Abstract
Objective
While the molecular events controlling insulin secretion from β-cells have been documented in detail, the exact mechanisms governing glucagon release by α-cells are understood only partially. This is a critical knowledge gap, as the normal suppression of glucagon secretion by elevated glucose levels fails in type 2 diabetes (T2D) patients, contributing to hyperglycemia through stimulation of hepatic glucose production. A critical role of glycolytic flux in regulating glucagon secretion was supported by recent studies in which manipulation of the activity and expression of the glycolytic enzyme glucokinase altered the setpoint for glucose-suppression of glucagon secretion (GSGS). Given this precedent, we hypothesized that genetic activation of glucokinase specifically in α-cells would enhance GSGS and mitigate T2D hyperglucagonemia.
Methods
We derived an inducible, α-cell-specific glucokinase activating mutant mouse model (GckLoxPGck∗/LoxPGck∗; Gcg-CreERT2; henceforth referred to as “α-mutGCK”) in which the wild-type glucokinase gene (GCK) is conditionally replaced with a glucokinase mutant allele containing the ins454A activating mutation (Gck∗), a mutation that increases the affinity of glucokinase for glucose by almost 7-fold. The effects of α-cell GCK activation on glucose homeostasis, hormone secretion, islet morphology, and islet numbers were assessed using both in vivo and ex vivo assays. Additionally, the effect of α-cell GCK activation on GSGS was investigated under diabetogenic conditions of high-fat diet (HFD) feeding that dysregulate glucagon secretion.
Results
Our study shows that α-mutGCK mice have enhanced GSGS in vivo and ex vivo, independent of alterations in insulin levels and secretion, islet hormone content, islet morphology, or islet number. α-mutGCK mice maintained on HFD displayed improvements in glucagonemia compared to controls, which developed the expected obesity, glucose intolerance, elevated fasting blood glucose, hyperinsulinemia, and hyperglucagonemia.
Conclusions
Using our novel α-cell specific activation of GCK mouse model, we have provided additional support to demonstrate that the glycolytic enzyme glucokinase is a key determinant in glucose sensing within α-cells to regulate glucagon secretion. Our results contribute to our fundamental understanding of α-cell biology by providing greater insight into the regulation of glucagon secretion through α-cell intrinsic mechanisms via glucokinase. Furthermore, our HFD results underscore the potential of glucokinase as a druggable target which, given the ongoing development of allosteric glucokinase activators (GKAs) for T2D treatment, could help mitigate hyperglucagonemia and potentially improve blood glucose homeostasis.
Keywords: Islet, α-Cell, Glucokinase, GCK, Glucagon, GSGS
Abbreviations: T2D, Type 2 Diabetes Mellitus; GCG, glucagon; CreERT2, tamoxifen-inducible Cre recombinase-estrogen receptor fusion protein; GCK, wild-type glucokinase gene; Gck∗, Gck mutant allele containing ins454A activating mutation; α-mutGCK, GckLoxPGck∗/LoxPGck∗ Gcg-CreERT2; GSIS, glucose-stimulated insulin secretion; GSGS, glucose-suppression of glucagon secretion; EdU, Ethynyl deoxyuridine; CgA, Chromogranin A; HFD, high-fat diet; GKAs, glucokinase activators
Highlights
-
•
Inducible and cell type-specific point mutation in glucokinase enables analysis of glucose suppression of glucagon secretion.
-
•
Glycolytic flux through glucokinase determines the set-point for glucagon secretion in pancreatic α-cells.
-
•
Pancreatic α-cells are a physiologically relevant target of glucokinase activator drugs.
1. Introduction
Maintaining blood glucose homeostasis is a central metabolic challenge in mammals and is regulated mainly by the opposing but coordinated action of glucagon-producing α-cells and insulin-producing β-cells within the endocrine pancreas [1]. Disturbances in this regulatory network cause metabolic disorders, such as type 2 diabetes mellitus (T2D), whose prevalence, comorbidities, and medical costs constitute a significant public health concern [2,3]. Although the defining physiological abnormalities underlying T2D are an inappropriately low insulin secretory response to elevated glucose levels and insulin resistance, patients with T2D also exhibit hyperglucagonemia, which exacerbates hyperglycemia through stimulation of hepatic glucose production [4,5]. Over the past few decades, there have been significant advances in our understanding of β-cell stimulus-secretion coupling, particularly in the cellular pathways involved in controlling glucose-stimulated insulin secretion (GSIS) and insulin action on its target cells [6]. However, the molecular mechanisms underlying dysregulated glucagon secretion in T2D remain poorly understood.
Recently, the contribution of elevated glucagon levels to hyperglycemia in T2D individuals has regained attention, due in part to the reported glucagon-suppressive effects of antidiabetic agents, such as dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists [7,8]. In addition, ablation of glucagon receptor signaling by administration of glucagon receptor antagonists has been shown to improve glycemic control in several animal models of diabetes [9,10]. Collectively, these studies have led to investment in the development of agents targeting inhibition of glucagon secretion or action [[11], [12], [13]]. However, there is still no consensus regarding the relative contribution of nutrients, hormones, and cellular pathways to the regulation of glucagon secretion from α-cells, with multiple fundamentally different mechanisms having been suggested [14,15]. For instance, autocrine regulation of glucagon secretion in α-cells by glucagon itself, as well as by glutamate that is co-released with glucagon, have been proposed to potentiate α-cell secretory activity by raising cAMP levels or [Ca2+]i, respectively [16,17]. Additionally, paracrine control of glucagon release in α-cells by neighboring β-cells and δ-cells is thought to also play a critical role. Thus, insulin, Zn2+, and GABA, all secreted by β-cells, have been reported to suppress glucagon secretion by hyperpolarizing α-cells and lowering [Ca2+]i [[18], [19], [20], [21]]. Lastly, a combination of insulin, as well as somatostatin – secreted by δ-cells – has likewise been proposed as a paracrine mediator of glucagon release by two independent signaling mechanisms that lower α-cell cAMP and decrease PKA phosphorylation [22].
Some studies have been carried out in recent years to investigate the role of glucokinase in glucose-suppression of glucagon secretion (GSGS). First, Basco et al. showed that mice with α-cell-specific ablation of GCK have impaired GSGS – which supports a direct effect of glucose levels in regulating glucagon secretion via cell-autonomous signaling mechanisms [23]. These authors concluded that defects in glucokinase expression or enzymatic activity may contribute to the aberrant glucagon secretion observed in T2D individuals, and that additional characterization of α-cell glucokinase activity and its regulation in diabetic conditions could provide novel insights into ameliorating T2D hyperglucagonemia. Second, a recent study by Moede et al. demonstrated that manipulation of glucokinase activity and expression in cultured single rat α-cells resulted in an altered glucose threshold for glucagon release, further strengthening the role of glucokinase in α-cell intrinsic glucose regulation [24]. While these studies have highlighted a link between α-cell glucokinase and regulation of glucagon secretion, it remains unknown whether genetic activation of glucokinase may enhance GSGS in vivo and mitigate hyperglucagonemia. In our work, we have addressed this question using a novel mouse model that permits inducible, α-cell-specific glucokinase activation to fully understand the α-cell-intrinsic role of glucokinase under both normal conditions and diabetogenic conditions of high-fat diet (HFD) feeding.
