Abstract
Objective
The study aimed to establish the spinal cord occupation rate of the dural sac during flexion and extension.
Methods
We measured the cross-sectional area of the dural sac and the spinal cord between C2/C3 and C7/T1 disc levels in 100 patients with cervical spondylotic myelopathy and 1211 asymptomatic subjects.
Results
The spinal cord occupation rate of the dural sac in the cross-sectional area was higher on extension than on flexion at the mid-lower cervical spine.
Conclusions
The spinal cord occupation rate of the dural sac in the cross-sectional area was highest at the C4/C5 and C5/C6 levels on extension.
Keywords: Spinal cord, Dural sac, Occupation rate, Cervical spondylotic myelopathy, Kinetic change, Asymptomatic subjects
1. Introduction
Cervical spondylotic myelopathy (CSM) is one of the most common causes of spinal cord dysfunction in adults, resulting in a progressive condition due to chronic compression of the cervical spinal cord.1,2 Static factors, such as congenital canal narrowing, degenerative intervertebral discs, osteophyte formation, and thickening of ligamentum flavum, plays a vital role in the pathogenesis of cervical myelopathy.3 However, dynamic factors induced by cervical spinal motion, such as anterior or posterior translation of vertebrae, disc protrusion, and buckling of the flavum, are known to contribute to the development and progression of neurological symptoms in CSM.4
Various radiological examinations, such as plain radiograph, computed tomography (CT), and magnetic resonance imaging (MRI), have been performed to evaluate the static structural abnormality of the cervical spine and spinal canal.5,6 To evaluate the dynamic factors in patients with CSM, several authors have reported the usefulness of kinetic MRI which demonstrates physiological alterations of the spinal canal and the spinal cord in different neck positions.7,8 Other studies have illustrated the efficacy of kinetic CT myelography in investigating dynamic factors in patients with CSM.9,10 In comparison with kinetic MRI, kinetic CT myelography offers several advantages, such as shorter scanning time, thinner axial slices, and high image resolution, particularly in bony or calcified compressive lesions.11,12
Although it is important to evaluate the relationship between the spinal cord and the dural sac (the contents and the container), no studies have investigated the spinal cord occupation rate of the dural sac in patients with CSM. Therefore, we conducted a prospective study to evaluate the anteroposterior diameter and the cross-sectional area of the dural sac and the spinal cord in CSM. The study aimed to establish the spinal cord occupation rate of the dural sac in these kinetic changes with flexion and extension for each level using multidetector-row CT (MDCT) and compare differences in imaging results between patients with CSM and healthy subjects.
2. Materials and methods
2.1. Study population
2.1.1. Patients with CSM
One hundred sixty-two consecutive patients who underwent laminoplasty at our institution for CSM were prospectively enrolled in this study. The exclusion criteria were as follows: (1) presence of an ossification of the posterior longitudinal ligament; (2) history of rheumatoid arthritis, cerebral palsy, or tumors; (3) spinal injuries; (4) destructive spondyloarthritis caused by hemodialysis; (5) previous cervical surgery; (6) severe kyphosis, spinal fusion with instrumentation; (7) thoracic spondylotic myelopathy; and (8) lumbar spinal canal stenosis. A total of 100 patients [61 males, 39 females; mean age, 63.8 ± 14.2 (range 23–93) years] were included in this study. All patients exhibited myelopathy that was confirmed by physical examination, and cord compression was present between the C2/C3 and C7/T1 levels using MRI and myelography. Furthermore, MDCT following myelography was preoperatively performed in both maximum passive flexion and extension. The aim of this study was explained to all patients prior to myelography and CT, and informed consent was obtained from all patients.
