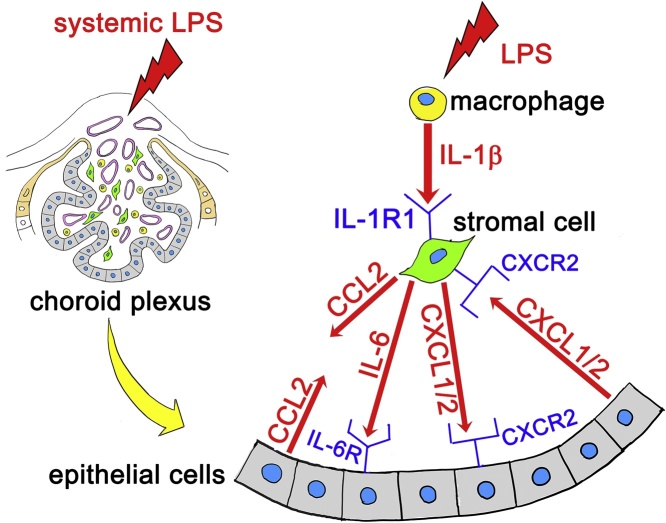
Graphical abstract
Keywords: Endotoxin, Sepsis, Encephalopathy, Choroid plexus, IL-1β
Highlights
-
•
Brain response to systemic inflammation is initiated by IL-1β from choroid plexus macrophages.
-
•
Choroid plexus stromal cells bear IL-1 receptors and participate in the immediate reaction to systemic inflammation.
-
•
This reaction is followed by elevated gene expression of various cytokines in the choroid plexus stroma and epithelium.
-
•
The choroid plexus immediate responses are relevant to understanding how sepsis-associated encephalopathy is initiated.
Abstract
Sepsis-associated encephalopathy (SAE) is characterized as diffuse brain dysfunction in patients with excessive systemic inflammatory reaction to an infection. In our previous studies using a mouse model of SAE with intraperitoneal injection of lipopolysaccharide (LPS), tissue concentrations of various cytokines were elevated in the entire brain parenchyma 4 and 24 h following LPS administration. Cytokines elevated at 4 h were produced by the choroid plexus, leptomeninges and vascular endothelium, while those at 24 h were produced by astrocytes. Interleukin (IL)-1β did not increase in the concentration in the brain parenchyma during the period from 1 to 24 h following LPS. In the present study, we hypothesized that the intracranial cells that initially respond to systemic inflammation are situated in the choroid plexus and produce IL-1β to initiate cytokine-mediated reactions. We quantified the transcript levels of related cytokines within the choroid plexus and specified the choroid plexus cells that are involved in the immediate cytokine-mediated responses. Mice received LPS or saline by intraperitoneal injection. Four hours after treatments, the choroid plexuses were isolated and subjected to cytokine gene expression analyses using real-time reverse transcription-polymerase chain reaction. Another group of mice was fixed at 1, 4 and 24 h after treatments and the expression of cytokines and receptors was studied with double immunohistofluorescence staining. The transcript levels of IL-1β, CC-motif ligand (CCL)2, CXC-motif ligand (CXCL)1, CXCL2 and IL-6 in the choroid plexus were significantly increased in mice treated with LPS compared to saline control. The IL-1β expression was remarkable in choroid plexus macrophages at 1 and 4 h but not in the brain parenchyma. Choroid plexus stromal cells expressed IL-1 receptor type 1 (IL-1R1). The IL-1R1-bearing stromal cells produced CCL2, CXCL1, CXCL2 and IL-6 at 4 h. Choroid plexus epithelial cells expressed CXCR2, a common receptor for CXCL1 and CXCL2. Choroid plexus epithelial cells also expressed CCL2, CXCL1 and CXCL2 at 4 h, and IL-1R1-bearing stromal cells expressed CXCR2. Therefore, in response to systemic LPS injection, one of the intracranial reactions was initiated within the choroid plexus using IL-1β derived from macrophages. The choroid plexus stromal cells subsequently had elevated expression of CCL2, CXCL1, CXCL2 and IL-6. The choroid plexus epithelial cells also had elevated expression of CCL2, CXCL1 and CXCL2. The presence of receptors for these cytokines on both epithelial and stromal cells raised the possibility of reciprocal interactions between these cells. The results suggested that the immediate early responses exerted by the choroid plexus are relevant to understanding how SAE is initiated in clinical settings.
1. Introduction
The brain and immune system interact with each other under inflammatory and healthy conditions. In our previous study using bone marrow chimeric mice, we determined the intracranial sites for interaction between immune cells and brain parenchymal cells. Based on the distribution of bone marrow-derived cells in the intracranial space, it has been revealed that the leptomeninges, choroid plexus stroma, attachments of choroid plexus, brain parenchymal perivascular space, circumventricular organs and astrocytic endfeet are the major histological architecture that gives a site for cell-cell interaction between immune and brain parenchymal cells [1]. It should be noted that the density of bone marrow-derived cells (mostly with myeloid differentiation) is highest in the choroid plexus stroma, followed by the leptomeninges [1]. Perivascular, leptomeningeal and choroid plexus macrophages transmit immune responses to the brain parenchyma at brain boundaries [2]. The choroid plexus also serves as a gateway for trafficking immune cells into the cerebrospinal fluid and a regulator for immune cell trafficking in disease and injury [3,4]
Clinical symptoms of sepsis-associated encephalopathy (SAE), or septic encephalopathy, include delirium, cognitive impairment and loss of consciousness, which represents diffuse brain dysfunction in patients with excessive systemic inflammatory reaction to pathogens [5,6]. SAE occurs in the absence of direct brain infection [7,8]. SAE is therefore a manifestation of brain-specific responses under peripheral inflammatory conditions. In our previous study [9,10], we created SAE models by intraperitoneal injection of lipopolysaccharide (LPS; a bacterial endotoxin) into mice at a concentration (3.0 mg/kg) known to cause sickness behavior [11]. By determining tissue concentrations of multiple cytokines, we reported that entire brain responds to endotoxin-induced systemic inflammation in the acute phase (4–24 h) after LPS injection with alteration in the concentrations of multiple cytokines that occurred in all brain areas. CC-motif ligand (CCL)2, CCL3, CXC-motif ligand (CXCL)1, CXCL2, CXCL9, interleukin (IL)-6 and tumor necrosis factor (TNF)-α are “early cytokines” whose concentrations increase at 4 h but return to ordinary concentrations by 24 h after LPS administration in all brain regions. Most of these cytokines are expressed by choroid plexus stromal and epithelial cells, leptomeningeal stromal cells, and brain parenchymal vascular endothelial cells [9]. Astrocytic endfeet bear the receptors for these cytokines. In contrast, CCL11, CXCL10 and G-CSF are “late cytokines” that keeps elevated concentrations until 24 h (and possibly longer) following LPS administration. These cytokines are expressed by astrocytes. Although IL-1β is well recognized as an important inflammatory mediator in peripheral tissues, IL-1β does not increase in the brain parenchyma in reactions to endotoxin-induced systemic inflammation in any brain area from 1 to 24 h after LPS administration.
