Abstract
Introduction
Curettage and dermabrasion are effective in the treatment of giant congenital melanocytic nevi (GCMN); however, local infection and hypertrophic scar formation are major issues. Thus, we applied cultured epithelial autografts (CEA) on skin defects after curettage or abrasion of GCMN and assessed the postoperative outcomes.
Methods
Seven nevi lesions of five patients (aged 3 months to 24 years) were treated with CEA after curettage or abrasion with a dermatome or a surgical bar, respectively. We assessed the postoperative outcomes, including CEA take ratio, erosion and/or ulcer formation in the acute phase, hospitalization days, Vancouver scar scale, and color improvement one year after the operation. In addition, a histological evaluation of a skin biopsy was performed over one year after the operation.
Results
The CEAs took well on the wound, and the wound surface was mostly epithelized by postoperative day 7 in all cases. While hypertrophic scar formation and slight pigmentation were observed in some lesions, the color was improved in all of the treated lesions. Histopathological examination revealed that the regenerated epidermis had stratified keratinocytes with rete ridges, and the dermal layer without nevus cells regenerated above the remaining dermis layer.
Conclusions
In this study, we found that early epithelialization and regeneration of the dermal layer was achieved after the application of CEA, suggesting that CEA could be an effective option after curettage or abrasion of GCMN.
Keywords: Giant congenital melanocytic nevus, Curettage, Cultured epidermis, Dermal regeneration
Abbreviations: GCMN, giant congenital melanocytic nevi; CMN, congenital melanocytic nevi; CEA, cultured epithelial autograft
Highlights
-
•
Cultured epithelial autograft (CEA) application is effective in the treatment of giant congenital melanocytic nevi.
-
•
The grafted CEA takes well on skin defects post curettage and abrasion.
-
•
CEAs can achieve rapid epithelization.
-
•
Normal dermal tissue regenerates after CEA application.
1. Introduction
Cultured epidermal autograft (CEA) is a cultivated keratinocyte sheet prepared using Green's method [1]. This development was a breakthrough in regenerative medicine and has been used for the coverage of burn wounds [2], partial thickness skin donor sites [3], and leg ulcers [4]. It has been reported that the take rate after CEA application is greatly influenced by the wound bed [5] and is reportedly low in full thickness skin defects, such as granulation tissue, adipose tissue, and fascia without dermis component [[6], [7], [8]]. Therefore, it is believed that the dermal component is essential for CEA application. Besides, wound infection and/or bacterial contamination reduce CEA take rate.
In 2016, CEA (JACEⓇ, Japan Tissue Engineering Co., Ltd. [J-TEC], Gamagori, Japan) was approved for the treatment of patients with Giant congenital melanocytic nevi (GCMN) in Japan. Congenital melanocytic nevi (CMN) are birthmarks resulting from the abnormal growth of cutaneous melanocytes and are reported to affect approximately 0.5%–31.7% of newborns [9]. GCMN are defined as CMN >20 cm in the greatest diameter in adults or ≥6 cm on the body or ≥9 cm on the head of neonates [10]. Larger CMNs are associated with an increased risk of malignant transformation to melanoma. It has been reported that malignant transformation presents before puberty or in childhood [11,12]. Therefore, treatment is targeted at effective removal of as many melanocytes as possible at an early age to decrease the risk of malignant transformation [13]. The most curative treatment is complete resection of a nevus lesion; however, the complete removal of a GCMN is impracticable in many cases due to the lack of skin for reconstruction.
In such cases, CEA is a good treatment option because it involves less donor site morbidity. As full thickness skin resection prevents CEA take, CEA is applied on the remaining dermal component after the superficial nevus tissue is partially resected as in curettage. In 1987, Moss reported that curettage is a surgical option to treat GCMN in the first few weeks of life [14]. With this technique, the superficial layer of GCMN can be easily removed on the cleavage plane using a curette. The application of CEA on partial thickness skin defects after curettage enables rapid epithelialization with less hypertrophic scar formation [15]. The wound surface after curettage of GCMN is an ideal recipient site for CEA application because the dermis component remains and infection or contamination is absent. Although few case reports have shown the effectiveness of CEA application for GCMN treatment [16,17], neither long-term follow-up nor histopathological evaluation after CEA application has been performed. Therefore, herein, we report the evaluation of our cases treated with CEA application along with the analysis of their long-term follow-up and histopathological evaluation.
In this study, we summarized the clinical course of seven consecutive operations in five patients treated with CEA in our hospital. We assessed the CEA take at early stage, color change and hypertrophic scar formation at six months after the operation in all the seven treated lesions, and histology of the biopsies taken from the treated area over one year after the CEA application in four lesions to evaluate the regeneration of epidermis and dermis.
