Abstract
Background
Military veterans living with post-traumatic stress disorder (PTSD) face significant physical and functional health disparities, which are often aggravated over time and in the context aging. Evidence has shown that physical activity can positively impact age-related health conditions, yet exercise trials in older adults with mental disorders are rare. Our study was a tailored and targeted pilot exercise intervention for older veterans with PTSD.
Methods
Fifty-four older veterans with PTSD (mean age = 67.4 years, 90.7% male, 85.2% non-white) were randomized to supervised exercise (n = 38) or wait-list usual care (n = 18) for 12 weeks. Physical activity (MET-min/wk) and aerobic endurance (assessed with the 6-minute walk test) were primary outcomes. Secondary outcomes were physical performance (strength, mobility, balance), cardiometabolic risk factors (eg, waist circumference), and health-related quality of life.
Results
At 12 weeks, a large effect of the intervention on physical activity levels (Cohen’s d = 1.37) was observed compared to wait-list usual care. Aerobic endurance improved by 69 m in the exercise group compared to 10 m in wait-list group, reflecting a moderate between-group effect (Cohen’s d = 0.50). Between-group differences on 12-week changes in physical performance, cardiometabolic risk factors, and health-related quality of life ranged from small to large effects (Cohen’s d = 0.28–1.48), favoring the exercise arm.
Conclusion
Participation in supervised exercise improved aerobic endurance, physical performance, and health-related clinical factors in older veterans with PTSD; a medically complex population with multiple morbidity. Group exercise is a low-cost, low-stigma intervention, and implementation efforts among older veterans with PTSD warrants further consideration.
Keywords: Metabolic syndrome, Health disparities, Mental health, Post-traumatic stress, Aerobic endurance
Post-traumatic stress disorder (PTSD) affects more than one-third of Vietnam-era veterans (1). These service members have endured 35–50 years with this chronic condition and experience an excess mortality rate three times higher than the general population (2,3). Veterans with PTSD also face decreased quality of life, obesity, and cardiovascular disease stemming from PTSD-related symptoms and unhealthy lifestyle factors (4–7). The idea that traumatic events can have a physical effect on people is not new. There is ample literature suggesting that traumatic stress starts a cascade of physiological and biological consequences (8–11), and diagnostic criteria for PTSD require that symptoms must also cause impairment (physical, social, emotional). Our group has previously shown that PTSD also accelerates functional decline, evidenced by impaired mobility, severe cardiovascular deconditioning, and muscle weakness in late life (6). To date, there has been little appreciation for the need to address these somatic comorbidities among older adults with chronic PTSD.
Exercise is a highly effective, pluripotent, and scientifically proven strategy for the prevention, treatment, and management of chronic physical and psychological health conditions (12–15). To date, only a few pilot studies of exercise and PTSD have been published (16–21), and nearly all had a major limitation: a singular focus on psychological outcomes. These studies do not measure the impact of exercise on physical health- and mobility-related outcomes that contribute to long-term impairment and disability in veterans with PTSD.
We are also unaware of any studies of exercise and PTSD conducted in older adults, representing a significant research gap. Exercise intervention is particularly relevant for older veterans, as they tend to be in poorer health with more comorbidities than their non-veteran counterparts (22). An emphasis on physical activity is consistent with both the values cultivated during military service as well as geriatric and recovery models of care that prioritize quality of life in the context of chronic disease. However, adherence to behavioral interventions in both the general population and patients with mental health disorders is a major issue. Veterans with PTSD engage in low levels of physical activity and spend much of their time in sedentary activities, adding to their risk of functional impairment (18,23,24).
Few studies of exercise and PTSD have been conducted, and among those studies, a majority focus on younger adults (17,19,20,25,26). These studies range in duration from 3 to 12 weeks and report moderate-to-large beneficial effects of exercise training on quality of life, PTSD and depression symptoms, sleep, and waist circumference. This literature also lacks reporting on functional and mobility outcomes, which is particularly important in the aged PTSD population who has demonstrated significant deconditioning and functional impairments reflective of accelerated aging (6,27). Only one of the aforementioned studies examined changes in physical function or clinical health indicators (eg, blood pressure) and reported small beneficial effects (20).
Studies of exercise for depression in older adults report that exercise sessions conducted in a group setting, and training programs that include both aerobic exercise and strength training, are the most effective treatments (vs home-based only, or resistance or aerobic training only) (13). Progressive, multicomponent exercise programs are also recommended for older adults and populations at risk for cardiometabolic disease (28). Given the high rate of comorbid depression, significant functional deficits, and clinical risk factors prevalent in this population, we adopted this program design to maximize impact among older adults with PTSD.
There is no reason to believe that the physiological benefit of exercise should be different in persons with PTSD compared to healthy individuals or those with other mental health conditions. However, psychological symptoms (eg, anxiety, depression), somatic symptoms (eg, pain, fatigue), side effects of psychotropic medications (eg, weight gain, sleep disturbance), and factors such as social avoidance and hyperarousal are challenges for many patients with PTSD. Owing to these comorbid challenges, results from lifestyle interventions in mentally healthy populations cannot be extrapolated to older populations with PTSD (29). This constellation of psychological and somatic symptoms in PTSD is also barrier to seeking mental health treatment (30). Exercise may have promise for attracting more veterans into care and improving overall health as a stand-alone or adjunctive behavioral treatment.
The objective of this study is to evaluate the effects of a tailored 12-week exercise intervention on physical function and cardiometabolic risk factors in older adults with PTSD. It was hypothesized that increased physical activity as well as improved physical performance and clinical health profiles would be observed among participants in the exercise group.
Methods
Study Design
The study used a randomized, two-group design comparing multicomponent exercise to usual care. A detailed description of study methods is published elsewhere (31), and CONSORT guidelines were used to guide study design and implementation. The institutional review board of the VA Medical Center in Durham, North Carolina, reviewed and approved this study. This exercise trial for older veterans with PTSD was registered in a public registry (ClinicalTrials.gov identifier NCT02295995).