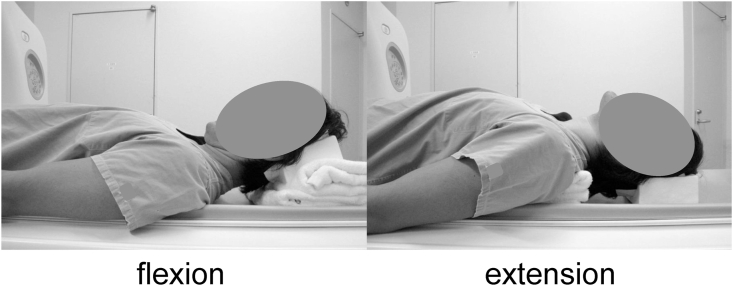
The neck posture was changed during flexion and extension during CT. On maximum passive flexion of the cervical spine, a higher pillow was placed under the posterior head, while on maximum passive extension, it was placed under the shoulder (Fig. 1).9 CT images (1-mm-thick axial helical) were obtained with sagittal and coronal reconstruction using a 64-line, multi-slice unit (Light Speed VCT; GE Healthcare Bio-Sciences, Piscataway, NJ, USA). It took about 5 s to perform MDCT.9,11,12
Fig. 1.
The neck posture during flexion and extension under computed tomography. Flexion: higher pillow supporting the posterior head was used. Extension: shoulder pillow was used.
2.1.2. Asymptomatic subjects (control)
This study prospectively enrolled 1211 healthy volunteers [606 males, 605 females; mean age 49.5 ± 16.8 (range, 20–79) years] as asymptomatic subjects. Subjects with a history of brain or spinal surgery; comorbid neurologic diseases, such as cerebral infarction or neuropathy; symptoms related to sensory or motor disorders (e.g., numbness, clumsiness, motor weakness, and gait disturbances); or the presence of severe neck pain were excluded. The exclusion criteria also included pregnant women and individuals who received worker's compensation or presented with symptoms after a motor vehicle accident. There were at least 100 males and 100 females in each decade of life between the third and eighth decades. The institutional review board approved this project, and we obtained written informed consent from patients with CSM and asymptomatic subjects before examination.
MRI was performed in a neutral position using a 1.5-Tesla superconductive magnet (Signa Horizon Excite HD version 12; GE Healthcare, UK).13 Scans were taken at slice thicknesses of 3 and 4 mm in the sagittal and axial planes, respectively. In the sagittal scans, T1-weighted images (fast spin echo repetition time [TR], 450 ms; echo time [TE], 8.1 ms) and T2-weighted images (fast spin echo TR, 3,500 ms; TE, 102 ms) were obtained. The axial scans were performed using T2-weighted images (fast spin echo TR, 4,000 ms; TE, 102 ms).
2.2. Measurement and data analysis
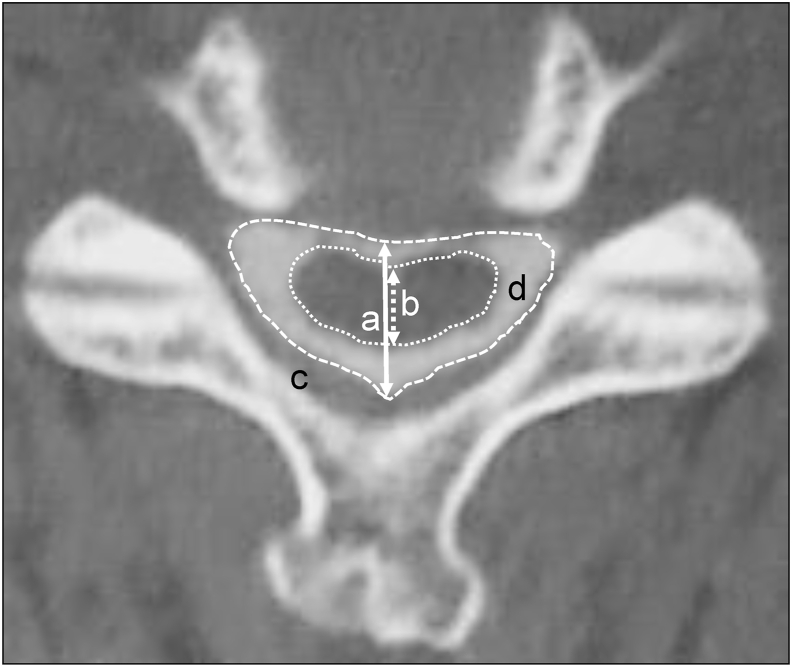
The anteroposterior diameter and the cross-sectional area of the dural sac and the spinal cord were determined on flexion and extension under CT and on neutral position under MRI. The axial images were taken perpendicularly as much as possible and at each disc level from C2/C3 to C7/T1 (Fig. 2). All images were transferred to a computer as Digital Imaging and Communications in Medicine (DICOM) data to measure the anteroposterior diameter and the cross-sectional area using an imaging software (Osiris 4; Icestar Media Ltd, Essex, UK). From these data, the spinal cord occupation rate in the dural sac was calculated using the formula: [(diameter or area of the spinal cord)/(diameter or area of the dural sac) × 100].13
Fig. 2.
