Abstract
Background
Guidelines recommend the PRECISE-DAPT (PD) score to adapt duration of dual antiplatelet therapy due to bleeding risk. However, there is first evidence that PD predicts mortality and ischemic events as well.
Methods
We investigated PD Score in 994 patients after percutaneous coronary intervention (PCI). PD was correlated with clinically frequently used scores. Major adverse cardiac and cerebrovascular events (MACCE) and Thrombolysis in Myocardial Infarction (TIMI) bleeding were assessed during one-year follow-up.
Results
524 patients had PD < 25 and 470 patients PD ≥ 25 (47%). Rate of major and minor bleeding was higher in the PD ≥ 25 group (major bleeding: Hazard ratio [HR] 2.9, 95% confidence interval [Cl] 1.01–8.16, p = 0.049; minor bleeding: HR 3.94, 95% Cl 1.36–9.19, p = 0.0096). Rate of MACCE, death and myocardial infarction were higher as well (MACCE: HR 2.0, 95% Cl 1.52–2.71, p < 0.0001; death: HR 3.9, 95% Cl 2.12–5.68, p < 0.0001; MI: HR 2.1, 95% Cl 1.26–3.43, p = 0.0041). Rate of stroke/transient ischemic attack did not differ between groups. Discriminative potency to predict major and minor bleeding, MACCE, death and MI were high with nearly equal cut-off values calculated by Youden’s index (YI) (major bleeding: Area under the curve [AUC] 0.66; p = 0.026; YI 32; minor bleeding: AUC 0.72; p = 0.001; YI 28; MACCE: AUC 0.62; p < 0.0001; YI 24).
Conclusion
In our cohort, PD score predicted bleeding moderately in post-PCI patients. In this study, ischemic events were predicted as well. Adaption of antiplatelet therapy duration by PD score is accurate. Nevertheless, it should be well-balanced with patient-related risk for ischemic events.
Keywords: Bleeding, DAPT, MACCE, Scores, TIMI
Abbreviations: AF, Atrial fibrillation; DAPT, Dual antiplatelet medication; GRACE, Global Registry of Acute Coronary Events; MACCE, Major adverse cardiac and cerebrovascular events; PARIS, Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients; PD, PRECISE-DAPT, PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Antiplatelet Therapy; PCI, percutaneous coronary intervention; ROC, Receiver operating characteristic; TIA, Transient ischemic attack; TIMI, Thrombolysis in myocardial infarction; STEMI, ST-elevation myocardial infarction
1. Introduction
Antiplatelet treatment is the backbone in secondary prevention after stent implantation in patients with coronary artery disease [1]. However, the optimal duration and regime of antithrombotic medication is challenging in individualized medicine. Especially, with regard to comorbidities as atrial fibrillation (AF) that require further antithrombotic strategies with oral anticoagulation as this has been shown to influence platelet reactivity as well [2]. Therefore, well-balanced stratification between risk for bleeding and ischemic events is crucial. A multiplicity of risk scores is already available. However, a user-friendly, ‘one-fits-all’ approach is still missing. Scores that predict ischemic complications frequently also predict bleeding complications and vice versa. Current guidelines recommend adaption of dual antiplatelet therapy (DAPT) based on the PRECISE-DAPT (PD) score (PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Antiplatelet Therapy) [3], [4]. However, there is first evidence that mortality and ischemic events are predicted as well [5]. In this analysis, we aimed to investigate the prognostic value of PD Score in comparison with frequently used scores in clinical routine for the determination of bleeding or ischemic complications.
2. Methods
Commonly used scores (GRACE [Global Registry of Acute Coronary Events] [6], DAPT-[7], NCDR mortality and NCDR bleeding[8], HASBLED[9], CHA2DS2-VASc[10], ABC stroke[11], PARIS [Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients] bleeding score, PARIS thrombotic score[12], PRECISE-DAPT Score [13]) were investigated in 994 patients after percutaneous coronary intervention (PCI) from 04/2015–05/2016.
One-year follow up was conducted. Major adverse cardiac and cerebrovascular events (MACCE) defined by mortality of any cause, myocardial infarction and stroke or transient ischemic attack (TIA) as well as Thrombolysis in Myocardial Infarction (TIMI) major and minor bleeding events were assessed (Suppl. Fig. 1).