2. Materials and methods
2.1. Mice
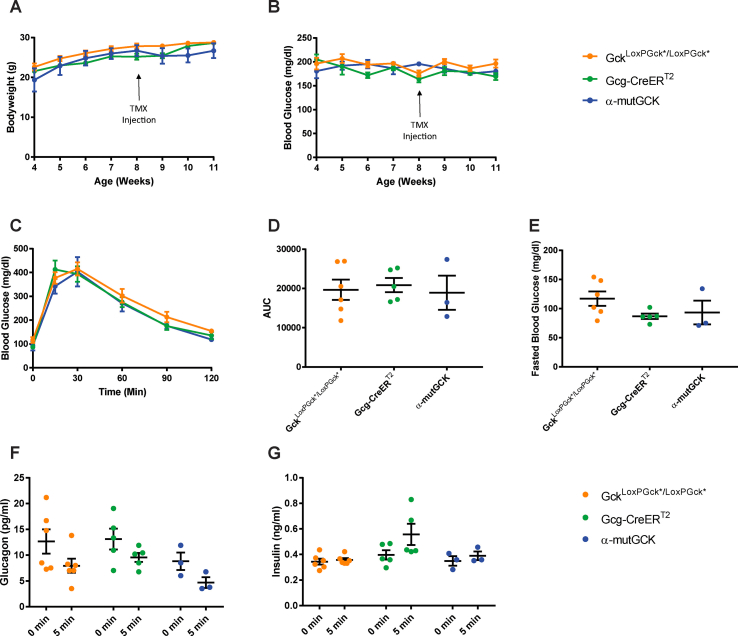
The derivation of the GckLoxPGck∗ and Gcg-CreERT2 alleles have been reported previously [25,26]. α-mutGCK were homozygous for the GckLoxPGck∗ allele. All mice were maintained on a mixed genetic background and housed on a standard 12-h light/12-h dark cycle with ad libitum access to food and water. Bodyweight and ad libitum blood glucose levels were measured weekly beginning at 4 weeks of age. To induce Cre activity, tamoxifen (Sigma–Aldrich #T5648) at 50 μg/g bodyweight was administered to 8-week-old mice via 3 intraperitoneal (i.p.) injections at 24-h intervals. In vivo metabolic studies were conducted two weeks following the final tamoxifen injection to ensure sufficient α-cell-specific genetic modification. At this time, any intestinal L- and K-cells that may have undergone Cre-mediated mutation of Gck have been replaced by wild-type cells continuously being produced in the intestinal crypt [26]. All animal procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
2.2. Glucose tolerance and insulin tolerance tests
For glucose tolerance tests, mice underwent a 16-h overnight fast and then received an i.p. injection of 1 mg/g bodyweight of d-glucose in sterile phosphate-buffered saline (PBS). Measurements of blood glucose were taken at 0, 15, 30, 60, 90, and 120 min post-injection using an automatic glucometer (Zoetis). The area under the curve was calculated using GraphPad PRISM software (v.7.0), with the time zero glucose measurement as the baseline. For assessment of glucose-stimulated insulin secretion (GSIS) and glucose-suppression of glucagon secretion (GSGS), blood was collected from the tail vein at 0- and 5-min post-injection and placed on dry ice with aprotinin (Sigma Aldrich #A6279; final concentration: 0.167 mg/ml) to prevent glucagon degradation. Plasma insulin and glucagon concentrations were quantified using enzyme-linked immunosorbent assay (ELISA, Crystal Chem #90080; Crystal Chem #81518).
For insulin tolerance tests, mice underwent a 4-h fast and then received an i.p. injection of 0.75 U/kg bodyweight of insulin. Measurements of blood glucose were taken at 0, 15, 30, 60, and 90, min post-injection using an automatic glucometer (Zoetis). To assess glucagonemia under conditions of insulin-induced hypoglycemia, blood was collected from the tail vein at 0 and 30 min post-injection, and glucagon concentrations were quantified as described above.
2.3. Islet isolation and culture
Mouse islets were isolated using published protocols [27]. Islet isolation required ductal inflation of the pancreas followed by digestion with collagenase (Roche #11213873001) at 37 °C. Islets were then enriched via density gradient centrifugation with Ficoll–Paque (GE 45-001-751) and underwent 3 rounds of hand-picking to separate islets from exocrine tissue. Islets were cultured for three days prior to static batch incubations. The culture media consisted of RPMI 1640 (11 mM glucose; Thermo #21870076) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate (Thermo #11360070), 10 mM HEPES, and 1% antibiotic antimycotic reagent (ThermoFisher Scientific #15240096). The pH of the culture media was adjusted to 7.3–7.4.
2.4. Static batch incubation
Batches of 15 size-matched islets were pre-incubated in Krebs buffer containing 9 mM glucose for 1 h, washed and re-incubated with 9 mM glucose, followed by incubations with varying concentrations of glucose for a period of 30 min each, in the presence of a physiological 4 mM amino acid mixture. At the end of the experiment, islets were incubated with 30 mM KCl to confirm islet viability. After each incubation, islets were centrifuged, and supernatants removed and stored at −80 °C with aprotinin (Sigma Aldrich #A6279; final concentration: 0.025 mg/ml) to prevent glucagon degradation. Finally, the islets were lysed using NP-40 lysis buffer (0.005% NP-40; pH 8.8) for total hormone content analysis. Static batch incubation studies were performed in triplicate. Insulin and glucagon concentrations were quantified using ELISA (Crystal Chem #90080; Crystal Chem #81518).
2.5. Immunofluorescent analysis
Pancreata were dissected, fixed in 4% paraformaldehyde (PFA) overnight at 4 °C, embedded in paraffin, and cut into 6-μm sections. To obtain the maximal footprint from each mouse pancreas, three consecutive sections spaced 250 μm apart from each other were stained. Slides were deparaffinized in xylene and rehydrated through a series of ethanol washes. Slides were subjected to antigen retrieval for 2 h in 10 mM citric acid buffer (pH 6.0). Ethynyl deoxyuridine (EdU) labeling was employed to detect cells that had entered or completed S-phase. EdU (Thermo # E10187) was supplied continuously for 7 days in drinking water at a concentration of 1 mg/ml. EdU+ cells were labeled using the Click-iT EdU Alexa Fluor 647 imaging kit (Invitrogen #C10640) according to the manufacturer's instructions. Sections were blocked using CAS-Block (Invitrogen #008120), and primary antibodies were diluted in CAS-Block and applied overnight at 4 °C. Slides were washed in PBS and incubated with the appropriate secondary antibodies diluted in CAS-Block for 2-h at RT. Primary and secondary antisera information is provided in Supplementary Tables 1 and 2, respectively. Hoechst 33342 (Thermo #H3570) was used to counterstain nuclei. Staining was visualized using a Keyence BZ-X800 fluorescence microscope, and images were analyzed using FIJI software. α-cell proliferation was calculated as the number of EdU+, glucagon+ cells normalized to the total number of glucagon+ cells. α-cell percentage was calculated as the number of CgA+, glucagon+ cells normalized to the number of CgA+ cells. These sections were also co-stained for insulin to assess β-cell proliferation and β-cell percentage using analogous methods.