2. Methods
2.1. Patients
We treated seven lesions of five patients with GCMN using CEA from April 2016 to March 2018 at Kyoto University Hospital. All surgeries were performed by the same surgeon. This study was approved by the Ethics committee of Kyoto University Graduate School and Faculty of Medicine (permit no. R2806), and conducted in accordance with the principles mentioned in the Declaration of Helsinki and its later amendments. Written informed consent was obtained from the patients or their legal guardians. Data on age, sex, anatomical location of nevi, surgical procedure, and the number of CEA used for coverage are summarized in Table 1.
Table 1.
Detailed patient characteristics.
| Op# | Age | Sex | Location | Modality | CEA (sheets) | Observational period (months) | Hospital stay (days) | Erosion/ulcer formation | VSS | Color improvement | Biopsy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 y | M | Left ear (front) | Curettage, TPS | 1 | 39 | 17 | + | 0 | Good | − |
| 2 | 1 y | M | Left ear (back) | Curettage, TPS | 1 | 36 | 15 | + | 0 | Good | − |
| 3 | 3 m | F | Head | Curettage | 2 | 40 | 17 | + | 0 | Excellent | + |
| 4 | 2 y | F | Left buttock | Curettage | 1 | 34 | 19 | − | 2 | Fair | + |
| 5 | 2 y | F | Left thigh | Curettage, TPS Dermatome shaving |
2 | 31 | 15 | − | 4 | Good | + |
| 6 | 3 m | F | Forehead | Curettage, TPS | 2 | 30 | 14 | + | 4 | Good | + |
| 7 | 24 y | F | Right shoulder | Dermatome shaving | 8 | 20 | 14 | + | 6 | Fair | − |
TPS: TPS System for electric surgical bar, CEA: cultured epithelial autografts, VSS: Vancouver scar scale, m: months, y: year, F: female, M: male, Op#: operation number.
Operation #1 and 2 were performed on the same patient as were operation #4 and 5.
2.2. Preparation of CEA (JACEⓇ)
A small skin biopsy, approximately 1–2 cm2 in size, was harvested to prepare the CEA (JACEⓇ) from an intact skin apart from the nevi site more than three weeks before the operation. The skin was sent to a tissue culture laboratory of J-TEC, and the CEA was prepared using Green's method [1,18] with some modifications. The CEA was backed with a 10 cm × 8 cm carrier, aseptically packaged, and delivered to our hospital on the day of the operation.
2.3. Surgical procedure
The superficial layer of a nevus was partially removed by curettage using a sharp curette or by abrasion using an electric dermatome (Zimmer® Dermatome AN; Zimmer Biomet, Warsaw, USA) or an electrical surgical bar (TPS system; Stryker, Kalamazoo, USA) until the color of the target nevus was removed macroscopically. However, full thickness resection was avoided to preserve the deeper part of the dermis as a recipient wound bed for CEA application, even if nevus tissue obviously remained. After hemostasis by electrocoagulation and irrigation with normal saline solution, the CEA was applied onto the skin defects. The applied CEA was covered with a non-adhesive wound dressing (UrgoTul®; Urgo Medical, Paris, France) as a contact layer. This dressing was fixed using a skin stapler or surgical suture followed by a tie-over dressing (Supplement data 1). The tie-over dressing was kept for seven days unless accidental contamination or infection was observed. At the first dressing change on day 7, all of the dressing, including the contact layer, was removed for all patients. The wound was subsequently covered by UrgoTul® and vaseline gauze, and the dressings were changed daily or every other day. All the patients stayed in the hospital for at least two weeks after the operation; patients were discharged when >95% of the wounds were epithelialized and patients or their parents learned the dressing change technique.
2.4. Evaluation of the wounds
The following parameters were evaluated in all seven lesions: take ratio of CEA, erosion and/or ulcer formation in the acute phase, hospitalization days, Vancouver scar scale (VSS), and color improvement in late phase. VSS is a scar assessment tool consisting of four parameters: Pigmentation (0–2), Vascularity (0–3), Pliability (0–5), and Height (0–3). The maximum score of 13 corresponds to the most severe hypertrophic scar [19]. Histopathological examinations were performed in four lesions to evaluate the regeneration of the epidermis, dermis, and residual nevus cells over one year after surgery.