The architecture comprised of stromal and epithelial cells of the choroid plexus, leptomeningeal stromal cells, vascular endothelial cells and astrocytic endfeet, as stated previously, serves as the interface where systemic inflammation-associated reactions are transmitted to the brain parenchyma. Such types of brain–immune interactions occur without abundant infiltration of the brain parenchyma by inflammatory cells. Rather, they occur by taking advantage of the close anatomical apposition of stromal cells of the choroid plexus and leptomeninges as well as vascular endothelial cells to astrocytic endfeet [12,13]. However, it remains to be clarified how systemically administered endotoxin induces cytokine-mediated responses of choroid plexus stromal and epithelial cells and leptomeningeal stromal cells, which subsequently stimulate astrocytes to produce their own cytokines.
The choroid plexus is situated within the brain ventricle and is well known to produce cerebrospinal fluid. During brain organogenesis, specialized segments are formed on the neural tube where the ventricle is located adjacent to the dorsal border and the brain parenchymal tissue becomes very thin. From such special regions, the pia protrudes into the ventricle together with subarachnoid tissue containing a convolution of fenestrated capillaries and matrix to form the stroma [12,13]. Thus, the choroid plexus stroma has the same tissue components as the leptomeninges, a part of which is derived from the neural crest. The choroid plexus epithelium, on the other hand, is a monolayered continuation of ependyma. The choroid plexus, as a whole, is a convolution consisting of highly vascularized leptomeningeal tissue of neural crest and mesenchymal origin and monolayered ependymal tissue of neuroectodermal origin. Importantly, there are many macrophages in the choroid plexus stroma and occasionally on the ventricular side of the choroid plexus epithelium (epiplexus cells).
Macrophages in various organs are the primary responders to endotoxin in vivo ([14] and produce IL-1β [[15], [16], [17]]. Systemic administration of a pyrogenic dose of endotoxin into rodents has been reported to lead to IL-1β expression in the brain [18], especially in macrophages of the leptomeninges and choroid plexus, in brain parenchymal perivascular macrophages and cells with ramifying processes surrounding the blood vessels corresponding to “vessel-associated microglia” [19], and in microglia of the circumventricular organs [20,21]. However, it has been controversial whether parenchymal microglia are the initial responders to endotoxin-induced systemic inflammation.
In the present study, based on our previous findings that IL-1β is not included in the cytokines produced by the brain parenchyma during the acute phase (until 24 h after systemic LPS injection) and the fact that resident macrophages respond to endotoxin by producing IL-1β in peripheral tissues, we formulated the following hypothesis. When the intracranial tissues respond to systemically injected endotoxin, the initial responder cells are situated in the choroid plexus and leptomeninges and produce IL-1β to stimulate neighboring cells within the choroid plexus and leptomeninges. This process leads to subsequent changes in the cytokine profile of the brain parenchyma. We examined this hypothesis using a mouse model of SAE with real-time reverse transcription-polymerase chain reaction (RT-PCR) gene expression analysis of isolated choroid plexuses, and with double immunohistofluorescence staining.
2. Experimental procedures
2.1. Animals
Eight-week-old male C57BL/6NCrSlc (B6) mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Mice received intraperitoneal injection of 3 mg/kg LPS from E. coli O55:B5 (Sigma, St. Louis, MO, USA) that was dissolved in physiological saline at a total volume of 7.5 mL/kg. As the control, mice received intraperitoneal injection of 7.5 mL/kg physiological saline. For the real-time RT-PCR, mice were examined at 4 h after injection of LPS (n = 4) or saline (n = 4). For the double immunohistofluorescence staining, they were examined at 1 (n = 12), 4 (n = 13), and 24 h (n = 8) after LPS injection, and at 1 h after saline injection (n = 15). All mice were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). All experiments were approved by the Institutional Animal Care and Use Committee of the Kyorin University Faculty of Health Sciences (Protocol “I17-08-02”)
2.2. Extraction of RNA from the choroid plexus
Individual mice were deeply anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and blood was flushed by transcardial perfusion with nuclease-free phosphate-buffered saline (PBS). After brains were removed, the choroid plexuses were dissected out from both lateral, third and fourth ventricles on ice, snap frozen in liquid nitrogen and stored frozen at −80 °C. The frozen choroid plexus tissues were then submerged in RNAlater-ICE Frozen Tissue Transition Solution (Invitrogen-Thermo Fisher Scientific, Waltham, MA, USA) and stored frozen at −20 °C for 40 h prior to RNA extraction. Total RNA was extracted from homogenized choroid plexus tissue using NucleoSpin RNA (Macherey-Nagel, Duren, Germany) according to the manufacturer’s protocols.
2.3. Real-time RT-PCR quantification assays
Two hundred nanograms of total RNA extracted from individual choroid plexuses were reverse transcribed to cDNA using SuperScript III Reverse Transcriptase (Invitrogen). Real-time quantitative RT-PCR (qRT-PCR) was performed using TaqMan Fast Advanced Master Mix (Applied Biosystems-Thermo Fisher Scientific) and a TaqMan primer/probe set for each of the following targets (all from Applied Biosystems): Il-1b, Mm01336189_m1; Ccl2, Mm00441242_m1; Cxcl1 Mm04207460_m1; Cxcl2, Mm00436450_m1; Il6, Mm00446190_m1; Gapdh, Mm99999915_g1. Reactions were run according to the manufacturer’s protocols on a 7500 Fast Real-Time PCR System (Applied Biosystems). Gapdh was used as a reference gene. Relative transcript levels were analyzed using the ΔΔCT method. Four mice were used per group and all samples were assayed in duplicate.
2.4. Frozen tissue section preparation
Mice were deeply anesthetized and blood was flushed by transcardial perfusion with PBS. They were then perfused with 120 mL of 4% paraformaldehyde at a rate of 8 mL/min. Heads of the mice were soaked in the same fixative at 4 °C overnight. After each brain was removed, the cerebrum was coronally sliced so that coronal sections through the hippocampus (containing the choroid plexuses of the third and lateral ventricles) were obtained. The cerebellum with the brain stem was bisected in the sagittal direction so that parasagittal sections through the choroid plexus of the fourth ventricle were obtained. The spleens were also removed from all mice examined. In addition, the livers were obtained from mice examined at 1 h after treatment with LPS or physiological saline. These tissues were cryo-protected with 30 % sucrose, embedded in Tissue-Tek™ Optimal Cutting Temperature Compound (OCT, Sakura Finetek Japan, Tokyo, Japan), and frozen in normal hexane cooled with dry ice. The frozen tissue blocks that contained the cerebrum, cerebellum and spleen (plus liver in mice at 1 h after treatment) were kept at −80 °C until they were cut into 14-μm thick sections using a cryostat (CM3050 S; Leica, Wetzlar, Germany). Immediately after sections were mounted on coated slide glasses, they were air-dried, then vacuum dried using a Buchi V-100 Vacuum Pump (Fisher Scientific, Leicestershire, UK) and stored at −20 °C until use.
