Abstract
This study aimed to research the post-treatment quality of life (QOL) between radiotherapy (RT)- and operation (OP)-treated early cervical cancer survivors, using separate questionnaires for physicians and patients. We administered an observational questionnaire to patients aged 20–70 years old with Stages IB1–IIB cervical cancer who had undergone RT or OP and without recurrence as outpatients for ≥6 months after treatment. We divided 100 registered patients equally into two treatment groups (n = 50 each). The average age was 53 and 44 years in the RT and OP groups, respectively. The RT group included 34 and 66% Stage I and II patients, respectively, whereas the OP group included 66 and 34% Stage I and II patients, respectively. The OP group included 58% of patients with postoperative RT. Combination chemotherapy was performed in 84 and 48% of patients in the RT and OP groups, respectively. On the physicians’ questionnaire, we observed significant differences in bone marrow suppression (RT) and leg edema (OP). On the patients’ questionnaire, significantly more patients had dysuria and leg edema in the OP group than in the RT group, and severe (Score 4–5) leg edema was significantly higher in the post-operative RT group than in the OP only group. The frequency of sexual intercourse decreased after treatment in both groups. On the patients’ questionnaire, there were no significant differences between the two groups regarding sexual activity. These findings are useful to patients and physicians for shared decision-making in treatment choices. The guidance of everyday life and health information including sexual life after treatment is important.
Keywords: uterine cervical cancer, radiotherapy, surgery, quality of life, questionnaire, sexuality
INTRODUCTION
Radiotherapy (RT) results at early-stage uterine cervical cancer are comparable to those of surgery [1]. However, in Japan, surgery-preferring gynecologists are responsible for determining treatment policies, therefore, most patients with stages IB–IIB cervical cancer were indicated for radical hysterectomy (RH) until recently. Conversely, because the National Cancer Institute (NCI) alert [2] recommends concurrent chemoradiation therapy [CCRT] for locally advanced cervical cancer, patients with stages IB2–IIB disease who were previously indicated for surgery are also increasly indicated for CCRT in Japan [3]. In the 2016 annual report of the Committee on Gynecologic Oncology, ~90% (3737/4164) of patients with stage I disease (18% [659/3737] received postoperative RT) and 47% (846/1804) with stage II disease (50% [420/846] received postoperative RT) underwent surgery, and only 9% (386/4164) and 52% (934/1804) of patients with stage I and II disease received radical RT, respectively [3]. From 1975 to 2000, the age of cervical cancer patients peaked at >75 years; however, from 2004, it peaked in an earlier bracket at 35–44 years, indicating that the incidence of cervical cancer is increasing among young Japanese women [4].
Posttreatment quality of life (QOL) is an important factor to consider before patients undergo treatment for uterine cervical cancer. Those with early cervical cancer have more than one treatment option; it is thus important that they understand the post-treatment change in QOL for each modality. Although the change in QOL after treatment is one of the crucial deciding factors in treatment selection, relevant information is limited because very few studies on this issue have been conducted in Japan [5, 6]. The long-term survival of young patients with cervical cancer highlights the importance of the late adverse events of treatment, particularly considering the increasing number of younger patients [2]. It is important to survey long-term survivors to understand the real-world situation with respect to adverse events associated with treatment and to determine the effects of different treatment approaches on QOL. Thus, the aim of the present study was to evaluate the incidence of adverse events and compare the differences in QOL between cervical cancer patients who underwent RT and radical operation (OP). These findings might help patients in the selection of treatment modalities. We present a multi-institutional study conducted by the Japanese Radiation Oncology Study Group (JROSG) gynecologic cancer committee.