Setting and Participants
Study eligibility criteria were (a) veteran status, (b) current diagnosis of PTSD, (c) 60 years or older, and (d) live within 50 miles of the VA medical center. All participants were screened for PTSD using the Clinician-Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V; CAPS-5) (32)), and only those who met diagnostic criteria were eligible to participate. Exclusions are described in detail elsewhere (31); in summary, these included hospitalizations for cardiovascular events in the past 3 months, current participation in PTSD-oriented therapy (ie, Prolonged Exposure or Cognitive Processing Therapy), significant cognitive impairment or dementia, and active substance abuse or dependence.
Participants were primarily recruited using Durham VA electronic health records to identify patients with PTSD. These patients were mailed an introductory letter describing the study and then screened by telephone to further assess eligibility. All participants who were eligible based on the screening call were scheduled for an in-person appointment with a study team member at the Durham VA to complete the consent process, additional screening assessments, and baseline testing.
Randomization
Group allocation was concealed from the participant and researcher until all baseline assessment were completed. Participants were randomized to supervised exercise or wait-list usual care (WL), using a 2:1 randomization scheme, respectively. The different sample ratios between the exercise arm and the usual care arm do minimally impact the power to detect between-group differences. However, we decided at the outset of this pilot study that this small loss in power (~5%) was balanced against the increased ability to (a) understand more deeply study issues, which may arise in a pilot of a new protocol, and (b) gather additional information on exercise participants. We used a simple randomization scheme, whereby individuals selected a slip of paper indicating “PA” (physical activity) or “UC” (usual care) from a sealed box.
Exercise Intervention
A detailed description of the exercise battery and programming elements is described elsewhere (31). Briefly, supervised exercise sessions were offered three times weekly (M,W,F) at a community-based fitness facility located in close proximity to the Durham VA Medical Center. The exercise program was multicomponent and included aerobic, muscle strengthening, flexibility, and balance training exercises. Modalities included body weight, free weight, cables, and exercise bands; treadmills and stationary bikes were used for aerobic training. The program aimed to have participants engaged in moderate-intensity exercise for at least 150 min/wk, in line with current recommendations (28).
Exercise sessions lasted 60–90 minutes depending on individual pace and prescription, but sessions were only offered between 9:00 and 10:30 am. Patients were provided an exercise card/log detailing their individualized exercise prescription, which was developed by the exercise physiologist following evaluations of functional status and patient-reported impairments. Individual prescription and progression were developed using American College of Sports Medicine guidelines and evaluated weekly by the exercise physiologist. The exercise leader worked one on one with participants during the introduction to the program (~2 weeks), after which participants were expected to exercise independently using the exercise prescription card, with occasional feedback and guidance from the instructor. The staff-to-participant ratio for the program ranged from 1:1 up to 1:5 and typically included two instructors. Although participants exercised in a common space, at a common time, each completed their own individual routine and did not participate in any formal, instructor-led group classes. Patients rated and recorded the intensity of effort on their exercise card, using the Borg CR10 Rating of Perceived Exertion Scale (33), a visual analog scale ranging from 0 (“rest”) to 10 (“maximal”). On this scale, the selected training intensity corresponded to a value of 3–5 (“moderate to hard”).
Wait-List Usual Care
Participants randomized to the WL group continued to receive the standard of care provided in their usual VA primary, women’s health, mental health, or geriatrics clinics. The control group did not receive exercise training. To reduce attrition, control group participants were offered the intervention after the trial; 9 of 17 WL completers chose to engage in supervised exercise at that time.
Measures
Assessments were completed in person at baseline and 12 weeks. All measures were administered by a trained research assistant at the Durham VA Medical Center, using standardized testing protocols. Study staff who conducted the baseline visit were blinded to participants’ randomization assignment until the end of the appointment. Owing to the nature of the study, neither participants nor researchers were blinded to treatment allocation during intervention delivery or outcome assessment. To maximize fidelity and reduce potential bias, both the researcher and the patient were blinded to previous results on testing, and research checklists and scripts were used to standardize testing procedures.
Screening Assessments
Trauma history/PTSD symptom screen
The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5) (34) was used during the phone screen as an initial indicator of trauma history and PTSD symptom prevalence over the past month. The PC-PTSD is a four-item screener, with one question reflecting each PTSD symptom cluster. Individuals who answered affirmatively to any of the questions were deemed eligible for further screening at the in-person baseline appointment. The PC-PTSD-5 has demonstrated excellent validity in military veterans (34).
Alcohol and substance abuse
The Alcohol Use Disorders Identification Test-Concise (AUDIT-C) questionnaire (35) was used to assess alcohol consumption. Total scores range from 0 to 12. Positive results for alcohol dependence included scores ≥3 for men and ≥2 for women (unless all points were from question 1: “How often do you have a drink containing alcohol?”). The Drug Abuse Screening Test (DAST-10) (36) questionnaire was used to assess recreational drug use (excluding alcohol and tobacco). Total scores range from 0 to 10, and positive results for substance abuse included scores ≥3. Both AUDIT-C and DAST-10 have demonstrated satisfactory reliability and validity (35,37).
PTSD diagnosis
The Clinician-Administered PTSD Scale for DSM-V (CAPS-5; 32) is the gold-standard diagnostic measure for PTSD. The CAPS-5 was only administered at baseline, as a diagnostic screening tool (yes/no PTSD). The CAPS-5 is a structured interview and was administered by trained research personnel to determine current (past month) diagnosis of PTSD. The CAPS-5 contains 20 items, corresponding to the DSM-V diagnosis for PTSD, and assesses symptom severity based on the frequency and intensity of the symptoms. Severity ratings range from 0 (“absent”) to 4 (“extreme/incapacitating”), with a score of 2 (moderate/threshold) indicating a clinically significant problem. PTSD symptom clusters include re-experiencing (Criterion B), avoidance (Criterion C), negative alterations in cognitions or mood (Criterion D), and hyperarousal (Criterion E). The DSM-V PTSD diagnostic rule requires (a) at least one Criterion B symptom; (b) at least one Criterion C symptom; (c) at least two Criterion D symptoms; (d) at least two Criterion E symptoms; (d) Criterion F is met (disturbance has lasted ≥1 month); and Criterion G is met (disturbance causes either clinically significant distress or functional impairment). Once a patient met criteria (severity rating ≥2) for a particular symptom cluster, the interviewer skipped to the next section/cluster of the CAPS-5. This means that a total score for the CAPS-5 was not calculated. The CAPS-5 has high internal consistency and inter-rater reliability (32).