The CEA take was assessed on day 7. Erosion or ulceration was observed in the treated area after complete epithelialization was achieved. VSS and color improvement for the treated area were assessed from the photos taken one year (allowance of 11–13 months) after the operation. Color improvement was assessed using the following four grades, according to a previous study [20]: excellent: identical to uninvolved skin; good: marked improvement; fair: slight improvement; and poor: no improvement.
The assessments of CEA take, VSS, and color improvement were performed by two independent plastic surgeons. When there was a difference of opinion between the two surgeons, consensus was reached through discussion.
Skin biopsies were harvested over one year (12–26 months) after the operation in four lesions, and hematoxylin and eosin (HE) stained sections were prepared.
Additionally, immunohistochemically stained sections with anti-Sox10 antibody and anti-melan A antibody were prepared in one lesion (Op#3). Sox10 and melan A were used to recognize cells of melanocytic differentiation. Morphology of the epidermal layer, rete ridge formation, dermis regeneration, and remaining nevus cells were evaluated in HE stained sections, and nevus cells were confirmed in the immunostained sections.
3. Results
3.1. Patients
Seven lesions in five patients (aged 3 months to 24 years) were treated (Table 1). Fig. 1 highlights the case presentation of the patient who underwent Op#3. The follow-up period was 20–40 months (mean: 32.8 months). Regarding the resection modality, curettage was adopted in six lesions. When curettage was ineffective, an electric surgical bar (TPS) and dermatome shaving were utilized in four and two lesions, respectively. CEA was applied on the wound surface with no difficulty in all of the lesions and the number of CEA used was decided according to the size of the implantation area, reaching a maximum of eight sheets.
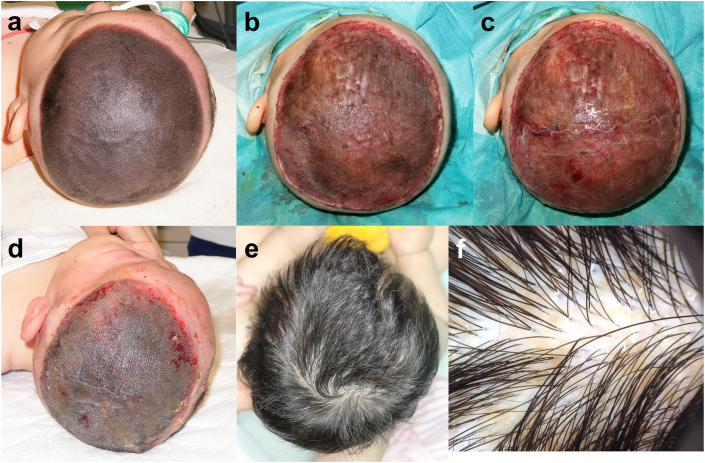
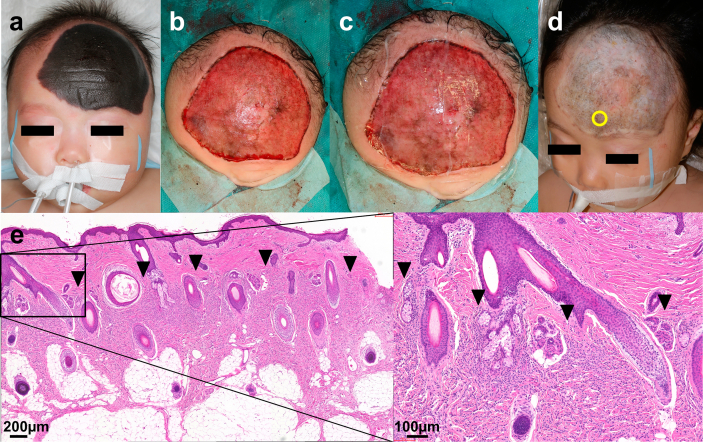
Fig. 1.
Clinical course of Operation #3. The nevus was 13 cm × 11 cm in size and had dense hair. (a) Pre-operation (the patient's hair was clipped). (b) After curettage. The central part was removed with curettage and the peripheral region was dermabraded with TPS. (c) CEA was applied on the wound bed after curettage. (d) Seven days after the operation. The CEA took well and the wound epithelialized. (e) One year after the operation. The patient's hair had grown without any retardation. (f) Dermoscopic view at 1 year after the operation. Only slight pigmentation was observed. CEA: cultured epidermal autograft.