2.5. Immunohistofluorescence staining
Frozen sections were soaked in Tris buffered saline with Tween 20 (TBS-T) for 10 min before being subjected to immunofluorescence staining. Following preincubation with blocking solution (1% bovine serum albumin in TBS-T), sections were incubated with primary antibodies overnight at 4 °C. Primary antibodies used are shown in Table 1. Primary antibodies combinations for double immunofluorescence staining are shown in Table 2. Following the incubation with primary antibodies, sections were incubated with donkey anti-goat, anti-rabbit, or anti-rat IgG secondary antibodies conjugated with Alexa Fluor 568 or 488 (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), in accordance with the species origin of primary antibodies for 60 min at room temperature. 4′, 6-diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining. Sections were then coverslipped with Fluorescence Mounting Medium (DAKO, Agilent, Santa Clara, CA, USA). Fluorescent images were obtained with a BZ-X710 microscope (Keyence, Osaka, Japan) that was equipped with structured illumination.
Table 1.
List of antibodies.
| Antibody against | Description | Host | Dilution | Source |
|---|---|---|---|---|
| α-SMA | smooth muscle cell | rabbit | 1:100 | AnaSpec (Fremont, CA,USA) |
| CCL2 | cytokine | rabbit | 1:100 | abcam (Cambridge, UK) |
| CD31 | vascular endothelium | rat | 1:200 | BMA Biomedicals (Augst, Switzerland) |
| CXCL1 | cytokine | goat | 1:100 | R&D Systems (Minneapolis, MN, USA) |
| CXCL2 | cytokine | rabbit | 1:100 | AbD Serotec, Bio-Rad (Hercules, CA, USA) |
| CXCR2 | cytokine receptor | rat | 1:50 | R&D Systems |
| ER-TR7 | fibroblast | rat | 1:200 | abcam |
| F4/80 | macrophage | rat | 1:100 | abcam |
| Iba-1 | microglia | rabbit | 1:1000 | Wako (Osaka, Japan) |
| IL-1R1 | cytokine receptor | goat | 1:20 | R&D Systems |
| IL-1RAcP | receptor accessory protein | goat | 1:100 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| IL-1β | cytokine | goat | 1:200 | R&D Systems |
| IL-6 | cytokine | rabbit | 1:100 | Bioss (Woburn, MA, USA) |
| IL-6R | cytokine receptor | goat | 1:20 | R&D Systems |
| NG2 | pericyte | rabbit | 1:100 | Merck Millipore (Burlington, MA, USA) |
| S-100 | arachnoid cell | rabbit | 1:100 | gift from Dr. K Katoh |
| transthyretin | choroid plexus epithelium | rabbit | 1:200 | Proteintech (Rosemont, IL, USA) |
Table 2.
Combinations of primary antibodies for double immunofluorescence staining.
| Antibody for cytokine (host) | Antibodies for cell markers (host) |
| IL-1β (goat) | F4/80 (rat) |
| IL-1β (goat) | Iba-1 (rabbit) |
| IL-1β (goat) | transthyretin (rabbit) |
| IL-1β (goat) | CD31 (rat) |
| IL-1β (goat) | NG2 (rabbit) |
| Antibody for receptor (host) | Antibodies for cell markers (host) |
| IL-1R1 (goat) | transthyretin (rabbit) |
| IL-1R1 (goat) | ER-TR7 (rat) |
| IL-1R1 (goat) | S100 (rabbit) |
| IL-1R1 (goat) | NG2 (rabbit) |
| IL-1R1 (goat) | F4/80 (rat) |
| IL-1R1 (goat) | α-SMA (rabbit) |
| IL-1R1 (goat) | CD31 (rat) |
| IL-1R1 (goat) | Iba-1 (rabbit) |
| Antibodies for receptors (host) | Antibodies for cytokines (host) |
| IL-1R1 (goat) | IL-6 (rabbit) |
| IL-1R1 (goat) | CCL2 (rabbit) |
| IL-1R1 (goat) | CXCL2 (rabbit) |
| IL-1RAcP (goat) | CXCL2 (rabbit) |
| CXCR2 (rat) | CXCL1 (goat) |
| Antibody for receptor (host) | Antibody for receptor (host) |
| IL-1R1 (goat) | CXCR2 (rat) |
2.6. Statistical analysis
Relative transcript levels of cytokines were analyzed with non-repeated two-way analysis of variance (ANOVA) using STATISTICA (StatSoft, Tulsa, OK, USA). The mean ΔCT values were compared with the statistical design in which the main effects were cytokine species (Il1b, Ccl2, Cxcl1, Cxcl2 and Il6) and treatments (saline and LPS). Post-hoc tests were performed using Tukey’s procedure.
3. Results
3.1. Endotoxemia-induced changes in the cytokine transcript levels of the choroid plexus
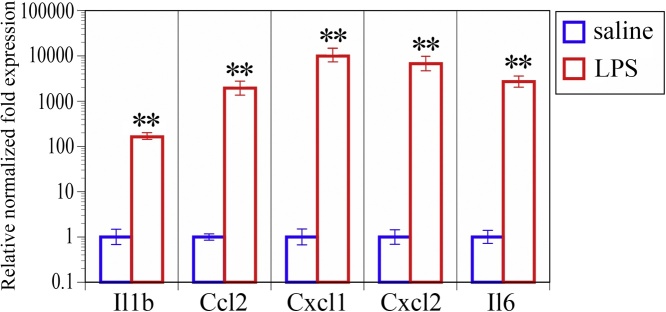
Our qRT-PCR study indicated that the relative transcript levels of IL-1β increased approximately 170-fold in the choroid plexus at 4 h after intraperitoneal LPS injection compared to saline injection (Fig. 1). Approximately 1900-fold increase, 9900-fold increase, 6700-fold increase and 2700-fold increase in the transcript levels of CCL2, Cxcl1, Cxcl2 and Il6, respectively were detected in choroid plexuses isolated from LPS-treated mice compared with saline control (Fig. 1). All of these systemic inflammation-induced changes in cytokine gene expression levels were statistically significant.
Fig. 1.
Changes in cytokine transcript levels. To visually compare the LPS-treated group with the saline control group, the transcript levels of five cytokines are graphically represented with average relative normalized fold expression and error bars (based on SEM of ΔCT) according to the previously described method [40]. n = 4 per experimental group, ∗∗p < 0.001.
