Abstract
Eurasian avian-like H1N1 (EA H1N1) swine influenza virus (SIV) outside European countries was first detected in Hong Kong Special Administrative Region (Hong Kong, SAR) of China in 2001. Afterwards, EA H1N1 SIVs have become predominant in pig population in this country. However, the epidemiology and genotypic diversity of EA H1N1 SIVs in China are still unknown. Here, we collected the EA H1N1 SIVs sequences from China between 2001 and 2018 and analyzed the epidemic and phylogenic features, and key molecular markers of these EA H1N1 SIVs. Our results showed that EA H1N1 SIVs distributed in nineteen provinces/municipalities of China. After a long-time evolution and transmission, EA H1N1 SIVs were continuously reassorted with other co-circulated influenza viruses, including 2009 pandemic H1N1 (A(H1N1)pdm09), and triple reassortment H1N2 (TR H1N2) influenza viruses, generated 11 genotypes. Genotype 3 and 5, both of which were the reassortments among EA H1N1, A(H1N1)pdm09 and TR H1N2 viruses with different origins of M genes, have become predominant in pig population. Furthermore, key molecular signatures were identified in EA H1N1 SIVs. Our study has drawn a genotypic diversity image of EA H1N1 viruses, and could help to evaluate the potential risk of EA H1N1 for pandemic preparedness and response.
Electronic supplementary material
The online version of this article (10.1007/s12250-020-00257-8) contains supplementary material, which is available to authorized users.
Keywords: Eurasian avian-like H1N1 (EA H1N1) swine influenza viruses (SIV), Epidemiology, Genotypes, Molecular markers
Introduction
Pigs are considered as “mixing vessel” of human and avian influenza viruses, since their respiratory tract contained both human-type and avian-type receptors (Ito et al. 1997; Suzuki et al. 1997). When infecting more than one co-criculating viruses, reassortments occurred and novel reassortant viruses were generated (Scholtissek et al. 1985). Currently, several lineage of swine influenza viruses (SIVs) were circulating in pigs globally, including classical swine influenza viruses (CS), Eurasian avian-like influenza viruses (EA), triple reassortment influenza viruses (TR), 2009 pandemic H1N1 viruses (A(H1N1)pdm09) and several reassorted SIVs (Chen et al. 2013; Smith et al. 2009; Yang et al. 2016).
The EA SIVs, with all eight genes from avian influenza virus gene pool, were firstly recognized in 1979 in Europe (Pensaert et al. 1981; Scholtissek et al. 1983). Since 1979, EA SIVs appeared to have a selective advantage over CS H1N1 and had replaced the CS SIVs in European swine populations (Campitelli et al. 1997). In China, CS H1N1 viruses were the predominant viruses in pigs before 2002. Since 2002, the prevalence of EA H1N1 SIVs were continuously increasing (Vijaykrishna et al. 2011). At present, EA H1N1 SIVs were predominant in pigs in China. Reassorments among EA SIVs and other co-circulated SIVs were continuously occurred and various genotypes were generated. Recent studies reported that the EA H1N1 SIVs circulated in pigs in China can bind to human-type receptors and most of them can transmitted via respiratory droplets among ferrets model (Yang et al. 2016). In addition, the majority of human population had low immunity to EA H1N1 viruses (Vijaykrishna et al. 2011). Actually, human infections with EA H1N1 SIVs have been reported in Europe and Asia (Gregory et al. 2003; Yang et al. 2012). In Chinese mainland, different genotypes EA H1N1 SIVs have been reported to infect humans (Wang et al. 2013; Xie et al. 2018; Yang et al. 2012; Zhu et al. 2016, 2019). Thus, the EA H1N1 SIVs were proposed as one of the most potential zoonotic influenza viruses to cause the next pandemic (Yang et al. 2016).
Previous studies on SIVs in China revealed that after a long-time evolution and transmission, the reassortant EA SIVs were dominant and the number of reassortant viruses was increasing during 2007–2009 (Vijaykrishna et al. 2011). In 2010, two novel double reassortants EA H1N1 SIVs were isolated (Sun et al. 2016). The EA H1N1 SIVs variants were reassorted with A(H1N1)pdm09 or TR H1N2 viruses, which were transmitted efficiently from pig to pig and from pig to ferret (Zhu et al. 2011). The surveillance in pigs from 2010 to 2013 in China isolated 139 EA H1N1 SIVs from ten provinces, which were divided into 5 genotypes (Yang et al. 2016). Up to now, no reports have updated the epidemiology and genotypic diversity of EA SIVs in pigs in China. Thus, we performed a systematic analysis on the epidemic characteristics, the variety and prevalence of genotypes and the key molecular markers of EA SIVs in China.