Computed tomography images in the axial plane following myelography. a: Anteroposterior diameter of the dural sac. b: Anteroposterior diameter of the spinal cord. c: Cross-sectional area of the dural sac. d: Cross-sectional area of the spinal cord.
2.3. Statistical analysis
All data were analyzed using an SPSS statistical software (version 25; SPSS, Inc., Chicago, IL, USA). All values were expressed as mean ± standard deviation. Parametric analysis using paired t-test was used to analyze the differences between flexion and extension, while nonparametric analysis using Mann–Whitney U test was used to evaluate the differences between the two groups. A p value of <0.05 was considered to be statistically significant.
3. Results
The anteroposterior diameter of the dural sac was significantly shorter on extension than on flexion in all segments, except at the C2/C3 level (p < 0.01). In patients with CSM and asymptomatic subjects, the anteroposterior diameter of the dural sac decreased from the C2/C3 level to the C5/C6 level and increased gradually below the C5/C6 level. The anteroposterior diameter of the dural sac at the C5/C6 level was shortest in all segments in both groups. Patients with CSM showed significantly shorter diameter of the dural sac than asymptomatic subjects in all segments (p < 0.0001) (Table 1).
Table 1.
The anteroposterior diameter of dural sac and spinal cord on each position.
| Level | CSM patients |
Asymptomatic subjects |
|
|---|---|---|---|
| Flexion (mm) | Extension (mm) | Neutral on MRI (mm) | |
| The anteroposterior diameter of dural sac | |||
| C2/C3 | 10.5 ± 1.3 | 10.4 ± 1.5 | 12.4 ± 1.6 |
| C3/C4 | 8.9 ± 1.4 | 8.6 ± 1.6 | 10.6 ± 1.4 |
| C4/C5 | 8.7 ± 1.6 | 7.7 ± 1.8 | 10.3 ± 1.5 |
| C5/C6 | 8.4 ± 1.6 | 7.1 ± 1.5 | 9.9 ± 1.5 |
| C6/C7 | 9.0 ± 1.4 | 7.8 ± 1.5 | 10.3 ± 1.5 |
| C7/T1 | 10.3 ± 1.3 | 9.6 ± 1.3 | 11.6 ± 1.4 |
| The anteroposterior diameter of spinal cord | |||
| C2/C3 | 6.5 ± 0.9 | 6.3 ± 0.9 | 7.7 ± 0.8 |
| C3/C4 | 5.7 ± 1.2 | 5.3 ± 1.2 | 6.9 ± 0.9 |
| C4/C5 | 5.2 ± 1.1 | 4.5 ± 1.4 | 6.6 ± 1.0 |
| C5/C6 | 4.8 ± 0.9 | 4.1 ± 1.0 | 6.3 ± 0.9 |
| C6/C7 | 5.0 ± 0.8 | 4.7 ± 1.0 | 6.2 ± 0.9 |
| C7/T1 | 5.2 ± 0.8 | 4.9 ± 0.7 | 6.1 ± 0.7 |
Values given are mean ± standard deviation (SD) unless otherwise specified.
CSM indicates cervical spondylotic myelopathy.
The anteroposterior diameter of the spinal cord was significantly shorter on extension than on flexion in all segments (p < 0.01). The anteroposterior diameter of the spinal cord decreased from the C2/C3 level to the C5/C6 level and increased gradually below the C5/C6 level in patients with CSM. In asymptomatic subjects, the anteroposterior diameter of the spinal cord decreased gradually from the C2/C3 level to the C7/T1 level. The diameter of the spinal cord in patients with CSM was significantly shorter than that in asymptomatic subjects in all segments (p < 0.0001) (Table 1).
