Abstract
Circular RNAs (circRNAs) belong to a class of non-coding RNAs with diverse biological functions. However, little is known about their roles in case of pseudorabies virus (PrV) infection. Here, we analyzed the expression profile of host circRNAs from a virulent PrV type II strain DX (PrV-DX) infected and an attenuated gE/TK deficient (gE−TK−PrV) strain of PrV infected PK-15 cells. CircRNAs were identified by find_circ and analyzed with DESeq 2. Compared with the mock cells, 449 differentially expressed (DE) circRNAs (233 down-regulated and 216 up-regulated) from PrV-DX infected and 578 DE circRNAs (331 down-regulated and 247 up-regulated) from gE−TK− PrV infected PK-15 cells were identified. In addition, 459 DE circRNAs (164 down-regulated and 295 up-regulated) between the PrV-DX and gE−TK−PrV infected cells were identified. The expression patterns of 13 circRNAs were validated by reverse transcription quantitative real-time PCR (RT-qPCR) and results were similar as of RNA-seq. The putative target miRNA binding sites of DE circRNAs were predicted by using miRanda and psRobot. The circRNA-miRNA-mRNA network was constructed and certain miRNAs that have possible roles in antiviral immune response, such as miR-210 and miR-340, were predicted. GO and KEGG pathway analysis demonstrated that DE circRNAs were enriched in the processes such as cellular metabolism, protein binding, RNA degradation and regulation of actin cytoskeleton. Collectively, these findings might provide the useful information for a better understanding of mechanisms underlying the interaction between PrV-II and host cells.
Electronic supplementary material
The online version of this article (10.1007/s12250-020-00255-w) contains supplementary material, which is available to authorized users.
Keywords: Pseudorabies virus infection, RNA-seq, CircRNA
Introduction
Pseudorabies virus (PrV), the causative pathogen for Aujeszky’s disease, belongs to Alphaherpesvirinae subfamily. PrV has a double-stranded DNA genome of more than 140 kb size and is packaged by the icosahedral capsid, protein tegument and lipid envelope (Engel et al. 2015). PrV can infect most mammals, including pigs, dogs, horses and rodents (Cramer et al. 2011; Kimman et al. 1991; Muller et al. 2011). Pseudorabies can be controlled using attenuated Bartha-based vaccines. However, since the latter part of 2011, pseudorabies outbreaks have occurred in many vaccinated farms in China. Phylogenetic analysis divided the Chinese strains into genotype II (PrV-II), and European/American strains in genotype I, which contains Bartha-K61 (Ye et al. 2015). Notably, PrV is becoming a potential threat to the public health as recently, a human infected with this virus was reported showing symptoms of endophthalmitis. This indicates that PrV could also have a zoonotic route of transmission (Ai et al. 2018). PrV infection causes neurological disorders, respiratory disease and abortion, resulting in high fatality as well as enormous losses to the swine industry (Sun et al. 2016). The infection starts in the oropharyngeal mucosa and nasal epithelial cells (Masic et al. 1965), then, spreads to the peripheral nervous system (PNS) and eventually establishes lifelong latency via retrograde transport to the peripheral ganglia (Hafezi et al. 2012; Pomeranz et al. 2005).
Recent studies showed that non-coding RNAs play important role in virus life cycle (Damas et al. 2019). As a key member of non-coding RNAs, circRNA was first discovered in RNA viruses via electron microscopy (Wang and Fang 2018), and represented as newly identified single-stranded non-coding covalently closed circular molecules existing in almost all species (Salzman et al. 2012). CircRNAs are comparatively more stable than linear RNA by resisting RNase mediated degradation (Jeck et al. 2013; Qu et al. 2015). The diverse biological functions of circRNA were continuously discovered from the past decade (Li et al. 2018a, b). Researchers found that circRNAs could act as miRNA sponges and regulate gene expression in distinct physiological and pathophysiological conditions (Li et al. 2017a, b). Studies suggest that circRNAs might act as diagnostic or predictive biomarkers (Meng et al. 2017). Furthermore, circRNAs might also play role in cancer progression (Guarnerio et al. 2016), physiological development (Liu et al. 2019) and immune response (Chen et al. 2017; Li et al. 2017a, b). The expression pattern of circRNAs induced by simian virus 40 had been investigated previously (Shi et al. 2017), Circular RNAs were also found to be involved in antiviral signaling pathway (Ma et al. 2018; Tagawa et al. 2018). But, still the circRNAs expression profiles in PrV infected cells has not been studied in detail.
To investigate the expression profiles of host circRNAs, microRNA (miRNA) and mRNA during PrV-II infection, high-throughput RNA sequencing was performed on the samples infected with virulent and attenuated PrV-II strain compared with mock controls. The expression levels of 13 randomly-selected circRNAs were confirmed by RT-qPCR. The competing endogenous RNA (ceRNA) network and co-expression network was constructed. Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of differentially expressed RNAs were also performed to predict their potential role during the PrV infection. The results of the present study will be useful for a better understanding of the mechanisms underlying the interaction between the PrV and host immune system.
Materials and Methods
Cells Culture and Viruses
PK-15 cells, maintained in our laboratory, was cultured at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (Gibco, CA, USA) containing 10% heat-inactivated fetal bovine serum (Biological Industries, Israel) and antibiotics (100 μg/mL streptomycin and 100U/mL penicillin). The virulent PrV-II strain DX (PrV-DX) and attenuated gE/TK gene-deleted strain gE−TK−PrV, was constructed in our laboratory (Jin et al. 2020) (US Patent No.15918547).
Infection and RNA Extraction
The PK-15 cells were infected with PrV-DX and gE−TK−PrV (MOI = 5) for 11 h. Total RNA was isolated from infected as well as uninfected cells using SuPerfecTRI™ Total RNA Isolation reagent (Pufei, Shanghai, China) according to the manufacturer’s instructions. Agarose gel electrophoresis was performed to detect the degradation of total RNA. The concentration and purity of RNA were checked by the Qubit R RNA Assay Kit (Life Technologies, CA, USA) and Nanodrop (Thermol Fisher, USA).