3.2. Surgical procedure
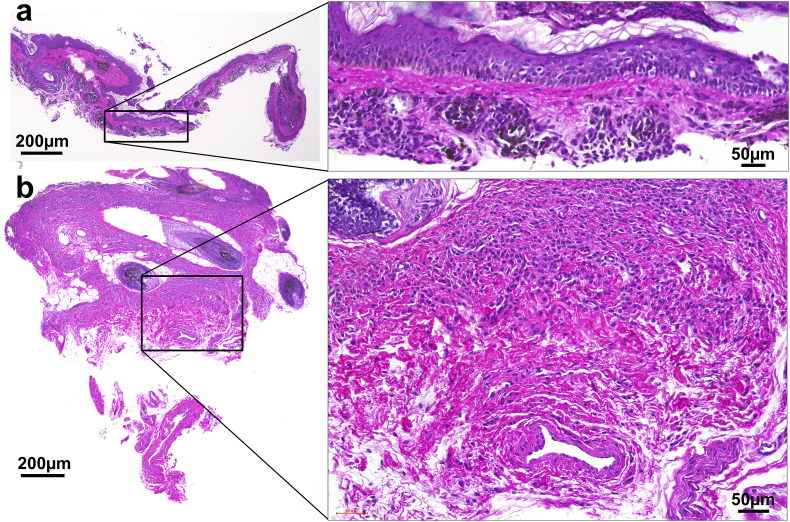
Figure 2 shows the HE stained sections of biopsies harvested from the curetted specimen and the wound bed after curettage in Op#3. The resected specimen by curettage was approximately 0.3–0.4 mm in thickness, when measured in this specimen, and composed of the entire epidermis and the superficial part of the upper dermis, which contained crowded nevus cell nests and melanin pigmentation. The deep part of reticular dermis, including hair follicles, remained in the wound bed, and a large number of nevus cell nests also remained. CEA was applied onto the exposed dermis layer as shown in Fig. 2.
Fig. 2.
Hematoxylin and eosin stained sections of biopsies harvested from the curetted specimen and the wound bed after curettage. (a) The curetted specimen. The epidermis and the superficial layer of the dermis, approximately 0.3 mm in thickness, were removed with curettage. (b) The wound bed after curettage. The nevus cell nests remained in the wound bed. Cultured epidermis was applied on the top of the wound.
3.3. Postoperative course
In the first week after the operation, there was minimal wound exudate and dressing changes were unnecessary. Upon removal of the initial dressing on day 7, we judged that the CEA application took well in >95% of the areas, patients were without complications, such as graft loss, allergy, and local infection, and initial epithelialization was achieved.
After epithelialization was achieved, the treated area was covered with gauze and bandages for protection from accidental injury. Nevertheless, erosion and/or ulcer formation occurred in five cases between two weeks to three months after CEA implantation. The erosion and/or ulcer healed with conservative treatment with Vaseline or wound dressings. Hospitalization days ranged from 14 to 19 days (mean: 15.9 days). Upon patient discharge, most of the treated lesions were epithelialized, even though small erosions or ulcers remained in some cases.
In Operations 5 and 6 (Op#5 and #6), the scar was slightly pigmented and hard, and the VSS score was 4 (Pigmentation, 2; Pliability, 2). In Op#7 of an adult case, an obvious scar with an irregular and rugged surface formed to make the VSS score 6 (Vascularity, 1; Pliability, 4; Height, 1). As to the color improvement evaluated one year after the operations, one, four, and two lesions were graded as excellent, good, and fair, respectively.
3.4. Histopathological evaluation
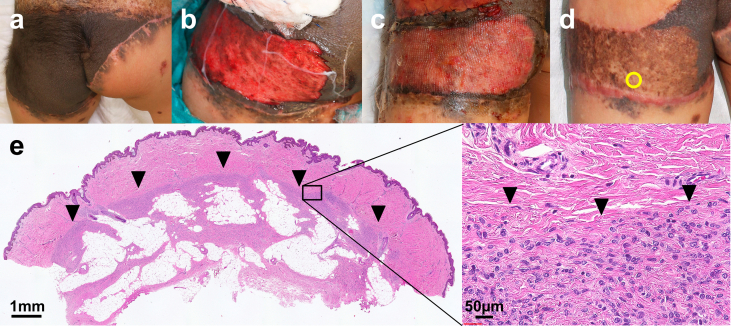
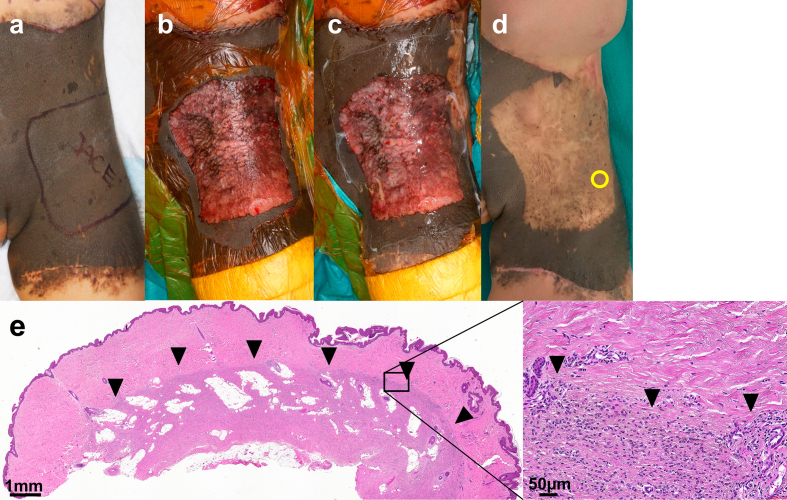
A biopsy of the scar was harvested from four lesions (Op#3, 4, 5, 6) between 13 and 26 months after the operation (Supplement data 2–4). In all of the biopsied lesions, the epidermis was well regenerated with stratified keratinocytes and rete ridges, and no subepidermal bulla was observed. The superficial dermal tissue regenerated upon the remaining deep dermal layer containing nevus cell nests.