MATERIALS AND METHODS
Patients
The present study was approved by the Institutional Review Boards (IRB) of all 12 institutions that participated in this study. The inclusion criteria were: (i) histologically confirmed FIGO Stages IB1–IIB cervical carcinoma; (ii) radical RT (combined external RT with intracavitary brachytherapy) or RH with or without postoperative RT: with or without chemotherapy; (iii) age = 20–70 years; (iv) recurrence-free; (v) final treatment was 6 months prior; (vi) performance status (PS) score of 0 or 1; (vii) could read and understand the questionnaire; (viii) no serious organ dysfunction and no psychological disease; and (ix) provision of written informed consent. The exclusion criteria were: (i) cervical stump cancer; (ii) use of conization or laser ablation technique and any other surgery except for RH; (iii) active double cancer patients who were treated and did not develop a recurrence within 5 years of treatment completion; and (iv) were judged unsuitable for inclusion in the study by a physician. We considered that PS ≤ 1 (0 or 1) is appropriate for comparing the RT group with the OP group because we sometimes encounter patients with PS ≥2 in the RT group, on the other hand surgery is usually difficult for patients with PS ≥2. The planned number of patients required for this study was 50 patients each in the RT and OP groups, total 100 patients.
At each institution, consecutive patients who met the eligibility criteria and obtained consent from the physician were enrolled between the January 2012 and April 2014. Patients in the OP group had undergone surgery between 22 August 1990 and 25 September 2009, and patients in the RT group had undergone radiotherapy between 12 December 1998 and 12 November 2008.
We conducted the questionnaire survey only once. The timing of evaluation after treatment differs for each patient. Therefore, after the evaluation, how the patient’s quality of life has changed is unknown except for items of dysuria described below from patients’ questionnaires.
Patient recruitment
The recruitment procedure for this study varied by institution: in some institutions, the radiation oncologists asked the gynecologists to recruit all OP patients, while in others if the patients received post-operative RT the radiation oncologists were in charge of patient recruitment. The 21 patients who were treated with surgery alone without post-operative RT in the OP group were directly recruited by gynecologists (14 patients from 4 institutions), or referred by gynecologist to the outpatient department of radiation therapy, or the radiation oncologist went to the outpatient department of gynecology to recruit the patient (7 patients from 2 institutions). Radiation oncologists alone recruited and assessed both RT patients and OP patients in 7 institutions (in one institution only RT patients were recruited), of which in 6 institutions, radiation oncologists also recruited and assessed postoperative RT patients. In 3 institutions, patient recruitment was a joint effort between the radiation oncologists and gynecologists In 1 institution, radiation oncologist referred to the gynecologist for both recruitment and assessment of RT and OP patients. No patient was recruited in 1 institution. Hence, patients were recruited jointly by radiation oncologists and gynecologists in 4 institutions.
Overall 21 physicians including 14 radiation oncologists and 7 gynecologists cooperated in recruiting the patients and filling out the questionnaires. A total of 78 patients were recruited by radiation oncologists and 22 were recruited by gynecologists. Of the 50 RT patients, 49 were recruited by radiation oncologists and 1 patient was recruited by a gynecologist, and in the 50 OP patients, 29 were recruited by radiation oncologists and 21 by gynecologists.
Overall, 55 patients were recruited by the same physicians (23 RT patients and 32 OP patients), of which 49 patients (22 RT patients and 27 OP patients) were recruited by 5 radiation oncologists and the rest (1 RT, 5 OP) by a gynecologist. Of the remaining 45 patients, 29 patients (27 RT patients, 2 OP patients) were recruited by 8 radiation oncologists and the other 16 patients (OP patients only) by 6 gynecologists.
QOL assessment
We assessed the patients’ QOL using The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire (QLQ)–Cervical Cancer Module (CX24) [7]. We obtained permission from the EORTC to translate and validate the QLQ–CX 24 for use in the Japanese population. Concurrently, while preparing the Japanese version of the QLQ–CX 24, our working group considered original questionnaires based on the literature, guidelines and clinical experience to ensure that the QOL was evaluated according to actual clinical practice in Japan.
We found that questions on treatment-specific adverse events and sexual life were not identified in the preparation of the Japanese version of EORTC QLQ–CX 24. Thus, we agreed to develop a questionnaire consistent with Japanese culture and lifestyle as follows.
