses a risk for potential drug-drug interactions leading to adverse effects or therapeutic failure. For instance, there have been reports of akathisia and parkinsonian symptoms during co-administration of fluoxetine and risperidone in patients with additional risk factors, probably due to inhibition of CYP2D6 by fluoxetine [55]. Also, there is consistent evidence that paroxetine and fluoxetine may reduce the effectiveness of the anticancer agent tamoxifen, by decreasing the formation of its active metabolite endoxifen [122]. Fluvoxamine is a potent inhibitor of CYP1A2 and CYP2C19, but it also inhibits CYP3A4 and CYP2C9 [123]. It has the potential, therefore, to modify the PKs of many drugs largely metabolized by these routes [112], [114], [115]. For example, there is experimental evidence that fluvoxamine increases human plasma (Cmax) melatonin levels by 6-fold and the area under the curve (AUC) by 9-fold [124]. It is reported that a single-dose of 16 mg of ramelteon added to fluvoxamine increased ramelteon's AUC by 190-fold, and Cmax by 70-fold [125]. Recently, Anderson et al. [126] hypothesized that fluvoxamine might also exert beneficial effects in COVID patients through its well-characterized ability to substantially increase nighttime plasma levels of melatonin. Fluvoxamine and fluoxetine may substantially increase the bleeding risk associated with warfarin (a CYP2C9 substrate) through the inhibition of the CYP2C9-mediated oxidative metabolism of the more biologically active (S)-enantiomer of warfarin, especially in elderly patients [127]. Also, SSRIs that inhibit CYP2C19, such as fluvoxamine and fluoxetine, can reduce the conversion of clopidogrel to its active metabolite, thereby reducing the concentration of the active antiplatelet agent in the blood [128]. Theoretically, CYP3A4-inhibiting SSRIs may increase the risk of bleeding when combined with direct oral anticoagulants (such as rivaroxaban and apixaban), particularly intracranial hemorrhage. In patients with diabetes mellitus, concomitant use of fluvoxamine/fluoxetine and sulfonylureas may result in hypoglycemia due to the inhibition of CYP2C9-mediated metabolism of sulfonylureas by fluvoxamine/fluoxetine [129]. Fluvoxamine potently inhibits the in vitro metabolism of caffeine and it reduced caffeine, a CYP1A2 substrate, clearance by 80% and extended the half-life in humans from 5 to 31 h [130]. In addition, fluvoxamine inhibits the clearance of theophylline, another CYP1A2 substrate, therefore, theophylline dosage should be reduced to one-third of the usual daily maintenance dose [131]. Fluvoxamine has also been shown to increase plasma concentrations of clozapine by up to 10-fold, primarily because of inhibition of CYP1A2 [132]. Other SSRIs, including citalopram, escitalopram, are weak inhibitors of CYP2D6 and are less likely to interact with other drugs, while sertraline may cause significant inhibition of this isoform only at high doses (at least 150 mg daily) [133]. However, the three SSRIs (citalopram, escitalopram, and sertraline), at usual therapeutic dosages, which only have minor inhibitory effect of CYPs are potentially subjected to drug interactions when co-administered with other potent CYP inhibitors. For example, co-administration of cimetidine, a potent inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4, increases the steady-state concentration of citalopram by 41% [134]. It is also worth noting that all six SSRIs have QT-prolonging capabilities [135]. However, citalopram and escitalopram prolong the QT interval to the greatest extent at therapeutic doses, and currently the UK Medicines and Healthcare products Regulatory Agency has released warnings issued to their proarrhythmic potential [136]. Also, SSRIs are weakly bound primarily to alpha-1-acid glycoprotein. Perhaps for this reason, even the highly protein bound SSRIs do not significantly increase the free fraction of concomitantly administered drugs that are highly protein bound [137].
9. Drug-drug interactions between SSRI drugs and COVID-19 treatments
Patients with COVID-19 are at high risk for drug-drug interactions because they commonly receive multiple medications. Drug-drug interactions can lead to serious and potentially lethal adverse events. Therefore, identifying and minimizing the effects of any harmful drug interactions should be an essential goal in COVID-19 therapy. Table 2 shows interactions between SSRI drugs and the main drugs used to treat SARS-CoV-2 [138], [139], [140], [141]. The most problematic COVID-19 drugs for co-administration with SSRIs were found to be azithromycin, atazanavir, chloroquine, hydroxychloroquine and lopinavir/ritonavir in terms of both pharmacokinetic as well as serious pharmacodynamic drug interactions, including QT prolongation and torsades de pointes (TdP).
Table 2.
Interactions between drugs commonly used to treat SARS-CoV-2 and SSRIs.