Materials and Methods
Surveillance Data and Genomic Sequences
The genomic sequences of Eurasian avian-like swine influenza A virus isolated in China were searched from the public database GenBank and Global Initiative on Sharing All Influenza Data (GISAID) databases from 2001 to 2018, including 293 HA, 274 NA, 271 PB2, 273 PB1, 269 PA, 272 NP, 276 M, 272 NS. A total of 266 full genomic sequences of EA SIVs were downloaded (Supplementary Table S1). Spatial and temporal data of these 266 EA SIVs were collected and analyzed.
Phylogenetic Analysis
All available EA H1N1 SIVs genomic sequences were aligned with MAFFT v7.222 (Kazutaka and Standley 2013). Phylogenetic trees were generated by applying maximum likelihood (ML) method with general time-reversible (GTR) model. The robustness of ML topology was determined by 1000 bootstrap replicates.
Genotypic Diversity
To identify the genotypic diversity of EA H1N1 SIVs, genotypes were defined based on the clade distributions of their internal gene segments. From the ML phylogenies, each gene segment was assigned to the specific lineages to generate a genotype.
Genotypes Definition
According to the ML phylogenies, each gene segment was categorized into lineages circulating in swine in China, as follows: EA H1N1, A(H1N1)pdm09, CS H1N1, and TR H1N2. Then the eight gene segments of each EA H1N1 virus could be derived from different lineages. The gene combination of distinct lineages was classified as a specific genotype.
Results
The Description Analysis of EA SIVs Sequences in China during 2001–2018
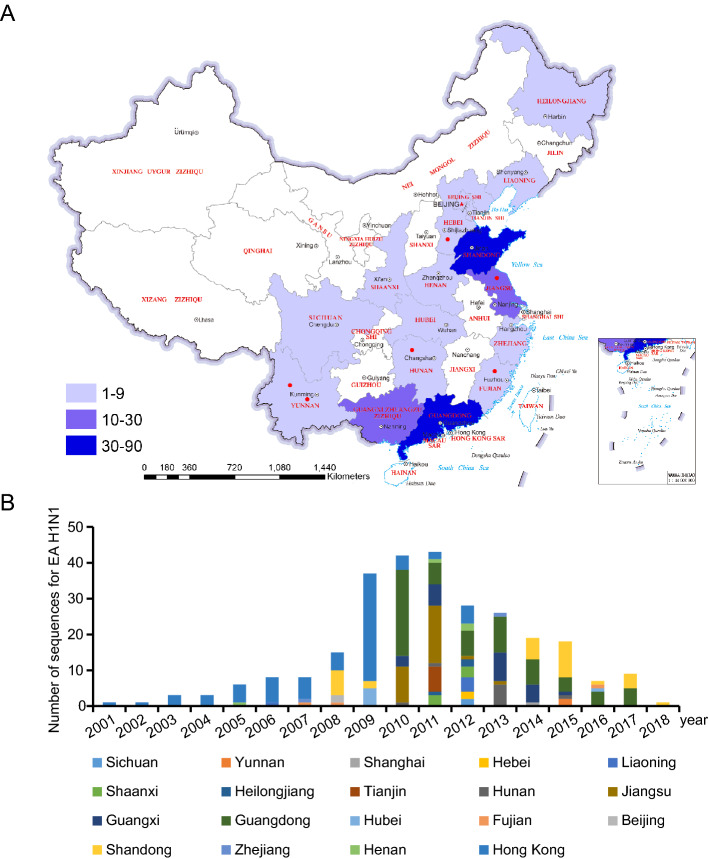
In all 293 HA sequences, 93.9% (275) of EA SIVs were EA H1N1 SIVs and 6.1% (18) were EA H1N2 SIVs. From 2001 to 2018, 275 EA H1N1 SIVs sequences were collected from nineteen provinces/municipalities of China (Fig. 1A). Hong Kong contributed the most EA H1N1 SIVs sequences, followed by Guangdong and Shandong provinces. The first EA H1N1 sequence was reported in Hong Kong in early 2001. Subsequently, more EA H1N1 SIVs sequences were reported and peaked during 2009–2011 (Fig. 1B). Human cases infected with EA H1N1 SIVs were reported from Jiangsu, Hebei, Hunan, Yunnan, and Fujian provinces (Table 1). These results indicated that the EA H1N1 SIVs were currently prevalent in pigs and sporadically cause human infections in China.
Fig. 1.
The description analysis of EA H1N1 SIVs sequences in China during 2001–2018. A Geographic distributions of EA H1N1 SIVs sequences in China. EA H1N1 SIVs affected regions are highlighted in blue. Blue from light to dark indicated the virus EA H1N1 SIVs number increased from 1 to 90. The red circles indicated human cases of infection with EA H1N1 SIVs. B Temporal distribution of EA H1N1 SIVs sequences in swine in China during 2001–2018.