Cognitive status
The Short Portable Mental Health Status Questionnaire (38) is a brief, 10-item screener that is widely used to detect cognitive impairment. A positive screen for mild cognitive impairment included ≥3 errors.
Participant characteristics
We collected patient-reported demographic and clinical characteristics, including age, sex, race/ethnicity, income, education level, and comorbid health conditions.
Primary outcomes
Physical activity levels were measured with the Aerobics Center Longitudinal Study Physical Activity Survey (39), a self-report measure of physical activity (eg, stair climbing, household activities, walking) and exercise habits (eg, weight training, swimming, treadmill) performed regularly (at least once a week) during the past 2 months. Participants were instructed to record the number of sessions per week, the average duration per session, and the average distance per session (as appropriate) for each activity. These data were then used to calculate metabolic equivalents of energy expenditure in reported activities (MET-min/wk).
Aerobic endurance, an indicator of cardiorespiratory fitness, was assessed with the 6-minute walk test (6MWT; 40), according to standard procedures. This is a self-paced assessment, and participants were instructed to walk as far and as fast as possible in 6 minutes, taking rests as necessary. The number of meters walked was recorded at baseline and 12 weeks. Greater distances walked reflect better aerobic endurance. This test has demonstrated excellent reliability and validity in older adults with chronic health conditions (41).
Secondary outcomes
We objectively assessed physical function using a battery of performance tests. Both rapid- and usual-pace gait speed (m/sec) were assessed using a 4-m walk, adapted from the Short Physical Performance Battery (42). Two timed trials of each condition were completed, with the best (fastest) time for each condition retained for analyses. Lower body strength was measured as the number of completed stands in 30 seconds, as described by Rikli and Jones (43). The 8-foot up-and-go test was used to measure balance and mobility, with the best time of two trials recorded for analyses. Balance was assessed using the time single leg stand test, which measures the time participants are able to stand unassisted on one leg up to 30 seconds with eyes open, as described by Yoshimura and coworkers (44). The performance tests were administered in a standardized sequence to minimize fatigue effects and maximize recovery between tests; tests were administered in the same order for all patients (balance, gait speed, chair stand, 8-foot up-and-go, and 6MWT).
Health-related quality of life was measured using the Medical Outcomes Study 36-item Short-Form Survey (SF-36; 45). The SF-36 is a widely used measure of general health that has been validated in diverse populations, is considered to be a reliable indicator of health status, and is sensitive to change. The SF-36 includes a Physical Health Component Score (PCS), which is the weighted sum of four subscales, including pain, physical function, general health, and role-physical. Total scores range from 0 to 100, with higher scores indicating higher quality of life in the physical domains.
A series of clinical health indicators were measured at baseline and 12 weeks. Waist circumference (cm), an indicator of cardiometabolic disease risk, was measured using a cloth measuring tape positioned at the top of the hip bone and wrapped fully around the waist. Participants were instructed not to hold their breath, and measures of the waist were recorded to the nearest 0.5 cm. Participants’ height was measured using a wall-mounted Seca stadiometer, and body weight (kg) was measured using a calibrated digital scale; these measurements were then used to calculate body mass index (BMI, kg/m2). A pulse oximeter was used to measure resting heart rate (beats per minute or bpm), and resting blood pressure (mm Hg) was taken manually following standard clinical procedures.
Participant characteristics
We collected patient-reported demographic and clinical characteristics, including age, sex, race/ethnicity, income, education level, depressive symptoms, and comorbid health conditions. PTSD symptom severity and depression were assessed using the PTSD Checklist for DSM-V (46) and the Patient Health Questionnaire-9 (47), respectively.
Statistical Analyses
Statistical analysis was conducted on a sample size of 46 (out of 54). Our prespecified intention-to-treat analysis was defined as participants who were randomly assigned to treatment and for whom 12-week data were available. No attempt was made to impute missing variables for participants who only completed baseline assessments. Data analysis was performed using PASW Statistics 18.0 (Chicago, IL) and SAS 9.3. We compared baseline characteristics of the two groups using independent samples t tests for continuous data and Pearson chi-square tests for categorical data.
The primary aim of this pilot study is to determine the feasibility and acceptability of structured exercise in older adults with late-life PTSD and is not powered to detect significant differences between the groups. As such, we estimate possible effects rather than formal hypothesis testing to infer the size and direction of treatment effect, though caution is still warranted in interpreting results due to small overall sample size. The between-group difference at 12 weeks (mean difference [MD] and 95% confidence interval [CI]) was calculated for all outcome measures. Cohen’s d effect sizes were calculated as the difference in means between the two groups divided by the SD and is interpreted as d = 0.20 (small), d = 0.50 (medium), and d = 0.80 (large).