RNA-seq and Data Analysis
Three biological replicates of the virus infected and control samples were used for miRNA, circRNA and mRNA sequencing. Sequencing libraries of circRNA were created after the depletion of rRNA and linear RNA by NEBNext Ultra Directional RNA Library Prep Kit for Illumina according to the manufacturer’s instructions. The miRNA sequencing libraries were generated by using the Small RNA Sample Pre Kit (Illumina, San Diego, CA, USA) and the mRNA were enriched by magnetic beads containing Oligo(dT). After the sequencing libraries were qualified, RNA-seq was performed using an Illumina HiSeq to get the raw data (raw reads). To get the clean data (clean reads), reads containing poly-N or adapter sequence and reads of low-quality were removed. Paired-end reads were aligned to the pig genome. CircRNA were identified using find_circ (Memczak et al. 2013) and miRNA were identified using bowtie (Langmead et al. 2009) tools, respectively. Differentially expressed (DE) transcripts were assigned significant when the P value < 0.05. The relative circRNAs and miRNAs amplification was estimated by using transcript per million tags (TPM) normalization (Zhou et al. 2010). DEseq 2 (Love et al. 2014) was used to determine the DE circRNAs and DE miRNAs with the P-value < 0.05.
Quantitative Real-Time PCR Analysis
The reliability of RNA-seq data was validated by RT-qPCR for the selected circRNAs. Briefly, RNA was isolated from virus infected cells and digested by RNaseR for 1 h according to the instruction. The reverse transcription kit was used to synthesize cDNA according to the manufacturer’s instruction. The RT-qPCR was performed in a 20 μL reaction volume, including 10 μL SYBR Green Master Mix, 0.4 μL PCR Primer (forward and reverse respectively), 7.2 μL nuclease-free water and 2 μL cDNA. The reaction was carried out at 95 °C for 3 min, followed by 95 °C 10 s and 60 °C 34 s for 45 cycles. GAPDH was used as a reference house-keeping gene. Three independent wells were performed for each sample. Primers used in RT-qPCR are shown in Supplementary Table S1. (Thermol Fisher, USA).
GO and KEGG Pathway Analysis
GO (http://www.geneontology.org/) analysis was performed using GOseq to annotate the genes under the category of cellular component, biological process and molecular function (Young et al. 2010). Kyoto encyclopedia of genes and genomes pathway enrichment using hypergeometric test was also conducted to predict the involvement of cellular pathways targeted by ncRNAs expressed during PrV infection (Kanehisa et al. 2008). The pathways of GO and KEGG with the corrected P-value < 0.05 were chosen to be significantly enriched.
Competing Endogenous RNA (ceRNA) Network Analysis
The miRanda and psRobot were used to search the potential miRNA response elements (MREs) of mRNA and circRNA. The same miRNA binding site on both mRNA and circRNA was presumed to be a potential circRNA-miRNA-mRNA interaction axis. Cytoscape was used to construct the ceRNA network.
Results
Differentially Expressed CircRNAs and miRNAs
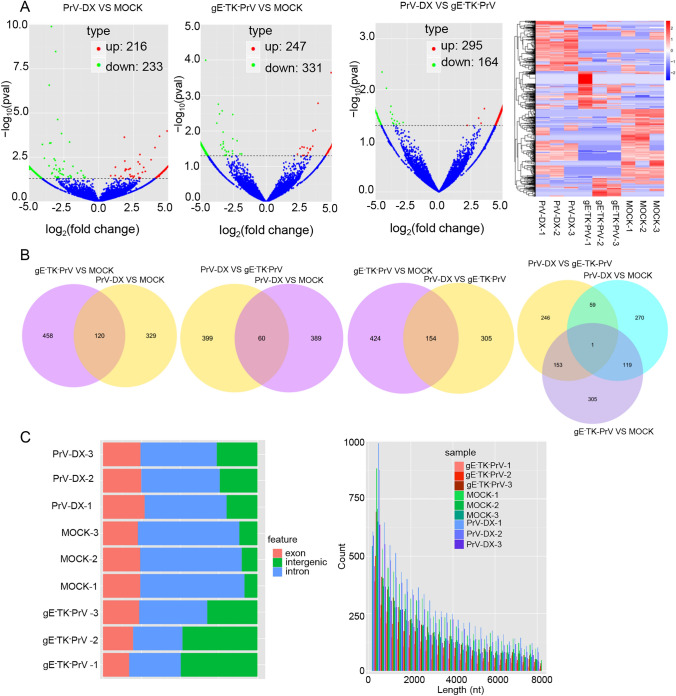
To determine whether circRNAs and miRNAs are involved during the PrV-II infection, the PK-15 cells infected with a virulent PrV-DX strain and the attenuated gE−TK−PrV strain together with the uninfected control group were analyzed using RNA-seq. Data were processed using bioinformatics software DESeq 2 and TPM by the criteria of P-value < 0.05 considered as significant. The results showed that there were total 233 down-regulated and 216 up-regulated circRNAs in PrV-DX infected cells, 331 down-regulated and 247 up-regulated circRNAs in gE−TK−PrV infected cells and the 459 DE circRNAs (164 down-regulated and 295 up-regulated) between the PrV-DX and gE−TK−PrV infected cells (Fig. 1A and 1B). The source and target genes of DE circRNAs between PrV-DX and gE−TK−PrV infected cells were intersected with the DE mRNAs (Supplementary Figure S1A). Genomic features and length distribution of novel circRNAs has been shown in Fig. 1C. Results showed that most of the circRNAs were encoded from the intron regions having size less than 2000 nt. DE miRNAs analysis of PrV-DX infected cells showed 44 down-regulated and 49 up-regulated miRNAs containing 7 and 10 novel miRNAs from each of them respectively (Supplementary Figure S1B and S1C). In case of gE−TK−PrV infected cells, there were 52 down-regulated and 43 up-regulated miRNAs with 8 and 10 novel miRNAs from each of them respectively. A total of 12 miRNAs, of which 6 were down-regulated with 4 novel and 6 were up-regulated with 1 novel miRNA, were differentially expressed in PrV-DX versus gE−TK−PrV infected cells (Supplementary Figure S1B and S1C).