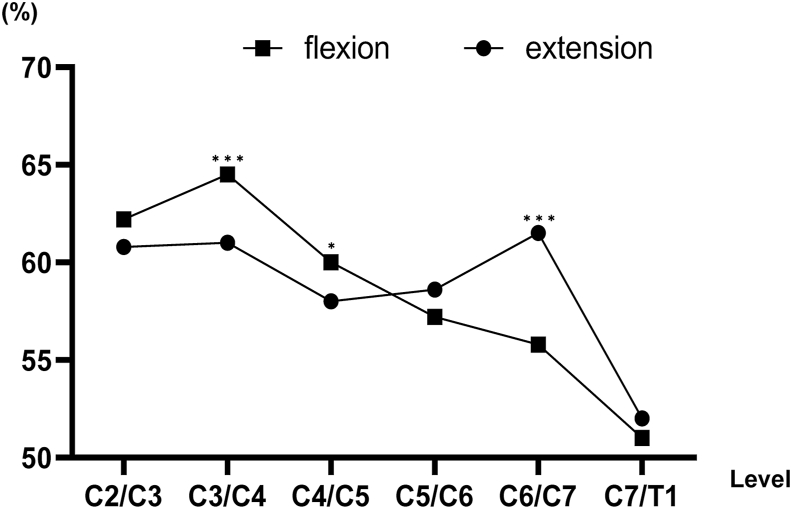
The spinal cord occupation rate of the dural sac in the anteroposterior diameter was higher on flexion than on extension from the C2/C3 level to the C4/C5 level. In contrast, the spinal cord occupation rate of the dural sac in the anteroposterior diameter was higher on extension than on flexion from the C5/C6 level to the C7/T1 level. The C3/C4 level was the highest on flexion, while the C6/C7 level was the highest on extension (Fig. 3). The spinal cord occupation rate of the dural sac in the anteroposterior diameter was nearly the same between patients with CSM and asymptomatic subjects from the C2/C3 level to the C7/T1 level, but not at the C4/C5 and C5/C6 levels (Table 2).
Fig. 3.
The spinal cord occupation rate of the dural sac in the anteroposterior diameter on flexion and extension. The spinal cord occupation rate was significantly lower on extension than on flexion at the C3/C4 and C4/C5 levels. Conversely, it was significantly lower on flexion than on extension at the C6/C7 level. ***p < 0.0001, *p < 0.05.
Table 2.
The spinal cord occupation rate of dural sac in anteroposterior diameter on each position.
| Level | CSM patients |
Asymptomatic subjects |
|
|---|---|---|---|
| Flexion (%) | Extension (%) | Neutral on MRI (%) | |
| C2/C3 | 62.2 ± 7.1 | 60.8 ± 7.2 | 62.3 ± 7.7 |
| C3/C4 | 64.5 ± 6.7 | 61.0 ± 9.6 | 65.8 ± 8.8 |
| C4/C5 | 60.0 ± 8.5 | 58.0 ± 9.9 | 64.7 ± 9.2 |
| C5/C6 | 57.2 ± 8.4 | 58.6 ± 9.8 | 64.6 ± 8.9 |
| C6/C7 | 55.8 ± 6.2 | 61.5 ± 11.3 | 61.1 ± 9.1 |
| C7/T1 | 51.0 ± 6.4 | 52.0 ± 9.1 | 53.7 ± 7.8 |
Values given are mean ± standard deviation (SD) unless otherwise specified.
CSM indicates cervical spondylotic myelopathy.
The cross-sectional area of the dural sac was significantly smaller on extension than on flexion from the C4/C5 level to C7/T1 level (p < 0.01), except at the C2/C3 and C3/C4 levels. The cross-sectional area of the dural sac decreased from the C2/C3 level to the C5/C6 level and increased gradually below the C5/C6 level in both groups. The C5/C6 level was the smallest in both groups. The cross-sectional area of the dural sac in patients with CSM was significantly smaller than that of asymptomatic subjects (p < 0.0001) (Table 3).
Table 3.