Results
Recruitment and Participant Characteristics
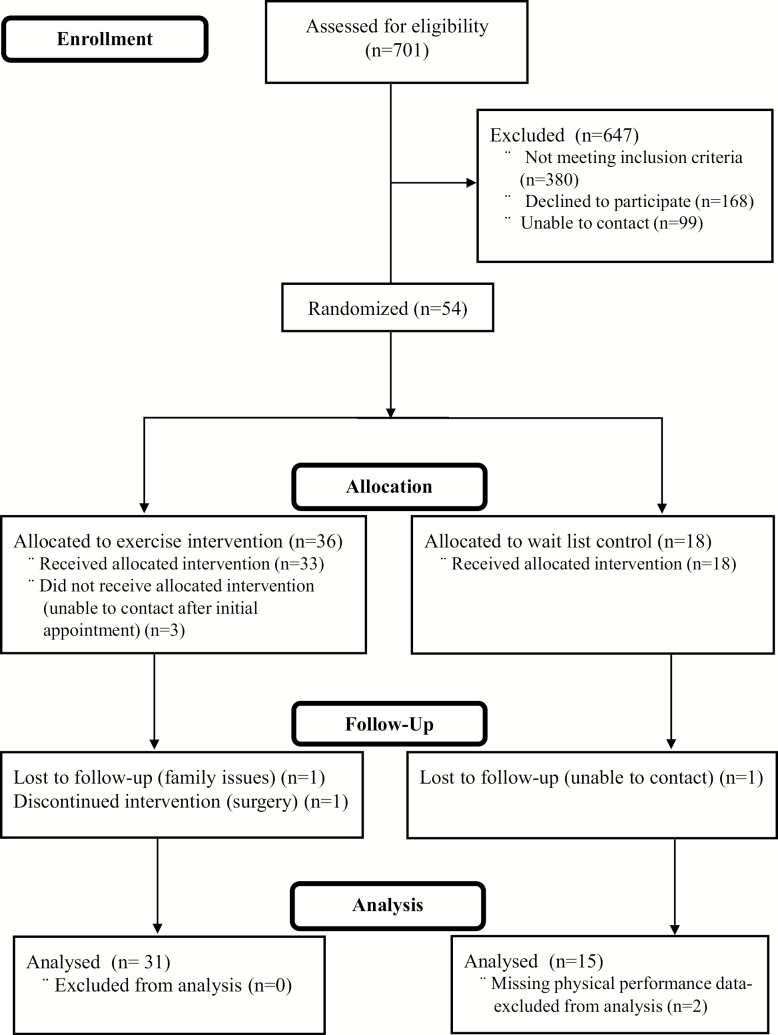
The recruitment period started November 2015 and was originally planned to last 18 months, but was extended to 2 years due to various life events of the principal investigator who caused delays in recruitment. Figure 1 shows the flow of participants through the study. In all, 701 patients were screened for eligibility, of which 380 were excluded. The most common reasons for ineligibility were psychotic illness and active alcohol or substance abuse. Among potentially eligible participants, 166 individuals declined to participate, and 99 were unable to be contacted by our research team. The remaining 54 participants were eligible and randomized (36 to exercise, 18 to WL); 89% completed the 12-week study. Overall, the dropout rate did not differ between groups (χ2 (1) = .84, p = 36). Sensitivity analyses between dropouts and those who completed the 12-week assessments revealed no significant baseline differences between groups in age, race, number of chronic conditions, or PTSD symptom severity, although BMI was higher among dropouts (35.2 kg/m2vs 29.9 kg/m2, p < .05). Two individuals had missing data on 12-week assessments, leaving 48 participants with complete data.
Figure 1.
Participant flow through the study.
The severity of PTSD reported by the sample was high (mean score on the PTSD Checklist for DSM-V = 40.9), and average duration of symptoms was 45 years. Baseline mental health characteristics and changes with exercise in this pilot trial have been reported previously (48). Participant characteristics are given in Table 1. The sample was predominantly male (90.7%) and were predominantly Vietnam-era veterans (88.9%), with a mean age of 67 years (range 60–78 years). Participants reported significant mental (87% comorbid depression) and physical (average 3.6 comorbid health conditions) health burden. The most common physical health comorbidities were hypertension (79.6%), hyperlipidemia (53.6%), and diabetes (50.0%). We had great success recruiting minority patients to participate (83% African American), who have higher rates of PTSD than Caucasians. The mean baseline BMI was 30.5 kg/m2, and 83.3% of the sample were overweight or obese. There were no significant differences between groups on any observed (Table 1) or measured (Table 2) variables at baseline.
Table 1.
Participant Characteristics at Baseline
| Variable | Exercise (n = 36) Mean (SD) |
WL (n = 18) Mean (SD) |
Statistic p Value |
|---|---|---|---|
| Age (y) | 67.7 (3.2) | 66.9 (4.3) |
t = 0.77 p = .44 |
| Male gender, n (%) | 34 (94%) | 15 (83) | χ 2 = 1.8 p = .18 |
| African American race, n (%) | 33 (92) | 13 (72) | χ 2 = 1.8 p = .06 |
| Hispanic ethnicity, n (%) | 0 | 0 | NA |
| ≤ High school education, n (%) | 6 (17) | 2 (11) | χ 2 = 0.29 p = .59 |
| Number of comorbidities | 3.7 (1.6) | 3.9 (1.8) |
t = −0.28 p = .78 |
| Body mass index (kg/m2) | 30.6 (5.9) | 30.4 (4.4) |
t = 0.12 p = .91 |
| Desired, n (%) | 6 (16.7) | 3 (16.7) | |
| Overweight, n (%) | 12 (33.3) | 5 (27.8) | |
| Obese, n (%) | 18 (50.0) | 10 (55.5) |
Note: NA = Not applicable; SD = Standard deviation.
Table 2.