Fig. 1.
Differentially expressed (DE) circRNAs in PrV-DX and gE−TK−PrV infected PK-15 cells using RNA-seq. A Volcano plots of DE circRNAs. The red dots correspond to up-regulated circRNAs, the green dots correspond to down-regulated circRNAs and the blue dots represent the circRNAs without statistically significant difference. Heatmap. Clustering map shows the differential expression profile of circRNAs among different groups. B Venn diagram of the DE circRNAs in and among different groups. The intersections of the circles represent the simultaneous dyregulated transcripts in different groups. C Genomic feature and length distribution of novel circRNAs. The red part is exon, green part is intergenic, and blue part is intron.
Validation of the Differentially Expressed Transcripts by RT-qPCR
To verify the results of high-throughput sequencing, representative circRNAs expression levels were detected by RT-qPCR. The ssc-circ-18815, ssc-circ-20488, and ssc-circ-22498 from PrV-DX infected cells, and ssc-circ-03155, ssc-circ-18151, and ssc-circ-29164 from gE−TK−PrV infected cells were significantly up-regulated compared to the uninfected control. Whereas, ssc-circ-03247, ssc-circ-26237, ssc-circ-09616, ssc-circ-16387, ssc-circ-26189, ssc-circ-26246, ssc-circ-28418 were down-regulated in both of the PrV-DX and gE−TK−PrV infected PK-15 cells (Fig. 2). The RT-qPCR results of the selected circRNAs were consistent with the results of RNA-seq. In addition, the expression of several selected DE miRNAs (ssc-let-7a, miR-146b, miR-210, miR-194a and miR-340 and miR-21) and mRNAs (MAP3K1 and ZNF10) were also validated and were consistent with the results of RNA-seq (Supplementary Figure S2).
Fig. 2.
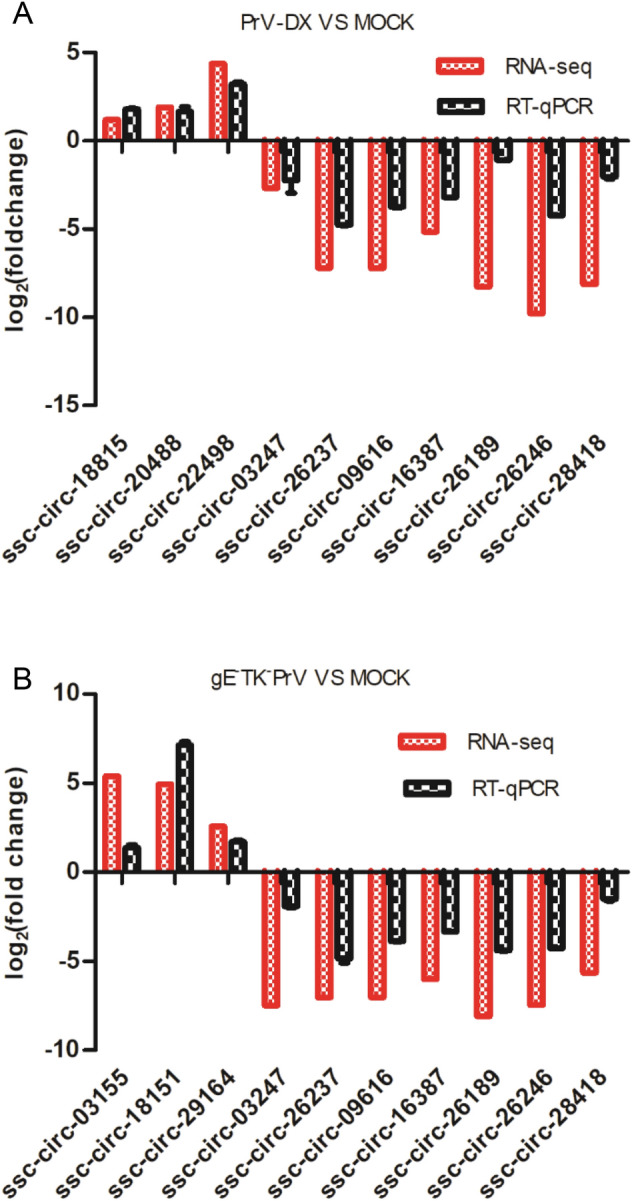
RT-qPCR validation of selected circRNAs. The randomly selected circRNAs from PrV-DX versus MOCK (A) and gE−TK−PrV versus MOCK (B) were monitored by RT-qPCR. The expression patterns of these transcripts determined by RT-qPCR are consistent with the results of RNA-seq.