We asked the patients about their surgical wound, included diarrhea and constipation as separate questions, and added a question regarding hematuria as a late adverse event of RT. Since dysuria may change immediately after treatment and over time, we asked for the most severe symptom immediately after treatment and then whether it improved. We evaluated abdominal pain using five grades: (i) slight pain; (ii) light pain; (iii) moderate pain; (iv) strong pain; (v) very intense pain, because it was subjective to each patient. With respect to sexual life, we asked whether the patients had a partner (Q21). In the EORTC QLQ–CX 24, sexual life during the past 4 weeks post-treatment is assessed; however, because Japanese sexual life is generally considered to be less active than that of the Westerners [8], we set this period at 1 year. We added a question on bleeding in addition to pain as a possible concern during sexual intercourse (Q23). Regarding the changes in sexual activity after treatment (Q32–45), we referred to a study by Sakurai et al. [9]. Finally, we created a 45-item QOL questionnaire for the present study (Table 1). A Japanese version of this QOL questionnaire is attached as Supplementary Table 1, see online supplementary material. We explained the background of the questionnaire development to the EORTC and obtained permission to use our modified version. The EORTC translation team leader instructed us to state that we referred to Greimel et al.’s paper [7]. A Japanese QOL questionnaire has been developed previously [10].
Table 1.
Patient questionnaire
|
|
|
|
|
|
Our patients completed the Japanese version questionnaires in a private room and in compliance with the Japan Council for Quality Health Care standards.
Collection of clinical information
The physicians confirmed that the patients met all the selection criteria and obtained written consent forms from each patient. The questionnaire we created for this study included 104 questions for physicians to collect information on (i) patient characteristics, (ii) treatment methods (radiation therapy, surgery, postoperative radiotherapy, combination chemotherapy), (iii) recurrences, (iv) adverse events, and (v) medical advice on their sexual life (Table 2). The Japanese version of this physicians’ questionnaire is provided as Supplementary Table 2, see online supplementary mateial.
Table 2.
Physician questionnaire
|
|
|
|
The physicians who participated in the study were 11 males and 10 females.
After-treatment medical guidance on patients sexual activity by healthcare professionals
We confirmed whether the physician or a nurse provided guidance to the patients about sexual activity after the treatment of cervical cancer at each institution.
Statistical analysis
We performed t-tests or Wilcoxon’s rank sum tests to compare continuous variables, and χ2-tests or Fisher’s exact tests were performed to compare categorical variables. We conducted a univariate analysis using χ2-tests or Fisher’s exact tests and a multivariate logistic regression analysis using a stepwise selection method to identify the factors associated with adverse events (14 variables) and QOL (45 variables) depending on the treatment technique (RT and OP). Factors that reached the 0.25 level of significance by stepwise procedure were included in the multivariate logistic regression analysis. All data were analyzed using JMP version 9 software (SAS Institute, Cary, NC, USA). For all analyses, P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 100 patients were registered from January 2012 to April 2014, with 50 patients each in the RT and the OP group (Table 3). There was no difference in the survey time from the treatment between the two groups (P = 0.346). In the RT group there were respectively 34 and 66% of patients with Stage I and II, while in OP group we had respectively 66 and 34. There were more stage I tumors in the OP group than in the RT group, and more stage II tumors in the RT group than in the OP group (P = 0.001). The patients in the OP group were younger than those in the RT group (P < 0.001). We have not investigated the body mass index of patients in both groups.
Table 3.