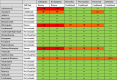 |
Risk Rating: Zone Red: Extremely significant interaction. These drugs should not be co-administered; Zone Orange: Potential interaction which may require a dose adjustment or close monitoring; Zone Yellow: Potential interaction likely to be of weak intensity. Additional action/monitoring or dosage adjustment unlikely to be required; Zone Green: No clinically significant interaction or does not require any action.
Type of interaction: The heart symbol (♥): One or both drugs may cause QT prolongation and/or torsades des pointes (TdP). ECG monitoring is advised if co-administered; ↑/↓ Potential increased/decreased exposure of SSRIs; ↔ No significant effect on drug serum levels.
TdP Risk: Risk Categories for Drugs that prolong QT and TdP: Known Risk of TdP (these drugs prolong the QT interval and are clearly associated with a known risk of TdP, even when taken as recommended); Possible Risk of TdP (these drugs can cause QT prolongation but currently lack evidence for risk of TdP when taken as recommended); Conditional Risk of TdP (these drugs are associated with TdP but only under certain conditions of their use (e.g. excessive dose, in patients with conditions such as hypokalemia, or when taken with interacting drugs) or by creating conditions that facilitate or induce TdP (e.g. by inhibiting metabolism of QT-prolonging drugs or by causing an electrolyte disturbance that induce TdP); Not classified (this drug has been reviewed but the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories. This is not an indication that this drug is free of a risk of QT prolongation or torsades de pointes since it may not have been adequately tested for these risks in patients) according to CredibleMeds®.
10. Conclusion
Based on current knowledge concerning SARS-CoV-2, drugs that combine anti-inflammatory and antiviral effects and have a favorable adverse effects profile, should be the most promising therapeutic strategies to fight this viral infection. In this context, SSRIs are not only inexpensive and widely available drugs with a safe tolerability profile (even in elderly patients) but significantly fit in this profile of effects. Hence, in this review, we critically analyze the preclinical and clinical evidence of SSRIs against COVID-19 and discuss the aspects over their safety and efficacy. Among all SSRI drugs, fluoxetine show a promising drug against COVID-19 by reducing the secretion of pro-inflammatory chemokine/cytokines (such as IL-6, TNF-α, CCL-2) and modulating immune system responsiveness to infection. Furthermore, fluoxetine has antiviral properties against a range of viruses (in vitro and in vivo) and is effective against SARS-CoV-2 infection in the cell culture models. Therefore, in light of current literature and the above discussion, we propose that the use of fluoxetine in combination with other agents could yield more effective outcomes through its immunomodulatory, anti-inflammatory and antiviral effects (Fig. 1).
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Johns Hopkins University and Medicine. Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html/; 2021 [accessed 14 January 2021].
- 3.Xiang Y.-T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T., et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astuti I., Ysrafil Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machitani M., Yasukawa M., Nakashima J., Furuichi Y., Masutomi K. RNA-dependent RNA polymerase, RdRP, a promising therapeutic target for cancer and potentially COVID-19. Cancer Sci. 2020;111:3976–3984. doi: 10.1111/cas.v111.1110.1111/cas.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung M.K., Karnik S., Saef J., Bergmann C., Barnard J., Lederman M.M., et al. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song P., Li W., Xie J., Hou Y., You C. Cytokine Storm Induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40 doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamrozik E., Selgelid M.J. COVID-19 human challenge studies: ethical issues. Lancet Infect Dis. 2020;20:e198–e203. doi: 10.1016/S1473-3099(20)30438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pashaei Y. Analytical methods for the determination of remdesivir as a promising antiviral candidate drug for the COVID-19 pandemic. Drug Discov Ther. 2020;14:273–281. doi: 10.5582/ddt.2020.03097. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar C., Mondal M., Torequl Islam M., Martorell M., Docea A.O., Maroyi A., et al. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.572870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attademo L., Bernardini F. Are dopamine and serotonin involved in COVID-19 pathophysiology? Eur J Psychiatry. 