2.6. HFD studies
Baseline metabolic measurements of GTT, GSIS, and GSGS were performed in 9- to 10-week-old male mice. One week following the metabolic measures, these mice were switched to HFD (60% fat/kcal; Research Diets #D12492) or maintained on standard chow for 16 weeks. After 16 weeks, metabolic measurements of GTT, GSIS, and GSGS were obtained to validate the effect of HFD feeding on glucose tolerance and hormone secretion. One week following these metabolic measures, mice were administered tamoxifen via 3 i.p. injections at 24-h intervals to induce Cre activity. Mice continued the same diet for an additional 4 weeks, after which metabolic measurements of GTT, GSIS, and GSGS were performed.
2.7. Data analysis
Data are presented as mean ± SEM and were analyzed using GraphPad Prism software (v.7.0). Statistical analysis was performed using an unpaired two-tailed Student's t-test when two groups were compared, one-way analysis of variance (ANOVA) with post hoc Bonferroni test when more than two groups were compared, and two-way ANOVA with post hoc Bonferroni test when two conditions were involved. Values are considered statistically significant when p < 0.05.
3. Results
3.1. Determination of the effects of GCK activation in α-cells on glucose homeostasis in vivo
To determine the consequences of GCK activation on GSGS, we generated an α-cell specific glucokinase mutant mouse that was homozygous for the mutant GCK allele (GckLoxPGck∗/LoxPGck∗; Gcg-CreERT2; henceforth referred to as “α-mutGCK”). In this model, the wild-type glucokinase gene is conditionally replaced with a glucokinase mutant allele containing the ins454A-activating mutation (Gck∗), previously demonstrated to increase the affinity of glucokinase for glucose by almost 7-fold and lower the threshold for glucose-stimulated insulin secretion (GSIS) [28]. Mice homozygous for the GckLoxPGck∗ allele but lacking the Cre transgene served as controls. Mice with only the Gcg-CreERT2 allele, previously demonstrated to exhibit comparable pancreatic glucagon mRNA expression, pancreatic glucagon protein levels, and plasma glucagon levels relative to wild-type mice, were also utilized as controls [26]. Gcg-CreERT2 was previously shown to be highly effective at recombining loxP-flanked targets in α-cells, with YFP expression in 95% of pancreatic α-cells of Gcg-CreERT2; Rosa-LSL-YFP mice following tamoxifen administration [26].
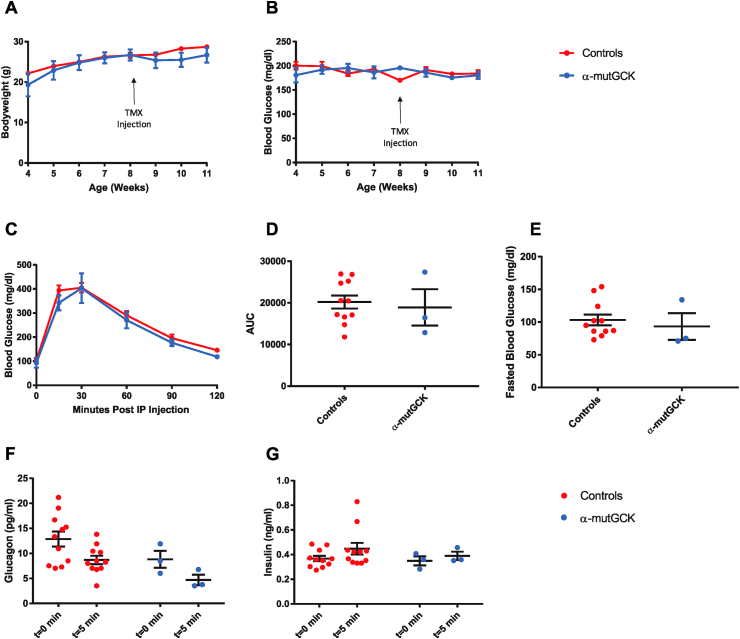
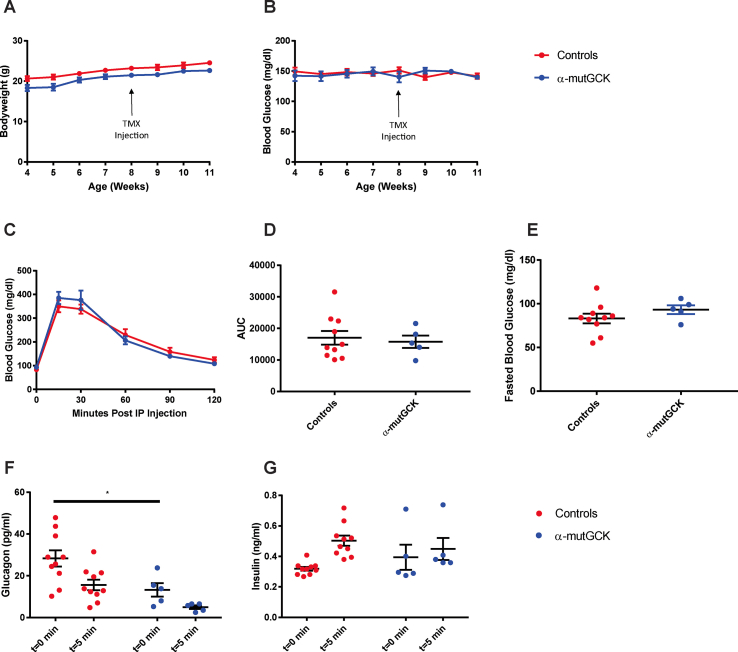
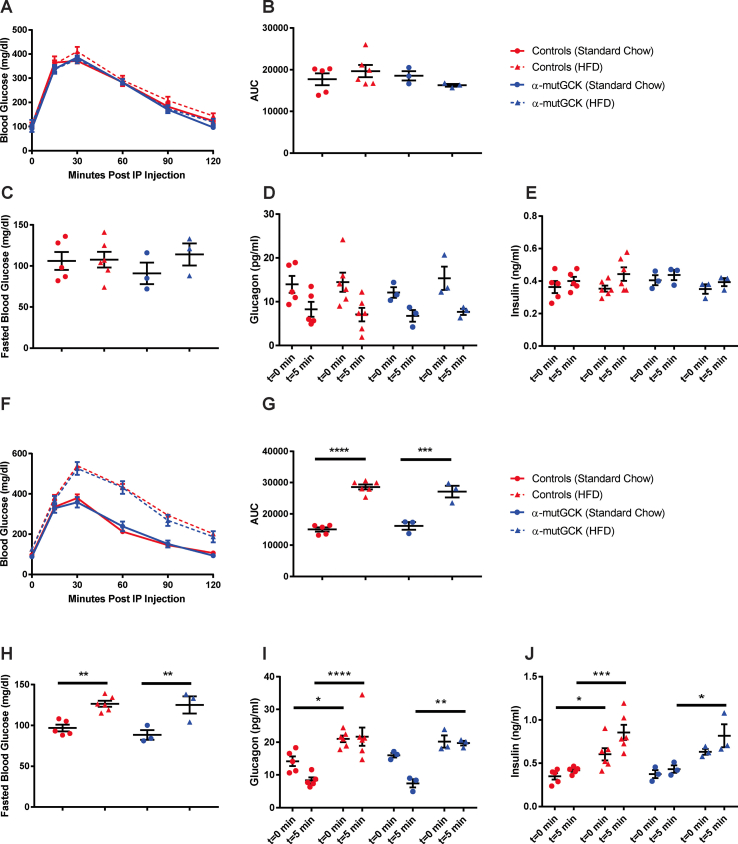
α-mutGCK and control mice displayed similar body weight and ad libitum blood glucose levels pre- and post-tamoxifen administration (Figure 1A, B for male mice and Supplemental Figure 1A, B for female mice). Intraperitoneal (i.p.) glucose tolerance tests conducted 2–3 weeks after the final tamoxifen injection revealed no differences between α-mutGCK and control mice (Figure 1C for male mice and Supplemental Figure 1C for female mice), as also confirmed by area under the curve (AUC) analysis (Figure 1D for male mice and Supplemental Figure 1D for female mice). Measurements of fasted blood glucose levels prior to i.p. glucose administration similarly revealed no significant differences (Figure 1E for male mice and Supplemental Figure 1E for female mice). Assessment of glucagonemia during the glucose tolerance test showed a trend toward the hypothesized reduction in glucagon levels pre- and post-i.p. glucose administration in α-mutGCK mice relative to control mice (Figure 1F for male mice and Supplemental Figure 1F for female mice), supporting a role for glycolytic flux through glucokinase in α-cells in the control of glucagon secretion. As expected from an α-cell specific activation of GCK, insulin levels during the GTT showed no significant differences between α-mutGCK and control mice (Figure 1G for male mice and Supplemental Figure 1G for female mice).