The cross-sectional area of dural sac and spinal cord on each position.
| Level | CSM patients |
Asymptomatic subjects |
|
|---|---|---|---|
| Flexion (mm2) | Extension (mm2) | Neutral on MRI (mm2) | |
| The cross-sectional area of dural sac | |||
| C2/C3 | 153.5 ± 19.5 | 159.8 ± 22.9 | 208.0 ± 39.2 |
| C3/C4 | 122.1 ± 17.4 | 120.6 ± 18.2 | 170.7 ± 32.1 |
| C4/C5 | 114.2 ± 19.5 | 102.6 ± 19.8 | 164.5 ± 32.7 |
| C5/C6 | 105.4 ± 14.8 | 90.3 ± 15.0 | 152.7 ± 30.8 |
| C6/C7 | 111.6 ± 17.0 | 100.1 ± 18.0 | 153.3 ± 30.7 |
| C7/T1 | 114.0 ± 17.5 | 107.0 ± 18.0 | 160.3 ± 29.7 |
| The cross-sectional area of spinal cord | |||
| C2/C3 | 61.7 ± 7.1 | 60.5 ± 6.9 | 75.7 ± 8.7 |
| C3/C4 | 56.1 ± 6.0 | 53.9 ± 7.1 | 75.0 ± 9.8 |
| C4/C5 | 50.9 ± 7.5 | 46.7 ± 7.4 | 75.5 ± 11.1 |
| C5/C6 | 45.9 ± 7.5 | 40.4 ± 6.5 | 69.8 ± 11.5 |
| C6/C7 | 43.9 ± 6.4 | 41.5 ± 7.4 | 62.4 ± 10.1 |
| C7/T1 | 40.1 ± 5.3 | 38.0 ± 4.9 | 52.3 ± 7.3 |
Values given are mean ± standard deviation (SD) unless otherwise specified.
CSM indicates cervical spondylotic myelopathy.
The cross-sectional area of the spinal cord at all segments was significantly smaller on extension than on flexion, except at the C2/C3 level (p < 0.01). In both groups, the cross-sectional area of the spinal cord tended to gradually decrease from the C2/C3 level to the C7/T1 level. The C7/T1 level was the smallest in both groups. The cross-sectional area of the spinal cord in patients with CSM was significantly smaller than that of asymptomatic subjects (p < 0.0001) (Table 3).
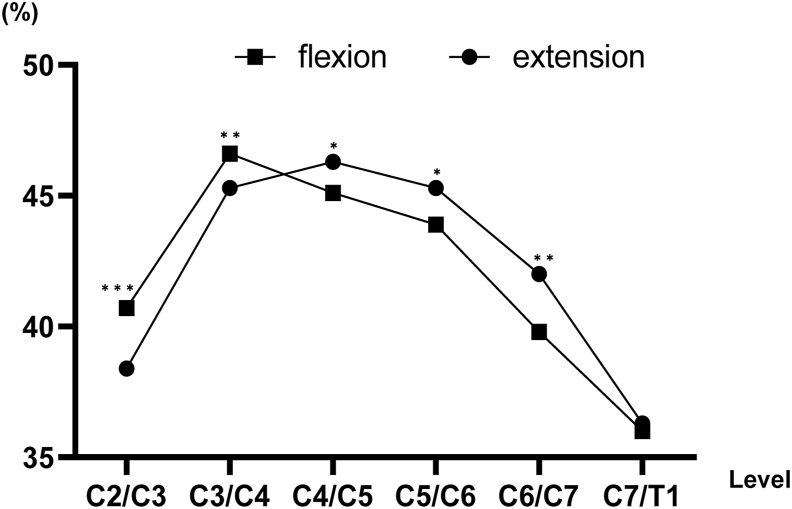
The spinal cord occupation rate of the dural sac in the cross-sectional area was higher on flexion than on extension from the C2/C3 level to the C3/C4 level. Conversely, the spinal cord occupation rate of the dural sac in the cross-sectional area was higher on extension than on flexion from the C4/C5 level to the C6/C7 level. The spinal cord occupation rate of the dural sac in the cross-sectional area on extension increased from the C2/C3 level to the C4/C5 level and decreased gradually below the C4/C5 level (Fig. 4). The spinal cord occupation rate of the dural sac in the cross-sectional area was nearly the same between patients with CSM and asymptomatic subjects from the C2/C3 level to the C7/T1 level (Table 4).
Fig. 4.