2021;35:62–63. doi: 10.1016/j.ejpsy.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yohn C.N., Gergues M.M., Samuels B.A. The role of 5-HT receptors in depression. Mol brain. 2017;10:1–12. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger M., Gray J.A., Roth B.L. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.1108020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herr N., Bode C., Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:48. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan M., Ding L., Wang D., Han J., Gao P. Serotonin: A potent immune cell modulator in autoimmune diseases. Front Immunol. 2020;11:186. doi: 10.3389/fimmu.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arreola R., Becerril-Villanueva E., Cruz-Fuentes C., Velasco-Velázquez M.A., Garcés-Alvarez M.E., Hurtado-Alvarado G., et al. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:1–21. doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller T., Dürk T., Blumenthal B., Grimm M., Cicko S., Panther E., et al. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS ONE. 2009;4(7):e6453. doi: 10.1371/journal.pone.0006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soga F., Katoh N., Inoue T., Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- 23.Vašíček O., Lojek A., Číž M. Serotonin and its metabolites reduce oxidative stress in murine RAW264.7 macrophages and prevent inflammation. J Physiol Biochem. 2020;76:49–60. doi: 10.1007/s13105-019-00714-3. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell P.J., Wang X., Leon-Ponte M., Griffiths C., Pingle S.C., Ahern G.P., et al. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sternberg E., Wedner H., Leung M., Parker C. Effect of serotonin (5-HT) and other monoamines on murine macrophages: modulation of interferon-gamma induced phagocytosis. J Immunol. 1987;138:4360–4365. [PubMed] [Google Scholar]
- 26.Sternberg E., Trial J., Parker C. Effect of serotonin on murine macrophages: suppression of Ia expression by serotonin and its reversal by 5-HT2 serotonergic receptor antagonists. J Immunol. 1986;137:276–282. [PubMed] [Google Scholar]
- 27.Arzt E., Costas M., Finkielman S., Nahmod V.E. Serotonin inhibition of tumor necrosis factor-alpha synthesis by human monocytes. Life Sci. 1991;48:2557–2562. doi: 10.1016/0024-3205(91)90612-f. [DOI] [PubMed] [Google Scholar]
- 28.Kubera M., Kenis G., Bosmans E., Scharpé S., Maes M. Effects of serotonin and serotonergic agonists and antagonists on the production of interferon-gamma and interleukin-10. Neuropsychopharmacology. 2000;23:89–98. doi: 10.1016/S0893-133X(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 29.Katoh N., Soga F., Nara T., Tamagawa-Mineoka R., Nin M., Kotani H., et al. Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin Exp Immunol. 2006;146:354–361. doi: 10.1111/j.1365-2249.2006.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang R., Patel D., Morris J.J., Rutschman R.L., Murray P.J. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 31.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menard G., Turmel V., Bissonnette E.Y. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol. 2007;150:340–348. doi: 10.1111/j.1365-2249.2007.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadirci E., Halici Z., Bayir Y., Albayrak A., Karakus E., Polat B., et al. Peripheral 5-HT7 receptors as a new target for prevention of lung injury and mortality in septic rats. Immunobiology. 2013;218:1271–1283. doi: 10.1016/j.imbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Liu M.-W., Su M.-X., Wang Y.-H., Wei W., Qin L.-F., Liu X.u., et al. Effect of melilotus extract on lung injury by upregulating the expression of cannabinoid CB2 receptors in septic rats. BMC Complement Altern Med. 2014;14 doi: 10.1186/1472-6882-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cloëz-Tayarani I., Petit-Bertron A.F., Venters H.D., Cavaillon J.M. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine 2A receptors. Int Immunol. 2003;15:233–240. doi: 10.1093/intimm/dxg027. [DOI] [PubMed] [Google Scholar]
- 36.Dürk T, Panther E, Müller T, Sorichter S, Ferrari D, Pizzirani C, et al. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol 2005;17:599–606. https://doi.org/10.1093/intimm/dxh242. [DOI] [PubMed]
- 37.Kubera M., Maes M., Kenis G., Kim Y.K., Lasoń W. Effects of serotonin and serotonergic agonists and antagonists on the production of tumor necrosis factor alpha and interleukin-6. Psychiatry Res. 2005;134:251–258. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 38.Ayaz G., Halici Z., Albayrak A., Karakus E., Cadirci E. Evaluation of 5-HT7 receptor trafficking on in vivo and in vitro model of lipopolysaccharide (LPS)-induced inflammatory cell injury in rats and LPS-treated A549 cells. Biochem Genet. 2017;55:34–47. doi: 10.1007/s10528-016-9769-2. [DOI] [PubMed] [Google Scholar]