Statistical analyses were conducted using IBM SPSS©-Software (New York, USA) and GraphPad-Prism© statistical software (GraphPad software Inc, San Diego). Normal distribution was calculated by Shapiro-Wilks test. Correlation between scores was calculated by Pearson correlation. For patients’ characteristics, quantitative variables are presented as mean (±SD), qualitative variables are presented as percentages and differences were analyzed by t-Student and Chi-square tests. Hazard ratios (HR) with 95% confidence interval (CI) and log-rank test were used for risk prediction of MACCE and bleeding during follow-up. Prediction of MACCE and bleeding was analyzed after adjustment for differentiating patient characteristics that are not included as parameter in the PD score (obesity, male gender, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, prior stroke, former smoking, severity of CAD, reduced EF, chronic coronary syndrome, NSTEMI, STEMI, radial approach, clopidogrel, prasugrel, ticagrelor, VKA, diuretics, statins, proton pump inhibitors, metamizole, ACE inhibitors). Receiver operating characteristic (ROC) analysis and Youden’s J statistic were conducted to evaluate predictive potency of each score for the detection of MACCE and bleeding. P values below 0.05 were defined significant. This study was approved by the University of Düsseldorf Ethics Committee and conformed to the Declaration of Helsinki.
3. Results
3.1. Study population
Mean age was 69.8 ± 11.8 years, 26.5% were obese, 69.5% were male, 97.9% had arterial hypertension, 84.4% chronic kidney disease and 34.8% diabetes mellitus type II. 35.8% patients had chronic coronary syndrome, 16.4% had ST-elevation myocardial infarction (STEMI) and 45.2% had non-STEMI for PCI indication. 96.3% of patients received aspirin, 98.4% P2Y12 inhibition and 21.2% oral anticoagulation. Duration of antiplatelet therapy and anticoagulation was chosen at the discretion of the treating interventionalist. 524 patients had PD < 25 and 470 patients PD ≥ 25. Patients with PD ≥ 25 were slightly older, were more often male, had more frequently diabetes, chronic obstructive pulmonary disease (COPD), atrial fibrillation, chronic kidney disease, prior stroke and were more often ongoing smokers. Moreover, severity of coronary-artery disease and rate of reduced ejection fraction were higher in PD ≥ 25. Regarding laboratory parameters, hemoglobin and glomerular filtration rate were lower whereby creatinine, C - reactive protein, leukocytes were higher in PD ≥ 25 (Table 1). Concerning antiplatelet therapy, PD ≥ 25 patients were more often on clopidogrel and had more often triple therapy and vitamin K-antagonists (VKA). They were less often on prasugrel or ticagrelor medication and had less often six months DAPT duration. Regarding other medication, they had less often ACE-inhibitors, statins and proton-pump inhibitors. Radial approach was less often in the PD ≥ 25 group (table 2).
Table 1.
Patients’ characteristics.
| All (n = 994) |
PD < 25 (n = 524) |
PD ≥ 25 (n = 470) |
p-Value |
|
|---|---|---|---|---|
| Patients characteristics | ||||
| Age (years) - mean ± SD | 69.77 ± 11.78 | 63.74 ± 10.27 | 76,5 ± 9.5 | <0.0001 |
| BMI > 30 – no. (%) | 262 (26.5%) | 157 (30.1%) | 105 (22.5%) | 0.007 |
| Male – no. (%) | 691 (69.5%) | 408 (77.9%) | 283 (60.2%) | <0.001 |
| Co-morbidities no. (%) | ||||
| Hypertension | 971 (97.9%) | 509 (97.3%) | 462 (98.5%) | 0.196 |
| Diabetes | 345 (34.8%) | 153 (29.3%) | 192 (40.9%) | <0.0001 |
| COPD | 136 (13.7%) | 57 (10.9%) | 79 (16.8%) | 0.007 |
| Atrial fibrillation | 225 (22.7%) | 71 (13.6%) | 154 (32.8%) | <0.0001 |
| Liver Disease | 13 (1.8%) | 7 (1.8%) | 6 (1.7%) | 0.923 |
| CKD | 837 (84.4%) | 373 (71.3%) | 464 (98.9%) | <0.0001 |
| Prior MI | 196 (19.7%) | 112 (21.4%) | 84 (17.9%) | 0.161 |
| Prior PCI | 364 (36.7%) | 199 (38.0%) | 165 (35.1%) | 0.337 |
| Prior Stroke | 78 (7.9%) | 25 (4.8%) | 53 (11.3%) | <0.0001 |
| Prior CABG | 113 (11.4%) | 52 (10%) | 61 (13%) | 0.135 |