Table 1.
Human cases infected with EA H1N1 SIVs in China.
| Year | Province | Strain name | Genotype | References |
|---|---|---|---|---|
| 2011 | Jiangsu | A/Jiangsu/1/2011 | 1 | Yang et al. (2012) |
| 2012 | Hebei | A/Hebei-Yuhua/SWL1250/2012 | 1 | Wang et al. (2013) |
| 2015 | Hunan | A/Hunan/42443/2015 | 3 | Zhu et al. (2016) |
| 2015 | Yunnan | A/Yunnan-Longyang/SWL1982/2015 | 3 | Zhu et al. (2019) |
| 2015 | Yunnan | A/Yunnan-Wuhua/SWL1869/2015 | 3 | Zhu et al. (2019) |
| 2016 | Fujian | A/Fujian-Cangshan/SWL624/2016 | 5 | Xie et al. (2018) |
Phylogenetic Analysis of EA H1N1 SIVs
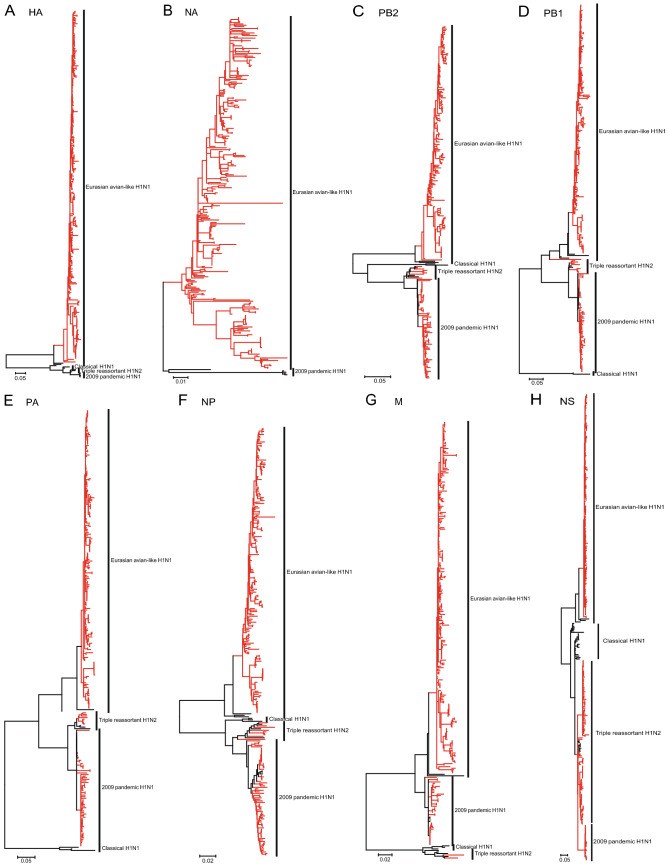
To determine the evolution of EA H1N1 SIVs in China during 2001–2018, phylogenetic analyses of all eight gene segments were performed. Phylogenetic tree of hemagglutinin (HA) showed that the EA H1N1 SIVs isolated from pigs in China formed a monophyletic group (Fig. 2A, red branches). We used the swine H1 clade classification tool to classify the clade of HA (Anderson et al. 2016) and found that all the HA sequences of EA H1N1 SIVs were classified as 1C.2.3. In contrast, the other genes of EA H1N1 SIVs, including the neuraminidase (NA), basic polymerase 2 (PB2), basic polymerase 1 (PB1), polymerase (PA), nucleoprotein (NP), matrix (M), and nonstructural protein (NS), were demonstrated distinct diversity (Fig. 2B–2H). The NA genes were derived from EA H1N1 and A(H1N1)pdm09. Origins of the PB2, PB1, PA, NP and M genes included EA H1N1, A(H1N1)pdm09 and TR H1N2. The NS genes were derived from EA H1N1, A(H1N1)pdm09, and TR H1N2 respectively. These results indicated that dynamic reassortments occurred between the EA H1N1 and co-circulated SIVs in pigs.
Fig. 2.
Phylogenies of HA (A), NA (B), PB2 (C), PB1 (D), PA (E), NP (F), M (G), and NS (H) of EA H1N1 SIVs from 2001 to 2018. The phylogenetic analysis was performed by MEGA 7.0 with maximum likelihood (ML) method. The bootstrap was 1000. The red indicated the sequences of EA H1N1 SIVs. The black indicated the reference sequences.