The spinal cord occupation rate of the dural sac in the cross-sectional area on flexion and extension. The spinal cord occupation rate was significantly lower on extension than on flexion at the C2/C3 and C3/C4 levels. Conversely, it was significantly lower on flexion than on extension from the C4/C5 level to the C6/C7 level. ***p < 0.0001, **p < 0.01, *p < 0.05.
Table 4.
The spinal cord occupation rate of dural sac in cross-sectional area on each position.
| Level | CSM patients |
Asymptomatic subjects |
|
|---|---|---|---|
| Flexion (%) | Extension (%) | Neutral on MRI (%) | |
| C2/C3 | 40.7 ± 5.7 | 38.4 ± 5.3 | 37.4 ± 6.8 |
| C3/C4 | 46.6 ± 6.2 | 45.3 ± 6.2 | 45.1 ± 8.0 |
| C4/C5 | 45.1 ± 6.2 | 46.3 ± 7.0 | 47.0 ± 8.7 |
| C5/C6 | 43.9 ± 6.9 | 45.3 ± 6.5 | 46.7 ± 8.4 |
| C6/C7 | 39.8 ± 6.3 | 42.0 ± 6.7 | 41.8 ± 8.5 |
| C7/T1 | 36.0 ± 7.6 | 36.3 ± 6.5 | 33.5 ± 6.6 |
Values given are mean ± standard deviation (SD) unless otherwise specified.
CSM indicates cervical spondylotic myelopathy.
4. Discussion
To date, there have been no studies assessing the spinal cord occupation rate of the dural sac in patients with CSM. Therefore, this study prospectively evaluated the spinal cord occupation rate of the dural sac during kinetic changes associated with flexion and extension, in terms of the anteroposterior diameter and the cross-sectional area in patients with CSM using MDCT following myelography. The present study found that the anteroposterior diameter of the dural sac and the spinal cord was significantly shorter on extension than on flexion. The cross-sectional area of the dural sac and the spinal cord was significantly smaller on extension than on flexion. This study also illustrated that the spinal cord occupation rate of the dural sac in the anteroposterior diameter and the cross-sectional area was higher on extension than on flexion at mid-lower cervical spine region.
The second aim of the present study was to compare the difference in the cervical spine imaging between patients with CSM and healthy subjects. This study confirmed that the anteroposterior diameter of the dural sac and the spinal cord in patients with CSM were significantly shorter than those in asymptomatic subjects. The current study also revealed that the cross-sectional area of the dural sac and the spinal cord in patients with CSM were significantly smaller than that in asymptomatic subjects. The spinal cord occupation rate of the dural sac in the anteroposterior diameter and the cross-sectional area was nearly the same between patients with CSM and asymptomatic subjects, except with the anteroposterior diameter at the C4/C5 and C5/C6 levels. From the results of this study, we assumed that the spinal cord of patients with CSM was compressed together with the dural sac. Thus, the spinal cord occupation rate of the dural sac in patients with CSM was similar to that in asymptomatic subjects. To the best of our knowledge, this is the first study to elucidate the spinal cord occupation rate with flexion and extension in patients with CSM in comparison to healthy subjects.
Cervical spine degeneration is common in both symptomatic and asymptomatic adults.14,15 Patients often experience CSM with apparent clinical signs, even if they have only mild spinal cord compression on MRI in a neutral position. MRI results during flexion and extension is helpful because dynamic factors also play a vital role in the pathogenesis of cervical degenerative disease. Numerous previous studies have described normal or abnormal cervical kinematics using different imaging techniques and measurements.7,8 Overall, segmental motion of the cervical spine is the least at the C2/C3 level and greatest at the C4/C5 and C5/C6 levels.9 The mobility of segments with severe cord compression and moderate disc degeneration tend to be lower compared to segments with severe cord compression and severe disc degeneration, and a significant difference has been observed in the mobility of the C5/C6 segment.9
CT following conventional myelography or MRI has been able to obtain only static factors.6 Functional MDCT following myelography is potentially a superior approach in the use of diagnostic imaging equipment for obtaining kinetic factors.9 Although in MDCT dural puncture and radiation exposure are required, there are certain advantages of spatial high-resolution MDCT.10 Furthermore, the aggravation of symptoms is unlikely due to a very short exposure time.11,12 Even patients with pacemakers can receive MDCT. In this study, no patients exhibited neurological deterioration during the functional MDCT examinations. In contrast, it takes longer to perform functional MRI, during which time symptoms can deteriorate.7 More attention should be drawn to dynamic factors in the diagnosis of CSM in patients with a narrow spinal canal.