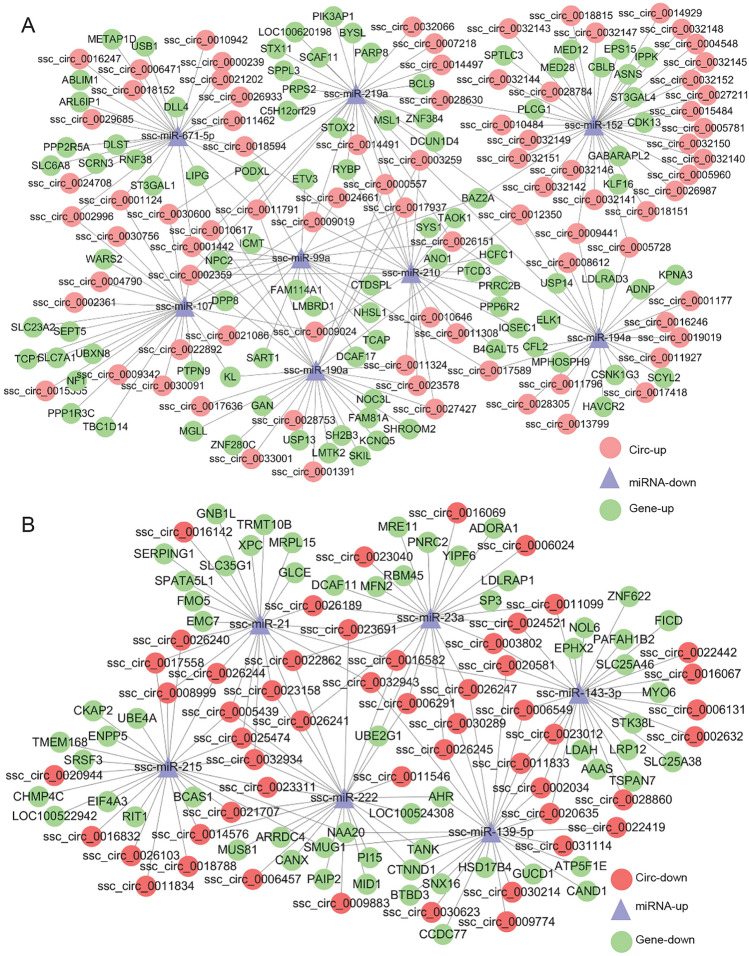
The Interaction Network of CircRNA-miRNA-Target Gene
To predict the potential functions of circRNAs, a co-expression network of circRNA-miRNA-target gene was constructed using Cytoscape tool. Within the ceRNA network, circRNA indirectly regulate the expression of target genes by regulating the miRNA through their microRNA response elements (Hansen et al. 2013). Of 14 detected miRNA-mediated circRNA-mRNA competing triplets, 8 miRNAs were down-regulated (Fig. 3A) and 6 were up-regulated (Fig. 3B). In addition, in the network between virulent and attenuated PrV infected cell’s ncRNAs there were 5 up-regulated (Supplementary Figure S3A) and 2 down-regulated miRNAs (Supplementary Figure S3B). These miRNAs such as miR-139-5p, miR-143-3p, miR-146b, miR-215, miR-194a, miR-210 and miR-340 (Curtale et al. 2013; Hoshina et al. 2016; Hou et al. 2018; Tian and He 2018; Wang et al. 2017a, b; Wu et al. 2012; Zhang et al. 2010; Zou et al. 2016), have possible roles in antiviral immune response.
Fig. 3.
The interaction network of circRNA-miRNA-target gene. A circRNA-miRNA-mRNAs regulatory network of up-down-up in infected PK-15 cells. B circRNA-miRNA-mRNAs regulatory network of down-up-down among infected PK-15 cells.
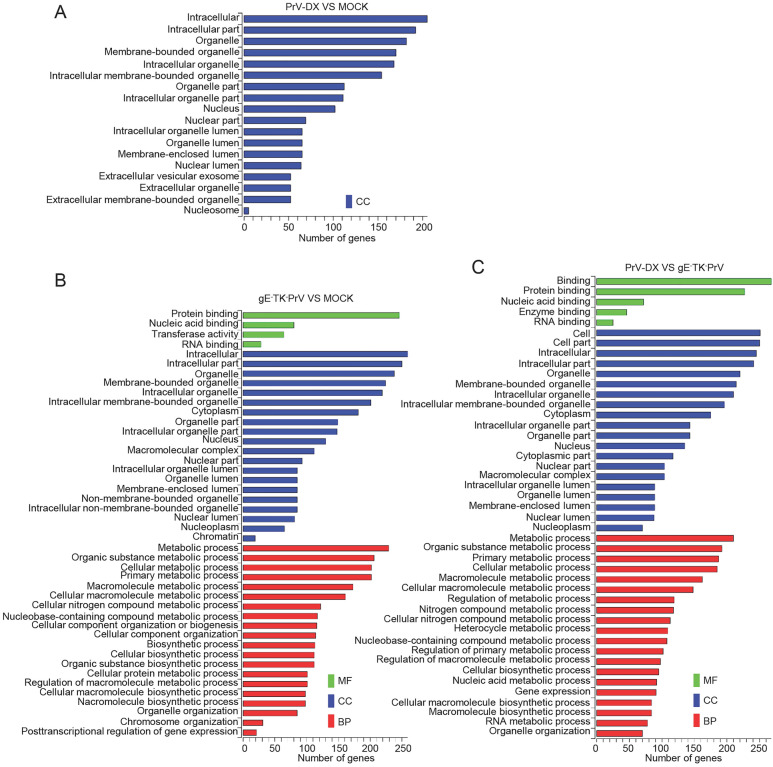
GO and KEGG Analysis of DE CircRNAs and miRNAs
GO and KEGG pathway enrichment was performed to predict the gene function of DE circRNAs and miRNAs. The target genes from the interaction network of circRNA-miRNA-target gene were used in this analysis. Three different categories, biological process (BP), cellular component (CC) and molecular function (MF) of GO annotation were taken into account (Fig. 4). The identified CC terms were intracellular, intracellular part, organelle, membrane-bounded organelle and intracellular organelle. The identified BP terms were metabolic process, cellular metabolic process, primary metabolic process and organic substance metabolic process. The identified MF mainly included protein binding and nucleic acid binding. The GO analysis of DE miRNAs was similar to those of DE circRNAs (Supplementary Fiureg S4).
Fig. 4.
GO analyses of Differentially expressed circRNAs. GO term from biological process (BP), cellular component (CC) and molecular function (MF).