| Former nicotine abusus | 128 (12.9%) | 71 (13.6%) | 57 (12.1%) | 0.497 |
| Ongoing nicotine abusus | 198 (19.9%) | 142 (27.2%) | 56 (11.9%) | <0.0001 |
| Type of CAD | ||||
| 1-vessel disease | 159 (16%) | 98 (18.7%) | 61 (13.0%) | <0.0001 |
| 2-vessel disease | 162 (16.3%) | 101 (19.3%) | 61 (13.0%) | |
| 3-vessel disease | 669 (67.3%) | 323 (61.6%) | 346 (73.6%) | |
| EF < 40% | 241 (24.3%) | 109 (20.8%) | 132 (28.1%) | <0.0001 |
|
Laboratory- Parameters - mean ± SD | ||||
| Hemoglobin[mg/dl] | 13.22 ± 2.05 | 14.08 ± 1.48 | 12.26 ± 2.16 | <0.0001 |
| Thrombocytes[x1000µl-1] | 236.6 ± 76.24 | 231.32 ± 64.98 | 242.46 ± 86.76 | 0.023 |
| Cholesterol[mg/dl] | 181.30 ± 52.02 | 181.45 ± 45.08 | 181.02 ± 63.11 | 0.946 |
| Triglycerides[mg/dl] | 160.79 ± 130.14 | 163.08 ± 130.48 | 156.66 ± 129.94 | 0.658 |
| Low Density Lipoprotein[mg/dl] | 115.32 ± 46.62 | 115.92 ± 44.94 | 114.12 ± 50.07 | 0.748 |
| High Density Lipoprotein[mg/dl] | 48.31 ± 17.82 | 48.22 ± 17.85 | 48.49 ± 17.85 | 0.901 |
| Lipoprotein(a) | 95.66 ± 151.31 | 90.26 ± 139.88 | 105.89 ± 172.48 | 0.691 |
| Creatinine [mg/dl] | 1.25 ± 1.06 | 0.96 ± 0.25 | 1.58 ± 1.45 | <0.0001 |
| GFR [ml/min] | 66.15 ± 23.08 | 80.75 ± 15.56 | 49.91 ± 18.86 | <0.0001 |
| HbA1c [%] | 6.29 ± 2.34 | 5.56 ± 2.40 | 7.6 ± 1.67 | 0.112 |
| C-reactive Protein [mg/dl] | 1.81 ± 3.91 | 0.96 ± 2.19 | 2.7 ± 4.93 | <0.0001 |
| Leukocytes [x1000/nl] | 9.27 ± 4.83 | 8.46 ± 3.09 | 10.17 ± 6.09 | <0.0001 |
BMI = Body Mass Index; CABG = Coronary artery bypass grafting; CAD = coronary artery disease; CKD = Chronic kidney disease; COPD = Chronic obstructive pulmonary disease; EF = Ejection fraction; GFR = Glomerular filtration rate; MI = Myocardial infarction; PCI = Percutaneous coronary intervention; PD = PRECISE DAPT; SD = Standard deviation
Table 2.
Co-Medication and procedural details.
| All (n = 994) |
PD < 25 (n = 524) |
PD ≥ 25 (n = 470) |
p-Value |
|
|---|---|---|---|---|
| Co-medication – no. (%) | ||||
| Aspirin | 957 (96.3%) | 511 (97.5%) | 446 (94.9%) | 0.338 |
| Clopidogrel | 629 (63.3%) | 287 (54.8%) | 342 (72.8%) | <0.0001 |
| Prasugrel | 84 (8.5%) | 75 (14.3%) | 9 (1.9%) | <0.0001 |
| Ticagrelor | 264 (26.6%) | 159 (30.3%) | 105 (22.3%) | 0.007 |
| Non-Vitamin K antagonists | 64 (6.4%) | 32 (6.1%) | 32 (6.8%) | 0.593 |
| Vitamin K antagonists | 59 (5.9%) | 20 (3.8%) | 39 (8.3%) | 0.002 |
| ß-blockers | 29 (2.9%) | 16 (3.1%) | 13 (2.8%) | 0.823 |
| ACEI /AT II RBs | 182 (18.3%) | 61 (11.6%) | 121 (25.7%) | <0.0001 |
| Digitalis | 766 (77.1%) | 416 (79.4%) | 350 (74.5%) | 0.196 |
| Statins | 794 (79.9%) | 443 (85.4%) | 351 (74.7%) | 0.007 |
| PP-inhibitors | 31 (3.1%) | 10 (1.9%) | 21 (4.5%) | 0.017 |
| Triple Therapy* | 225 (22.6%) | 71 (13.5%) | 154 (32.3%) | <0.0001 |
| 12 months DAPT | 479 (48.2%) | 250 (47.7%) | 229 (48.7%) | 0.7511 |
| 6 months DAPT | 239 (24.1%) | 181 (34.6%) | 58 (12.3%) | <0.0001 |
| 1–3 months DAPT | 51 (5.1%) | 22 (4.2%) | 29 (6.1%) | 0.0604 |
| Procedural Details | ||||
| Radial Approach | 335 (33.7%) | 213 (40.6%) | 122 (26.0%) | <0.0001 |
| Intracoronary Medication | 53 (5.3%) | 31 (5.9%) | 22 (4.7%) | 0.387 |
| Naive Vessels | 748 (75.3%) | 391 (74.6%) | 357 (76.0%) | 0.625 |
| Angioseal | 192 (19.3%) | 109 (20.8%) | 83 (17.7%) | 0.210 |
| Culprit Lesion | 903 (90.8%) | 476 (90.8%) | 427 (90.9%) | 0.995 |
| Multivessel PCI | 90 (9.1%) | 48 (9.2%) | 42 (8.9%) | 0.902 |
| Type of stent | ||||
| Scaffold | 22 (22.0%) | 18 (3.4%) | 4 (0.9%) | 0.031 |
| DES | 875 (88.0%) | 458 (87.4%) | 417 (88.7%) | |
| BMS | 76 (7.6%) | 36 (6.9%) | 40 (8.5%) | |
ACEI /AT II RBs = Angiotensin-converting enzyme inhibitors/angiontensin II receptor blockers; BMS = Bare metal stent; DAPT = Dual antiplatelet therapy; DES = Drug eluting stent; PP = Proton Pump; PCI = Percutaneous coronary intervention; *four weeks triple therapy followed by eleven months of dual therapy with oral anticoagulation and P2Y12 inhibition, followed by oral anticoagulation alone.