3.2. Time-dependent changes in IL-1β expression following LPS challenge
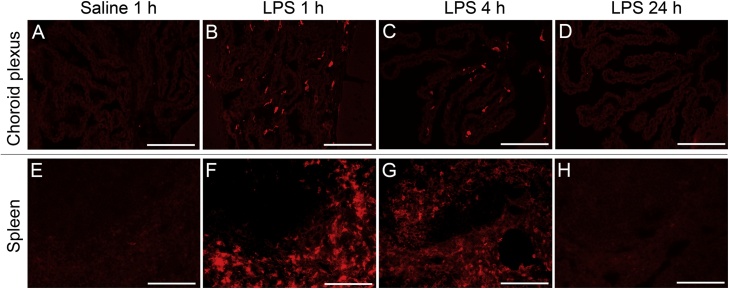
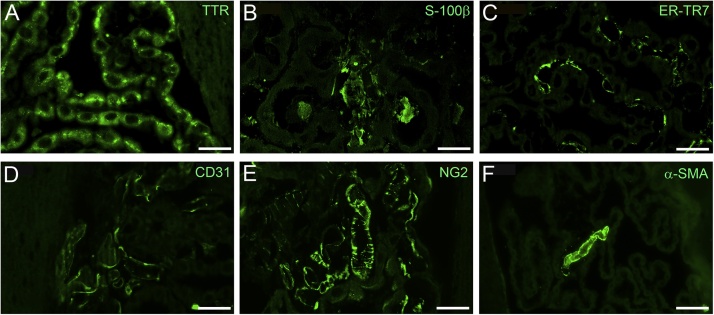
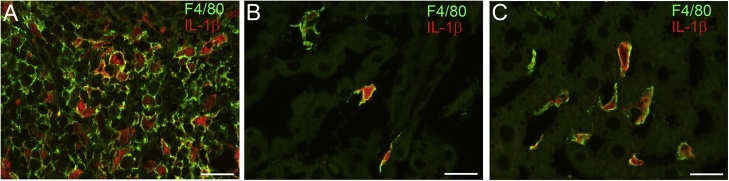
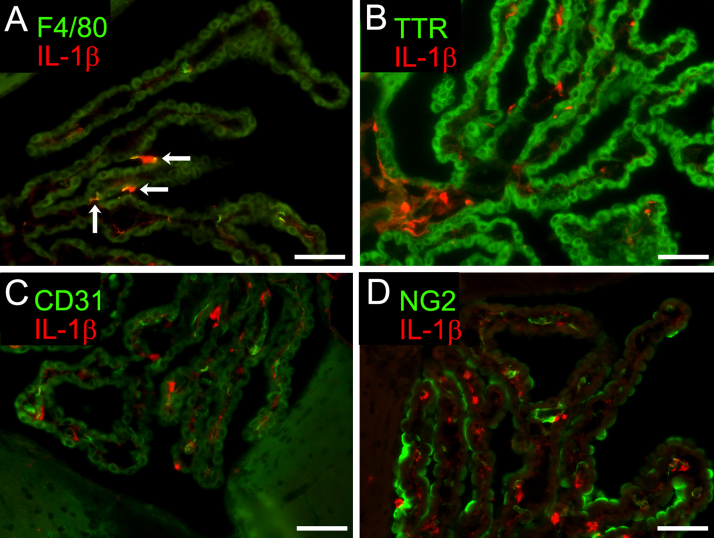
Our immunohistofluorescence study indicated that the intracranial expression of IL-1β was remarkable in the choroid plexus and leptomeninges but not in the brain parenchyma. IL-1β expression in the choroid plexus appeared 1 and 4 h after LPS injection and then disappeared by 24 h (Fig. 2). This time course was the same as in the spleen (Fig. 2). There was no IL-1β expression in the choroid plexus, spleen or liver after saline injection. The cell type identification of choroid plexus cells was performed with immunohistofluorescence staining for choroid plexus epithelial cells (transthyretin as a marker), arachnoid cells (S-100β), reticular fibroblastic cells (ER-TR7), vascular endothelial cells (CD31), pericytes (NG2), smooth muscle cells (α-smooth muscle actin, α-SMA) (Fig. 3A–F, respectively) and macrophages (Iba-1 and F4/80). The choroid plexus cells that produced IL-1β were macrophages, as evidenced by the double immunofluorescence for IL-1β and F4/80 or IL-1β and Iba-1 (Fig. 4). As in the spleen and liver, choroid plexus macrophages responded immediately with IL-1β production (Fig. 4). Other choroid plexus cells rarely expressed IL-1β in response to systemic LPS injection (Supplementary Fig. 1).
Fig. 2.
Time-dependent changes in IL-1β expression following LPS injection. Photomicrographic images of immunohistofluorescence staining for IL-1β in the choroid plexus (A-D) and spleen (E-H) of mice treated with saline (A and E) or LPS (B-D and F-H) are shown. In the choroid plexus, no cells expressed IL-1β in saline-treated mice (A), whereas IL-1β expression appeared 1 h after LPS injection (B), lasted up to 4 h (C) and then disappeared by 24 h (D). This time course was the same as in the spleen. There were no IL-1β-expressing cells (E), whereas IL-1β expression appeared 1 h (F) and 4 h (G) after LPS injection and then disappeared by 24 h (H). Bars =50 μm.
Fig. 3.
Cell type identification of choroid plexus cells. Immunohistofluorescence staining was performed to identify choroid plexus epithelial cells (transthyretin, TTR as a marker; A), arachnoid cells (S-100β; B), reticular fibroblastic cells (ER-TR7; C), vascular endothelial cells (CD31; D), pericytes (NG2; E) and smooth muscle cells (α-smooth muscle actin, α-SMA; F). Bars =20 μm for A-E and 50 μm for F.
Fig. 4.
Cell type identification of IL-1β expressing cells. Double immunohistofluorescence staining for IL-1β (red) and F4/80 (green) confirmed that cells expressing IL-1β in response to systemic LPS injection were macrophages in the spleen (A) and choroid plexus (B), and Kupffer cells (or macrophages) in the liver (C). These cells expressed IL-1β 1 h after LPS injection. Note the high rate of IL-1β-immunopositive cells among all macrophages in all three different tissue types. Bars =20 μm.