Group Means and Differences Between Group Means for All Outcomes
| Outcome | Baseline | Change | Difference Between Groups | Cohen’s d | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean Change | 95% CI | ||
| Physical activity (MET-min/wk) | |||||||
| Exercise group | 365.8 | 95.2 | +1,588.6 | 192.2 | |||
| Control group | 349.4 | 122.2 | +325.9 | 178.8 | 1262.7 | 675.1 to 1,850.9 | 1.37 |
| Aerobic endurance (6MWT, m) | |||||||
| Exercise group | 462.1 | 22.3 | +69.0 | 29.8 | |||
| Control group | 465.9 | 32.0 | +10.1 | 21.7 | 58.9 | −53.4 to 440.2 | 0.50 |
| Gait speed (m/s) | |||||||
| Usual gait speed | |||||||
| Exercise group | 1.10 | 0.03 | +0.07 | 0.03 | |||
| Control group | 1.12 | 0.05 | −0.01 | 0.06 | 0.08 | −0.04 to 0.20 | 0.40 |
| Rapid gait speed (m/s) | |||||||
| Exercise group | 1.57 | 0.29 | +0.16 | 0.05 | |||
| Control group | 1.74 | 0.40 | −0.13 | 0.66 | 0.27 | 0.11 to 0.40 | 1.11 |
| 30-sec chair stands | |||||||
| Exercise group | 10.3 | 3.1 | +3.29 | 0.4 | |||
| Control group | 10.4 | 3.6 | −0.27 | 0.6 | 3.56 | −4.9 to −2.2 | 1.48 |
| 8-foot up-and-go (sec) | |||||||
| Exercise group | 6.0 | 1.7 | −0.69 | 0.14 | |||
| Control group | 5.5 | 1.1 | −0.13 | 0.66 | −0.57 | −1.6 to 0.5 | 0.28 |
| Single leg stand (sec) | |||||||
| Exercise group | 16.6 | 10.6 | +2.34 | 1.18 | |||
| Control group | 22.0 | 8.4 | −4.37 | 1.35 | 6.71 | 2.9 to 10.5 | 1.10 |
| SF-36 Physical Health Component Score | |||||||
| Exercise group | 39.8 | 9.8 | +2.15 | 1.38 | |||
| Control group | 43.8 | 8.2 | −1.85 | 2.20 | 4.00 | −1.0 to 9.0 | 0.48 |
| Waist circumference (cm) | |||||||
| Exercise group | 110.5 | 16.6 | −2.09 | 0.70 | |||
| Control group | 108.2 | 12.5 | +0.22 | 1.28 | −2.31 | −0.46 to 5.0 | 0.51 |
| Weight (kg) | |||||||
| Exercise group | 95.91 | 3.72 | +0.39 | 0.35 | |||
| Control group | 90.69 | 4.21 | +0.05 | 0.53 | 0.34 | −0.89 to 1.59 | 0.16 |
| BMI (kg/m2) | |||||||
| Exercise group | 30.57 | 0.98 | 0.17 | 0.14 | |||
| Control group | 30.38 | 1.05 | −0.09 | 0.20 | 0.27 | −0.21 to 0.74 | 0.34 |
| Resting HR (bpm) | |||||||
| Exercise group | 72.31 | 2.27 | −2.06 | 1.18 | |||
| Control group | 69.22 | 2.24 | +1.06 | 2.78 | −3.12 | −8.34 to 2.09 | 0.33 |
| Systolic blood pressure | |||||||
| Exercise group | 132.92 | 3.14 | −4.93 | 3.06 | |||
| Control group | 127.61 | 2.98 | +4.29 | 3.17 | −9.23 | −18.81 to 0.35 | 0.61 |
| Diastolic blood pressure | |||||||
| Exercise group | 76.94 | 1.79 | −2.13 | 1.38 | |||
| Control group | 74.39 | 1.85 | −0.41 | 2.53 | −1.72 | −7.02 to 3.59 | 0.19 |
Note: Higher scores reflect better function/health with the exception of 8-foot up-and-go and waist circumference. Cohen’s d effect sizes interpreted as d = 0.20 (small), d = 0.50 (medium), and d = 0.80 (large). No significant between-group differences were observed for any of the variables at baseline. BMI = body mass index; HR = heart rate; 6MWT = 6-minute walk test.
Primary Outcomes
Feasibility and acceptability
Participation in the exercise program among these older veterans with PTSD was very high, with 89% completing the 12-week program and attending 82% of the sessions offered. In evaluating the data on a dose–response relationship, there were small-to-moderate correlations between number of sessions completed and change in outcome measures, though none were statistically significant. Analysis of the relationship between baseline levels of the outcome variables and the number of sessions attended showed significant positive associations with balance and gait speed (rs = 0.35 and 0.42, ps < .05) and significant negative associations with waist circumference, body weight, and BMI (rs = −0.40 to −0.43, ps < .05).
Intervention Effects
Between-group effect sizes for pre-post changes are given in Table 2. Substantial improvements in physical activity levels (MD = 1262.7, d = 1.37 [95% CI: 675.1, 1850.9) were observed for the exercise group, compared with the WL group. Participants in the exercise group demonstrated an increase in 1,588.6 MET-min/wk in physical activity from baseline to postintervention (434% increase from baseline), and patients in the WL condition showed an increase in 325.9 MET-min/wk (93% increase from baseline). A substantial portion of the increase in activity minutes among exercise group participants was accounted for by changes in strengthening activities, with a greater than 10-fold increase from 57 MET-min/wk at baseline to 758 MET-min/wk at 12 weeks (data not shown). Among those in the exercise group, 97% achieved the recommended therapeutic dose of 500–1,000 MET-min/wk by the end of 12 weeks compared to 29% among those in the WL.
Moderate between-group differences were observed for the 6MWT. Performance on the 6MWT improved by 69 m in the exercise group compared to 10 m in the WL group (MD = 58.93, d = 0.50 [95% CI: −16.3, 134.2]). At baseline, participants scored at the 10th percentile of population norms; those in the exercise group improved performance to the 37th percentile at 12 weeks, but performance remained relatively stable in the WL group. A change in 6-minute walk distance of 50 m is considered substantial meaningful change (49), and 70% met or exceeded this criteria in the exercise group.
Secondary Outcomes
Changes in physical function performance
Between-group effect sizes for pre-post changes are given in Table 2. The treatment groups differed with respect to changes in physical function performance. Between-group differences across the performance assessments ranged from small to large (ds = 0.28–1.48), favoring the exercise arm. Large between-group differences were observed for rapid gait speed (MD = 58.93, d = 0.50 [95% CI: −16.3, 134.2]), single leg balance stand (MD = 6.71, d = 1.10 [95% CI: 2.9, 10.5]), and 30-second chair stands (MD = 3.56, d = 1.48 [95% CI: −4.9, −2.2). The most marked between-group differences were observed for muscle strength, as measured by the 30-second chair stand test. At baseline, participants scored at the 11th percentile of population norms; those in the exercise group improved performance to the 41st percentile at 12 weeks, but performance remained stable in the WL group. Participants in the exercise condition also improved rapid-paced walking speed (+0.16 m/s; clinically meaningful difference = 0.1 m/s, 50) and balance (+2.34 seconds) compared to those in the WL group who showed declines in walking speed (−0.13 m/s) and balance performance (−4.37 seconds). Small between-group differences were observed for usual gait speed (MD = 0.08, d = 0.40 [95% CI: −0.04, 0.20]) and 8-foot up-and-go test (MD = 6.71, d = 0.28 [95% CI: −1.6, 0.5]), favoring the exercise arm.