3.2. Correlation with frequently used scores
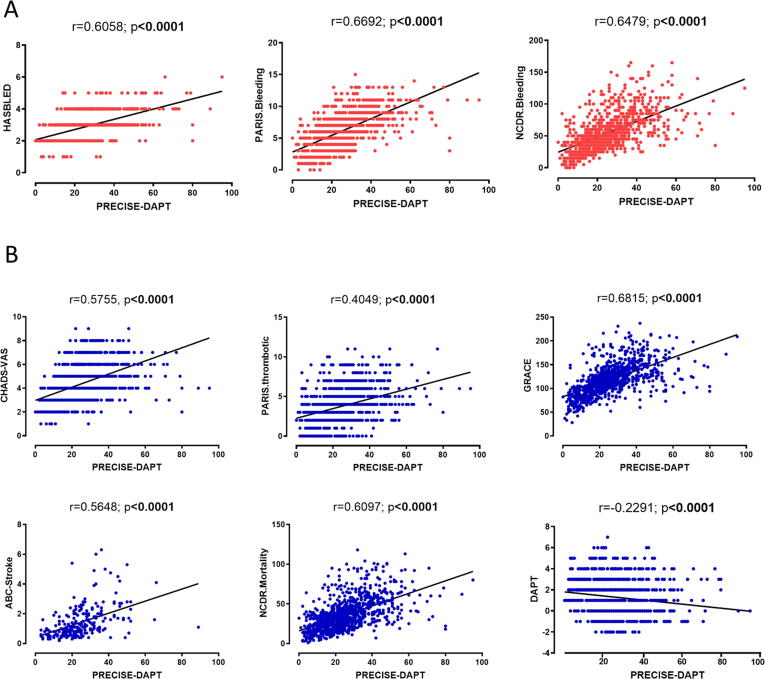
PD correlated well with bleeding risk prediction scores (HASBLED: r = 0.6058, p < 0.0001; PARIS bleeding: r = 0.6692, p < 0.0001; NCDR-bleeding: r = 0.6479, p < 0.0001, Fig. 1A). Moreover, PD correlated as well with risk prediction scores for ischemic events (CHA2DS2-VASc: r = 0.5755, p < 0.0001; PARIS-thrombotic: r = 0.4049, p < 0.0001; GRACE: r = 0.6815, p < 0.0001; ABC-stroke: r = 0.5648, p < 0.0001; NCDR-mortality: r = 0.6097, p < 0.0001). A negative correlation was revealed for the DAPT score (r = -0.2291, p < 0.0001, Fig. 1B).Fig. 2.
Fig. 1.
The PRECISE-DAPT score correlated highly positively with hitherto scores for (A) bleeding prediction (HASBLED, PARIS bleeding and NCDR bleeding score) and (B) ischemic events prediction (CHA2DS2-VASc, PARIS thrombotic, GRACE, ABC-Stroke, NCDR-mortality and DAPT-Score). N = 994, Pearson correlation; Shapiro Wilks test revealed normal distribution, r-value and significance level as indicated. DAPT = Dual antiplatelet therapy; GRACE = Global Registry of Acute Coronary Events; MACCE = Major adverse cardiac and cerebrovascular events; NCDR = National Cardiovascular Data Registry; PARIS = Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients; PD = PRECISE-DAPT = PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Antiplatelet Therapy.
Fig. 2.
Kaplan Meier Kurves with log-rank analysis for occurrence of (A) major and (B) minor bleeding, (C) major adverse cerebro- and cardiovascular events (MACCE) and its single components (D) all-cause mortality, (E) myocardial infarction (MI) and (F) stroke/transient ischemic attack (TIA). N = 994, log-rank test for hazard ratio (HR) determination with 95% confidence interval (CI), Shapiro-Wilks test showed normal distribution. MACCE = Major adverse cerebro- and cardiovascular events; MI = Myocardial infarction; TIA = Transient ischemic attack; TIMI = Thrombolysis in Myocardial infarction.