- 39.Mota C.M.D., Borges G.S., Amorim M.R., Carolino R.O.G., Batalhão M.E., Anselmo-Franci J.A., et al. Central serotonin prevents hypotension and hypothermia and reduces plasma and spleen cytokine levels during systemic inflammation. Brain Behav Immun. 2019;80:255–265. doi: 10.1016/j.bbi.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Saper C.B., Romanovsky A.A., Scammell T.E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidibe S., Saal F., Rhodes-Feuillette A., Lagaye S., Pelicano L., Canivet M., et al. Effects of serotonin and melanin on in vitro HIV-1 infection. J Biol Regul Homeost Agents. 1996;10:19–24. [PubMed] [Google Scholar]
- 42.Evans D.L., Lynch K.G., Benton T., Dubé B., Gettes D.R., Tustin N.B., et al. Douglas, Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatry. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellstrand K., Hermodsson S. Enhancement of human natural killer cell cytotoxicity by serotonin: role of non-T/CD16+ NK cells, accessory monocytes, and 5-HT1A receptors. Cell Immunol. 1990;127:199–214. doi: 10.1016/0008-8749(90)90125-B. [DOI] [PubMed] [Google Scholar]
- 44.Manéglier B, Guillemin G, Clayette P, Rogez-Kreuz C, Brew B, Dormont D, et al. Serotonin decreases HIV-1 replication in primary cultures of human macrophages through 5-HT1A receptors. Br J Pharmacol 2008;154:174–182. https://doi.org/10.1038/bjp.2008.80. [DOI] [PMC free article] [PubMed]
- 45.Bouma E.M., van de Pol D.P., Sanders I.D., Rodenhuis-Zybert I.A., Smit J.M. Serotonergic drugs inhibit Chikungunya infection at different stages of the cell entry pathway. J Virol. 2020;94 doi: 10.1128/JVI.00274-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mainou B.A., Ashbrook A.W., Smith E.C., Dorset D.C., Denison M.R., Dermody T.S., et al. Serotonin receptor agonist 5-nonyloxytryptamine alters the kinetics of reovirus cell entry. J Virol. 2015;89:8701–8712. doi: 10.1128/JVI.00739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szabo A., Gogolak P., Koncz G., Foldvari Z., Pazmandi K., Miltner N., et al. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci Rep. 2018;8 doi: 10.1038/s41598-018-20173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson G., Reiter R.J. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol. 2020;30 doi: 10.1002/rmv.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahrampour Juybari K., Pourhanifeh M.H., Hosseinzadeh A., Hemati K., Mehrzadi S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020;287:198108. doi: 10.1016/j.virusres.2020.198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X., Zhang L., Lei Y., Liu X., Zhou X., Liu Y., et al. Meta-analysis of infectious agents and depression. Sci Rep. 2015;4 doi: 10.1038/srep04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hampton T. How depressed mood may develop after viral infection. JAMA. 2016;315:2267. doi: 10.1001/jama.2016.6852. [DOI] [Google Scholar]
- 52.Mahalakshmi A.M., Ray B., Tuladhar S., Bhat A., Paneyala S., Patteswari D., et al. Does COVID-19 contribute to development of neurological disease? Immun Inflamm Dis. 2021;9:48–58. doi: 10.1002/iid3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skånland S.S., Cieślar-Pobuda A. Off-label uses of drugs for depression. Eur J Pharmacol. 2019;865:172732. doi: 10.1016/j.ejphar.2019.172732. [DOI] [PubMed] [Google Scholar]
- 54.Hiemke C., Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28. doi: 10.1016/S0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 55.Low Y., Setia S., Lima G. Drug-drug interactions involving antidepressants: focus on desvenlafaxine. Neuropsychiatr Dis Treat. 2018;14:567. doi: 10.2147/NDT.S157708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson J.M. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;03:22–27. doi: 10.4088/PCC.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank M.G., Hendricks S.E., Johnson D.R., Wieseler J., Burke W.J. Antidepressants augment natural killer cell activity: in vivo and in vitro. Neuropsychobiology. 1999;39:18–24. doi: 10.1159/000026555. [DOI] [PubMed] [Google Scholar]
- 58.Kook A.I., Mizruchin A., Odnopozov N., Gershon H., Segev Y. Depression and immunity: the biochemical interrelationship between the central nervous system and the immune system. Biol Psychiatry. 1995;37:817–819. doi: 10.1016/0006-3223(95)00038-I. [DOI] [PubMed] [Google Scholar]