Figure 1.
Determination of the effects of α-cell GCK activation on glucose homeostasis in adult male mice. (A) Body weights of α-cell GCK mutant and control mice (n = 4 for α-mutGCK, n = 11 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (B)Ad libitum blood glucose for α-cell GCK mutant and control mice (n = 4 for α-mutGCK, n = 11 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (C) Intraperitoneal glucose tolerance test (1 g/kg bodyweight) (n = 3 for α-mutGCK, n = 11 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (D) Area under the curve (AUC) and (E) fasted blood glucose measurements from intraperitoneal glucose tolerance test in (C). (F) Plasma glucagon and (G) plasma insulin in mice fasted or 5 min after glucose injection in (C).
Of note, in the above experiments, we pooled GckLoxPGck∗/LoxPGck∗ mice and Gcg-CreERT2 mice into one “control” group due to their similar phenotypes. The results from these experiments conducted in male mice, which are illustrated in Figure 1, with separated control groups for GckLoxPGck∗/LoxPGck∗ mice and Gcg-CreERT2 mice can be found in Supplemental Figure 5. From this point onward, the “control” group will continue to pool together data from both GckLoxPGck∗/LoxPGck∗ mice and Gcg-CreERT2 mice.
3.2. α-Cell GCK activation enhances glucose-suppression of glucagon secretion ex vivo
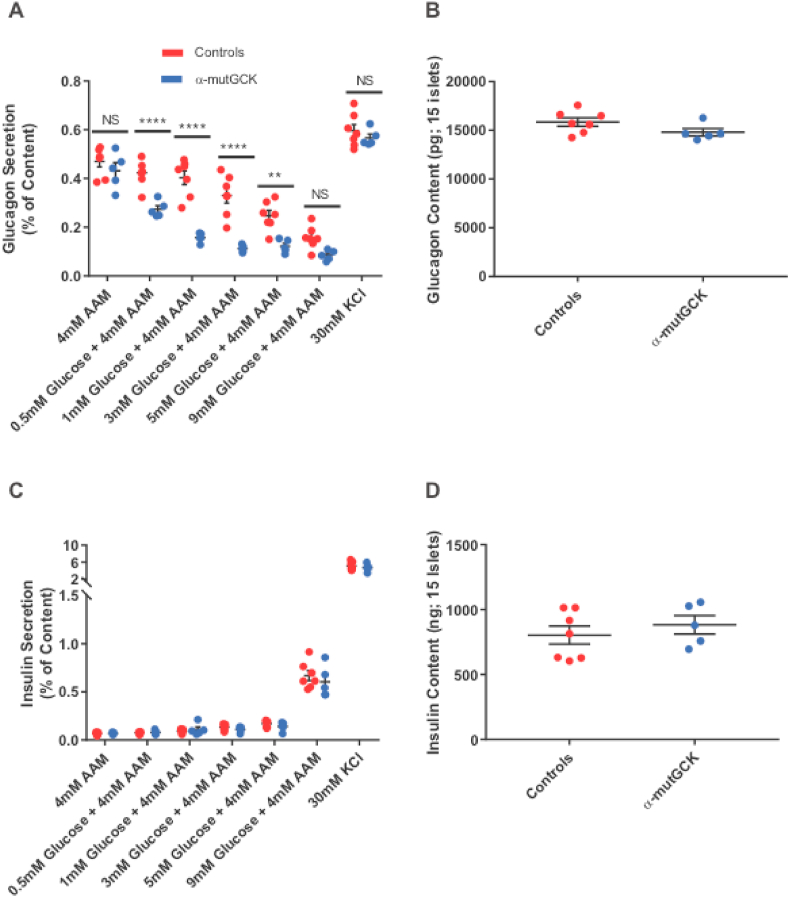
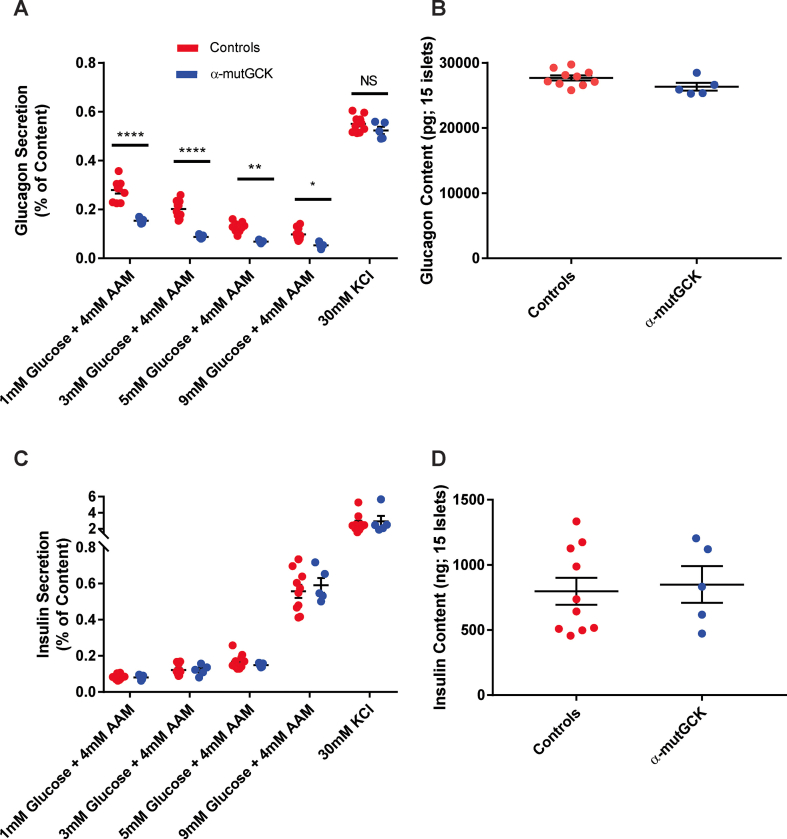
Next, we performed static batch incubation experiments in isolated islets to assess hormone secretion in response to varying concentrations of glucose, while maintaining islets in a medium containing physiological 4 mM amino acid levels. Our data show that when incubated with 1 mM, 3 mM, and 5 mM glucose, α-mutGCK islets displayed a significant decrease in glucagon secretion relative to control islets, indicating a left-shift of the dose response curve for glucose suppression of glucagon secretion (Figure 2A for male mice and Supplemental Figure 2A for female mice). There was also a downward trend of glucagon secretion at 9 mM glucose, although this was not statistically significant in male mice. Depolarization of islets with 30 mM KCl in the absence of glucose resulted in a similar secretory response across all groups, indicating that basic electrical stimulus-secretion coupling in α-cells remained unchanged. Additionally, the changes in islet glucagon secretion occurred independently of alterations in islet glucagon content, further confirming the functional integrity of α-mutGCK islets relative to control islets (Figure 2B for male mice and Supplemental Figure 2B for female mice). Given the proposed role of insulin as a paracrine mediator of glucagon release [18,19], we wanted to rule out that these observed changes in glucagon secretion were a secondary consequence of altered insulin level within the islet. We found that the reduction of glucagon secretion in α-mutGCK islets ex vivo was independent of significant changes in glucose-stimulated insulin secretion and insulin content (Figure 2C, D for male mice and Supplemental Figure 2C, D for female mice).