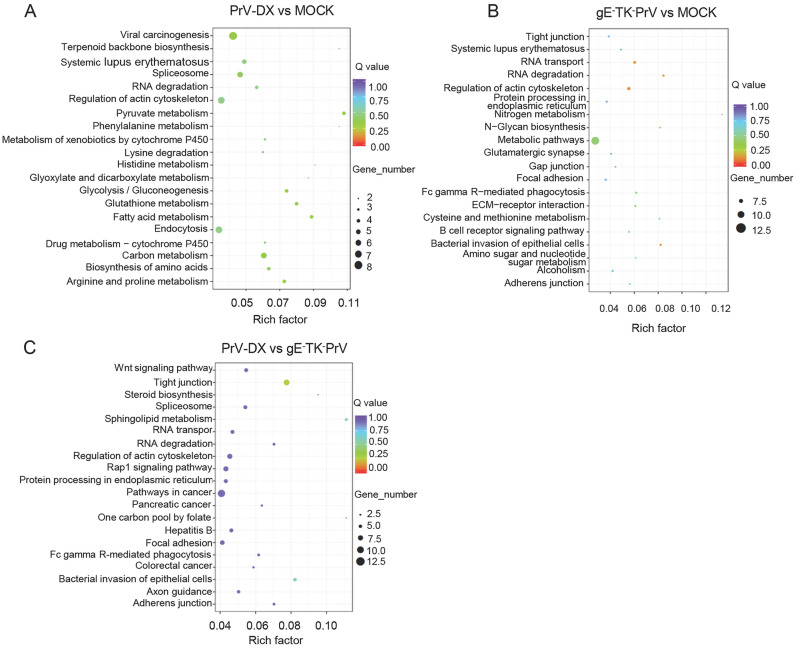
As a major public database on pathways, KEGG analysis was used to predict the biochemical pathways targeted by the DE circRNAs. Results showed that the DE circRNAs from infected and mock cells (Fig. 5A and 5B) involved in the pathways such as RNA degradation and regulation of actin cytoskeleton. DE circRNAs from PrV-DX and gE−TK−PrV infected cells (Fig. 5C) involved in the pathways such as Wnt signaling pathway and tight junction. The KEGG analysis of DE miRNAs were shown in Supplementary Figure S5.
Fig. 5.
KEGG enrichment analyses of differentially expressed circRNAs. A KEGG enrichment analyses of DE circRNAs from PrV-DX versus MOCK. B KEGG enrichment analyses of DE circRNAs from gE−TK−PrV versus MOCK. C KEGG enrichment analyses of DE circRNAs from PrV-DX vs gE−TK−PrV.
To investigate more about the virus virulence, the intersection of the source and target genes of DE circRNAs from PrV-DX and gE−TK−PrV infected cells were enriched by GO and KEGG analysis (Supplementary Figure S6A and S6B). Results showed that intracellular, intracellular part, metabolic process were mainly enriched by GO analysis. Regarding to the result of KEGG analysis, pathways such as Wnt signaling pathway, tight junction and TGF-beta signaling pathway are enriched.
Discussion
PrV is considered to act as a model virus to study of herpesvirus biology (Enquist 1999). Due to its possible zoonotic transmission (Yang et al. 2019), PrV-II got attention in recent years. Previous studies indicated that the noncoding RNAs including miRNAs and lncRNAs play important role during PrV infection (Huang et al. 2014; Nishitsuji et al. 2016). CircRNAs are important member of the class of non-coding RNAs, having critical roles in regulation of gene expression (Li et al. 2015), disease progression (Peng et al. 2017) and immune response (Li et al. 2017a, b). During the infection of herpes simplex virus 1, there is a change in the expression profiles of host circRNAs (Shi et al. 2018). However, the expression profile alteration of host circRNAs and the circRNA-miRNA-mRNA axis after PrV-II infection has not been studied so far. In the present study, 449 and 578 DE circRNAs were detected in virulent PrV-DX strain and gE and TK genes deleted attenuated strain of gE−TK−PrV infected cells respectively. Similarly, 93 and 95 miRNAs as well as 7199 and 7149 DE mRNAs were also detected. Notably, the DE transcripts between virulent and attenuated strains may be useful to understand the role of ncRNAs in determining the virulence property of PrV-II strain. The intersection of the source and target genes of DE circRNAs from PrV-DX and gE−TK−PrV infected cells (Supplementary Figure S5A) show that most of the source genes are included in the target genes. This accord with one of the circRNA characteristics: regulating the transcription of their parental genes (Li et al. 2015). In addition, not all the source and target genes are differently expressed, indicating that except for the regulation of source and target genes, these DE circRNAs may play roles by other means. For example, they may encode a protein (Zhang et al. 2018a, b), interact with a protein (Yang et al. 2017) or change the expression of other genes (Zhang et al. 2017). To the best of our knowledge, this is the first comprehensive report about the DE circRNA, miRNA and mRNA during PrV infection.
The GO annotation analysis indicated that the DE circRNAs and miRNAs in PrV-II infected cells involved in the protein binding, nucleic acid binding and transferase activity, which was similar to those of DE circRNAs identified during hepatitis B virus (Wang et al. 2018) and human papilloma virus (Wang et al. 2017a, b) infected cells. Actually, the function of protein binding was critical for viral infection (Sathiyamoorthy et al. 2017). These results indicated that DE ncRNAs might contribute to PrV-II infection. Furthermore, KEGG analyses showed that the pathways of RNA transport, RNA degradation and regulation of actin cytoskeleton were specifically enriched in infected cells compared to the uninfected control cells. This suggests that the DE transcripts might be involved in the replication of viral genome.