- 59.Mizruchin A., Gold I., Krasnov I., Livshitz G., Shahin R., Kook A.I. Comparison of the effects of dopaminergic and serotonergic activity in the CNS on the activity of the immune system. J Neuroimmunol. 1999;101:201–204. doi: 10.1016/S0165-5728(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 60.Park E.J., Lee J.H., Jeong D.C., Han S.I., Jeon Y.W. Natural killer cell activity in patients with major depressive disorder treated with escitalopram. Int Immunopharmacol. 2015;28:409–413. doi: 10.1016/j.intimp.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 61.Benton T, Lynch K, Dubé B, Gettes DR, Tustin NB, Lai JP, et al. Selective serotonin reuptake inhibitor suppression of HIV infectivity and replication. Psychosom Med 2010;72:925. https://doi.org/10.1097/PSY.0b013e3181f883ce. [DOI] [PMC free article] [PubMed]
- 62.Pellegrino T.C., Bayer B.M. Modulation of immune cell function following fluoxetine administration in rats. Pharmacol Biochem Behav. 1998;59:151–157. doi: 10.1016/S0091-3057(97)00382-1. [DOI] [PubMed] [Google Scholar]
- 63.Pellegrino T.C., Bayer B.M. Specific serotonin reuptake inhibitor-induced decreases in lymphocyte activity require endogenous serotonin release. NeuroImmunoModulation. 2000;8:179–187. doi: 10.1159/000054278. [DOI] [PubMed] [Google Scholar]
- 64.Canan F., Ataoglu A. Effect of escitalopram on white blood cells in patients with major depression. J Clin Med Res. 2009;1:290. doi: 10.4021/jocmr2009.12.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang K.-A., Kim J.a., Kim S., Joo Y., Shin K.Y., Kim S., et al. Therapeutic potentials of neural stem cells treated with fluoxetine in Alzheimer's disease. Neurochem Inter. 2012;61:885–891. doi: 10.1016/j.neuint.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez M.E., Martinez-Fong D., Perez-Tapia M., Estrada-Garcia I., Estrada-Parra S., Pavón L. Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur Neuropsychopharmacol. 2010;20:88–95. doi: 10.1016/j.euroneuro.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Taler M., Gil-Ad I., Lomnitski L., Korov I., Baharav E., Bar M., et al. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur Neuropsychopharmacol. 2007;17:774–780. doi: 10.1016/j.euroneuro.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. 2001;16:95–103. doi: 10.1002/hup.v16:110.1002/hup.191. [DOI] [PubMed] [Google Scholar]
- 70.Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 71.Słuzewska A., Rybakowski J., Laciak M., Mackiewicz A., Sobieska M., Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- 72.Sharma G., Champalal Sharma D., Hwei Fen L., Pathak M., Bethur N., Pendharkar V., et al. Reduction of influenza virus-induced lung inflammation and mortality in animals treated with a phosophodisestrase-4 inhibitor and a selective serotonin reuptake inhibitor. Emerg Microbes Infect. 2013;2:1–9. doi: 10.1038/emi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obuchowicz E., Bielecka A.M., Paul-Samojedny M., Pudełko A., Kowalski J. Imipramine and fluoxetine inhibit LPS-induced activation and affect morphology of microglial cells in the rat glial culture. Pharmacol Rep. 2014;66:34–43. doi: 10.1016/j.pharep.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Shenoy A.R., Dehmel T., Stettner M., Kremer D., Kieseier B.C., Hartung H.P., et al. Citalopram suppresses thymocyte cytokine production. J Neuroimmunol. 2013;262:46–52. doi: 10.1016/j.jneuroim.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Tucker P., Ruwe W.D., Masters B., Parker D.E., Hossain A., Trautman R.P., et al. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Roumestan C., Michel A., Bichon F., Portet K., Detoc M., Henriquet C., et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8 doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11:eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alboni S., Poggini S., Garofalo S., Milior G., El Hajj H., Lecours C., et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun. 2016;58:261–271. doi: 10.1016/j.bbi.2016.07.155. [DOI] [PubMed] [Google Scholar]
- 79.Fukushima S., Kurganov E., Hiratsuka D., Miyata S. Effect of fluoxetine on proliferation and/or survival of microglia and oligodendrocyte progenitor cells in the fornix and corpus callosum of the mouse brain. Pharmacol Rep. 2020;72:340–349. doi: 10.1007/s43440-020-00079-1. [DOI] [PubMed] [Google Scholar]