3.3. Expression of IL-1β receptor
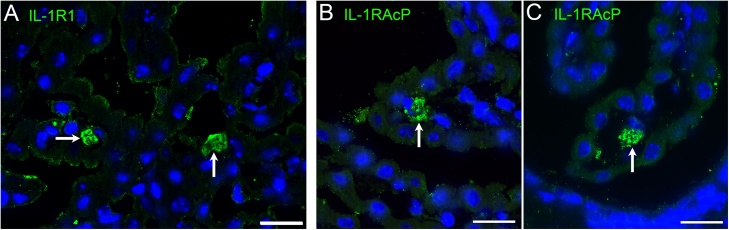
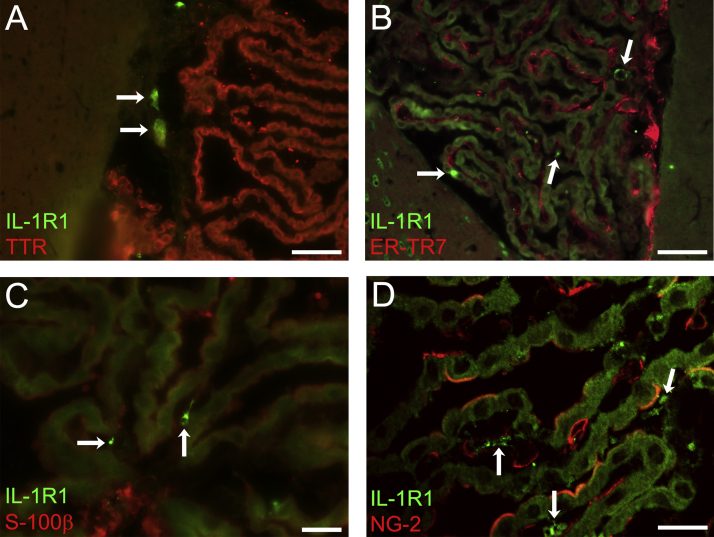
Immunofluorescence staining revealed the presence of cells that expressed IL-1 receptor type I (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP or IL-1R3) in the choroid plexus stroma (Fig. 5). The morphology and distribution of IL-1R1-immunopositive cells and IL-1RAcP-immunopositive cells were similar. IL-1R1 expression was constitutively seen in both LPS- and saline-injected mice. We tried to determine the cell type for IL-1 receptor-bearing choroid plexus cells using double immunofluorescence staining. However, neither macrophages nor epithelial cells nor any of the stromal cells examined were immunopositive for IL-1R1 or IL-1RAcP (Supplementary Fig. 2). Therefore, the exact cell type of stromal cells that expressed IL-1R1 or IL-1RAcP remains to be identified.
Fig. 5.
Presence of IL-1β receptor-expressing cells in the choroid plexus stroma. Immunohistofluorescence staining revealed that some choroid plexus stromal cells expressed IL-1 receptor type I (IL-1R1) (A, arrows) and IL-1 receptor accessory protein (IL-1RAcP or IL-1R3) (B and C, arrows). The morphology and distribution of IL-1R1-immunopositive cells and IL-1RAcP-immunopositive cells were similar. The photomicrographic images were taken using sections from mice fixed 4 h after LPS injection. Nuclei were counterstained with DAPI. Bars =20 μm.
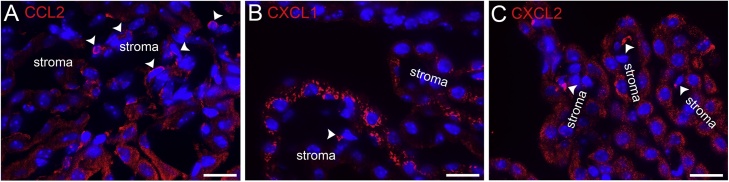
3.4. Cytokine expression at 4 h after LPS challenge
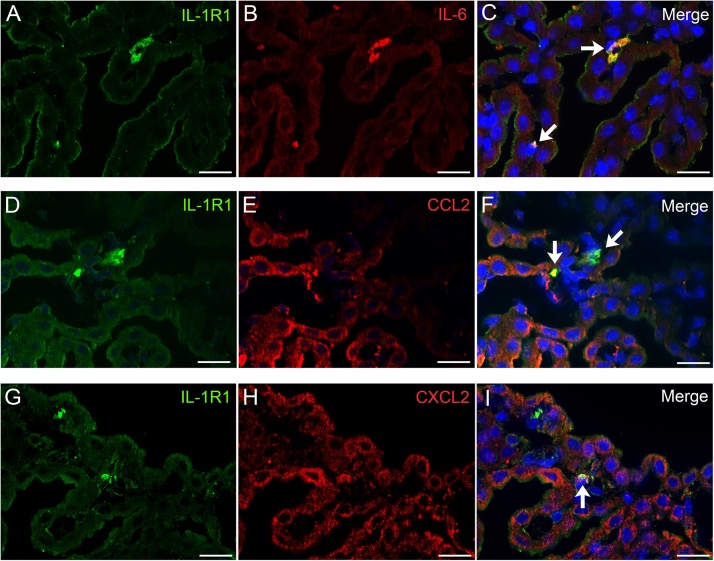
CCL2, CXCL1 and CXCL2 were expressed by both epithelial and stromal cells of the choroid plexus 4 h after LPS injection (Fig. 6). IL-6, in contrast, was expressed chiefly by stromal cells of the choroid plexus (Fig. 7B). Double immunofluorescence analysis revealed that IL-1R1-bearing choroid plexus stromal cells were immunopositive for CCL2, CXCL2 and IL-6 (Fig. 7).
Fig. 6.
Cytokines expressed in the choroid plexus 4 h after systemic endotoxin injection. Immunohistofluorescence staining revealed that CCL2 (A), CXCL1 (B) and CXCL2 (C) were expressed by both epithelial cells and stromal cells of the choroid plexus 4 h after LPS injection. The majority of epithelial cells were immunopositive for these cytokines. Stromal cells were occasionally immunopositive (arrow heads). Nuclei were counterstained with DAPI. Bars =20 μm.
Fig. 7.
Cytokine expression of IL-1R1-bearing stromal cells of the choroid plexus.
Double immunofluorescence staining revealed that IL-1R1-bearing stromal cells of the choroid plexus expressed IL-6 (A–C), CCL2 (D–F) and CXCL2 (G–I) 4 h after LPS injection. Note that almost no epithelial cells expressed IL-6, whereas CCL2 and CXCL2 were expressed in both epithelial and stromal cells of the choroid plexus. Nuclei were counterstained with DAPI. Bars =20 μm.
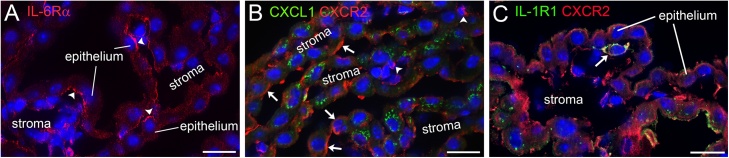
3.5. Expression of cytokine receptors other than IL-1R1
IL-6 receptor α subunit (IL-6Rα) expression was detected in choroid plexus epithelial cells and was more marked on the basal side (stromal side), which contrasted with the IL-6 expression by the stromal cells (Fig. 8A). This suggested that choroid plexus stromal cells expressing IL-6 could perform cell-cell interactions with epithelial cells via IL-6-IL-6 receptor signaling.