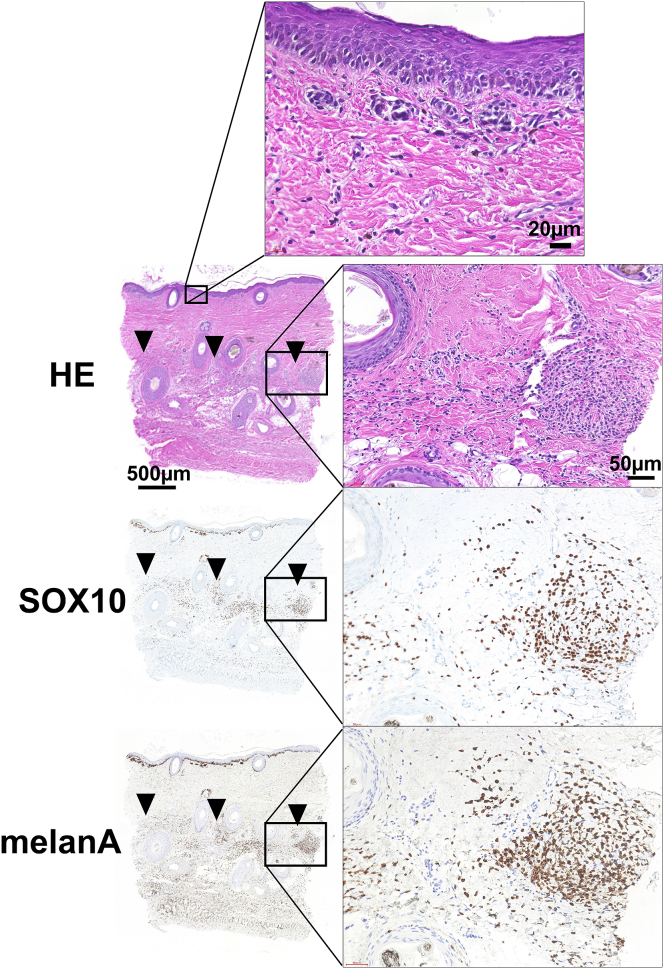
The regenerated superficial dermal tissue was composed of mature collagen bundles and contained neither nevus cell migration nor melanin deposition. As a result, a clear boundary was observed dividing the upper part of the regenerated dermis without nevus cells and the deeper part of the remaining dermis with a large number of nevus cells. In some cases, remaining nevus cells and melanin pigmentation were also observed at dermo-epidermal junction. Fig. 3 shows the HE or immunochemically stained (anti-Sox10 or anti-melan A) sections of the skin biopsy from the treated area with curettage and CEA grafting, harvested one year after the operation.
Fig. 3.
Hematoxylin and eosin stained and immunohistochemically stained sections of the scar biopsy harvested 1 year after the curettage and cultured epidermal autograft application. The epidermis was composed of well stratified keratinocytes with rete ridge formation. The dermis with dense collagen fibers regenerated in the superficial layer. Black arrowheads indicate the remaining nevus cell nests. The nevus cells were immunohistochemically positive for SOX10 and melan-A.
3.5. Long-term follow-up
During the follow-up period, no malignant change was observed in any of the treated lesions.
