Abstract
We here present a female patient who is a recipient of liver transplantation from a cadaveric donor. She developed abdominal pain, nausea, vomiting, and diarrhea for two weeks of duration, after four months of the transplant. Upper gastrointestinal (GI) endoscopy and stool analysis for ova and parasite showed Necator americanus / Ancylostoma duodenale (Hookworm) ova /larvae and Strongyloides stercoralis Larvae. She had a dramatic clinical response to Ivermectin and Albendazole combination, which was given until the clearance of her stool exam. She was discharged from the hospital in good condition, and her infection is considered as a donor-derived transmission of these parasites. To the best of our knowledge, this is the first case report of Strongyloides stercoralis and hookworm co-infection in a liver transplant patient. Parasitic infection should be considered in the differential diagnosis of diarrheal illness of cadaveric transplant patients, even if it is not prevalent in the region.
Keywords: Strongyloides, Hookworms, Liver transplant patient
Introduction
Strongyloides stercoralis and hookworms are parasitic intestinal nematodes that belong to the group of soil-transmitted helminths (STH) [1]. Strongyloides stercoralis had significant morbidity but are mostly neglected, while Hookworm causes public health burden among Soil-transmitted helminths but non-endemic in our area. Both parasites have the same infection route, i.e., skin penetration [1]. Hookworm is one of the most common roundworms of humans, and infection is caused by the nematode parasites Necator americanus and Ancylostoma duodenale. Hookworm infections usually occur in areas where human feces are used as fertilizer or where defecation onto soil happens [2].
Ancylostoma duodenale and Necator americanus, are worldwide in areas with warm, moist climates and are widely overlapping. Necator americanus was widespread in the Southeastern United States until the early 20th century [2].
We describe an unusual case of an immunocompromised patient (liver transplant recipient) presenting with GI symptoms found later to have parasitic Co-infection.
Case presentation
A 53-year-old Kuwaiti female, known case of Diabetes mellites, Ulcerative colitis, and liver cirrhosis due to Autoimmune liver disease, undergone a successful cadaveric liver transplant from a Bangladesh-born donor. The patient's immunosuppressive regimen after transplant consisted of Mycophenolate Mofetil, Tacrolimus, and Prednisolone.
No other details from the donor were available except he was admitted as a case of motor vehicle accident then became brain dead, his hospitalization was for one week, and during this week he had been managed for his multiple trauma. The pre-transplant screening investigation did not include Strongyloides serology.
Four months after the transplant, she presented to the hospital with abdominal pain, nausea, vomiting, and diarrhea for two weeks of duration, the diarrhea was watery with no blood or mucus, about 4–6 times/day, no history of eating from outside or any family member had the same symptoms, no respiratory or genitourinary symptoms, no animal or plants exposure. She never traveled to an area where strongyloidiasis or Hookworms were known to be endemic.
The immunosuppressive regimen: (Mycophenolate Mofetil (750 mg PO twice daily) Tacrolimus (6 mg PO once daily) and Prednisolone (25 mg PO once daily)).
The physical exam was notable only for minimal tenderness in the epigastrium without any organomegaly or masses; her chest examination was within normal. No skin rash was noted.
She was admitted from the clinic for further workup and endoscopy. Stool analysis for ova and parasites was done for her, which was showing (Many Necator americanus / Ancylostoma duodenale (Hookworm) OVA, and Larvae Seen, Moderate Strongyloides stercoralis Larvae Seen). Strongyloides larvae were identified on a wet mount of a stool specimen by their morphological characteristics of a large genital primordium and short buccal capsule (Fig. 1).
Fig. 1.
Stool analysis for ova and parasites showing (Necator americanus / Ancylostoma duodenale (Hookworm) ova and larvae, and Strongyloides stercoralis Larvae).
The method used was the formalin–ethyl acetate sedimentation technique. The microscopic examination was performed by a correctly calibrated microscope on Fresh stool processed directly for a (liquid or semi-liquid) sample and used a wet mount of the sediment.