Fig. 8.
Expression of cytokine receptors other than IL-1R1 in the choroid plexus.
(A) Immunohistofluorescence staining revealed that IL-6 receptor α subunit (IL-6Rα) expression was detected in choroid plexus epithelial cells, especially on the basal side (or stromal side) (arrow heads).
(B) Double immunohistofluorescence staining revealed that CXCR2, a common receptor for both CXCL1 and CXCL2, was most frequently expressed by choroid plexus epithelial cells (arrows). CXCR2 was less frequently expressed by choroid plexus stromal cells (arrow heads). CXCL1 was expressed chiefly by choroid plexus epithelial cells. Note that CXCR2 and its ligand CXCL1 were expressed in close apposition to each other.
(C) Double immunohistofluorescence staining revealed that IL1R1-bearing stromal cells were sometimes immunopositive for CXCR2, indicating the presence of stromal cells that bear both IL-1R1 and CXCR2 (arrow).
Nuclei were counterstained with DAPI for A–C. Bars =20 μm for A-C.
CXCR2, a common receptor for both CXCL1 and CXCL2, was most frequently expressed by choroid plexus epithelial cells (Fig. 8B). CXCR2 was less frequently expressed by choroid plexus stromal cells. CXCR2 and its ligand CXCL1 were expressed in close apposition to each other, suggesting that CXCL1 and CXCR2 can bind within the choroid plexus. In addition, CXCR2-expressing stromal cells were sometimes immunopositive for IL-1R1, indicating the presence of stromal cells that bear both receptors for IL-1 and for CXCL1 or CXCL2 (Fig. 8C).
4. Discussion
4.1. Contribution of the choroid plexus to initial responses to systemic inflammation
We modeled SAE by injecting a pyrogenic dose of endotoxin intraperitoneally into mice. In the choroid plexuses isolated at 4 h after LPS injection, transcript levels of IL-1β, CCL2, CXCL1, CXCL2 and IL-6 were significantly elevated in response to endotoxin-induced systemic inflammation. The intracranial protein expression of IL-1β was remarkable in the choroid plexus and leptomeninges but not in the brain parenchyma. IL-1β expression in the choroid plexus appeared 1 and 4 h after systemic endotoxin injection and then disappeared by 24 h. The time course was the same as in the spleen. Macrophages in the choroid plexus stroma expressed IL-1β and served as one of the immediate responders to acute systemic inflammation, as in the spleen and liver. IL-1β expression by macrophages was also remarkable in the leptomeninges, which is reasonable because the tissue components are shared by leptomeninges and choroid plexus stroma as stated previously [13]. Endotoxin-induced IL-1β expression by macrophages of the choroid plexus and leptomeninges as well as perivascular macrophages of the brain parenchyma has been reported to occur 4 h after endotoxin injection [22]. Although the same researchers reported that some parenchymal microglia also expressed IL-1β at 8 and 24 h after endotoxin administration, the present study indicated that parenchymal microglial reaction with IL-1β expression did not occur until 24 h after endotoxin administration. Since it has been reported that the elevation of IL-1β in the brain occurs 28 h after endotoxin administration [11], we consider that the production of IL-1β by brain parenchymal microglia could occur later than the time window examined in the present study.
Located in close apposition to macrophages, stromal cells were observed to express IL-1R1 and IL-1RAcP, indicating that these receptor-bearing stromal cells can be stimulated by IL-1β-expressing macrophages. Although the exact cell-typing of IL-1R1-bearing cells was not successful in the present study, these cells were different from smooth muscle cells, vascular endothelial cells, reticular fibroblastic cells and arachnoid cells. Importantly, IL-1R1-bearing stromal cells increased their expression of IL-6, CCL2, CXCL1 and CXCL2 at 4 h after systemic endotoxin administration, which occurred later than the time point at which macrophages started to express IL-1β. CCL2, CXCL1 and CXCL2 were also expressed by choroid plexus epithelial cells 4 h after endotoxin injection.
CCL2, previously known as monocyte chemoattractant protein-1 (MCP-1), serves as a chemokine that recruits monocytes/macrophages from the blood stream. Previous studies have indicated that the direct recruitment of monocytes into the brain in West Nile encephalitis is dependent on the expression of CCR2, a receptor for CCL2, on monocytes [23]. Choroid plexus CCL2 may be a link between blood-borne pro-inflammatory mediators and the brain during peripheral inflammation [24]. Thus, an increased number of monocytes may have been recruited from the blood stream to the choroid plexus stroma in the present mouse model of SAE, with some being further attracted by epithelial cells to become epiplexus cells.
CXCR2, a common receptor that binds to both CXCL1 and CXCL2, was expressed by choroid plexus epithelial cells. The proximity of receptors and ligands guaranteed the potential intercellular interaction between the choroid plexus epithelial and stromal cells via CXCL1-CXCR2 signaling. It is known that choroid plexus epithelial cells possess the capacity to produce CXC chemokines including CXCL1 and CXCL2, which is enhanced by IL-1β and other mediators in neuroinflammatory conditions [25]. Thus, we consider that the endotoxin-induced expression of CXCL1 and CXCL2 by choroid plexus epithelial cells is a manifestation of immunological activation of the choroid plexus. Interestingly, the choroid plexus stromal cells bearing IL-1R1 occasionally expressed CXCR2 at the same time. Therefore, some stromal cells that were activated by macrophages via IL-1β-IL-1R1 signaling and subsequently stimulated epithelial cells via CXCL1-CXCR2 signaling may also have been stimulated by epithelial cells in a reciprocal manner.
IL-6Rα was chiefly expressed on the stromal side of choroid plexus epithelial cells, which suggested that epithelial cells were stimulated by stromal cells expressing IL-6 via IL-6-IL-6 receptor signaling. It is well known that IL-6 is promptly and transiently produced in response to tissue injuries, and contributes to acute phase immunological reactions [26]. Thus, we consider that the endotoxin-induced expression of IL-6 by choroid plexus stromal cells is another manifestation of immunological activation of the choroid plexus.
The data from the present study are consistent with the following events (Fig. 9). When endotoxin is injected systemically, macrophages of the choroid plexus stroma are some of the initial cells that responded to endotoxin stimulation. Macrophages produce IL-1β, which may stimulate neighboring stromal cells via IL-1R1. Stimulated stromal cells produce CCL2, CXCL1, CXCL2 and IL-6. CXCL1, CXCL2 and IL-6 can bind to receptors on choroid plexus epithelial cells, which also produce CCL2, CXCL1 and CXCL2. At least CXCL1 and CXCL2, produced by the epithelial cells, can reciprocally activate stromal cells via ligand-receptor signaling. These potential stromal-epithelial interactions may contribute to keeping the choroid plexus immunologically activated until the brain parenchymal astrocytes receive cytokines derived from the choroid plexus and leptomeninges. Thereafter, astrocytes become activated to change the brain parenchymal cytokine microenvironment as we previously reported [9]. The potential routes along which the immunologically activated choroid plexus could stimulate parenchymal astrocytes are discussed elsewhere [9,12,13].