The intersection of the source and target genes of DE circRNAs from PrV-DX and gE−TK−PrV infected cells were also enriched by GO and KEGG analysis (Supplementary Figure S5C). Metabolic process, intracellular and intracellular part are mainly enriched by GO analysis. For KEGG analysis, such as Wnt signaling pathway, Tight junction and TGF-beta signaling pathway are enriched. According to the report: Wnt signaling was demonstrated to implicate in the Virulence of Influenza Virus (Forero et al. 2015); Tight junction may influence the virus infection by affecting the entry of virus (Luo et al. 2017); TGF-beta signaling pathway can regulate INF and the function of Mitochondria (Grunwell et al. 2018). Except the displayed pathways, other pathways such as Apoptosis (Pontes et al. 2016) and NF-kappa B signaling pathway (Diel et al. 2011) were also enriched and were related to virus virulence. The results remind us that the DE circRNAs between PrV-DX and gE−TK−PrV may affect the virulence of PrV-II by changing the epigenetic modification, signal transduction or metabolic process in these pathways.
An important function of circRNA is acting as the miRNA sponges and modulating the activity of miRNA on their target genes. In detail, miR-21, which was demonstrated to restrain PrV replication (Huang et al. 2014), was up-regulated in the virulent PrV-DX compared to attenuated gE−TK−PrV infected cells (Fig. 3), suggesting that it may relate to the viral virulence. Additionally, ssc-let-7a, which was up-regulated during PrV infection, may alter the expression of MAP3K1 and ZNF10 genes, which have role in apoptosis (Cao et al. 2015) and interact with NF-кB (Nishitsuji et al. 2015). DNM1L and SOD1, the potential target genes of ssc-miR-146b, have role in mitophagy (Zhang et al. 2018a, b) and neuropathology (Yang et al. 2014). Moreover, ssc-miR-210 (Guethlein et al. 2007), ssc-miR-194a (Li et al. 2018a, b) and ssc-miR-340 (Zhou et al. 2016), which were down-regulated during viral infection, may bind to the DE circRNAs and participate in the antiviral, viral replication or immune response. Hence, the detailed information about the target genes of DE circRNAs might be useful to understand the virulence factors of PrV-II or the process of viral infection. These results will be useful to understand the role of circRNAs during PrV pathogenesis.
To conclude, the profiles of DE noncoding RNAs and their target genes in PrV-DX and gE−TK−PrV infected PK-15 cells were analyzed by high-throughput RNA sequencing. The DE circRNAs were identified and their potential functions were predicted by GO, KEGG and circRNA-miRNA-target gene interaction network, indicating that the DE circRNAs might play critical roles during the viral infection. These results will be useful for understanding the effects and mechanisms of circRNAs in PrV pathogenesis and host response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Tianqi Yu for the assistance of data analysis. This work was supported by the National Key Research & Development Program of China (2016YFD0500102), Key Research & Development Program of Zhejiang Province (Grant No. 2020C02011) and the Fundamental Research Funds for the Central Universities (2017FZA6018).
Author Contributions
JZ designed the study and supervised the experiment. YJ and YY prepared the samples. HL validated and analyzed the RNA-seq data and drafted the manuscript. WD and WT helped to analyze the data. JZ finalized the manuscript. All authors read and approved the final version of the manuscript.
Data Availability
The data of RNA-seq was deposited in the Gene Expression Omnibus (GEO) database with the Accession Number of GSE139424.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflicts of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects.
References
- Ai JW, Weng SS, Cheng Q, Cui P, Li YJ, Wu HL, Zhu YM, Xu B, Zhang WH. Human endophthalmitis caused by pseudorabies virus infection, China, 2017. Emerg Infect Dis. 2018;24:1087–1090. doi: 10.3201/eid2406.171612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wu W, Zhou X, Liu K, Li B, Huang X, Zhang Y, Liu H. Let-7 g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary. Int J Biochem Cell Biol. 2015;68:148–157. doi: 10.1016/j.biocel.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Chen YG, Kim MV, Chen X, Batista PJ, Aoyama S, Wilusz JE, Iwasaki A, Chang HY. Sensing self and foreign circular RNAs by intron identity. Mol Cell. 2017;67:228–238. doi: 10.1016/j.molcel.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SD, Campbell GA, Njaa BL, Morgan SE, Smith SN, McLin WT, Brodersen BW, Wise AG, Scherba G, Langohr IM, Maes RK. Pseudorabies virus infection in Oklahoma hunting dogs. J Vet Diagn Invest. 2011;23:915–923. doi: 10.1177/1040638711416628. [DOI] [PubMed] [Google Scholar]
- Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci U S A. 2013;110:11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas ND, Fossat N, Scheel T. Functional interplay between RNA viruses and non-coding RNA in mammals. Noncoding RNA. 2019;5:7. doi: 10.3390/ncrna5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel DG, Luo S, Delhon G, Peng Y, Flores EF, Rock DL. Orf virus ORFV121 encodes a novel inhibitor of NF-kappaB that contributes to virus virulence. J Virol. 2011;85:2037–2049. doi: 10.1128/JVI.02236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel EA, Song R, Koyuncu OO, Enquist LW. Investigating the biology of alpha herpesviruses with MS-based proteomics. Proteomics. 2015;15:1943–1956. doi: 10.1002/pmic.201400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist LW. Life beyond eradication: veterinary viruses in basic science. Arch Virol Suppl. 1999;15:87–109. doi: 10.1007/978-3-7091-6425-9_7. [DOI] [PubMed] [Google Scholar]