3.6. Case presentation (Op#3)
The patient who underwent Op#3 had a GCMN (13 cm × 11 cm) on her head from birth. The nevus was dark black in color and had dense hair (Fig. 1a). We performed partial resection of her nevi with CEA application at the age of three months. Most of the nevi (specifically, central part of the nevi excluding the peripheral lesion of approximately 2 cm width) was partially removed using the curettage technique (Fig. 1b). Biopsies were harvested from the curetted material and the remaining wound bed to investigate the curettage layer (Fig. 2). The other peripheral lesion of the nevi underwent dermabrasion with an electric surgical bar (TPS) because the nevus tissue was rigid and could not be removed by curettage in this area. CEA was applied onto the wound surface (Fig. 1c), covered with UrgoTul® as a contact layer, and fixed with a skin stapler. Subsequently, the wound was covered with wet cotton gauze and fixed with a bandage.
The dressing was removed seven days later. The applied CEA took well on the wound, and the entire wound was epithelialized (Fig. 1d). However, thick slough developed on the entire healed scalp soon and caused cracks throughout the slough. We removed the slough with scissors, thus leaving erosions, which healed over the course of several days. The healed area never generated another slough, and her hair grew without any retardation.
At one year after the operation, no hypertrophic scar formation was observed (VSS score: 0) and re-pigmentation was minimal (color improvement: excellent) (Fig. 1e). Dermoscopic observation is shown in Fig. 1f. No alopecia developed, and the hair shaft had intact appearance without hair fragility or growth cycle disorder. We obtained a skin biopsy from the area treated with CEA one year after the operation.
4. Discussion
The take rate of CEA has been reported to be unstable. In burns patients, factors such as improper wound bed preparation, CEA placement timing, and wound infections are reported to be contributors to CEA failure [21]. Conversely, the wound surface after curettage of GCMN is suitable for CEA application because the dermis remains and infection or contamination is absent. Previous studies have reported successful CEA application; prompt epithelialization was achieved on the wound surface after curettage or abrasion with a YAG Laser [15] and after curettage and Q-switched Ruby Laser Irradiation [16]. Similar to these reports, CEA application was successful in all the seven lesions treated in this study. At day 7, the applied CEA took well and epithelialization was achieved in >95% of the area in all the lesions. However, the newly formed epidermis was fragile and subject to injury, leaving erosions and/or ulcers. It has been reported that an applied CEA differentiates into normal epidermal strata but lacks rete ridges by six days postgrafting; however at 6–12 months, the applied CEA develops rete ridges and a neodermis with normal stromal and vascular organization [22]. Besides, the fragility after CEA application is caused by the immature functional status of the regenerating dermo-epidermal junction; basement membrane formation and full maturation of anchoring fibrils requires more than one year to achieve [23]. In our cases, this fragility spontaneously ameliorated and the frequency of ulceration gradually reduced in the three months after surgery. One year after the CEA application, histopathological evaluation revealed that the generated epidermis was composed of firmly stratified keratinocytes with well generated rete ridges and epidermal-dermal connection was matured with no subepidermal bulla, which was consistent with the clinical durability of regenerated epidermis.
Regarding the dermis, a mature collagen layer with sufficient thickness was observed in the superficial layer of all the biopsied lesions. It is supposed that this dermal layer regenerated on the wound bed after curettage and CEA application. Moreover, nevus cells and melanin deposition were rarely seen in the regenerated superficial dermis, but numerous pigmented nevus cell nests remained in the deeper layer. Thus, the regenerated dermal layer covered the remaining pigmented nevus cells, and, therefore, could contribute to the clinical improvement in color of GCMN after treatment with curettage and CEA application. The mechanism whereby CEA influences dermal regeneration is unclear.
Besides, in some cases, a small number of nevus cells was observed at dermo-epidermal junction. As the nevus cell nests at superficial layer were initially resected by curettage, it is supposed that these recurrent nevus cells were derived from deeper part, i.e., the remained nevus cells around the deep part of hair follicles might migrate and redistribute to superficial layer with migrating keratinocytes.
This study highlights the potential of CEA as one of the coverage methods of skin defects after GCMN treatment. The CEA applied on the wounds after curettage and dermabrasion of GCMN took well on the wound, enabled early epithelialization, and decreased wound exudation and local infections without complications. These clinical advantages would allow for a deeper curettage or dermabrasion to be performed in cases with subsequent CEA application. On the other hand, although the reduction of the number of nevus cells may reduce the risk of malignancy [24], the possibility of malignant transformation from the remaining nevus cells in deep layer remains. Therefore, this treatment should be considered only when complete resection of the entire nevus without severe cosmetic morbidities is impractical. Besides, long-term follow-up is necessary after this treatment and CEA has drawbacks such as extensively high cost and delay of three weeks for autograft cultivation. As to the limitation of this study, we did not show statistical significance of the effectiveness of CEA application because of the small number of cases. Large-scale examinations of the functional and cosmetic long-term outcomes of CEA application should be conducted in future studies.