Fig. 9.
Possible cytokine-mediated reactions by the choroid plexus stroma and epithelium in response to LPS-induced systemic inflammation.
When LPS was injected systemically, macrophages of the choroid plexus stroma initially responded to endotoxin by producing IL-1β. IL-1β potentially stimulated neighboring stromal cells via receptors (the presence of IL-1R1 and IL-1RAcP on stromal cells was observed in the present study). IL-1R1-bearing stromal cells produced CCL2, CXCL1, CXCL2 and IL-6. These cytokines were potentially received by epithelial cells via receptors (the presence of CXCR2 and IL-6Rα on epithelial cells was observed). Epithelial cells also produced CCL2, CXCL1 and CXCL2. Epithelial cell-derived cytokines potentially stimulated stromal cells in a reciprocal fashion via ligand-receptor signaling (the presence of CXCR2 on IL-1R1-bearing stromal cells was observed).
4.2. Choroid plexus macrophages versus brain parenchymal microglia
Although previous studies have reported that brain parenchymal microglia express toll-like receptor (TLR)4 ([27], it has been controversial whether microglia are the initial responders to endotoxin-induced systemic inflammation. In the very acute phase of endotoxemia, endotoxin enters the tissue of various organs and stimulates macrophages via TLR4. In the present study, the liver Kupffer cells and spleen macrophages clearly expressed IL-1β. However, the brain microglia did not express IL-1β, consistent with our previous study that revealed no increase in the brain parenchymal tissue concentration of IL-1β before 24 h after endotoxin injection [9]. This is understandable, given that the endotoxin does not cross the blood brain barrier (BBB) [28]. As such, at least during the acute phase (1–24 h) following a single shot of endotoxin, it is unlikely that the endotoxin directly stimulates the brain parenchymal microglia. Rather, one of the initial responder cells was the tissue resident macrophages that populated the choroid plexus and leptomeninges. The findings are consistent with the literature indicating that TLR4 expression is maximal in the choroid plexus and leptomeninges ([27], although TLR4 is expressed to some extent in brain parenchymal perivascular cells and microglia, especially in the circumventricular organs.
4.3. IL-1 receptors in choroid plexus cells versus brain parenchymal microglia
The intracranial responder cells could also be stimulated by circulating IL-1β, rather than by endotoxin [29]. This is unlikely, however, since IL-1β cannot passively transported across the BBB given the molecular size and hydrophilic property. Instead, peripheral IL-1β needs to cross the BBB actively to bring about sickness behavior [30]. The cell types that express IL-1R1 in rodents have been controversial. The presence of IL-1R1 on microglia in vitro has been reported in several studies [31,32] but has not been replicated in other studies [33,34]. IL-1R1 expression by hippocampal neurons has been reported in some studies [35] but not in others [36,37]. It has been reported that IL-1R1 exists on brain endothelial cells [37] and selected neurons in the hypothalamus and circumventricular organs [36,38]. In a recent study, researchers created genetically modified mouse lines in which IL-1R1 receptors were overexpressed or deleted in a brain cell-type-specific manner. The study suggested that the IL-1R1 expression by glia and neurons is limited to low levels [39]. More importantly, these previous studies have consistently revealed that the IL-1R1 is most remarkably expressed in the choroid plexus and leptomeninges, followed by brain parenchymal perivascular macrophages and vascular endothelial cells [38,39]. In the present study, we observed that brain parenchymal vascular endothelial cells exhibited relatively weak IL-1R1 expression and that there was no obvious IL-1R1 immunoreactivity in hippocampal neurons or in parenchymal microglia, probably because the expression level in these cells was not sufficiently high to be detected by immunohistofluorescence. Therefore, we consider it important to note that the choroid plexus and leptomeninges, as opposed to the brain parenchyma, are the major sites for the initial responder macrophages to generate acute reactions to endotoxin-induced systemic inflammation.
4.4. Conclusion
When endotoxin is injected systemically, an immediate intracranial reaction is initiated within the choroid plexus via increased expression of IL-1β by macrophages, which occurs 1 h after endotoxin injection. Neighboring stromal cells of the choroid plexus are able to receive IL-1β, and such stromal cells subsequently have elevated expression of CCL2, CXCL1, CXCL2 and IL-6 at 4 h after endotoxin injection. At the same time, the choroid plexus epithelial cells also have elevated expression of CCL2, CXCL1 and CXCL2. The presence of receptors for IL-6, CXCL1 and CXCL2 on epithelial cells as well as the presence of receptors for CXCL1 and CXCL2 on stromal cells raises the possibility of cytokine-mediated reciprocal interactions between stromal and epithelial cells. Given that brain parenchymal concentrations of CCL2, CXCL1, CXCL2 and IL-6 are elevated at 4 h after endotoxin injection and astrocytes bearing receptors for these cytokines are activated at 24 h after endotoxin injection, the immediate early responses to systemic inflammation exerted by the choroid plexus are relevant to understanding how SAE is initiated in clinical settings.
Author roles
AS designed and coordinated the study. AS and SHI performed the experiments, analyzed the data, and drafted the manuscript. The authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We thank Asumi Ochiai, Reina Kazui, Fumika Sase and Haruka Sawamura for their technical contribution to experiments. Rabbit polyclonal antibody against S-100β was a gift from Dr. Kanefusa Katoh at Aichi Developmental Disability Center. This study was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS) (KAKENHI Nos.: 25290020 and 18K07072 to AS).