Changes in Health-Related Quality of Life
Between-group effect sizes for pre-post changes are given in Table 2. Small improvements in the SF-36 PCS were observed for the exercise group, compared with the WL group (MD = 4.00 d = 0.48 [95% CI: −1.0, 9.0]). Between-group differences across the four Physical Health Component Score subscales (bodily pain, physical function, general health, and role-physical) ranged from small to moderate (ds = 0.29–0.61), favoring the exercise arm (data not shown). A 1.0-unit change in SF-36 PCS is considered clinically meaningful (51), and scores improved, on average, 2.1 units in the exercise arm.
Changes in Clinical Health Indicators
Between-group effect sizes for pre-post changes are given in Table 2. Moderate between-group differences were observed for waist circumference (MD = −2.31, d = 0.51 [95% CI: −0.46, 5.0]). Waist circumference decreased an average 2 cm (0.8 inches) in the exercise group, compared to patients in the WL group where waist circumference remained stable for more than 12 weeks. Small-sized effects were observed for BMI (MD = +0.27, d = 0.34 [95% CI: −0.21, 0.74]) for more than 12 weeks; trending upward in the exercise group. Small between-group differences were also observed for resting heart rate, which dropped an average 2 bpm in the exercise group compared to a 1 bpm increase in WL (MD = −3.12, d = 0.33 [95% CI: −0.89, 1.59]). Moderate between-group differences were observed for systolic blood pressure, which decreased by nearly 5 mm Hg in the exercise group compared to a 4 mm Hg increase in WL (MD = −9.23, d = 0.61 [95% CI: −18.81, 0.35]). No between-group differences were observed for body weight or diastolic blood pressure.
Discussion
This pilot study investigated the feasibility of delivering a supervised exercise intervention to older veterans with PTSD and the potential impact on important functional and clinical health indicators. This is the first exercise trial to target older veterans with PTSD and is the first to report the impact of exercise training on physical and functional outcomes in this population with poor health and significant comorbidities. We observed high adherence and participation rates, evidence that this program was feasible and acceptable to patients. This pilot exercise intervention also demonstrated substantial improvements in self-reported physical activity and aerobic endurance, as well as functional outcomes, among older adults with PTSD. Metabolic syndrome is highly prevalent in this population, and we observed clinically relevant reductions in waist circumference and systolic blood pressure, two indicators of metabolic syndrome.
Despite being relatively young, participants in this study presented with high levels of physical inactivity and substantial functional impairment. Compared to age- and gender-matched population norms, they performed at just the 10th percentile for aerobic endurance (6MWT), the 15th percentile for lower body strength (chair stand test), and below average for gait speed and balance (43). Average group performance on the chair stand test, 6MWT, and the 8-foot up-and-go test also fall far below recommended standards for remaining physically mobile and independent in later life (52). Self-reported physical health was also poor in this population, averaging nearly nine points below population norms for the SF-36 (53). In the absence of health-promoting interventions, these individuals are at high risk for premature disability and frailty.
Warrior Wellness was a progressive, multicomponent exercise program, emphasizing strength training and aerobic activity to address the functional deficits and clinical health risk factors in this population. Despite the presence of multiple comorbidities and functional limitations, nearly 100% of patients were exercising at levels commensurate with public health guidelines for physical activity by the end of 12 weeks. Increases in physical activity were accompanied by clinically significant improvements in aerobic endurance, strength, and gait speed in this sample of older veterans with PTSD. The results of this pilot study suggest clinically meaningful benefit of exercise across multiple domains of function associated with independent living and quality of life; though additional larger studies are needed to robustly test these effects.
Older veterans with PTSD entered the study with high rates of overweight/obesity and substantial impairments across multiple domains of physical function, underscoring the critical need for health interventions in this population. Clinical trials in PTSD have largely focused on empirically supported psychotherapy treatments to reduce symptoms. There are few studies of trauma-focused therapy for PTSD in older adults, but based on the current evidence, psychotherapy alone is unlikely to improve cardiovascular health or induce clinically relevant functional improvements in older adults with PTSD (54).
Psychotherapy treatment options for PTSD also suffer poor engagement and adherence: One-third of patients drop out before completing a full course of treatment (55–57), and African American veterans are less likely to seek mental health treatment (1,58), despite having higher rates of PTSD and greater symptom severity than Caucasian veterans. The low adherence and racial disparities reported for psychotherapy among veterans with PTSD contrast sharply with what we observed in this study of supervised exercise where adherence was very high (89%) and we had great success recruiting minority veterans with PTSD to participate (83% African American). Exercise may have promise for attracting more veterans, particularly minorities, into care and for improving cardiometabolic health and physical functioning as a stand-alone or adjunctive behavioral treatment for late-life PTSD. Future studies in this area are warranted.
The main limitation of the current study is that generalizability may be limited by several factors, including small overall sample size, high exclusion rate, and majority representation of a specific cohort of veteran (Vietnam-era), all of which could impact participation, adherence, and intervention effects. Relatedly, the relatively low number of female veterans who participated in this pilot study (9.3%), although expected given the demographics of the older veteran population, is a limitation of the study and the field more generally that has largely studied younger male populations (59). The focus of this study on military veterans also limits the generalizability of conclusions to non-Veterans (ie, civilian older adults). Military veterans represent a unique population that has been exposed to a plethora of individual, psychological, and physical stressors (eg, musculoskeletal and overuse injuries) through military training and combat that are typically not germane to the general population. For example, 78% of this sample reported PTSD symptoms related to traumatic combat experiences. Military core values such as leadership and subordination, commitment, and duty also make this a unique population (60) and may contribute to higher rates of adherence and compliance compared to non-veterans. Although this has not been directly explored, other studies of similar duration in younger adults with PTSD (veterans and non-veterans) report a 75% completion rate (17,20), compared to 89% reported here.