Interestingly, the present study identified that a peak value of the spinal cord occupation rate of the dural sac in the anteroposterior diameter was found at the C3/C4 level on flexion and at the C6/C7 level on extension. Moreover, the results showed that the inflexion point of the spinal cord occupation rate of the dural sac in the anteroposterior diameter was between the C4/C5 and C5/C6 levels. We also demonstrated that the peak value of the spinal cord occupation rate of the dural sac in the cross-sectional area was found at the C3/C4 level on flexion and at the C4/C5 level on extension. The findings also showed that the inflexion point of the spinal cord occupation rate of the dural sac in the cross-sectional area was between the C3/C4 and C4/C5 levels.
There are several limitations in our study. First, imaging examinations were not unified; patients with CSM underwent CT, while asymptomatic subjects underwent MRI. This was done to reduce radiation exposure. Another reason was that kinetic MRI in patients with CSM leads to aggravation of symptoms when MRI is performed. Second, all changes noted in this study may vary according to age, gender, and many other parameters, and this study did not account for these variables. Third, each parameter was measured once in two different days, and average values were adopted. The measurements of patients with CSM were performed by an experienced spinal surgeon, and the measurements of asymptomatic subjects were performed by well-experienced radiology technologists with extensive knowledge on cervical osseous anatomy.13 Fourth, the study did not reveal any information regarding the relationship between the spinal cord occupation rate and clinical symptoms. More studies should be conducted to understand whether those changes have any effect on clinical symptoms and treatment options.3,4,10 This study revealed that at the C4/C5 and C5/C6 levels on extension, the spinal cord occupation rate of the dural sac in the cross-sectional area was highest. Therefore, the spinal cord may be more susceptible to compression at these levels. Analyzing these changes may increase our understanding of cervical spine disorder involving sudden flexion and extension of the neck. Also, such analysis may contribute to the research on cervical spinal stenosis.
The decision to choose surgical intervention for patients with CSM is based on the appropriate clinical diagnosis and confirmation by imaging studies. It is known that the amount of spinal cord compression depends on the neck position.9 Therefore, this study can provide new and important information with regard to cervical spinal cord occupation rate of the dural sac in these kinetic changes with flexion and extension.
5. Conclusion
The spinal cord occupation rate of the dural sac in the anteroposterior diameter and the cross-sectional area were established between patients with CSM and asymptomatic subjects. This study demonstrated that kinetic factors influence spinal cord occupation rate of the dural sac in patients with CSM.
Funding acknowledgement
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. The Institutional Review Board in our institution approved this study, and written informed consent was obtained from each patient before study participation. This study was supported by institutional funds and by grant research funds, which are intended for promoting hospital functions, of the Japan Labor Health and Welfare Organization (Kawasaki, Japan). Funds were used to pay for data collection and analysis. The study does not contain information about medical device(s)/drug(s).
Declaration of competing interest
The authors have no financial conflicts of interest.
Acknowledgments
We are grateful to Dr. Yasutsugu Yukawa of the Department of Orthopedic Surgery, Chubu Rosai Hospital; Dr. Kota Suda of the Department of Orthopedic Surgery, Hokkaido Chuo Rosai Hospital Sekison Center; Dr. Masatsune Yamagata of the Department of Orthopedic Surgery, Chiba Rosai Hospital; and Dr. Takayoshi Ueta of the Department of Orthopedic Surgery, Spinal Injuries Center for their great contribution in this study.