- 80.Tynan R.J., Weidenhofer J., Hinwood M., Cairns M.J., Day T.A., Walker F.R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26:469–479. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W., et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jablonski A.M., Warren L., Usenovic M., Zhou H., Sugam J., Parmentier-Batteur S., et al. Astrocytic expression of the Alzheimer’s disease risk allele, ApoEε4, potentiates neuronal tau pathology in multiple preclinical models. Sci Rep. 2021;11:1–18. doi: 10.1038/s41598-021-82901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedberg J.S., Aytan N., Cherry J.D., Xia W., Standring O.J., Alvarez V.E., et al. Associations between brain inflammatory profiles and human neuropathology are altered based on apolipoprotein E ε4 genotype. Sci Rep. 2020;10 doi: 10.1038/s41598-020-59869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C., Zhang M., Garcia G., Tian E., Cui Q., Chen X., et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell. 2021;28 doi: 10.1016/j.stem.2020.12.018. 331–42. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee K.M., Kim Y.K. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int Immunopharmacol. 2006;6:1298–1304. doi: 10.1016/j.intimp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Torrisi S.A., Geraci F., Tropea M.R., Grasso M., Caruso G., Fidilio A., et al. Fluoxetine and vortioxetine reverse depressive-like phenotype and memory deficits induced by Aβ1-42 oligomers in mice: a key role of transforming growth factor-β1. Front Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marques-Deak A, Neto FL, Dominguez W, Solis A, Kurcgant D, Sato F, et al. Cytokine profiles in women with different subtypes of major depressive disorder. J Psychiatr Res 2007;4:152–59. https://doi.org/10.1016/j.jpsychires.2005.11.003. [DOI] [PubMed]
- 89.Frick L.R., Palumbo M.L., Zappia M.P., Brocco M.A., Cremaschi G.A., Genaro A.M. Inhibitory effect of fluoxetine on lymphoma growth through the modulation of antitumor T-cell response by serotonin-dependent and independent mechanisms. Biochem Pharmacol. 2008;75:1817–1826. doi: 10.1016/j.bcp.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 90.Hernández M.E., Mendieta D., Martínez-Fong D., Loría F., Moreno J., Estrada I., et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. Eur Neuropsychopharmacol. 2008;18:917–924. doi: 10.1016/j.euroneuro.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Keaton S.A., Madaj Z.B., Heilman P., Smart L., Grit J., Gibbons R., et al. An inflammatory profile linked to increased suicide risk. J Affect Disord. 2019;247:57–65. doi: 10.1016/j.jad.2018.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amitai M., Taler M., Ben-Baruch R., Lebow M., Rotkopf R., Apter A., et al. Increased circulatory IL-6 during 8-week fluoxetine treatment is a risk factor for suicidal behaviors in youth. Brain Behav Immun. 2020;87:301–308. doi: 10.1016/j.bbi.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Kristiansen J.E., Hansen J.B. Inhibition of HIV replication by neuroleptic agents and their potential use in HIV infected patients with AIDS related dementia. Int J Antimicrob Agents. 2000;14:209–213. doi: 10.1016/S0924-8579(99)00157-0. [DOI] [PubMed] [Google Scholar]
- 94.Greeson J.M., Gettes D.R., Spitsin S., Dubé B., Benton T.D., Lynch K.G., et al. The selective serotonin reuptake inhibitor citalopram decreases human immunodeficiency virus receptor and coreceptor expression in immune cells. Biol psychiatry. 2016;80:33–39. doi: 10.1016/j.biopsych.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Letendre S.L., Marquie-Beck J., Ellis R.J., Woods S.P., Best B., Clifford D.B., et al. The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. J Neuroimmune Pharmacol. 2007;2:120–127. doi: 10.1007/s11481-006-9054-y. [DOI] [PubMed] [Google Scholar]
- 96.Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. 290ra89–290ra89 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 97.Zuo J., Quinn K.K., Kye S., Cooper P., Damoiseaux R., Krogstad P. Fluoxetine is a potent inhibitor of coxsackievirus replication. Antimicrob Agents Chemother. 2012;56:4838–4844. doi: 10.1128/AAC.00983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ulferts R., van der Linden L., Thibaut H.J., Lanke K.H.W., Leyssen P., Coutard B., et al. Selective serotonin reuptake inhibitor fluoxetine inhibits replication of human enteroviruses B and D by targeting viral protein 2C. Antimicrob Agents Chemother. 2013;57:1952–1956. doi: 10.1128/AAC.02084-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alidjinou E.K., Sané F., Bertin A., Caloone D., Hober D. Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine. Antiviral Res. 2015;116:51–54. doi: 10.1016/j.antiviral.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Bauer L., Manganaro R., Zonsics B., Strating J.R., El Kazzi P., Lorenzo Lopez M., et al. Fluoxetine inhibits enterovirus replication by targeting the viral 2C protein in a stereospecific manner. ACS Infect Dis. 2019;5:1609–1623. doi: 10.1021/acsinfecdis.9b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manganaro R., Zonsics B., Bauer L., Lorenzo Lopez M., Donselaar T., Zwaagstra M., et al. Synthesis and antiviral effect of novel fluoxetine analogues as enterovirus 2C inhibitors. Antivir Res. 2020;178:104781. doi: 10.1016/j.antiviral.2020.104781. [DOI] [PubMed] [Google Scholar]