Edited by Dr. A.M. Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.03.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Hasegawa-Ishii S., Shimada A., Inaba M., Li M., Shi M., Kawamura N., Takei S., Chiba Y., Hosokawa M., Ikehara S. Selective localization of bone marrow-derived ramified cells in the brain adjacent to the attachments of choroid plexus. Brain Behav. Immun. 2013;29:82–97. doi: 10.1016/j.bbi.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Goldmann T., Wieghofer P., Jordao M.J., Prutek F., Hagemeyer N., Frenzel K., Amann L., Staszewski O., Kierdorf K., Krueger M., Locatelli G., Hochgerner H., Zeiser R., Epelman S., Geissmann F., Priller J., Rossi F.M., Bechmann I., Kerschensteiner M., Linnarsson S., Jung S., Prinz M. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016;17(7):797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelhardt B., Wolburg-Buchholz K., Wolburg H. Involvement of the choroid plexus in central nervous system inflammation. Microsc. Res. Tech. 2001;52(1):112–129. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Meeker R.B., Williams K., Killebrew D.A., Hudson L.C. Cell trafficking through the choroid plexus. Cell Adh. Migr. 2012;6(5):390–396. doi: 10.4161/cam.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandijck D., Decruyenaere J.M., Blot S.I. The value of sepsis definitions in daily ICU-practice. Acta Clin. Belg. 2006;61(5):220–226. doi: 10.1179/acb.2006.037. [DOI] [PubMed] [Google Scholar]
- 6.Ziaja M. Septic encephalopathy. Curr. Neurol. Neurosci. Rep. 2013;13(10):383. doi: 10.1007/s11910-013-0383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhry N., Duggal A.K. Sepsis associated encephalopathy. Adv. Med. 2014;2014:16. doi: 10.1155/2014/762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green R., Scott L.K., Minagar A., Conrad S. Sepsis associated encephalopathy (SAE): a review. Front. Biosci. 2004;9:1637–1641. doi: 10.2741/1250. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa-Ishii S., Inaba M., Umegaki H., Unno K., Wakabayashi K., Shimada A. Endotoxemia-induced cytokine-mediated responses of hippocampal astrocytes transmitted by cells of the brain-immune interface. Sci. Rep. 2016;6:25457. doi: 10.1038/srep25457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa-Ishii S., Inaba M., Shimada A. Widespread time-dependent changes in tissue cytokine concentrations in brain regions during the acute phase of endotoxemia in mice. Neurotoxicology. 2020;76:67–74. doi: 10.1016/j.neuro.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Erickson M.A., Banks W.A. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav. Immun. 2011;25(8):1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimada A. Principles of neuroanatomical architecture supporting brain–immune cell–cell interactions. Clin. Exp. Neuroimmunol. 2020;11(1):5–15. doi: 10.1111/cen3.12559. [DOI] [Google Scholar]
- 13.Shimada A., Hasegawa-Ishii S. Histological architecture underlying brain-immune cell-cell interactions and the cerebral response to systemic inflammation. Front. Immunol. 2017;8:17. doi: 10.3389/fimmu.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stearns-Kurosawa D.J., Osuchowski M.F., Valentine C., Kurosawa S., Remick D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello C.A. Interleukin-1. Cytokine Growth Factor Rev. 1997;8(4):253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello C.A. IL-1: discoveries, controversies and future directions. Eur. J. Immunol. 2010;40(3):599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018;281(1):8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ban E., Haour F., Lenstra R. Brain interleukin 1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine. 1992;4(1):48–54. doi: 10.1016/1043-4666(92)90036-q. [DOI] [PubMed] [Google Scholar]
- 19.Koizumi T., Kerkhofs D., Mizuno T., Steinbusch H.W.M., Foulquier S. Vessel-associated immune cells in cerebrovascular diseases: from perivascular macrophages to vessel-associated microglia. Front. Neurosci. 2019;13:1291. doi: 10.3389/fnins.2019.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quan N., Whiteside M., Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83(1):281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 21.Van Dam A.M., Brouns M., Louisse S., Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588(2):291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 22.Van Dam A.M., Bauer J., Tilders F.J., Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65(3):815–826. doi: 10.1016/0306-4522(94)00549-K. [DOI] [PubMed] [Google Scholar]
- 23.Lim J.K., Obara C.J., Rivollier A., Pletnev A.G., Kelsall B.L., Murphy P.M. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J. Immunol. 2011;186(1):471–478. doi: 10.4049/jimmunol.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell K., Yang H.Y., Berk J.D., Tran J.H., Iadarola M.J. Monocyte chemoattractant protein-1 in the choroid plexus: a potential link between vascular pro-inflammatory mediators and the CNS during peripheral tissue inflammation. Neuroscience. 2009;158(2):885–895. doi: 10.1016/j.neuroscience.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szmydynger-Chodobska J., Gandy J.R., Varone A., Shan R., Chodobski A. Synergistic interactions between cytokines and AVP at the blood-CSF barrier result in increased chemokine production and augmented influx of leukocytes after brain injury. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakravarty S., Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 2005;25(7):1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banks W.A., Robinson S.M. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 2010;24(1):102–109. doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thibeault I., Laflamme N., Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J. Comp. Neurol. 2001;434(4):461–477. doi: 10.1016/s0165-5728(98)00224-0. [DOI] [PubMed] [Google Scholar]
- 30.Banks W.A. The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav. Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monif M., Reid C.A., Powell K.L., Drummond K.J., O’Brien T.J., Williams D.A. Interleukin-1beta has trophic effects in microglia and its release is mediated by P2X7R pore. J. Neuroinflammation. 2016;13(1):173. doi: 10.1186/s12974-016-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato A., Ohtaki H., Tsumuraya T., Song D., Ohara K., Asano M., Iwakura Y., Atsumi T., Shioda S. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J. Neuroinflammation. 2012;9:65. doi: 10.1186/1742-2094-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X., Quan N. Microglia and CNS Interleukin-1: beyond immunological concepts. Front. Neurol. 2018;9:8. doi: 10.3389/fneur.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X., Yamashita T., Chen Q., Belevych N., McKim D.B., Tarr A.J., Coppola V., Nath N., Nemeth D.P., Syed Z.W., Sheridan J.F., Godbout J.P., Zuo J., Quan N. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J. Neurosci. 2015;35(7):2860–2870. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.French R.A., VanHoy R.W., Chizzonite R., Zachary J.F., Dantzer R., Parnet P., Bluthe R.M., Kelley K.W. Expression and localization of p80 and p68 interleukin-1 receptor proteins in the brain of adult mice. J. Neuroimmunol. 1999;93(1–2):194–202. doi: 10.1016/s0165-5728(98)00224-0. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham E.T., Jr., Wada E., Carter D.B., Tracey D.E., Battey J.F., De Souza E.B. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J. Neurosci. 1992;12(3):1101–1114. doi: 10.1523/JNEUROSCI.12-03-01101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konsman J.P., Vigues S., Mackerlova L., Bristow A., Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J. Comp. Neurol. 2004;472(1):113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 38.Ericsson A., Liu C., Hart R.P., Sawchenko P.E. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J. Comp. Neurol. 1995;361(4):681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 39.Liu X., Nemeth D.P., McKim D.B., Zhu L., DiSabato D.J., Berdysz O., Gorantla G., Oliver B., Witcher K.G., Wang Y., Negray C.E., Vegesna R.S., Sheridan J.F., Godbout J.P., Robson M.J., Blakely R.D., Popovich P.G., Bilbo S.D., Quan N. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50(2):317–333. doi: 10.1016/j.immuni.2018.12.012. e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor S.C., Nadeau K., Abbasi M., Lachance C., Nguyen M., Fenrich J. The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends Biotechnol. 2019;37(7):761–774. doi: 10.1016/j.tibtech.2018.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.