- Forero A, Tisoncik-Go J, Watanabe T, Zhong G, Hatta M, Tchitchek N, Selinger C, Chang J, Barker K, Morrison J, Berndt JD, Moon RT, Josset L, Kawaoka Y, Katze MG. The 1918 influenza virus PB2 protein enhances virulence through the disruption of inflammatory and Wnt-mediated signaling in mice. J Virol. 2015;90:2240–2253. doi: 10.1128/JVI.02974-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwell JR, Yeligar SM, Stephenson S, Ping XD, Gauthier TW, Fitzpatrick AM, Brown L. TGF-beta1 suppresses the type I IFN response and induces mitochondrial dysfunction in alveolar macrophages. J Immunol. 2018;200:2115–2128. doi: 10.4049/jimmunol.1701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH, Pandolfi PP. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Guethlein LA, Older AA, Abi-Rached L, Parham P. Evolution of killer cell Ig-like receptor (KIR) genes: definition of an orangutan KIR haplotype reveals expansion of lineage III KIR associated with the emergence of MHC-C. J Immunol. 2007;179:491–504. doi: 10.4049/jimmunol.179.1.491. [DOI] [PubMed] [Google Scholar]
- Hafezi W, Lorentzen EU, Eing BR, Muller M, King NJ, Klupp B, Mettenleiter TC, Kuhn JE. Entry of herpes simplex virus type 1 (HSV-1) into the distal axons of trigeminal neurons favors the onset of nonproductive, silent infection. PLoS Pathog. 2012;8:e1002679. doi: 10.1371/journal.ppat.1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- Hoshina S, Sekizuka T, Kataoka M, Hasegawa H, Hamada H, Kuroda M, Katano H. Profile of exosomal and intracellular microRNA in gamma-herpesvirus-infected lymphoma cell lines. PLoS ONE. 2016;11:e162574. doi: 10.1371/journal.pone.0162574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Liao J, Zhang C, Sun C, Li X, Wang G. Elevated expression of miR-146, miR-139 and miR-340 involved in regulating Th1/Th2 balance with acute exposure of fine particulate matter in mice. Int Immunopharmacol. 2018;54:68–77. doi: 10.1016/j.intimp.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Huang J, Ma G, Fu L, Jia H, Zhu M, Li X, Zhao S. Pseudorabies viral replication is inhibited by a novel target of miR-21. Virology. 2014;456–457:319–328. doi: 10.1016/j.virol.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Zhang K, Huang W, Tang W, Li H, Dong W, Gu J, Zhou J. Identification of functional lncRNAs in pseudorabies virus type II infected cells. Vet Microbiol. 2020;242:108564. doi: 10.1016/j.vetmic.2019.108564. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimman TG, Binkhorst GJ, van den Ingh TS, Pol JM, Gielkens AL, Roelvink ME. Aujeszky’s disease in horses fulfils Koch’s postulates. Vet Rec. 1991;128:103–106. doi: 10.1136/vr.128.5.103. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- Li LJ, Zhao W, Tao SS, Leng RX, Fan YG, Pan HF, Ye DQ. Competitive endogenous RNA network: potential implication for systemic lupus erythematosus. Expert Opin Ther Targets. 2017;21:639–648. doi: 10.1080/14728222.2017.1319938. [DOI] [PubMed] [Google Scholar]
- Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin QF, Wei J, Yao RW, Yang L, Chen LL. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell. 2017;67:214–227. doi: 10.1016/j.molcel.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Li J, Lu M, Huang B, Lv Y. Porcine circovirus type 2 inhibits inter-beta expression by targeting Karyopherin alpha-3 in PK-15 cells. Virology. 2018;520:75–82. doi: 10.1016/j.virol.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71:428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Y, Yin J, Yan XY, Chen WJ, Jiang CY, Hu XS, Wang XY, Zhu JG, Yu ZB, Han SP. Profiles analysis reveals circular RNAs involving zebrafish physiological development. J Cell Physiol. 2019;234:15922–15933. doi: 10.1002/jcp.28250. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Guo L, Zhang J, Xu Y, Gu W, Feng L, Wang Y. Tight junction protein occludin is a porcine epidemic diarrhea virus entry factor. J Virol. 2017 doi: 10.1128/JVI.00202-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhao X, Zhang Z, Guo J, Guan L, Li J, Mi M, Huang Y, Tong D. Differentially expressed non-coding RNAs induced by transmissible gastroenteritis virus potentially regulate inflammation and NF-kappaB pathway in porcine intestinal epithelial cell line. BMC Genom. 2018;19:747. doi: 10.1186/s12864-018-5128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masic M, Ercegan M, Petrovic M. The significance of the tonsils in the pathogenesis and diagnosis of Aujeszyk’s disease in pigs. Zentralbl Vet B. 1965;12:398–405. [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011;156:1691–1705. doi: 10.1007/s00705-011-1080-2. [DOI] [PubMed] [Google Scholar]
- Nishitsuji H, Sawada L, Sugiyama R, Takaku H. ZNF10 inhibits HIV-1 LTR activity through interaction with NF-kappaB and Sp1 binding motifs. FEBS Lett. 2015;589:2019–2025. doi: 10.1016/j.febslet.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Nishitsuji H, Ujino S, Yoshio S, Sugiyama M, Mizokami M, Kanto T, Shimotohno K. Long noncoding RNA #32 contributes to antiviral responses by controlling interferon-stimulated gene expression. Proc Natl Acad Sci U S A. 2016;113:10388–10393. doi: 10.1073/pnas.1525022113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Chen G, Zhu Z, Shen Z, Du C, Zang R, Su Y, Xie H, Li H, Xu X, Xia Y, Tang W. Circular RNA ZNF609 functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-150-5p in Hirschsprung’s disease. Oncotarget. 2017;8:808–818. doi: 10.18632/oncotarget.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005;69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes MS, Van Waesberghe C, Nauwynck H, Verhasselt B, Favoreel HW. Pseudorabies virus glycoprotein gE triggers ERK1/2 phosphorylation and degradation of the pro-apoptotic protein Bim in epithelial cells. Virus Res. 2016;213:214–218. doi: 10.1016/j.virusres.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Chen J, Longnecker R, Jardetzky TS. The COMPLEXity in herpesvirus entry. Curr Opin Virol. 2017;24:97–104. doi: 10.1016/j.coviro.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Hu N, Li J, Zeng Z, Mo L, Sun J, Wu M, Hu Y. Unique expression signatures of circular RNAs in response to DNA tumor virus SV40 infection. Oncotarget. 2017;8:98609–98622. doi: 10.18632/oncotarget.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Hu N, Mo L, Zeng Z, Sun J, Hu Y. Deep RNA sequencing reveals a repertoire of human fibroblast circular RNAs associated with cellular responses to herpes simplex virus 1 infection. Cell Physiol Biochem. 2018;47:2031–2045. doi: 10.1159/000491471. [DOI] [PubMed] [Google Scholar]