Genotypic Diversity of EA H1N1 SIVs
Reassortant genotypes of the EA H1N1 SIVs could then be defined based on the clade distributions of their internal gene segments. The EA H1N1 SIVs isolated from both pigs and humans were classified into 11 distinct genotypes, from genotype 1 to genotype 11. Genotype 1 viruses accounted for 55.3% of the EA H1N1 SIVs. And the eight gene segments of this genotype viruses were exclusively originated from EA H1N1 (Fig. 3). EA H1N1 and TR H1N2 viruses produced double reassortment viruses and generated genotypes 2, 6, and 10 (Fig. 3). The introductions of the A(H1N1)pdm09 into pigs continuously provided their internal genes to EA H1N1 SIVs and generated novel genotypes 4, 7, and 8 (Fig. 3). Reassortments of EA H1N1, A(H1N1)pdm09 and TR H1N2 generated triple reassortment viruses and formed genotypes 3, 5, 9, and 11 (Fig. 3). These results indicated that the EA H1N1 SIVs circulated in China exhibited a high genotypic diversity.
Fig. 3.
Genotypes of EA H1N1 SIVs identified in China. The name of representative viruses are showed (left). All the eight gene segments of EA H1N1 SIVs are at the top of the graph. Origin of each gene segment is indicated by a colored block for representing the different swine influenza virus lineages. The 11 distinct genetic constellations are labeled as genotype 1 to 11. EA H1N1, Eurasian avian-like H1N1; A(H1N1)pdm09, 2009 pandemic H1N1; TR H1N2, triple reassortment H1N2.
Genotype 1 was widely prevalent from 2001 to 2013 (Fig. 4A). From 2009 to 2013, the genotypes 2 and 4 were co-circulated with genotype 1 (Fig. 4A). Since 2013, genotypes 3 and 5 have gradually replaced genotypes 1, 2, and 4 in swine populations (Fig. 4A). Guangdong Province had the largest number of genotypes, with a total of 7 genotypes (Fig. 4B). In addition, Hong Kong and Guangxi ranked second and third, respectively (Fig. 4B). This result indicated that genotype 3 and 5 have become predominant in pig population.
Fig. 4.
Development and prevalence of EA H1N1 genotypes during 2001–2018. A 11 distinct genotypes are listed on the left. The colored circles represent the corresponding genotypes and the isolated time. B The distribution of EA H1N1 genotypes in each province. The size of the circle indicated the number of EA H1N1 SIVs. The different colors represent the different genotypes which refer to A.
Molecular Characteristics of EA H1N1 SIVs
We next analyzed the molecular characteristics which were associated with mammalian adaptations, receptor binding ability, virulence or transmission and antiviral resistance of all EA H1N1 SIVs (Table 2).
Table 2.
Prevalence of key molecular markers in EA H1N1 SIVs in China.
| Gene | Phenotypic characteristic(s) | Mutation | Percentage | |
|---|---|---|---|---|
| HAa | Altered the receptor specificity | E190D | E (0%) | D (85.9%) |
| G225E | G (12.7%) | E (84.1%) | ||
| NAb | Resistance to NA inhibitors | H274Y | H (100%) | Y (0%) |
| N294S | N (100%) | S (0%) | ||
| PB2 | Altered the virulence in mice | D9N | D (98.1%) | N (1.1%) |
| L89V | L (0.4%) | V (98.9%) | ||
| E158G | E (100%) | G (0%) | ||
| Mammalian host adaption | D256G | D (100%) | G (0%) | |
| Enhance viral polymerase activity | T271A | T (68%) | A (30.8%) | |
| Enhance the 627 k and 701 N function | K526R | K (94.4%) | R (5.6%) | |
| Restored the polymerase activity | M535L | M (100%) | L (0%) | |
|
Enhance the viral polymerase activity, increase the virulence in mice |
Q591K | Q (66.2%) | K (0.4%) | |
| E627K | E (98.5%) | K (0.4%) | ||
| D701N | D (30%) | N (70%) | ||
| Altered the virulence in mice | A676T | A (2.2%) | T (96%) | |
| PB1 | Enhance the viral polymerase activity | L473V | L (0.4%) | V (99.6%) |
| Altered the virulence in mice | R198K | R (0.8%) | K (99.2%) | |
| PA | Enhance the viral polymerase activity | L336M | L (72.9%) | M (26.8%) |
|
Enhance the viral polymerase activity, increase the virulence in mice |
K356R | K (74.4%) | R (25.7%) | |
| Species-associated signature positions | S409N | S (17.5%) | N (81.0%) | |
| NP | Enhance the viral polymerase activity | A150R | A (0%) | R (98.9%) |
|
Enhance the viral polymerase activity, altered the virulence in mice |
N319K | N (85.6%) | K (14.4%) | |
| Q357K | Q (66.7%) | K (31.5%) | ||
| M1 | Increase the transmission in guinea pigs | P41A | P (0%) | A (98.6%) |
| Altered the virulence in mice | T215A | T (0%) | A (100%) | |
| M2 | Resistance to adamantine derivatives | S31N | S (0.7%) | N (98.9%) |
| NS1 | Altered the virulence in mice | D92E | D (97.1%) | E (1.5%) |
| Altered the antiviral response in host | N205S | N (10.2%) | S (54.2%) | |
| G210R | G (12.7%) | R (56.7%) | ||
aThe H3 numbering system was used.
bThe N2 numbering system was used.