The direct wet mount examination of the specimen is used for quantitative assessments of infection. All specimens are storage at 4 °C/3 days after examination performed as lab requirement. All Safety Standard protocols and adopting universal precautions were applied for the handling of patient samples, including Administrative and workplace controls, as an example of hand hygiene and Personal protective equipment (PPE) to eliminate and reduce the chemical/biological hazards.
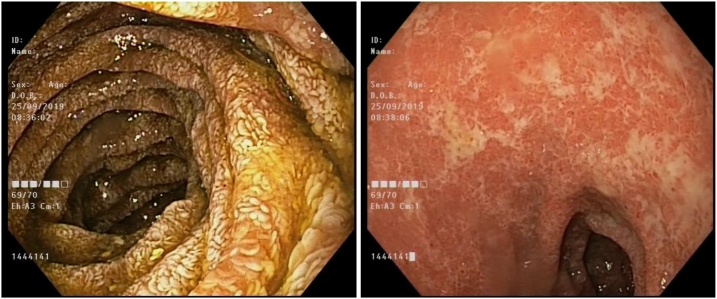
Upper GI endoscopy was done on the next day, showing (mild lower esophagitis, severe gastritis with multiple clean ulcers and multiple ulcers in the duodenum) duodenal aspirates, and multiple biopsies were taken (Fig. 2). Gastric ulcer body and Antral biopsy were showing Epstein-Barr virus (EBV)- negative post-transplant lymphoproliferative disorder; diffuse large B-cell lymphoma, non-germinal center subtype.
Fig. 2.
Esophagogastroduodenozscopy showing mild lower esophagitis, severe gastritis with multiple clean ulcers and multiple ulcers in the duodenum.
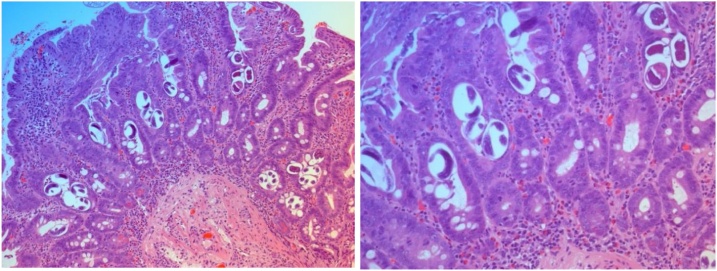
A duodenal biopsy was done, and it shows Strongyloides duodenitis (Fig. 3).
Fig. 3.
A) HE staining of duodenal biopsy: The mucosa showing numerous larvae WITH Subtotal villous atrophy, eosinophils infiltration. B) HE staining of duodenal biopsy: Numerous larvae are observed within the glands.
The patient was started on a combination of Ivermectin (12 mg orally daily) and Albendazole (400 mg orally Q12 h) and continued to complete ten days of treatment, during this period the patient improved from GI point of view, the Strongyloides and the hookworms were persistent in the stool for five days then cleared.
Her Immunosuppressive therapy was reduced, and Mycophenolate Mofetil was stopped. She was evaluated after finishing the antiprotozoal treatment by Hematology team regarding her finding of diffuse large B-cell lymphoma in the biopsies from the gastric ulcers, and start her on Rituximab. The patient was assessed in the clinic six months later, and she was in good health.
Discussion
Strongyloides stercoralis and hookworms are parasitic intestinal nematodes, and they are common in tropical and subtropical regions [1,3,4]. There are high numbers of immunocompromised patients throughout the world, including transplant recipients, which need a closer examination of parasitic infections like S. stercoralis and Hookworm infection particularly if the clinical presentation support that [5].
Strongyloidiasis has been reported in transplant recipient patients of either hematopoietic stem cells or solid organ transplants like kidneys, liver, heart, intestine, and pancreas [6]. The sources of infection in the recipient usually identified either as chronic preexisting infection in the recipient or from the transmission from the donor organ [7].