Figure 2.
Activation of α-cell GCK enhances glucose-suppression of glucagon secretion (GSGS) in isolated pancreatic islets from male mice.(A) Glucagon secretion and (B) content from isolated pancreatic islets in the presence of the indicated glucose concentrations with 4 mM amino acid mixture or 30 mM KCl. (C) Insulin secretion and (D) content from isolated pancreatic islets in the presence of the indicated glucose concentrations with 4 mM amino acid mixture or 30 mM KCl. (∗∗p < 0.01, ∗∗∗∗p < 0.0001 vs. genetically unmodified control). Analysis by two-way ANOVA with post hoc Bonferroni test.
3.3. Activation of α-cell GCK impairs the counter-regulatory response under conditions of insulin-induced hypoglycemia
Next, to explore whether insulin-induced hypoglycemia, which is a strong stimulator of counterregulatory glucagon secretion, is also affected by altered glucose sensing in α-mutGCK mice, we performed i.p. insulin tolerance tests 2–3 weeks after the final tamoxifen injection. As shown in Figure 3, α-mutGCK mice recover slightly slower from hypoglycemia relative to control mice (Figure 3A for male mice). Intriguingly, the lower blood glucose levels following the insulin injection correlated with significantly lower glucagon levels in α-mutGCK mice relative to control mice 30 min post-injection (Figure 3B). Female mice showed similar responses (Figure 3C, D).
Figure 3.
Activation of α-cell GCK impairs the counter-regulatory response during an insulin tolerance test.(A) Intraperitoneal insulin tolerance test (0.75 U/kg body weight) in adult male mice (n = 5 for α-mutGCK, n = 7 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (B) Plasma glucagon in mice fasted or 30 min after insulin injection in (A). (C) Intraperitoneal insulin tolerance test in adult female mice (0.75 U/kg bodyweight) (n = 3 for α-mutGCK, n = 7 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (D) Plasma glucagon in mice fasted or 30 min after insulin injection in (C). (∗p < 0.05, ∗∗p < 0.01 vs. genetically unmodified control). Analysis by two-way ANOVA with post hoc Bonferroni test.
3.4. Enhanced GSGS occurs independently of changes in islet morphology and number
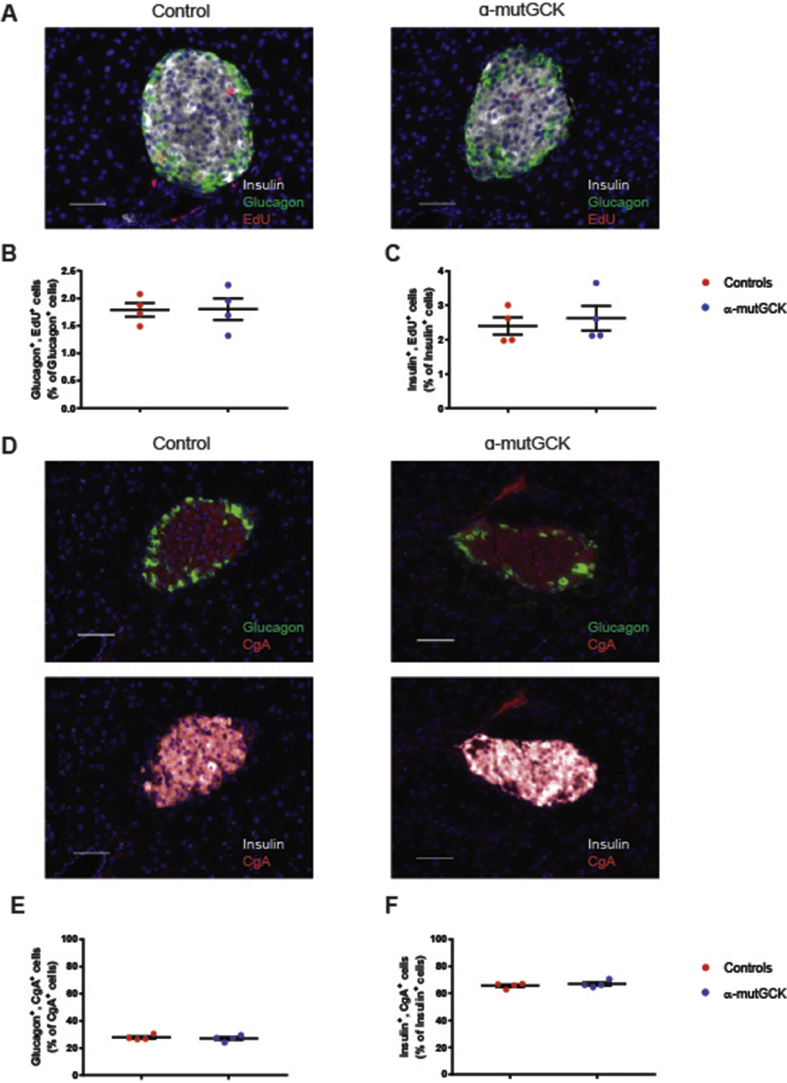
Next, we sought to determine whether changes in islet morphology and/or altered α-cell number could potentially account for the reduction in glucagon levels and secretion from α-mutGCK animals. Therefore, we performed immunofluorescent staining on pancreatic islet sections from α-mutGCK and control mice and quantified α-cell and β-cell proliferation using ethynyl deoxyuridine (EdU) labeling, and α-cell and β-cell percentage by co-staining with the pan-endocrine marker Chromogranin A (CgA). We observed no changes in the number of glucagon+, EdU+ cells, nor insulin+, EdU+ cells, in α-mutGCK islets relative to control islets, indicating no differences in α-cell and β-cell proliferation (Figure 4A–C). In addition, the number of glucagon+, CgA+ cells and insulin+, EdU+ cells were similar between α-mutGCK islets and control islets, signifying no differences in α-cell and β-cell percentage (Figure 4D–F). Taken together with the results from the in vivo and ex vivo measurements of hormone secretion, we demonstrate that the enhanced GSGS observed in α-mutGCK animals occurs independently of changes in islet morphology and α-cell numbers.