The influenza virus HA gene was a major factor that determines the receptor binding property and the host range. It was well known that E190D and G225E could increase the receptor-binding affinity to human type α-2,6-linked sialic acid receptors (Tumpey et al. 2007). And majority EA H1N1 SIVs contained HA-190D (85.9%) and 225E (84.1%) mutations. Studies have shown that adaptive mutations of influenza A viruses were identified mostly in viral polymerase complexes (Chen et al. 2007). Mutations PB2-E627K and D701N not only enhanced the viral polymerase activity but also increased the virulence of H7N9 and H5N1 avian influenza viruses in mammals (Chen et al. 2007; Steel et al. 2009; Zhu et al. 2015). The substitution PB2-D701N could also enhance viral replication and pathogenicity of EA H1N1 viruses in mice (Liu et al. 2018). 70.0% of the EA H1N1 SIVs posed N at position 701 in the PB2 protein. Whereas, 98.5% of all EA H1N1 SIVs at 627 site were E. Another PB2-Q591K mutation was shown to enhance polymerase activity, replication and virulence in mice in H5N1 influenza virus (Yamada et al. 2010). The percentage of 591K residue in EA H1N1 SIVs was 0.4%. In addition to key adaptive signatures in PB2 protein, several other mutations in PB1, PA and NP were implicated in enhanced viral polymerase activity including PB1-L473V (Xu et al. 2011), PA-K356R (Xu et al. 2016), NP-Q357K (Zhu et al. 2019) and so on. Theses mutations in EA H1N1 SIVs were PB1-L473V (99.6%), PA-K356R (25.7%), and NP-Q357K (31.5%), respectively. Furthermore, it was also reported that M1-P41A mutation in EA H1N1 SIVs increased the transmission in guinea pigs (Campbell et al. 2014). We found that 98.6% was A at position 41 in M1 protein in EA H1N1 SIVs. In our study, we analyzed the percentage of these mutations in all eight gene segments of EA H1N1 SIVs. We found that some molecular markers were already widespread in EA H1N1 SIVs, such as PB1-L473V (99.6%), and M1-P41A (98.6%). There were still some molecular markers including PB2-E627K (0.4%) and PB2-K526R (5.6%) that account for a very small proportion in EA H1N1 SIVs. Therefore, continuous surveillance needs to be implemented.
Antiviral drugs M2 and neur-aminidase inhibitors played important role in influenza treatment (Babu et al. 2001; Hay et al. 1985). It was known that M2-S31 N mutation was resistant to amantadine and rimantadine by changing in the transmembrane channel domain (Shiraishi et al. 2003). We found that 98.9% of EA H1N1 SIVs posed 31N in the M2 protein. It suggested that the EA H1N1 SIVs were resistant to amantadine and rimantadine widely. H274Y and N294S mutations caused resistance to neur-aminidase inhibitors (Ives et al. 2002). However, these substitutions were not observed in all EA H1N1 SIVs, which suggested the neur-aminidase inhibitors could be used for human infections with EA H1N1 viruses.
Discussion
The EA H1N1 SIV outside European countries was detected in Hong Kong in 2001. Currently, EA H1N1, CS H1N1, TR H1N2 and A(H1N1)pdm09 influenza viruses co-circulated in pigs in China (Chen et al. 2013). The co-circulation of these lineage viruses in pigs resulted in an increased number of novel reassortment viruses. A total of 11 different genotypes were identified. Genotype 1 viruses have all of their eight gene of avian-origin, which were widely prevalent from 2001 to 2013. Higher genetic diversity was detected between 2009 and 2014, with all genotypes were identified. This might have been caused by strengthened surveillance of SIVs since A(H1N1)pdm09 virus pandemic. During this period, genotype 1, 2, and 4 were prevalent in China, nevertheless, genotype 7–11 were detected only one time. Since 2013, genotype 3 and 5 have become increasingly prevalent and have a selective advantage over original EA H1N1 viruses. Although Hong Kong contributed the largest number of EA H1N1 viruses and had the second largest number of genotypes, most of the swine slaughtered in Hong Kong come from provinces in Chinese mainland (Vijaykrishna et al. 2011). This indicates most of swine influenza viruses detected in Hong Kong were imported from Chinese mainland.