Infection with Strongyloides or Hookworm in the immunocompetent host is associated with mild gastrointestinal symptoms. However, Strongyloidiasis in immunosuppressed patients, it has been known to cause a "hyper infection syndrome" with fatal complications [7,8]. In our patient here, either Reactivation of latent infection or transmission from donor organs have been suggested as possible causes initially.
Stool analysis for ova and parasite for our patient was negative four months pre-transplant, which supports that the patient getting the co-infection from the donor, also that donor is originally from an endemic area of this parasitic infection, which support our theory of that infection was transmitted from the donor. After reporting this to the transplant coordinator to trace other recipients, we discovered another recipient (Kidney transplant) from the same donor, up to our knowledge, they also had Strongyloides infection.
A study done in Dhaka, Bangladesh, confirms the high prevalence of S. stercoralis infection identified both by serological and coprological methods [9] and another study showing the aggregation of S.stercoralis infection may be related to risk factors or familial genetic predisposition to infection [10].
There are several reported cases of post-transplantation Strongyloidiasis infection in which the clinical circumstances and laboratory results strongly suggest donor transmission of Strongyloides [11]. Serological testing for Strongyloides is generally available. ELISA IgG antibody tests 90 % estimated sensitivity for patients with documented Strongyloides larvae in stool specimens. Some patients are from the group with 5 %–10 % with false-negative results, and this is maybe related to cross-react with other nematodes. So, clinical suspicion remains essential, and negative antibody testing should be taken cautiously with the risk factors of the patient [11]. This serological screening is not a part of the regular pre-transplant workup in Saudi Arabia as the disease is rare in the region.
Treatment for the Co-infection (Strongyloides and Hookworm) was recommended in some studies to be continued daily until the microscopic clearance of the larvae [11]. This is what our treatment team elected to use in treating the patient and showed a favorable outcome.
In conclusion, we need to have high anticipation for the parasitic infection in immunocompromised patients, especially the transplant recipients from donors who originated from an endemic area of parasitic infections. Early identification and treatment would result in a positive outcome. Strongyloides serology as a pre-transplant screening might be useful and should be considered for cadaveric or high-risk transplant donors.
References
- 1.Forrer A. Strongyloides stercoralis and hookworm co-infection: spatial distribution and determinants in Preah Vihear Province, Cambodia. Parasit Vectors. 2018;vol. 11(33):11–33. doi: 10.1186/s13071-017-2604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC . 2013. Global health, division of parasitic diseases. 10 January [Online] [Google Scholar]
- 3.Hamilton K.W. Donor-Derived Strongyloides stercoralis Infections in Renal Transplant Recipients. Transplantation. 2011;vol. 91(no. 9) doi: 10.1097/TP.0b013e3182115b7b. [DOI] [PubMed] [Google Scholar]
- 4.Requena-Méndez A. The American society of tropical medicine and hygiene. 2017. Evidence-based guidelines for screening and management of strongyloidiasis in non-endemic countries; pp. 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keiser P.B. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;vol. 17(1) doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos L.A. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep. 2011;vol. 13(1) doi: 10.1007/s11908-010-0150-z. [DOI] [PubMed] [Google Scholar]
- 7.Mani B.C. Case Reports in Transplantations; 2013. Strongyloides stercoralis and Organ Transplantation; p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winnicki W. Prevalence of Strongyloides stercoralis infection and hyperinfection syndrome among renal allograft recipients in Central Europe. Natureresearch J. 2018 doi: 10.1038/s41598-018-33775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.YasminSultana Strongyloidiasis in a high risk community of Dhaka, Bangladesh. ScienceDirect. 2012:756–762. doi: 10.1016/j.trstmh.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Conway D.J. Household aggregation of Strongyloides stercoralis infection in Bangladesh. Trans R Soc Trop Med Hyg. 1995;vol. 89(no. 3) doi: 10.1016/0035-9203(95)90527-8. [DOI] [PubMed] [Google Scholar]
- 11.Roxby A.C. Strongyloidiasis in transplant patients. Clin Infect Dis. 2009;vol. 49(9):1411–1423. doi: 10.1086/630201. [DOI] [PMC free article] [PubMed] [Google Scholar]