3.3. Outcomes
During one-year follow-up, MACCE occurred in 195 (19.6%) patients (all-cause mortality 71 [7.1%], MI 62 [6.2%], stroke/TIA 15 [1.5%]). 35 (3.5%) patients had a TIMI major or minor bleeding event (TIMI major 17 [1.7%], TIMI minor 18 [1.8%]).
Log-Rank revealed a higher incidence of major and minor bleeding events during one-year follow-up in patients with PD ≥ 25 (major bleeding: HR 2.9, 95% confidence interval Cl 1.01–8.16, p = 0.049; minor bleeding: HR 3.94, 95% Cl 1.36–9.19, p = 0.0096). MACCE, death and MI occurred more often as well (MACCE: HR 2.0, 95% Cl 1.52–2.71, p < 0.0001; death: HR 3.9, 95% Cl 2.12–5.68, p < 0.0001; MI: HR 2.1, 95% Cl 1.26–3.43, p = 0.0041). Occurrence of stroke was numerically higher in patients with PD ≥ 25 (HR 2.2, 95% CI 0.76–6.26, p = 0.1481). Multivariate analysis was conducted for bleeding and MACCE and revealed robust findings after adjustment for differentiating characteristics (supplement 1).
3.4. Discrimination potency and optimal cut-off values of PRECISE-DAPT for bleeding and ischemic risk
In our cohort, PD showed moderate discrimination indices for prediction of major and minor bleeding (major bleeding: Area under the curve [AUC] 0.66, p = 0.026; Youden-Index [YI] 32, sensitivity [sens] 0.59, specificity [spec] 0.73; minor bleeding: AUC 0.72, p = 0.001, YI 28, sens 0.72, spec 0.63). Moreover, discrimination index of MACCE, all-cause mortality and MI was high as well (MACCE: AUC 0.62, p < 0.0001, YI 24, sens 0.68, spec 0.54; all-cause mortality: AUC 0.73, p = 0.026, YI 29, sens 0.66, spec 0.69 MI: AUC 0.601, p = 0.008, YI 24, sens 0.69, spec 0.51). Discrimination of stroke/TIA was not significant (AUC 0.597, p = 0.291, YI 26, sens 0.65, spec 0.56, Fig. 3A).
Fig. 3.
Receiver operating statistics (ROC) analysis and Youden’s statistic for discrimination potency of the PRECISE-DAPT Score for (A) major and minor bleeding as well as for MACCE and its single components and (B) in comparison with hitherto scores for bleeding and ischemic risk prediction. AUC = area under the curve; DAPT = Dual antiplatelet therapy; GRACE = Global Registry of Acute Coronary Events; MACCE = Major adverse cardiac and cerebrovascular events; MACCE = Major adverse cerebro- and cardiovascular events; MI = Myocardial infarction; NCDR = National Cardiovascular Data Registry; PARIS = Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients; PD = PRECISE-DAPT = PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Antiplatelet Therapy; TIA = Transient ischemic attack.
Discrimination potency for MACCE and bleeding was calculated for all analyzed scores in this study. All scores except from the ABC stroke score predicted the occurrence of MACCE. The HASBLED, DAPT and PARIS thrombotic scores predicted MACCE solely with the highest discrimination index for the PARIS thrombotic score (HASBLED: AUC 0.552, p = 0.024, YI 3, sens 0.51, spec 0.42; DAPT score: AUC 0.55, p = 0.031, YI 2, sens 0.52, spec 0.57; PARIS thrombotic: AUC 0.61, p < 0.0001, YI 4, sens 0.66, spec 0.52). Bleeding prediction was not significant for these scores (HASBLED: AUC 0.59, p = 0.075, YI 3, sens 0.55, spec 0.56; DAPT Score: AUC 0.54, p = 0.464, YI 1, sens 0.457, spec 0.647; PARIS thrombotic: AUC 0.55, p = 0.305, YI 4, sens 0.66, spec 0.49). The PARIS bleeding, GRACE-, NCDR mortality, NCDR bleeding and CHA2DS2-VASc -Score predicted both MACCE (PARIS bleeding: AUC 0.62, p < 0.0001, YI 6, sens 0.73, spec 0.47; GRACE: AUC 0.65, p < 0.0001, YI 118, sens 0.62, spec 0.57; NCDR mortality: AUC 0.66, p < 0.0001, YI 36, sens 0.62, spec 0.60; NCDR bleeding: AUC 0.61, p < 0.001, YI 63, sens 0.49, spec 0.72; CHA2DS2-VASc: AUC 0.57, p = 0.002, YI 5, sens 0.54, spec 0.56) and bleeding significantly (PARIS bleeding: AUC 0.77, p < 0.0001, YI 7, sens 0.77, spec 0.57; GRACE: AUC 0.69, p < 0.0001, YI 130, sens 0.63, spec 0.70; NCDR mortality: AUC 0.67, p = 0.001, YI 35, sens 0.71, spec 0.55; NCDR bleeding: AUC 0.58, p < 0.0001, YI 53, sens 0.69, spec 0.55; CHA2DS2-VASc: AUC 0.56, p = 0.0045, YI 5, sens 0.54, spec 0.56, Fig. 3B).