Figure 4.
Mice with activated α-cell GCK have normal islet morphology.(A) Insulin, glucagon, and EdU shown by co-immunofluorescence (40×). Scale bar: 50 μm. (B) Glucagon+, EdU+ cells as a percentage of total Glucagon+ cells (n = 4 for each genotype). (C) Insulin+, EdU+ cells as a percentage of total Insulin+ cells. (n = 4 for each genotype) (D) Insulin, glucagon, and CgA shown by co-immunofluorescence (40×). Scale bar: 50 μm. (E) Glucagon+, CgA+ cells as a percentage of total CgA+ cells (n = 4 for each genotype). (F) Insulin+, CgA+ cells as a percentage of total CgA+ cells (n = 4 for each genotype).
3.5. α-Cell GCK activation mitigates HFD-induced hyperglucagonemia
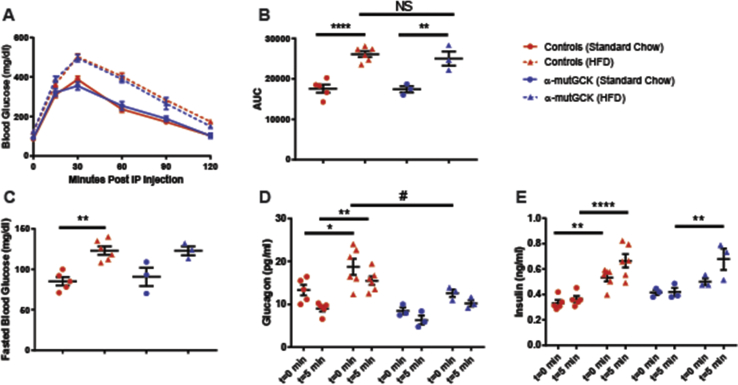
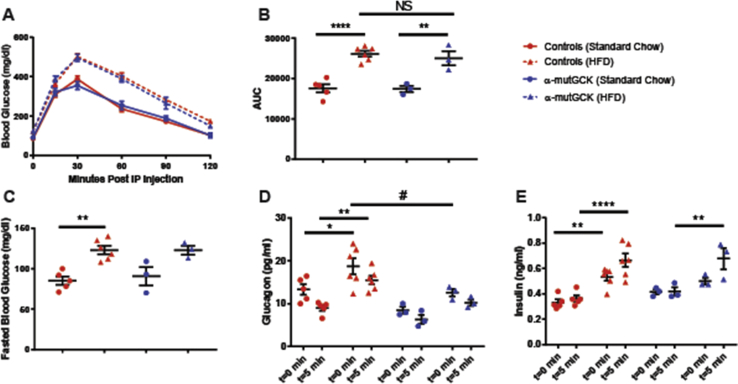
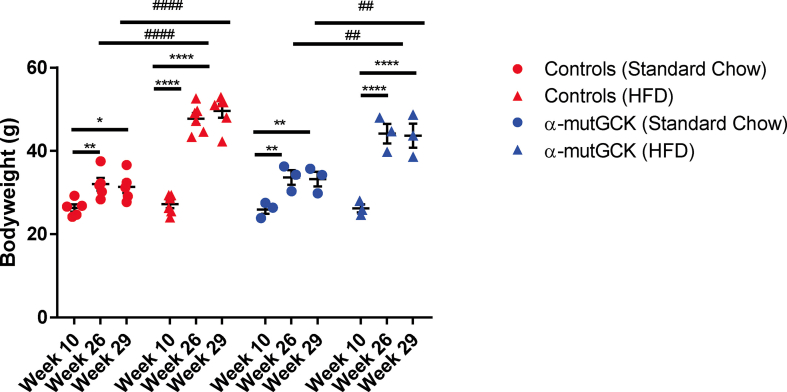
Next, we sought to determine whether α-cell GCK activation would be beneficial in a model of insulin resistance induced by an HFD feeding paradigm. We first placed α-mutGCK and control mice on 60% HFD for 16 weeks beginning at 10 weeks of age. A parallel group of mice was maintained on standard chow (SC). As expected, no differences were observed in glucose tolerance, fasting blood glucose, bodyweight, GSIS, and GSGS prior to the initiation of HFD (Supplemental Figures 3 and 4A–E). At the end of the 16 weeks, mice maintained on HFD developed obesity, glucose intolerance, elevated fasting blood glucose, hyperinsulinemia, and hyperglucagonemia relative to the SC-fed cohort (Supplemental Figures 3 and 4F–J). Following the 16 weeks of HFD feeding, we administered tamoxifen to all mice to induce Cre activity. In doing so, we observed an improvement in glucagonemia within α-mutGCK mice maintained on HFD relative to their genetically unmodified controls, highlighting a potential benefit of α-cell GCK activation on GSGS under diabetogenic conditions, although no significant effects were noted on bodyweight, glucose tolerance, fasted blood glucose, or hyperinsulinemia (Figure 5A–E, Supplemental Figure 3).
Figure 5.
α-Cell GCK activation mitigates HFD-induced hyperglucagonemia.(A) Intraperitoneal glucose tolerance test after α-cell GCK activation in HFD fed mice and SC controls (1 g/kg bodyweight) (HFD: n = 3 for α-mutGCK, n = 6 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2; Standard Chow Control: n = 3 for α-mutGCK, n = 5 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (B) Area under the curve (AUC) and (C) fasted blood glucose measurements for intraperitoneal glucose tolerance test in (A). (D) Plasma glucagon and (E) plasma Insulin in fasted or 5 min after glucose injection in (A). (∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001 vs. standard chow fed control; #p < 0.05 vs. genetically unmodified control). Analysis by two-way ANOVA with post hoc Bonferroni test.
4. Discussion and conclusions
Despite the fact that abnormal glucagon secretion contributes to the pathophysiology of T2D, the precise mechanisms regulating glucagon release from α-cells have remained elusive. Using our novel GCK activating mutation mouse model specifically targeting α-cells, we have shown conclusively that α-cell intrinsic glucose sensing via glucokinase is a key determinant of glucagon secretion. Although our findings do not exclude the possibility of additional forms of α-cell regulation, including paracrine, juxtracrine, and neuronal control (reviewed in [14]), our findings provide a significant contribution to our fundamental understanding of α-cell stimulus-secretion coupling.
Of note, while activation of GCK in pancreatic β-cells using the same point mutation caused a dramatic if transient increase in the proliferation rate [25], such an effect was not present in pancreatic α-cells (Figure 4A–C). Thus, while in rodent islets glycolytic flux, and thus workload, of β-cells represents a mitogenic stimulus, but this is not the case for α-cells. Instead, α-cell proliferation is stimulated dramatically by interruption of hepatic glucagon signaling, mediated by elevated plasma amino acids levels, in particular l-glutamine [29]. Thus, compensatory expansion of the two major endocrine cell types α- and β-cells appears to be an attempt to prevent super-physiologic levels of amino acids and glucose, respectively.