- 102.Medigeshi G.R., Kumar R., Dhamija E., Agrawal T., Kar M. N-Desmethylclozapine, fluoxetine, and salmeterol inhibit postentry stages of the dengue virus life cycle. Antimicrob Agents Chemother. 2016;60:6709–6718. doi: 10.1128/AAC.01367-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Young K.-C., Bai C.-H., Su H.-C., Tsai P.-J., Pu C.-Y., Liao C.-S., et al. Fluoxetine a novel anti-hepatitis C virus agent via ROS-, JNK-, and PPARβ/γ-dependent pathways. Antiviral Res. 2014;110:158–167. doi: 10.1016/j.antiviral.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 104.Gofshteyn J., Cárdenas A.M., Bearden D. Treatment of chronic enterovirus encephalitis with fluoxetine in a patient with X-linked agammaglobulinemia. Pediatr Neurol. 2016;64:94–98. doi: 10.1016/j.pediatrneurol.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 105.Schloer S., Brunotte L., Goretzko J., Mecate-Zambrano A., Korthals N., Gerke V., et al. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg Microbes Infect. 2020;9:2245–2255. doi: 10.1080/22221751.2020.1829082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zimniak M., Kirschner L., Hilpert H., Seibel J., Bodem J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.06.14.150490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rescher U., Schloer S., Brunotte L., Mecate-Zambrano A., Zheng S., Tang J., et al. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. BioRxiv. 2020 doi: 10.1101/2020.10.16.342410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoertel N, Rico MS, Vernet R, Beeker N, Jannot A-S, NEURAZ A, et al. Association between SSRI antidepressant use and reduced risk of intubation or death in hospitalized patients with coronavirus disease 2019: a multicenter retrospective observational study. MedRxiv 2020. https://doi.org/10.1101/2020.07.09.20143339.
- 109.Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 2020;324:2292-2300. https://doi.org/10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed]
- 110.Clinicaltrials.gov. Fluoxetine to reduce intubation and death after COVID19 infection, https://clinicaltrials.gov/ct2/show/NCT04342663/; 2021 [accessed 14 January 2021].
- 111.Foong A.L., Grindrod K.A., Patel T., Kellar J. Demystifying serotonin syndrome (or serotonin toxicity) Can Fam Physician. 2018;64:720–727. [PMC free article] [PubMed] [Google Scholar]
- 112.Bleakley S. Antidepressant drug interactions: evidence and clinical significance. Prog Neurol Psychiatry. 2016;20:21–27. doi: 10.1002/pnp.429. [DOI] [Google Scholar]
- 113.Racz R., Soldatos T.G., Jackson D., Burkhart K. Association between serotonin syndrome and second-generation antipsychotics via pharmacological target-adverse event analysis. Clin Transl Sci. 2018;11:322–329. doi: 10.1111/cts.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hemeryck A., Belpaire F.M. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drug Metab. 2002;3:13–37. doi: 10.2174/1389200023338017. [DOI] [PubMed] [Google Scholar]
- 115.Brown C. Overview of drug-drug interactions with SSRIs. US Pharm. 2008;33:3–19. [Google Scholar]
- 116.Shin J, Hills NK, Finley PR. Combining antidepressants with β-Blockers: evidence of a clinically significant CYP2D6 drug interaction. Pharmacotherapy 2020;40:507–16. https://doi.org/10.1002/phar.2406. [DOI] [PubMed]
- 117.Bahar MA, Kamp J, Borgsteede SD, Hak E, Wilffert B. The impact of CYP2D6 mediated drug-drug interaction: a systematic review on a combination of metoprolol and paroxetine/fluoxetine. Br J Clin Pharmacol 2018;84:2704–15. https://doi.org/10.1111/bcp.13741. [DOI] [PMC free article] [PubMed]
- 118.Richelson E. Pharmacokinetic drug interactions of new antidepressants: a review of the effects on the metabolism of other drugs. Mayo Clin Proc. 1997;72:835–847. doi: 10.4065/72.9.835. [DOI] [PubMed] [Google Scholar]
- 119.Preskorn S.H., Alderman J., Chung M., Harrison W., Messig M., Harris S. Pharmacokinetics of desipramine coadministered with sertraline or fluoxetine. J Clin Psychopharmacol. 1994;14:90–98. doi: 10.1097/00004714-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 120.Deodhar M., Rihani S.B.A., Darakjian L., Turgeon J., Michaud V. Assessing the mechanism of fluoxetine-mediated CYP2D6 inhibition. Pharmaceutics. 2021;13:148. doi: 10.3390/pharmaceutics13020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vuppalanchi R. Metabolism of Drugs and Xenobiotics. Practical Hepatic Pathology: A Diagnostic Approach E-Book: A Volume in the Pattern Recognition Series. 2011:45.