Another limitation is that we did not track medications that could impact changes in some secondary outcomes. Finally, we did not measure outcomes in long term, so we cannot say whether these changes were sustained past this brief intervention. It would also be of interest to know whether changes in physical activity behavior impacted other lifestyle behaviors (eg, diet, substance use) or biomarkers of cardiometabolic health (eg, inflammatory cytokines), and whether the effects of the exercise program differed for specific physical health comorbidities.
We previously reported improvements in PTSD and specific PTSD symptom clusters with exercise training in this sample (48). Considering that many of the impairing conditions associated with PTSD (eg, diabetes, physical deconditioning) are linked to lifestyle factors (eg, smoking, diet, exercise), treatment options that target exercise represent a promising approach for reducing physical comorbidity and functional impairment among older adults with PTSD.
Exercise is a low-cost and low-stigma health intervention that is relatively easy to implement and warrants consideration as a potent clinical intervention for older adults with PTSD. However, it is important to note that we employed several evidence-based strategies to enhance motivation and retention in these patients; these strategies should be considered (along with other design elements) in future clinical implementation efforts. The tailored and targeted strategies utilized in this study include psychoeducation around hyperarousal and vigilance in the exercise setting, peer support, physical function testing with patient feedback, and individualized exercise prescriptions, among others (31). Patient feedback about the program suggests that these elements were salient and effectual (61). In conclusion, supervised exercise training may represent an empowering adjunctive therapy to promote mobility, social engagement, and functional independence among older adults living with chronic PTSD.
Funding
This project is supported by the Department of Veterans Affairs, Rehabilitation Research and Development Service (2RX001316, K.S.H.). K.S.H. and M.C.M. also receive support from the Duke Claude D. Pepper Older Americans Independence Center (P30 AG028716).
Acknowledgments
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the Department of Veterans Affairs. The authors thank research team members Lisa Brown, MS, and Jessica McDermott, BS, for their help with conducting the study. The authors extend sincerest gratitude to the veterans who participated in the study and contributed so much to our understanding of health and health promotion in older adults living with PTSD.
Conflict of Interest
None reported.
References
- 1. Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma and the Vietnam War Generation. New York, NY: Brunner/Manzel; 1990. [Google Scholar]
- 2. Boscarino JA. Posttraumatic stress disorder and mortality among U.S. army veterans 30 years after military service. Ann Epidemiol. 2006;16:248–256. doi:10.1016/j.annepidem.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 3. Xue Y, Taub PR, Iqbal N, et al. Cardiac biomarkers, mortality, and post-traumatic stress disorder in military veterans. Am J Cardiol. 2012;109:1215–1218. doi:10.1016/j.amjcard.2011.11.063 [DOI] [PubMed] [Google Scholar]
- 4. Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39:61–78. doi:10.1007/s12160-010-9165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farr OM, Sloan DM, Keane TM, Mantzoros CS. Stress- and PTSD-associated obesity and metabolic dysfunction: a growing problem requiring further research and novel treatments. Metabolism. 2014;63:1463–1468. doi:10.1016/j.metabol.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall KS, Beckham JC, Bosworth HB, Sloane R, Pieper CF, Morey MC. PTSD is negatively associated with physical performance and physical function in older overweight military Veterans. J Rehabil Res Dev. 2014;51:285–295. doi:10.1682/JRRD.2013.04.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jakupcak M, Luterek J, Hunt S, Conybeare D, McFall M. posttraumatic stress and its relationship to physical health functioning in a sample of Iraq and Afghanistan War veterans seeking postdeployment VA health care. J Nerv Ment Dis. 2008;196:425–428. doi:10.1097/NMD.0b013e31817108ed [DOI] [PubMed] [Google Scholar]
- 8. Pagoto SL, Schneider KL, Bodenlos JS, et al. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity (Silver Spring). 2012;20:200–205. doi:10.1038/oby.2011.318 [DOI] [PubMed] [Google Scholar]
- 9. Jin H, Lanouette NM, Mudaliar S, et al. Association of posttraumatic stress disorder with increased prevalence of metabolic syndrome. J Clin Psychopharmacol. 2009;29:210–215. doi:10.1097/JCP.0b013e3181a45ed0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kubzansky LD, Koenen KC, Spiro A 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64:109–116. doi:10.1001/archpsyc.64.1.109 [DOI] [PubMed] [Google Scholar]
- 11. Stefanovics EA, Potenza MN, Pietrzak RH. The physical and mental health burden of obesity in U.S. veterans: results from the National Health and Resilience in Veterans Study. J Psychiatr Res. 2018;103:112–119. doi:10.1016/j.jpsychires.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 12. Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–193. [DOI] [PubMed] [Google Scholar]
- 13. Schuch FB, Morres ID, Ekkekakis P, Rosenbaum S, Stubbs B. Exercise works for depression: bridging the implementation gap and making exercise a core component of treatment. Acta Neuropsychiatr. 2017;29:124–126. doi:10.1017/neu.2017.1 [DOI] [PubMed] [Google Scholar]
- 14. Windle G, Hughes D, Linck P, Russell I, Woods B. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment Health. 2010;14:652–669. doi:10.1080/13607861003713232 [DOI] [PubMed] [Google Scholar]
- 15. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- 16. Davidson CL, Babson KA, Bonn-Miller MO, Souter T, Vannoy S. The impact of exercise on suicide risk: examining pathways through depression, PTSD, and sleep in an inpatient sample of veterans. Suicide Life Threat Behav. 2013;43:279–289. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: a randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345–352. doi:10.1016/j.jad.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 18. Hall KS, Hoerster KD, Yancy WS Jr. Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37:103–115. doi:10.1093/epirev/mxu011 [DOI] [PubMed] [Google Scholar]
- 19. Mehling WE, Chesney MA, Metzler TJ, et al. A 12-week integrative exercise program improves self-reported mindfulness and interoceptive awareness in war veterans with posttraumatic stress symptoms. J Clin Psychol. 2018;74:554–565. doi:10.1002/jclp.22549 [DOI] [PubMed] [Google Scholar]