References
- 1.Karadimas S.K., Erwin W.M., Ely C.G., Dettori J.R., Fehlings M.G. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013 Oct 15;38(22 Suppl 1):S21–S36. doi: 10.1097/BRS.0b013e3182a7f2c3. [DOI] [PubMed] [Google Scholar]
- 2.Nouri A., Tetreault L., Singh A., Karadimas S.K., Fehlings M.G. Degenerative cervical myelopathy: epidemiology, genetics and pathogenesis. Spine (Phila Pa 1976) 2015 Jun 15;40(12):E675–E693. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 3.Yu M., Tang Y., Liu Z., Sun Y., Liu X. The morphological and clinical significance of developmental cervical stenosis. Eur Spine J. 2015 Aug;24(8):1583–1589. doi: 10.1007/s00586-015-3896-z. [DOI] [PubMed] [Google Scholar]
- 4.Kadanka Z., Kerkovsky M., Bednarik J., Jarkovsky J. Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2007 Nov 1;32(23):2573–2577. doi: 10.1097/BRS.0b013e318158cda0. [DOI] [PubMed] [Google Scholar]
- 5.Takeshima T., Omokawa S., Takaoka T., Araki M., Ueda Y., Takakura Y. Sagittal alignment of cervical flexion and extension: lateral radiographic analysis. Spine (Phila Pa 1976) 2002 Aug 1;27(15):E348–E355. doi: 10.1097/00007632-200208010-00014. [DOI] [PubMed] [Google Scholar]
- 6.Imagama S., Matsuyama Y., Yukawa Y. Nagoya Spine Group. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br. 2010 Mar;92(3):393–400. doi: 10.1302/0301-620X.92B3.22786. [DOI] [PubMed] [Google Scholar]
- 7.Miura J., Doita M., Miyata K. Dynamic evaluation of the spinal cord in patients with cervical spondylotic myelopathy using a kinematic magnetic resonance imaging technique. J Spinal Disord Tech. 2009 Feb;22(1):8–13. doi: 10.1097/BSD.0b013e31815f2556. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Zeitoun D., Rangel A., Lazennec J.Y., Catonné Y., Pascal-Moussellard H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: about a prospective study of fifty patients. Spine (Phila Pa 1976) 2011 Aug 1;36(17):E1134–E1139. doi: 10.1097/BRS.0b013e3181f822c7. [DOI] [PubMed] [Google Scholar]
- 9.Machino M., Yukawa Y., Ito K., Nakashima H., Kato F. Dynamic changes in dural sac and spinal cord cross-sectional area in patients with cervical spondylotic myelopathy: cervical spine. Spine (Phila Pa 1976) 2011 Mar 1;36(5):399–403. doi: 10.1097/BRS.0b013e3181d2510b. [DOI] [PubMed] [Google Scholar]
- 10.Yoshii T., Yamada T., Hirai T. Dynamic changes in spinal cord compression by cervical ossification of the posterior longitudinal ligament evaluated by kinematic computed tomography myelography. Spine (Phila Pa 1976) 2014 Jan 15;39(2):113–119. doi: 10.1097/BRS.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 11.Kanbara S., Yukawa Y., Ito K., Machino M., Kato F. Dynamic changes in the dural sac of patients with lumber canal stenosis evaluated by multidetector-row computed tomography after myelography. Eur Spine J. 2014 Jan;23(1):74–79. doi: 10.1007/s00586-013-2873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita D., Yukawa Y., Nakashima H. Dynamic changes in the cross-sectional area of the dural sac and spinal cord in the thoracic spine. Eur Spine J. 2017 Jan;26(1):64–70. doi: 10.1007/s00586-015-4173-x. [DOI] [PubMed] [Google Scholar]
- 13.Kato F., Yukawa Y., Suda K., Yamagata M., Ueta T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: magnetic resonance imaging of over 1,200 asymptomatic subjects. Eur Spine J. 2012 Aug;21(8):1499–1507. doi: 10.1007/s00586-012-2176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swinkels R.A., Swinkels-Meewisse I.E. Normal values for cervical range of motion. Spine (Phila Pa 1976) 2014 Mar 1;39(5):362–367. doi: 10.1097/BRS.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 15.Machino M., Yukawa Y., Imagama S. Age-related and degenerative changes in the osseous anatomy, alignment, and range of motion of the cervical spine: a comparative study of radiographic data from 1016 patients with cervical spondylotic myelopathy and 1230 asymptomatic subjects. Spine (Phila Pa 1976) 2016 Mar;41(6):476–482. doi: 10.1097/BRS.0000000000001237. [DOI] [PubMed] [Google Scholar]