The prevalence of EA SIVs in pigs could cause the human infections. In China, human infections with EA H1N1 SIVs were reported occasionally. The first human infection with EA H1N1 SIVs was identified in Jiangsu Province in 2011 (Yang et al. 2012). Thereafter, another 3-year-old boy was identified as EA H1N1 SIV case in Hebei Province in 2012 (Wang et al. 2013), which belonged to genotype 1. In 2015, one and two human cases with EA H1N1 SIVs infections were reported in Hunan and Yunnan provinces, respectively (Zhu et al. 2016, 2019), which were classified as genotype 3. Fujian Province reported its first EA H1N1 human case in 2016 (Xie et al. 2018), which was listed as genotype 5. These were consistent with the prevalence of EA H1N1 SIVs circulating in pig populations during the same period.
The available full genome sequences may have some bias. Hong Kong contributed the largest number of EA H1N1 sequences in the public database. It might be due to systematic surveillance since 1998 in Hong Kong (Vijaykrishna et al. 2011). Since 2007, swine influenza virus surveillance began to been implemented in Guangdong, Guangxi, Shandong and other provinces in Northern China (He et al. 2018; Liu et al. 2009; Sun et al. 2016; Yang et al. 2016; Zhu et al. 2011). Therefore, although all EA H1N1 sequences from the public database have exclusively been included for analysis, they might not cover all of the evolutionary image of the viruses.
In our study, some important molecular signatures were analyzed. The specific amino acid mutations of HA could switch the receptor preference from avian-type receptors to human-type receptors. It was noted that majority of all EA H1N1 SIVs posed 190D (85.9%) and 225E (84.1%), which were preferentially bind to the human-type receptors and caused human infections. Some adaptive mutations including PB2-T271A, PB2-Q591K, PB2-E627K, and PB2-D701 N were able to enhance viral polymerase activity and further facilitated pathogenicity in mice (Bussey et al. 2010; Yamada et al. 2010; Zhu et al. 2015). The EA H1N1 viruses beard 271A (30.8%), 591K (0.4%), and 701N (70.0%) in the PB2 protein, respectively. Through the analysis of antiviral resistance molecular characteristics, we found that EA H1N1 SIVs were resistant to amantadine and rimantadine. However, they were sensitive to neur-aminidase inhibitors. Hence, we should pay more attention to these molecular signatures and identify their effects on pathogenicity, transmission and antiviral resistance of EA H1N1 SIVs.
Taken together, our finding suggested that dynamic reassortments among EA H1N1 SIVs and other swine influenza viruses were continuously occurring in pigs. Occasionally, it has caused human infections, therefore, we should strengthen the monitoring of EA H1N1 SIVs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1 The source and accession number of all the sequences (XLSX 49 kb)
Acknowledgements
We gratefully acknowledge the authors and laboratories for sharing the EA SIVs sequences in GISAID and GenBank database. The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of China CDC and other organizations. The study was supported by the National Nature Science Foundation of China (81961128002, 81971941, and 31761133003).
Author Contributions
YS, DW, and WZ designed the study; YS, WZ, ZF, LY, JL, and LZ analyzed the data and discussed the results; ZF wrote the manuscript; YS and WZ finalized the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declared that they have no conflicts of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Wenfei Zhu, Email: wenfei@cnic.org.cn.
Dayan Wang, Email: dayanwang@cnic.org.cn.
Yuelong Shu, Email: shuylong@mail.sysu.edu.cn.