- 20. Rosenbaum S, Sherrington C, Tiedemann A. Exercise augmentation compared with usual care for post-traumatic stress disorder: a randomized controlled trial. Acta Psychiatr Scand. 2015;131:350–359. doi:10.1111/acps.12371 [DOI] [PubMed] [Google Scholar]
- 21. Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75:964–974. doi:10.4088/JCP.13r08765 [DOI] [PubMed] [Google Scholar]
- 22. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi:10.1001/archinte.160.21.3252 [DOI] [PubMed] [Google Scholar]
- 23. Buckley TC, Mozley SL, Bedard MA, Dewulf AC, Greif J. Preventive health behaviors, health-risk behaviors, physical morbidity, and health-related role functioning impairment in veterans with post-traumatic stress disorder. Mil Med. 2004;169:536–540. doi:10.7205/milmed.169.7.536 [DOI] [PubMed] [Google Scholar]
- 24. de Assis MA, de Mello MF, Scorza FA, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics (Sao Paulo). 2008;63:473–478. doi:10.1590/s1807-59322008000400010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of Post-traumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230:130–136. doi:10.1016/j.psychres.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 26. Whitworth JW, Nosrat S, SantaBarbara NJ, Ciccolo JT. High intensity resistance training improves sleep quality and anxiety in individuals who screen positive for posttraumatic stress disorder: a randomized controlled feasibility trial. Ment Health Phys Act. 2019;16:43–49. [Google Scholar]
- 27. Westphal M, Olfson M, Gameroff MJ, et al. Functional impairment in adults with past posttraumatic stress disorder: findings from primary care. Depress Anxiety. 2011;28:686–695. doi:10.1002/da.20842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services. 2018. [Google Scholar]
- 29. Harada ND, Wilkins SS, Schneider B, et al. The influence of depression and PTSD on exercise adherence in older veterans. Mil Behav Health. 2013;1:146–151. doi:10.1080/21635781.2013.829400 [Google Scholar]
- 30. Kantor V, Knefel M, Lueger-Schuster B. Perceived barriers and facilitators of mental health service utilization in adult trauma survivors: a systematic review. Clin Psychol Rev. 2017;52:52–68. doi:10.1016/j.cpr.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 31. Hall KS, Morey MC, Beckham JC, et al. The Warrior Wellness Study: a randomized controlled exercise trial for older veterans with PTSD. Transl J Am Coll Sports Med. 2018;3:43–51. doi:10.1249/TJX.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–395. doi:10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998; 104. [Google Scholar]
- 34. Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and Evaluation Within a Veteran Primary Care Sample. J Gen Intern Med. 2016;31:1206–1211. doi:10.1007/s11606-016-3703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT Alcohol Consumption Questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi:10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 36. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–371. doi:10.1016/0306-4603(82)90005-3 [DOI] [PubMed] [Google Scholar]
- 37. Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32:189–198. doi:10.1016/j.jsat.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 38. Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi:10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 39. Kohl HW, Blair SN, Paffenbarger RS Jr, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi:10.1093/oxfordjournals.aje.a114915 [DOI] [PubMed] [Google Scholar]
- 40. Balke B. A simple field test for the assessment of physical fitness. CARI Rep. 1963;63:18. [PubMed] [Google Scholar]
- 41. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82:128–137. doi:10.1093/ptj/82.2.128 [DOI] [PubMed] [Google Scholar]
- 42. Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi:10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 43. Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults, ages 60–94. J Aging Phys Act. 1999;7:162–181. [Google Scholar]
- 44. Yoshimura N, Oka H, Muraki S, et al. Reference values for hand grip strength, muscle mass, walking time, and one-leg standing time as indices for locomotive syndrome and associated disability: the second survey of the ROAD study. J Orthop Sci. 2011;16:768–777. doi:10.1007/s00776-011-0160-1 [DOI] [PubMed] [Google Scholar]
- 45. Ware JE Jr, Bayliss MS, Rogers WH, Kosinski M, Tarlov AR. Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. JAMA. 1996;276:1039–1047. [PubMed] [Google Scholar]
- 46. Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28:1379–1391. doi:10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- 47. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi:10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2019. Epub ahead of print. https://www.ncbi.nlm.nih.gov/pubmed/31264055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 50. Chui K, Hood E, Klima D. Meaningful change in walking speed. Top Geriatr Rehabil. 2012;28:97–103. doi: 10.1097/TGR.0b013e3182510195 [Google Scholar]
- 51. Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health. 2013;16:993–1000. doi:10.1016/j.jval.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 52. Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–267. doi:10.1093/geront/gns071 [DOI] [PubMed] [Google Scholar]
- 53. Ware JE Jr, Kosinski M. SF-36 Physical and Mental Health Summary Scores: A Manual for Users of Version 1. et al. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 54. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74:e541–e550. doi:10.4088/JCP.12r08225 [DOI] [PubMed] [Google Scholar]
- 55. Imel ZE, Laska K, Jakupcak M, Simpson TL. Meta-analysis of dropout in treatments for posttraumatic stress disorder. J Consult Clin Psychol. 2013;81:394–404. doi:10.1037/a0031474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112–128. doi:10.1016/j.psychres.2019.02.027 [DOI] [PubMed] [Google Scholar]
- 57. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43. doi:10.12703/P7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41:71–83. doi:10.1017/S0033291710000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pebole MM, Hall KS. Physical activity promotion in women with PTSD: what we need for progress. Psychol Sport Exerc. 2019;41:127–129. [PMC free article] [PubMed] [Google Scholar]
- 60. Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635–639. doi:10.2147/AMEP.S89479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pebole MM, Hall KS. Insights following implementation of an exercise intervention in older veterans with PTSD. Int J Environ Res Public Health. 2019;16:2630. doi:10.3390/ijerph16142630 [DOI] [PMC free article] [PubMed] [Google Scholar]