4. Discussion
The major findings of this study were (A) that PRECISE-DAPT correlates with scores for prediction of bleeding, (B) that PRECISE-DAPT ≥ 25 was associated with higher rates of bleeding but also with enhanced risk for MACCE and its single components all-cause mortality and MI and (C) that discrimination potency of PRECISE-DAPT is moderate for bleeding prediction but also for MACCE with nearly equal cut-off values in our cohort.
The optimal DAPT duration is challenging. DAPT guidelines recommend shortening of DAPT duration down to three months after ACS based on the PRECISE-DAPT Score [1], [4], [13]. In this analysis, nearly half of patients had higher bleeding risk according to PD score and are recommended to receive shortened DAPT. Here, we could show that the PD score predicts bleeding with moderate accuracy – however, ischemic events were predicted as well. This is in line with first pilot studies investigating risk for ischemic events by the PD: Ando et al. revealed that a high PD was associated with higher long-term mortality after ACS [14]. This has also been shown by Morici et al. who investigated platelet count and use of PD score for prediction of mortality [15]. Cardiovascular events are the most common reason for mortality in western countries [16] and moreover, PD was shown to be associated with the degree of coronary stenosis in ACS [17]. Therefore, ischemic events might explain the high mortality in PD ≥ 25 patients. In our study, we show that MI was higher as well. This is in line with a recent analysis by Valgimgli et al. who revealed that PD also has a discrimination capacity for cardiovascular mortality, MI or stent thrombosis [5]. Based on these results, optimal DAPT duration in patients with high risk for thrombotic and bleeding events seems even more challenging in reference to scoring systems. In comparison, the American DAPT-guidelines do not include a score based decision for de-escalated DAPT. Instead, an individual assessment including clinical judgment and a balanced ratio for bleeding and ischemia is recommended [18]. This seems both pleasant and testing as it relies on the clinical assessment of the treating physician. However, a sub-study of the SMART-Date trial investigated shortening DAPT duration to 6 months with the view to bleeding and MACCE. The authors revealed a reduction in bleeding complications without any differences in MACCE [19]. Additionally, a recent meta-analysis investigated adaption of DAPT duration by PD after second-generation stent implantation: Bleeding complications were again reduced in patients with shortened DAPT whereby MACCE were unaffected [20]. This underlines the clinical usefulness of the score irrespectively from prediction of MACCE. It is crucial in this context that PD is based on a cohort with pre-dominant clopidogrel use for DAPT. However, ticagrelor and prasugrel are recommended over clopidogrel in ACS [1], [21]. Nevertheless, a current analysis by Valgimigli et al. investigated PD in DAPT with ticagrelor. In this cohort as well, PD was useful to predict major bleeding with the PARIS thrombotic score to complement for ischemic risk prediction [5].
As expected, PD predicted bleeding well in our analysis. This is in line with its generation approach and was already demonstrated in several studies [19], [22]. Additionally, its predictive ability was shown to be stable with regard to longitudinal changes [23]. Moreover, a simplified four-item PD score was already shown to categorize patients benefiting from prolonged DAPT without higher risk for bleeding [24], [25]. In our analysis, major bleeding only just reached statistical significance. This might be due to the fact that bleeding rates were rather low as they were defined by TIMI major and minor bleeding. Moreover, patient related factors such as co-morbidities, co-medication and clinical presentation in comparison with other studies might have influenced results. Nearly two thirds of patients were on proton pump inhibitors in our study. Moreover, due to its all-comers design, number of patients with ACS as index event rather low. However, in a post hoc sub-analysis of the Global Leaders trial, PD was demonstrated to predict short-term bleeding both for the over-all population and for the subgroup of ACS patients [26]. Interestingly, patients with PD > 25 had more often triple antiplatelet therapy in line with an increased rate of patients with AF. Triple therapy is a known risk factor for bleeding that will have intensified rate of bleeding in this cohort. However, DAPT duration is altered in these patients with four weeks of triple therapy followed by dual therapy with a P2Y12 inhibitor and oral anticoagulation. In case of high bleeding risk, a potential drop of aspirin is discussed as strategy to reduce rate of bleeding events [3], [4].