The preeminent role of glucokinase activity in regulating blood glucose homeostasis has made it an attractive pharmacological target for treatment of T2D patients. The potential of small molecule allosteric glucokinase activators (GKAs), previously demonstrated to lower the glucose S0.5 of glucokinase, have been investigated in their ability to improve glycemic control [30,31]. While existing data demonstrates the therapeutic benefit of GKAs on the β-cell (potentiating insulin secretion) and liver (increasing glycogen synthesis and decreasing hepatic glucose output) [32,33], our findings likewise suggest an additional benefit of these therapeutics in suppressing glucagon secretion from the α-cell. Of importance, the findings from our ex vivo batch incubation studies in α-mutGCK islets are consistent with results recently obtained from Moede et al., whereby modulation of glucokinase activity by pharmacological activators lowered the glucose threshold of glucagon release in single α-cells [24]. The results of α-cell GCK activation from our HFD feeding experiments also underscore the potential effect of these compounds on α-cell function by improving glucagonemia, which may provide additional benefits under conditions of insulin resistance. Given this precedent, evaluating α-cell function should be a key component of assessing allosteric glucokinase activators as treatment for T2D.
Although glucagon levels were noticeably reduced in α-mutGCK mice relative to genetically unmodified controls in our HFD feeding experiments, glucose tolerance and fasting blood glucose levels remained unchanged. These results suggest that, within rodents, the effects of insulin resistance within the HFD fed mice are dominant over the effects of glucagon. However, there are key differences in islet cell composition between human islets and rodent islets – notably, a much higher proportion of α-cells in human islets [34,35]. Consequently, it is possible that within humans, α-cells may have a larger role in blood glucose homeostasis.
Contributions
VB designed experiments, collected data, analyzed data, drafted the article, and revised the article. CLM conceived the study, designed experiments, and revised the article. AP collected data and approved the final version for publication. BG conceived the study and revised the article. KHK conceived the study, designed experiments, revised the article, and approved the final version for publication.
Acknowledgments
We would like to thank Dr. Franz Matschinsky for teaching us everything we know about glucokinase and for his continuous encouragement throughout this study. We thank the following core facilities at the University of Pennsylvania for their assistance: the Pancreatic Islet Cell Biology Core (supported by P30-DK19525) and the Molecular Pathology and Imaging Core (supported by P30-DK050306). We are grateful to the Transgenic and Chimeric Mouse Core of the University of Pennsylvania for generation of all transgenic lines (supported by NIH center grants P30DK050306, P30DK019525, and P30CA016520). We also thank Alanis Perez and Ashleigh Morgan for animal husbandry. This work was supported by the NIH T32 Predoctoral Training Grant in Pharmacology (T32GM008076) to VB and the Ruth L. Kirschstein National Research Service Award (NRSA) Individual Predoctoral Fellowship (F31 DK126231-01) to VB, and UC4 DK116271 to KHK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101193.
Contributor Information
Varun Bahl, Email: varunb@pennmedicine.upenn.edu.
Catherine Lee May, Email: catheril@pennmedicine.upenn.edu.
Alanis Perez, Email: Alanis.Perez@pennmedicine.upenn.edu.
Benjamin Glaser, Email: ben.glaser@mail.huji.ac.il.
Klaus H. Kaestner, Email: kaestner@pennmedicine.upenn.edu.
Conflicts of interest
The authors have no conflicts of interest to report.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Figure S1.
Determination of the effects of α-cell GCK activation on glucose homeostasis in adult female mice. (A) Body weights of α-cell GCK mutant and control mice (n = 5 for α-mutGCK, n = 10 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (B)Ad libitum blood glucose for α-cell GCK mutant and control mice (n = 5 for α-mutGCK, n = 10 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (C) Intraperitoneal glucose tolerance test (1 g/kg bodyweight) (n = 5 for α-mutGCK, n = 10 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (D) Area under the curve (AUC) and (E) fasted blood glucose measurements for intraperitoneal glucose tolerance test in (C). (F) Plasma glucagon and (G) plasma insulin in fasted or 5 min after glucose injection in (C). (∗p < 0.05 vs. genetically unmodified control). Analysis by two-way ANOVA with post hoc Bonferroni test.
Figure S2.
Activation of α-cell GCK enhances glucose-suppression of glucagon secretion (GSGS) in isolated pancreatic islets from adult female mice. (A) Glucagon secretion and (B) content from isolated pancreatic islets in the presence of the indicated glucose concentrations with 4 mM amino acid mixture or 30 mM KCl. (C) Insulin secretion and (D) content from isolated pancreatic islets in the presence of the indicated glucose concentrations with 4 mM amino acid mixture or 30 mM KCl. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001 vs. genetically unmodified control). Analysis by two-way ANOVA with post hoc Bonferroni test.
Figure S3.
HFD-induced obesity in male mice. Body weights of α-cell GCK mutant and control mice prior to HFD (week 10), immediately after HFD (week 26), and 2 weeks after the final tamoxifen injection (week 29) (HFD: n = 3 for α-mutGCK, n = 6 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2; Standard Chow Control: n = 3 for α-mutGCK, n = 5 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001 vs. week 10). (##p < 0.01, ####p < 0.0001 vs. standard chow fed control). Analysis by two-way ANOVA with post hoc Bonferroni test.
Figure S4.
HFD feeding induces hyperglucagonemia in male mice. (A) Intraperitoneal glucose tolerance test prior to HFD (1 g/kg body weight) (HFD: n = 3 for α-mutGCK, n = 6 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2; standard chow control: n = 3 for α-mutGCK, n = 5 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (B) Area under the curve (AUC) and (C) fasted blood glucose measurements for intraperitoneal glucose tolerance test in (A). (D) Plasma glucagon and (E) plasma insulin in fasted or 5 min after glucose injection in (A). (F) Intraperitoneal glucose tolerance test immediately after HFD (1 g/kg body weight) (HFD: n = 3 for α-mutGCK, n = 6 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2; Standard chow control: n = 3 for α-mutGCK, n = 5 total for GckLoxPGck∗/LoxPGck∗ and Gcg-CreERT2). (G) Area under the curve (AUC) and (H) fasted blood glucose measurements for intraperitoneal glucose tolerance test in (F). (I) Plasma glucagon and (J) plasma insulin in fasted or 5 min after glucose injection in (F). (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. standard chow fed control). Analysis by one-way or two-way ANOVA with post hoc Bonferroni test, as appropriate.
Figure S5.
Determination of the effects of α-cell GCK activation on glucose homeostasis in adult male mice (separated control groups from Figure 1). (A) Body weights of α-cell GCK mutant and control mice (n = 4 for α-mutGCK, n = 6 for GckLoxPGck∗/LoxPGck∗, and n = 5 for Gcg-CreERT2). (B)Ad libitum blood glucose for α-cell GCK mutant and control mice (n = 4 for α-mutGCK, n = 6 for GckLoxPGck∗/LoxPGck∗, and n = 5 for Gcg-CreERT2). (C) Intraperitoneal glucose tolerance test (1 g/kg body weight) (n = 3 for α-mutGCK, n = 6 for GckLoxPGck∗/LoxPGck∗, and n = 5 for Gcg-CreERT2). (D) Area under the curve (AUC) and (E) fasted blood glucose measurements from intraperitoneal glucose tolerance test in (C). (F) Plasma glucagon and (G) plasma insulin in mice fasted or 5 min after glucose injection in (C).