5. Conclusion
In this study, we found that CEA took well on the wound after curettage and abrasion. Additionally, early epithelialization and regeneration of the dermal layer was achieved after CEA application, suggesting that CEA could be an effective option after curettage or abrasion of GCMN.
Declaration of competing interest
The authors (MS and NM) were financially supported by Japan Tissue Engineering Co., Ltd. (J-TEC) for their collaborating research. However, the company did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We deeply thank Editage (http://www.editage.jp/) for their assistance in language editing of our manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2021.02.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
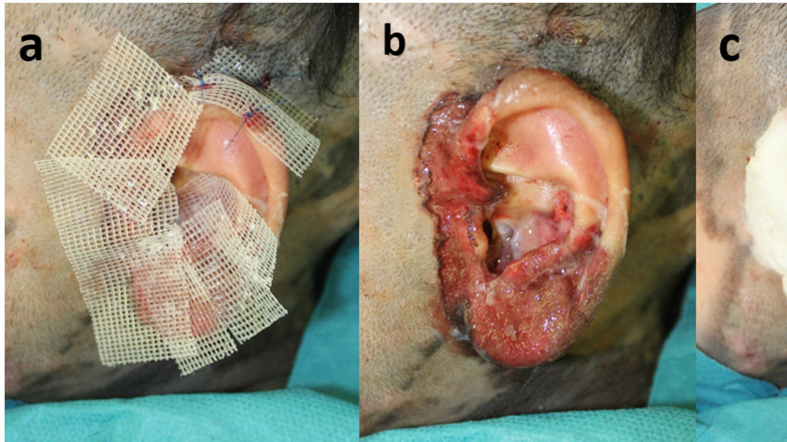
Supplement 1: Dressing method. (a) After curettage and cultured epidermal autograft application. (b) The applied CEA was covered with a non-adhesive wound dressing as a contact layer. (c) CEA covered with a non-adhesive wound dressing was fixed using surgical suture followed by a tie-over dressing.
figs2.
Supplement 2: Operation #4 in a 2-year-old female. (a) Pre-operation. (b) After curettage and cultured epidermal autograft application. (c) Seven days after the operation. The wound had completely epithelialized. (d) One year after the operation. Spotty re-pigmentation is observed. The yellow circle indicates the biopsy site. (e) Hematoxylin and eosin stained section of the specimen harvested from the scar 1 year after curettage and CEA application. Black arrowheads indicate the remaining nevus cell nests.
figs3.
Supplement 3: Operation #5 in a 2-year-old female (the same patient with Operation #4 shown in Supplement 1). (a) Pre-operation. (b) After curettage and dermabrasion with TPS. (c) After cultured epithelial autograft application. (d) Thirteen months after the operation. Spotty re-pigmentation was observed. The yellow circle indicates the biopsy site. (E) Hematoxylin and eosin stained section of the specimen harvested from the scar 13 months after CEA application. Black arrowheads indicate the remaining nevus cell nests.
figs4.
Supplement 4: Operation #6 in a 3-month-old female. (a) Pre-operation. (b) After curettage and dermabrasion with TPS. (c) After cultured epithelial autograft application. (d) Twenty-five months after the operation. Spotty re-pigmentation was observed. The yellow circle indicates the biopsy site. (e) Hematoxylin and eosin stained section of the specimen harvested from the scar 25 months after CEA application. Black arrowheads indicate the remaining nevus cell nests.