It is a common problem that bleeding scores predict ischemic events as well and vice versa. This might be due to the fact that included parameters are similar. Especially age is included in every score except from the PARIS thrombotic score. Moreover, any kind of thromboembolic event in the past or CKD represent frequently used parameters followed by ACS, diabetes, anemia, reduced ejection fraction or smoking. In comparison with the other analyzed scores, prediction of bleeding substantially differed in our study. The PARIS bleeding score showed the highest discrimination index followed by PD, GRACE – and NCDR mortality Score. The NCDR Bleeding and CHA2DS2-VASc Score predicted bleeding with moderate accuracy. The HASBLED-, DAPT-, PARIS thrombotic and ABC stroke score did not predict bleeding. Surprisingly, the CHA2DS2-VASc Score predicted bleeding even better than the HASBLED score. However, these scores as well as the ABC stroke score were established for patients with indication for oral anticoagulation and not for risk assessment post PCI. In our cohort, only one quarter of patients had AF and moreover, rate of stroke was low. These facts might have biased the results.
A prolonged DAPT is discussed in accordance to the DAPT score [7]. In our study, the DAPT score performed moderately in prediction of MACCE but did not predict the occurrence of bleeding. However, discrimination between bleeding and ischemic events by DAPT score is currently a matter of debate. In this context, a recent analysis of real world data did not reveal relevant differences in MI rates in patients stratified by DAPT score [27]. Moreover, a recent Swedish register study demonstrated that DAPT score did not discriminate well between bleeding and ischemic events [28]. The PARIS thrombotic score was established to calculate the risk of coronary thrombotic events after PCI [12]. Here, we could show that performance in prediction of MACCE was precise without prediction of bleeding. This is in line with the results by Valgimigli et al. [5].
Our study has several limitations. First, this is not a prospective-randomized trial. Moreover, patients with PD ≥ 25 had more co-morbidities and differed in laboratory parameters and co-medication. However, multivariate analysis was conducted to adjust for these differentiating characteristics. Nevertheless, further factors that have been assessed might have confounded the analysis as well. Additionally, our cohort represents a heterogeneous one with 60% of patients with acute coronary syndrome with higher mortality and thrombotic risk than CCS patients. Moreover, a substantial part of patients is in the need for triple therapy. Oral anticoagulants have been shown to influence platelet reactivity [2]. Therefore, further sub-analyses in these patients are needed. Finally, this study was not designed to investigate clinical outcome based on shortened DAPT duration. This would have applicated our findings more directly into routine clinical practice. However, our findings emphasize the importance of an individual based medicine instead of an objectified score related risk estimation.
5. Conclusion
With the aim of an objectified therapy, a multiplicity of scores is available in clinical routine. The recently developed PRECISE-DAPT score is easy to use and PD score has a high prediction value for bleeding prediction. In this study, we revealed that MACCE can be predicted as well. Hence, adaptation of antiplatelet therapy based on calculated PD score is a useful, objective tool to assess bleeding risk in post-PCI patients. Nevertheless, it should be made with caution under outweigh of ischemic complications with the view to an individualized patient related therapy.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgments
None.
Funding source
This work was supported by the Forschungskommission of the Medical Faculty of the Heinrich Heine University (No 29-2019, to L.D., No. 18-2019 to A.P., No. 15-2019 to S.A., 2018-32 to G.W) and by the German Research Foundation (PO 2247/2-1 to A.P. and SFB1116 to A.P.).
Ethics committee approval
The study conformed to the Declaration of Helsinki and was approved by the University of Düsseldorf Ethics Committee.
Conflict of interest/Disclosures
None.