- Sun Y, Luo Y, Wang CH, Yuan J, Li N, Song K, Qiu HJ. Control of swine pseudorabies in China: opportunities and limitations. Vet Microbiol. 2016;183:119–124. doi: 10.1016/j.vetmic.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Gao S, Koparde VN, Gonzalez M, Spouge JL, Serquina AP, Lurain K, Ramaswami R, Uldrick TS, Yarchoan R, Ziegelbauer JM. Discovery of Kaposi’s sarcoma herpesvirus-encoded circular RNAs and a human antiviral circular RNA. Proc Natl Acad Sci U S A. 2018;115:12805–12810. doi: 10.1073/pnas.1816183115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, He Z. miR-215 enhances HCV replication by targeting TRIM22 and inactivating NF-kappaB signaling. Yonsei Med J. 2018;59:511–518. doi: 10.3349/ymj.2018.59.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang L. Advances in circular RNAs and their roles in breast cancer. J Exp Clin Cancer Res. 2018;37:206. doi: 10.1186/s13046-018-0870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhao Y, Chen M, Cui J. Identification of novel long non-coding and circular RNAs in human papillomavirus-mediated cervical cancer. Front Microbiol. 2017;8:1720. doi: 10.3389/fmicb.2017.01720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang D, Xie G, Yin Y, Zhao E, Tao K, Li R. MicroRNA-152 regulates immune response via targeting B7-H1 in gastric carcinoma. Oncotarget. 2017;8:28125–28134. doi: 10.18632/oncotarget.15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Cui S, Zhao W, Qian Z, Liu H, Chen Y, Lv F, Ding HG. Screening and bioinformatics analysis of circular RNA expression profiles in hepatitis B-related hepatocellular carcinoma. Cancer Biomark. 2018;22:631–640. doi: 10.3233/CBM-170910. [DOI] [PubMed] [Google Scholar]
- Wu TH, Pan CY, Lin MC, Hsieh JC, Hui CF, Chen JY. In vivo screening of zebrafish microRNA responses to bacterial infection and their possible roles in regulating immune response genes after lipopolysaccharide stimulation. Fish Physiol Biochem. 2012;38:1299–1310. doi: 10.1007/s10695-012-9617-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang G, Sun H, Shu R, Liu T, Wang CE, Liu Z, Zhao Y, Zhao B, Ouyang Z, Yang D, Huang J, Zhou Y, Li S, Jiang X, Xiao Z, Li XJ, Lai L. Species-dependent neuropathology in transgenic SOD1 pigs. Cell Res. 2014;24:464–481. doi: 10.1038/cr.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZG, Awan FM, Du WW, Zeng Y, Lyu J, Wu GS, Yang W, Yang BB. The circular RNA interacts with STAT3, increasing its nuclear translocation and wound repair by modulating Dnmt3a and miR-17 function. Mol Ther. 2017;25:2062–2074. doi: 10.1016/j.ymthe.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Guan H, Li C, Li Y, Wang S, Zhao X, Zhao Y, Liu Y. Characteristics of human encephalitis caused by pseudorabies virus: a case series study. Int J Infect Dis. 2019;87:92–99. doi: 10.1016/j.ijid.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Ye C, Zhang QZ, Tian ZJ, Zheng H, Zhao K, Liu F, Guo JC, Tong W, Jiang CG, Wang SJ, Shi M, Chang XB, Jiang YF, Peng JM, Zhou YJ, Tang YD, Sun MX, Cai XH, An TQ, Tong GZ. Genomic characterization of emergent pseudorabies virus in China reveals marked sequence divergence: evidence for the existence of two major genotypes. Virology. 2015;483:32–43. doi: 10.1016/j.virol.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GL, Li YX, Zheng SQ, Liu M, Li X, Tang H. Suppression of hepatitis B virus replication by microRNA-199a-3p and microRNA-210. Antivir Res. 2010;88:169–175. doi: 10.1016/j.antiviral.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu H, Hou L, Wang G, Zhang R, Huang Y, Chen X, Zhu J. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16:151. doi: 10.1186/s12943-017-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Qin Y, Chen M. Viral strategies for triggering and manipulating mitophagy. Autophagy. 2018;14:1665–1673. doi: 10.1080/15548627.2018.1466014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F, Chen W, Gao X, Zhao K, Zhou H, Li Z, Ming L, Xie B, Zhang N. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37:1805–1814. doi: 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen J, Li Z, Li X, Hu X, Huang Y, Zhao X, Liang C, Wang Y, Sun L, Shi M, Xu X, Shen F, Chen M, Han Z, Peng Z, Zhai Q, Chen J, Zhang Z, Yang R, Ye J, Guan Z, Yang H, Gui Y, Wang J, Cai Z, Zhang X. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE. 2010;5:e15224. doi: 10.1371/journal.pone.0015224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Li C, Lu C, Zhang X, Pan Y, Liu X, Liu G, Zhao Z, Sun B. miRNA29 promotes viral replication during early stage of PRRSV infection in vitro. DNA Cell Biol. 2016;35:636–642. doi: 10.1089/dna.2015.3103. [DOI] [PubMed] [Google Scholar]
- Zou F, Mao R, Yang L, Lin S, Lei K, Zheng Y, Ding Y, Zhang P, Cai G, Liang X, Liu J. Targeted deletion of miR-139-5p activates MAPK, NF-kappaB and STAT3 signaling and promotes intestinal inflammation and colorectal cancer. FEBS J. 2016;283:1438–1452. doi: 10.1111/febs.13678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of RNA-seq was deposited in the Gene Expression Omnibus (GEO) database with the Accession Number of GSE139424.