References
- 1.Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979;76:5665–5668. [Google Scholar]
- 2.Gallico G.G., O'Connor N.E., Compton C.C., Kehinde O., Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 3.Madden M.R., Finkelstein J.L., Staiano-Coico L., Goodwin C.W., Shires G.T., Nolan E.E. Grafting of cultured allogenic epidermis on second and third degree burn wounds on 26 patients. J Trauma. 1986;26:955–962. doi: 10.1097/00005373-198611000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hefton J.M., Caldwell D., Biozes D.G., Balin A.K., Carter D.M. Grafting of skin ulcers with cultured autologous epidermal cells. J Am Acad Dermatol. 1986;14:399–405. doi: 10.1016/s0190-9622(86)70048-0. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura H., Matsushima A., Ueyama M., Kumagai N. Application of the cultured epidermal autograft "JACE®" for treatment of severe burns: results of a 6-year multicenter surveillance in Japan. Burns. 2016;42:769–776. doi: 10.1016/j.burns.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Odessey R. Addendum: multicenter experience with cultured epidermal autograft for treatment of burns. J Burn Care Rehabil. 1992;13:174–180. [PubMed] [Google Scholar]
- 7.Sood R., Balledux J., Koumanis D.J., Mir H.S., Chaudhari S., Roggy D. Coverage of large pediatric wounds with cultured epithelial autografts in congenital nevi and burns: results and technique. J Burn Care Res. 2009;30:576–586. doi: 10.1097/BCR.0b013e3181ac02de. [DOI] [PubMed] [Google Scholar]
- 8.Cirodde A., Leclerc T., Jault P., Duhamel P., Lataillade J.J., Bargues L. Cultured epithelial autografts in massive burns: a single-center retrospective study with 63 patients. Burns. 2011;37:964–972. doi: 10.1016/j.burns.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Alikhan A., Ibrahimi O.A., Eisen D.B. Congenital melanocytic nevi: where are we now? Part I. Clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67 doi: 10.1016/j.jaad.2012.06.023. 495.e1-17;quiz 512-4. [DOI] [PubMed] [Google Scholar]
- 10.Kopf A.W., Bart R.S., Hennessey P. Congenital nevocytic nevi and malignant melanomas. J Am Acad Dermatol. 1979;1:123–130. doi: 10.1016/s0190-9622(79)70009-0. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence C.M. Treatment options for giant congenital naevi. Clin Exp Dermatol. 2000;25:7–11. doi: 10.1046/j.1365-2230.2000.00560.x. [DOI] [PubMed] [Google Scholar]
- 12.Belysheva T.S., Vishnevskaya Y.V., Nasedkina T.V., Emelyanova M.A., Abramov I.S., Orlova K.V. Melanoma arising in a giant congenital melanocytic nevus: two case reports. Diagn Pathol. 2019;14:21. doi: 10.1186/s13000-019-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu C., Wang X.X., Luo X., Liu F., Zhou X.Y., Yang J. An alternative strategy treated giant congenital melanocytic nevi with epidermis and superficial dermis of the lesions. Medicine (Baltim) 2018;97 doi: 10.1097/MD.0000000000009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss A.L. Congenital "giant" naevus: a preliminary report of a new surgical approach. Br J Plast Surg. 1987;40:410–419. doi: 10.1016/0007-1226(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 15.Whang K.K., Kim M.J., Song W.K., Cho S. Comparative treatment of giant congenital melanocytic nevi with curettage or Er:YAG laser ablation alone versus with cultured epithelial autografts. Dermatol Surg. 2005;31:1660–1667. doi: 10.2310/6350.2005.31305. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto N., Kakudo N., Kako A., Nishimura K., Mitsui T., Miyake R. A case report of the first application of culture epithelial autograft (JACE®) for giant congenital melanocytic nevus after its approval in Japan. J Artif Organs. 2018;21:261–264. doi: 10.1007/s10047-017-1007-0. [DOI] [PubMed] [Google Scholar]
- 17.Maeda T., Morimoto N., Kakudo N., Kusumoto K. Efficacy of cultured epithelial autograft after curettage for giant melanocytic nevus of the head. Plast Reconstr Surg Glob Open. 2018;6 doi: 10.1097/GOX.0000000000001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rheinwald J.G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan T., Smith J., Kermode J., Mclver E., Courtemanche D.J. Rating the burn scar. J Burn Care Rehabil. 1990;11:256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Yanai A., Fukuda O., Soyano S., Takayama O., Kataigi T. Argon laser therapy of port-wine stains: effects and limitations. Plast Reconstr Surg. 1985;75:520–527. doi: 10.1097/00006534-198504000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Sood R., Roggy D., Zieger M., Balledux J., Chaudhari S., Koumanis D.J. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patinets: the Indiana University experience. J Burn Care Res. 2010;31:559–568. doi: 10.1097/BCR.0b013e3181e4ca29. [DOI] [PubMed] [Google Scholar]
- 22.Compton C.C. Cultured epithelial autografts: skin regeneration and wound healing. A long-term biopsy study. Skin Res. 1996;38:148–159. [Google Scholar]
- 23.Compton C.C., Gill J.M., Bradford D.A., Regauer S., Gallico G.G., O'Connor N.E. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989;60:600–612. [PubMed] [Google Scholar]
- 24.Viana A.C., Gontijo B., Bittencourt F.V. Giant congenital melanocytic nevus. An Bras Dermatol. 2013;88:863–878. doi: 10.1590/abd1806-4841.20132233. [DOI] [PMC free article] [PubMed] [Google Scholar]