References
- Anderson TK, Macken CA, Lewis NS, Scheuermann RH, Reeth KV, Brown IH, Swenson SL, Simon G, Saito T, Berhane Y, Ciacci-Zanella J, Pereda A, Davis CT, Donis RO, Webby RJ, Vincent AL. A phylogeny-based global nomenclature system and automated annotation tool for H1 hemagglutinin genes from swine influenza A viruses. MSphere. 2016;1:e00216–e00275. doi: 10.1128/mSphere.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu YS, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin TH, Hutchison TL, Elliott AJ, Parker CD. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2001;32:3482. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- Bussey KA, Bousse TL, Desmet EA, Kim B, Takimoto T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J Virol. 2010;84:4395–4406. doi: 10.1128/JVI.02642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Kyriakis CS, Marshall N, Suppiah S, Seladi-Schulman J, Danzy S, Lowen AC, Steel J. Residue 41 of the Eurasian avian-like swine influenza a virus matrix protein modulates virion filament length and efficiency of contact transmission. J Virol. 2014;88:7569–7577. doi: 10.1128/JVI.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campitelli L, Donatelli I, Foni E, Castrucci MR, Fabiani C, Kawaoka Y, Krauss S, Webster RG. Continued evolution of H1N1 and H3N2 influenza viruses in pigs in Italy. Virology. 1997;232:310–318. doi: 10.1006/viro.1997.8514. [DOI] [PubMed] [Google Scholar]
- Chen H, Bright RA, Subbarao K, Smith C, Cox NJ, Katz JM, Matsuoka Y. Polygenic virulence factors involved in pathogenesis of 1997 Hong Kong H5N1 influenza viruses in mice. Virus Res. 2007;128:159–163. doi: 10.1016/j.virusres.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang J, Qiao CL, Yang HL, Zhang Y, Xin XG, Chen HL. Co-circulation of pandemic 2009 H1N1, classical swine H1N1 and avian-like swine H1N1 influenza viruses in pigs in China. Infect Genet Evol. 2013;13:331–338. doi: 10.1016/j.meegid.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Gregory V, Bennett M, Thomas Y, Kaiser L, Wunderli W, Matter H, Hay A, Lin YP. Human infection by a swine influenza A (H1N1) virus in Switzerland. Arch Virol. 2003;148:793–802. doi: 10.1007/s00705-002-0953-9. [DOI] [PubMed] [Google Scholar]
- Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Wang GJ, Mo YN, Yu Q, Xiao X, Yang WJ, Zhao WF, Guo X, Chen Q, He JQ, Liang ML, Zhu J, Ding YB, Wei ZZ, Ouyang K, Liu F, Jian H, Huang WJ, Garcia-Sastre A, Chen Y. Novel triple-reassortant influenza viruses in pigs, Guangxi, China. Emerg Microbes Infect. 2018;7:85. doi: 10.1038/s41426-018-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Suzuki Y, Mitnaul L, Vines A, Kida H, Kawaoka Y. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- Ives JAL, Carr JA, Mendel DB, Tai CY, Lambkin R, Kelly L, Oxford JS, Hayden FG, Roberts NA. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antivir Res. 2002;55:307–317. doi: 10.1016/S0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Kazutaka K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Bi YH, Qin K, Fu GH, Yang J, Peng JS, Ma GP, Liu QF, Pu J, Tian FL. Emergence of European avian influenza virus-like H1N1 swine influenza A viruses in China. J Clin Microbiol. 2009;47:2643–2646. doi: 10.1128/JCM.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Zhu WF, Feng ZM, Gao RB, Guo JF, Li XY, Liu J, Wang DY, Shu YL. Substitution of D701N in the PB2 protein could enhance the viral replication and pathogenicity of Eurasian avian-like H1N1 swine influenza viruses. Emerg Microbes Infect. 2018;7:75. doi: 10.1038/s41426-018-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M, Ottis K, Vandeputte J, Kaplan MM, Bachmann PA. Evidence for the natural transmission of influenza A virus from wild ducts to swine and its potential importance for man. Bull World Health Organ. 1981;59:75–78. [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C, Bürger H, Bachmann PA, Hannoun C. Genetic relatedness of hemagglutinins of the H1 subtype of influenza a viruses isolated from swine and birds. Virology. 1983;129:521–523. doi: 10.1016/0042-6822(83)90194-0. [DOI] [PubMed] [Google Scholar]
- Scholtissek C, Bürger H, Kistner O, Shortridge KF. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985;147:287–294. doi: 10.1016/0042-6822(85)90131-X. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Mitamura K, Sakai-Tagawa Y, Goto H, Sugaya N, Kawaoka Y. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J Infect Dis. 2003;188:57–61. doi: 10.1086/375799. [DOI] [PubMed] [Google Scholar]