Authors’ contributions
L.D., S.A., N.C., R.M., S.Z. designed the study, analyzed and interpreted data and wrote the manuscript. C.H., P.M., D.Z., S.A., K.T., M.Be., M.Ba. collected data and revised the manuscript. G.W., T.Z., M.K. and A.P. supervised the study and revised the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100750.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Valgimigli M., Bueno H., Byrne R.A. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2017 doi: 10.1093/ejcts/ezx334. [DOI] [PubMed] [Google Scholar]
- 2.Petzold T., Thienel M., Dannenberg L.K. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa Driven Platelet Activation Via Protease Activated Receptor-1. Circ. Res. 2019 doi: 10.1161/CIRCRESAHA.119.315099. [DOI] [PubMed] [Google Scholar]
- 3.Knuuti J., Wijns W., Saraste A. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 4.Collet J.P., Thiele H., Barbato E. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa624. [DOI] [PubMed] [Google Scholar]
- 5.Bianco M., D'Ascenzo F., Raposeiras Roubin S. Comparative external validation of the PRECISE-DAPT and PARIS risk scores in 4424 acute coronary syndrome patients treated with prasugrel or ticagrelor. Int. J. Cardiol. 2020;301:200–206. doi: 10.1016/j.ijcard.2019.11.132. [DOI] [PubMed] [Google Scholar]
- 6.Granger C.B., Goldberg R.J., Dabbous O. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003;163(19):2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 7.Yeh R.W., Secemsky E.A., Kereiakes D.J. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA. 2016;315(16):1735–1749. doi: 10.1001/jama.2016.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson E.D., Dai D., DeLong E.R. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 2010;55(18):1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisters R., Lane D.A., Nieuwlaat R. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 10.Lip G.Y., Nieuwlaat R., Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 11.Hijazi Z., Lindback J., Alexander J.H. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur. Heart J. 2016;37(20):1582–1590. doi: 10.1093/eurheartj/ehw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baber U., Mehran R., Giustino G. Coronary Thrombosis and Major Bleeding After PCI With Drug-Eluting Stents: Risk Scores From PARIS. J. Am. Coll. Cardiol. 2016;67(19):2224–2234. doi: 10.1016/j.jacc.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 13.Costa F., van Klaveren D., James S. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389(10073):1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 14.Ando T., Nakazato K., Kimishima Y. The clinical value of the PRECISE-DAPT score in predicting long-term prognosis in patients with acute myocardial infarction. Int J Cardiol Heart Vasc. 2020;29 doi: 10.1016/j.ijcha.2020.100552. 100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morici N., Tavecchia G.A., Antolini L. Use of PRECISE-DAPT Score and Admission Platelet Count to Predict Mortality Risk in Patients With Acute Coronary Syndrome. Angiology. 2019;70(9):867–877. doi: 10.1177/0003319719848547. [DOI] [PubMed] [Google Scholar]
- 16.Mortality, G.B.D. and C. Causes of Death, Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, 2015. 385(9963): p. 117-71. [DOI] [PMC free article] [PubMed]
- 17.Long T., Peng L., Li F. Correlations of DAPT score and PRECISE-DAPT score with the extent of coronary stenosis in acute coronary syndrome. Medicine (Baltimore) 2018;97(39) doi: 10.1097/MD.0000000000012531. e12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine G.N., Bates E.R., Bittl J.A. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2016;152(5):1243–1275. doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Choi K.H., Song Y.B., Lee J.M. Clinical Usefulness of PRECISE-DAPT Score for Predicting Bleeding Events in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention: An Analysis From the SMART-DATE Randomized Trial. Circ Cardiovasc Interv. 2020;13(5) doi: 10.1161/CIRCINTERVENTIONS.119.008530. e008530. [DOI] [PubMed] [Google Scholar]
- 20.Jang J.Y., Jung H.W., Lee B.K. Impact of PRECISE-DAPT and DAPT Scores on Dual Antiplatelet Therapy Duration After 2nd Generation Drug-Eluting Stent Implantation. Cardiovasc. Drugs Ther. 2020 doi: 10.1007/s10557-020-07008-7. [DOI] [PubMed] [Google Scholar]
- 21.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 22.Choi S.Y., Kim M.H., Cho Y.R. Performance of PRECISE-DAPT Score for Predicting Bleeding Complication During Dual Antiplatelet Therapy. Circ Cardiovasc Interv. 2018;11(12) doi: 10.1161/CIRCINTERVENTIONS.118.006837. e006837. [DOI] [PubMed] [Google Scholar]
- 23.Pelliccia, F., V. Pasceri, G. Marazzi, et al., Predictive ability of longitudinal changes in PRECISE-DAPT score in patients on dual antiplatelet therapy: The RE-SCORE multicentre prospective registry. Eur J Prev Cardiol, 2020: p. 2047487320937846. [DOI] [PubMed]
- 24.Costa F., van Klaveren D., Colombo A. A 4-item PRECISE-DAPT score for dual antiplatelet therapy duration decision-making. Am. Heart J. 2020;223:44–47. doi: 10.1016/j.ahj.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Choi S.Y., Kim M.H., Yun S.C. Predicting bleeding risk by simplified PRECISE-DAPT score. Thromb. Res. 2020;195:72–73. doi: 10.1016/j.thromres.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima H., Gao C., Takahashi K. Comparative Assessment of Predictive Performance of PRECISE-DAPT, CRUSADE, and ACUITY Scores in Risk Stratifying 30-Day Bleeding Events. Thromb. Haemost. 2020;120(7):1087–1095. doi: 10.1055/s-0040-1712449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witberg G., Zusman O., Bental T. Validation of the DAPT score in real-world patients undergoing coronary stent implantation. Int. J. Cardiol. 2020;300:99–105. doi: 10.1016/j.ijcard.2019.08.044. [DOI] [PubMed] [Google Scholar]
- 28.Ueda P., Jernberg T., James S. External Validation of the DAPT Score in a Nationwide Population. J. Am. Coll. Cardiol. 2018;72(10):1069–1078. doi: 10.1016/j.jacc.2018.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.