References
- 1.Gerich J.E., Charles M.A., Grodsky G.M. Regulation of pancreatic insulin and glucagon secretion. Annual Review of Physiology. 1976;38:353–388. doi: 10.1146/annurev.ph.38.030176.002033. [DOI] [PubMed] [Google Scholar]
- 2.Kahn S.E., Zraika S., Utzschneider K.M., Hull R.L. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52:1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P., Zhang X., Brown J., Vistisen D., Sicree R., Shaw J. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Research and Clinical Practice. 2010 doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Burcelin R., Knauf C., Cani P.D. Pancreatic alpha-cell dysfunction in diabetes. Diabetes & Metabolism. 2008;34(Suppl 2):S49–S55. doi: 10.1016/S1262-3636(08)73395-0. [DOI] [PubMed] [Google Scholar]
- 5.Unger R.H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 6.Rorsman P., Braun M. Regulation of insulin secretion in human pancreatic islets. Annual Review of Physiology. 2013;75:155–179. doi: 10.1146/annurev-physiol-030212-183754. [DOI] [PubMed] [Google Scholar]
- 7.Lund A., Bagger J.I., Christensen M., Knop F.K., Vilsbøll T. Glucagon and type 2 diabetes: the return of the alpha cell. Current Diabetes Reports. 2014;14:1–7. doi: 10.1007/s11892-014-0555-4. [DOI] [PubMed] [Google Scholar]
- 8.Nauck M.A., Vilsboll T., Gallwitz B., Garber A., Madsbad S. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care. 2009;32:S223–S231. doi: 10.2337/dc09-S315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Harte F.P.M., Franklin Z.J., Irwin N. Two novel glucagon receptor antagonists prove effective therapeutic agents in high-fat-fed and obese diabetic mice. Diabetes Obesity and Metabolism. 2014;16:1214–1222. doi: 10.1111/dom.12360. [DOI] [PubMed] [Google Scholar]
- 10.McShane L.M., Franklin Z.J., O'Harte F.P.M., Irwin N. Ablation of glucagon receptor signaling by peptide-based glucagon antagonists improves glucose tolerance in high fat fed mice. Peptides. 2014;60:95–101. doi: 10.1016/j.peptides.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Hædersdal S., Lund A., Knop F.K., Vilsbøll T. The role of glucagon in the pathophysiology and treatment of type 2 diabetes. Mayo Clinic Proceedings. 2018 doi: 10.1016/j.mayocp.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Jiang G., Zhang B.B. Glucagon and regulation of glucose metabolism. American Journal of Physiology-Endocrinology and Metabolism. 2003;284:E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 13.Sloop K.W., Michael M.D., Moyers J.S. Glucagon as a target for the treatment of Type 2 diabetes. Expert Opinion on Therapeutic Targets. 2005;9:593–600. doi: 10.1517/14728222.9.3.593. [DOI] [PubMed] [Google Scholar]
- 14.Gylfe E. Glucose control of glucagon secretion-‘There's a brand-new gimmick every year’. Upsala Journal of Medical Sciences. 2016;121:120–132. doi: 10.3109/03009734.2016.1154905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker J.N., Ramracheya R., Zhang Q., Johnson P.R.V., Braun M., Rorsman P. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obesity and Metabolism. 2011;13:95–105. doi: 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Zhang Y., Gromada J., Sewing S., Berggren P.O., Buschard K. Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors. Molecular Endocrinology. 2005;19:198–212. doi: 10.1210/me.2004-0059. [DOI] [PubMed] [Google Scholar]
- 17.Cabrera O., Jacques-Silva M.C., Speier S., Yang S.N., Köhler M., Fachado A. Glutamate is a positive autocrine signal for glucagon release. Cell Metabolism. 2008;7:545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin I., Gromada J., Gjinovci A., Theander S., Wollheim C.B. β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama H., Hisatomi A., Orci L., Grodsky G.M., Unger R.H. Insulin within islets is a physiologic glucagon release inhibitor. Journal of Clinical Investigation. 1984;74:2296–2299. doi: 10.1172/JCI111658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slucca M., Harmon J.S., Oseid E.A., Bryan J., Robertson R.P. ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes. 2010;59:128–134. doi: 10.2337/db09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rorsman P., Berggren P.O., Bokvist K., Ericson H., Möhler H., Ostenson C.G. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 22.Elliott A.D., Ustione A., Piston D.W. Somatostatin and insulin mediate glucose-inhibited glucagon secretion in the pancreatic α-cell by lowering cAMP. American Journal of Physiology. Endocrinology and Metabolism. 2015;308:E130. doi: 10.1152/ajpendo.00344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basco D., Zhang Q., Salehi A., Tarasov A., Dolci W., Herrera P. alpha-cell glucokinase suppresses glucose-regulated glucagon secretion. Nature Communications. 2018;9:546. doi: 10.1038/s41467-018-03034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moede T., Leibiger B., Sanchez P.V., Daré E., Köhler M., Muhandiramlage T.P. Glucokinase intrinsically regulates glucose sensing and glucagon secretion in pancreatic alpha cells. Scientific Reports. 2020;10 doi: 10.1038/s41598-020-76863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tornovsky-Babeay S., Weinberg-Corem N., Schyr R.B.H., Avrahami D., Lavi J., Feleke E. Biphasic dynamics of beta cell mass in a mouse model of congenital hyperinsulinism: implications for type 2 diabetes. Diabetologia. 2020 doi: 10.1007/s00125-021-05390-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackermann A.M., Zhang J., Heller A., Briker A., Kaestner K.H. High-fidelity glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Molecular Metabolism. 2017;6:236–244. doi: 10.1016/j.molmet.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Good A.L., Cannon C.E., Haemmerle M.W., Yang J., Stanescu D.E., Doliba N.M. JUND regulates pancreatic β cell survival during metabolic stress. Molecular Metabolism. 2019;25:95–106. doi: 10.1016/j.molmet.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayed S., Langdon D.R., Odili S., Chen P., Buettger C., Schiffman A.B. Extremes of clinical and enzymatic phenotypes in children with hyperinsulinism caused by glucokinase activating mutations. Diabetes. 2009;58:1419–1427. doi: 10.2337/db08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean E.D., Li M., Prasad N., Wisniewski S.N., Deylen A.V., Spaeth J. Interrupted glucagon signaling reveals hepatic α cell Axis and role for L-glutamine in α cell proliferation. Cell Metabolism. 2017;25:1362–1373. doi: 10.1016/j.cmet.2017.05.011. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matschinsky F.M. GKAs for diabetes therapy: why no clinically useful drug after two decades of trying? Trends in Pharmacological Sciences. 2013;34:90–99. doi: 10.1016/j.tips.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Matschinsky F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nature Reviews Drug Discovery. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 32.Matschinsky F.M., Wilson D.F. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Frontiers in Physiology. 2019;10:148. doi: 10.3389/fphys.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimsby J., Sarabu R., Corbett W.L., Haynes N.E., Bizzarro F.T., Coffey J.W. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 34.Cabrera O., Berman D.M., Kenyon N.S., Ricordi C., Berggren P.O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quesada I., Tudurí E., Ripoll C., Nadal Á. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. Journal of Endocrinology. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.