- Smith GJD, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JSM, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Steel J, Lowen AC, Mubareka S, Palese P, Baric RS. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627 K or 627E/701N. PLoS Pathog. 2009;5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Wang XH, Li XL, Zhang L, Li HH, Lu C, Yang CL, Feng J, Han W, Ren WK, Tian XX, Tong GZ, Wen F, Li ZJ, Gong XQ, Liu XM, Ruan BY, Yan MH, Yu H. Novel triple-reassortant H1N1 swine influenza viruses in pigs in Tianjin, Northern China. Vet Microbiol. 2016;183:85–91. doi: 10.1016/j.vetmic.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Horiike G, Yamazaki Y, Kawabe K, Masuda H, Miyamoto D, Matsuda M, Nishimura S-I, Yamagata T, Ito T, Kida H, Kawaoka Y, Suzuki Y. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 1997;404:192–196. doi: 10.1016/S0014-5793(97)00127-0. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, Pappas C, Cox NJ, Swayne DE, Palese P, Katz JM. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- Vijaykrishna D, Smith GJD, Pybus OG, Zhu H, Bhatt S, Poon LLM, Riley S, Bahl J, Ma SK, Cheung CL, Perera RAPM, Chen H, Shortridge KF, Webby RJ, Webster RG, Guan Y, Peiris JSM. Long-term evolution and transmission dynamics of swine influenza A virus. Nature. 2011;473:519–522. doi: 10.1038/nature10004. [DOI] [PubMed] [Google Scholar]
- Wang DY, Qi SX, Li XY, Guo JF, Tan MJ, Han GY, Liu YF, Lan Y, Yang L, Huang WJ, Cheng YH, Zhao X, Bai T, Wang Z, Wei HJ, Xiao N, Shu YL. Human infection with Eurasian avian-like influenza A(H1N1) virus, China. Emerg Infect Dis. 2013;19:1709–1711. doi: 10.3201/eid1910.130420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JF, Zhang YH, Zhao L, Xiu WQ, Chen HB, Lin Q, Weng YW, Zheng KC. Emergence of Eurasian avian-like swine influenza A (H1N1) virus from an adult case in Fujian Province, China. Virol Sin. 2018;33:282–286. doi: 10.1007/s12250-018-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Hu WB, Xu K, He YX, Wang TY, Chen Z, Li TX, Liu JH, Buchy P, Sun B. Amino acids 473 V and 598P of PB1 from an avian-origin influenza A virus contribute to polymerase activity, especially in mammalian cells. J Gen Virol. 2011;93:531–540. doi: 10.1099/vir.0.036434-0. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang X, Gao W, Wang C, Wang J, Sun H, Sun Y, Guo L, Zhang R, Chang K-C, Liu J, Pu J, Lyles DS. Prevailing PA mutation K356R in avian influenza H9N2 virus increases mammalian replication and pathogenicity. J Virol. 2016;90:8105–8114. doi: 10.1128/JVI.00883-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Hatta M, Staker BL, Watanabe S, Imai M, Shinya K, Sakai-Tagawa Y, Ito M, Ozawa M, Watanabe T, Sakabe S, Li C, Kim JH, Myler PJ, Phan I, Raymond A, Smith E, Stacy R, Nidom CA, Lank SM, Wiseman RW, Bimber BN, O’Connor DH, Neumann G, Stewart LJ, Kawaoka Y. Biological and structural characterization of a host-adapting amino acid in influenza virus. PLoS Pathog. 2010;6:e1001034. doi: 10.1371/journal.ppat.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Qiao CL, Tang X, Chen Y, Xin XG, Chen HL. Human infection from avian-like influenza A (H1N1) viruses in pigs, China. Emerg Infect Dis. 2012;18:1144–1146. doi: 10.3201/eid1807.120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Chen Y, Qiao CL, He XJ, Zhou H, Sun Y, Yin H, Meng SS, Liu LP, Zhang QY, Kong HH, Gu CY, Li CJ, Bu ZG, Kawaoka Y, Chen HL. Prevalence, genetics, and transmissibility in ferrets of Eurasian avian-like H1N1 swine influenza viruses. Proc Natl Acad Sci U S A. 2016;113:392–397. doi: 10.1073/pnas.1522643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HC, Zhou BP, Fan XH, Lam TTY, Wang J, Chen A, Chen XC, Chen HL, Webster RG, Webby R, Peiris JSM, Smith DK, Guan Y. Novel reassortment of Eurasian avian-like and pandemic/2009 influenza viruses in swine: infectious potential for humans. J Virol. 2011;85:10432–10439. doi: 10.1128/JVI.05352-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WF, Li L, Yan ZG, Gan TH, Li LF, Chen RR, Chen RD, Zheng ZY, Hong WS, Wang J, Smith DK, Guan Y, Zhu HC, Shu YL. Dual E627K and D701N mutations in the PB2 protein of A(H7N9) influenza virus increased its virulence in mammalian models. Sci Rep. 2015;5:14170. doi: 10.1038/srep14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WF, Zhang H, Xiang XY, Zhong LL, Yang L, Guo JF, Xie YR, Li FC, Deng ZH, Feng H, Huang YW, Hu SX, Xu X, Zou XH, Li XD, Bai T, Chen YK, Li Z, Li JH, Shu YL. Reassortant Eurasian avian-like influenza A(H1N1) virus from a severely ill child, Hunan Province, China, 2015. Emerg Infect Dis. 2016;22:1930–1936. doi: 10.3201/eid2211.160181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WF, Feng ZM, Chen YK, Yang L, Liu J, Li XY, Liu SL, Zhou LJ, Wei HJ, Gao RB, Wang DY, Shu YL. Mammalian-adaptive mutation NP-Q357K in Eurasian H1N1 swine influenza viruses determines the virulence phenotype in mice. Emerg Microbes Infect. 2019;8:989–999. doi: 10.1080/22221751.2019.1635873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 The source and accession number of all the sequences (XLSX 49 kb)