- 122.Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354 doi: 10.1136/bmj.i5309. [DOI] [PubMed] [Google Scholar]
- 123.Brøsen K. Some aspects of genetic polymorphism in the biotransformation of antidepressants. Therapie. 2004;59:5–12. doi: 10.2515/therapie:2004003. [DOI] [PubMed] [Google Scholar]
- 124.Papagiannidou E., Skene D.J., Ioannides C. Potential drug interactions with melatonin. Physiol Behav. 2014;131:17–24. doi: 10.1016/j.physbeh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 125.Zammit G.K. Ramelteon: a novel hypnotic indicated for the treatment of insomnia. Psychiatry (Edgmont) 2007;4:36. [PMC free article] [PubMed] [Google Scholar]
- 126.Anderson G.M. Fluvoxamine, melatonin and COVID-19. Psychopharmacology. 2021;238:611. doi: 10.1007/s00213-020-05753-z. 10.1007/s00213-020-05753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spina E, Barbieri MA, Cicala G, Bruno A, de Leon J. Clinically relevant drug interactions between newer antidepressants and oral anticoagulants. Expert Opin Drug Metab Toxicol 2020;16:31–44. https://doi.org/10.1080/17425255.2020.1700952. [DOI] [PubMed]
- 128.Bykov K, Schneeweiss S, Donneyong MM, Dong Y-H, Choudhry NK, Gagne JJ. Impact of an interaction between clopidogrel and selective serotonin reuptake inhibitors. Am J Card 2017;119:651–7. https://doi.org/10.1016/j.amjcard.2016.10.052. [DOI] [PubMed]
- 129.Madsen H., Enggaard T.P., Hansen L.L., Klitgaard N.A., Brøsen K. Fluvoxamine inhibits the CYP2C9 catalyzed biotransformation of tolbutamide. Clin Pharmacol Ther. 2001;69:41–47. doi: 10.1067/mcp.2001.112689. [DOI] [PubMed] [Google Scholar]
- 130.DeVane CL. Antidepressant-drug interactions are potentially but rarely clinically significant. Neuropsychopharmacology. 2006;31:1594–604. https://doi.org/10.1038/sj.npp.1301069. [DOI] [PubMed]
- 131.Figgitt DP, McClellan KJ. Fluvoxamine. Drugs 2000;60:925–54. https://doi.org/10.2165/00003495-200060040-00006. [DOI] [PubMed]
- 132.Hoffelt C., Gross T. A review of significant pharmacokinetic drug interactions with antidepressants and their management. Ment Health Clin. 2016;6:35–41. doi: 10.9740/mhc.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther 2008;30:1206–27. https://doi.org/10.1016/s0149-2918(08)80047-1. [DOI] [PubMed]
- 134.Priskorn M., Larsen F., Segonzac A., Moulin M. Pharmacokinetic interaction study of citalopram and cimetidine in healthy subjects. Eur J Clin Pharmacol. 1997;52:241. doi: 10.4088/jcp.v61n0809. [DOI] [PubMed] [Google Scholar]
- 135.Beach SR, Kostis WJ, Celano CM, Januzzi JL, Ruskin JN, Noseworthy PA, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTC prolongation. J Clin Psychiatry 2014;75:441–49. https://doi.org/10.4088/JCP.13r08672. [DOI] [PubMed]
- 136.Assimon MM, Brookhart MA, Flythe JE. Comparative cardiac safety of selective serotonin reuptake inhibitors among individuals receiving maintenance hemodialysis. J Am Soc Nephrol 2019;30:611–23. https://doi.org/10.1681/ASN.2018101032. [DOI] [PMC free article] [PubMed]
- 137.Wyska E. Pharmacokinetic considerations for current state-of-the-art antidepressants. Expert Opin Drug Metab Toxicol. 2019;15:831–847. doi: 10.1080/17425255.2019.1669560. [DOI] [PubMed] [Google Scholar]
- 138.QT/TdP Risk Categories for Drugs. https://www.crediblemeds.org/index.php/drugsearch/; 2021 [accessed 14 January 2021].
- 139.Bishara D., Kalafatis C., Taylor D. Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents. Ther Adv Psychopharmacol. 2020;10:1–15. doi: 10.1177/2045125320935306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liverpool COVID-19 Interactions. https://www.covid19-druginteractions.org/checker/; 2021 [accessed 14 January 2021].
- 141.Ostuzzi G, Papola D, Gastaldon C, Schoretsanitis G, Bertolini F, Amaddeo F, et al. Safety of psychotropic medications in people with COVID-19: evidence review and practical recommendations. BMC Med 2020;18:215. https://doi.org/10.1186/s12916-020-01685-9. [DOI] [PMC free article] [PubMed]