Patients’ characteristics and treatment methods
| RT (n = 50) | OP (n = 50) | P –value | ||
|---|---|---|---|---|
| Age at treatment, yearsa | 53 ± 12 (26–70) | 44 ± 10 (26–67) | <0.001 | |
| Age at investigation, yearsa | 56 ± 13 (27–76) | 48 ± 10 (30–69) | 0.001 | |
| Years from treatment, yearsa | 3.2 ± 2.5 | 3.8 ± 3.7 | 0.346 | |
| Stage | I | 17 (34%) | 33 (66%) | |
| II | 33 (66%) | 17 (34%) | 0.001 | |
| Maximum tumor diameter (cm)a,* | 4.4 ± 1.04 | 3.4 ± 1.25 | 0.004 | |
| Pelvic lymph nodes metastasis ≥1 cm | ||||
| Yes | 19 (38%) | 9 (18%) | ||
| No | 31 (62%) | 41 (82%) | 0.026 | |
| PS | 0 | 43 (86%) | 46 (92%) | |
| 1 | 7 (16%) | 4 (8%) | NSe | |
| Coexisting illnessb | ||||
| Yes | 9 (18%) | 5 (10%) | ||
| No | 41 (82%) | 45 (90%) | NS | |
| Previous abdominal surgeryc | ||||
| Yes | 3(6%) | 3(6%) | ||
| No | 47 (94%) | 47(94%) | NS | |
| Marriage historyd | ||||
| Yes | 47 (94%) | 46 (92%) | ||
| No | 3 (6%) | 4 (8%) | ||
| Delivery history | ||||
| Yes | 36 (73%) | 44 (92%) | 0.018 | |
| No | 13 (27%) | 4 (8%) | ||
| Number of deliveries | 2 (0–3) | 2 (0–4) | ||
| Postoperative RT (PO-RT) | — | 29 (58%) | ||
| PO-RT alone | — | 11 (22%) | ||
| PO-RT + chemotherapy | — | 18 (36%) | ||
| Surgery alone | — | 14 (28%) | ||
| Surgery + chemotherapy | — | 7 (14%) | ||
| Combination of chemotherapy | 42 (84%) | 25 (50%) | <0.001 |
aMeans and standard deviations, otherwise numbers and proportions.
bE.g. diabetes mellitus, hypertension.
cE.g. appendectomy.
dIncluding common-law marriage.
eNS = not significant.
Treatment
The RT group received a combination of external beam RT (EBRT) to the pelvic cavity and high dose rate intracavitary brachytherapy (HDR-ICBT). EBRT and HDR-ICBT were administered in accordance with the guidelines for RT included in General Rules for Clinical and Pathological Study of Uterine Cervical Cancer in Japan [11]. In the early part of EBRT, the median 30.4 Gy was delivered to the whole pelvis. Thereafter, the remaining the median 20 Gy was administered to the same whole-pelvic field with central shield. EBRT was given using the four-field box technique with 3D conformal radiation therapy (3D-CRT) for 37 patients (74%), and the parallel-opposed (anteroposterior–posteroanterior) technique for the other 13 patients (26%). Intensity modulated radiation therapy (IMRT) was not used. Eleven (22%) patients received boost irradiation with mean dose of 6.8 Gy, while 6 (12%) patients received extended field irradiation with whole pelvis and para-aortic region. The median number of HDR-ICBT fractions was 4, and the median dose at Point A/fraction was 6 Gy. The mean overall treatment time was 47.3 days. In 42 (84%) patients who received combined chemotherapy, cisplatin (CDDP) was used in 40 cases, mitomycin C in 6 cases and nedaplatin in 2 cases.
All of the patients in the OP group underwent RH, pelvic lymphadenectomy and bilateral salpingo-oophorectomy. The number of dissected pelvic lymph nodes was 7–120 (mean 38), and the number of metastatic pelvic lymph nodes was 0–20 (mean 1.08). One or both ovaries were preserved in 6 (12%) patients; all patients were in stage IB, and the mean patient age was 35 years (range, 27–48 years). In total, 29 (58%) patients received postoperative RT, 18 of whom also received chemotherapy. Of the 18 patients who received postoperative RT and chemotherapy, 5 received the neo-adjuvant chemotherapy (NAC) → OP→postoperative RT regimen and 13 received the OP→postoperative CCRT regimen. Meanwhile, 14 (28%) patients received surgery alone, and 7 (14%) patients received surgery + chemotherapy (2 patients received NAC, 4 received adjuvant chemotherapy and 1 received NAC and adjuvant chemotherapy). Of the 25 (50%) patients who were treated with combined chemotherapy, 12 were treated with CDDP. (Table 3).
Adverse events according to the physicians’ questionnaire
The RTOG/EORTC classification lists the acute and late adverse events for RT patients, which do not apply to patients who have undergone surgery. So, we used the CTCAE classification to describe the late adverse events in OP patients.
Based on the physicians’ questionnaire, there were significantly more cases of bone marrow suppression (BMS) and diarrhea in the RT group than in the OP group as per the univariate analysis, and there were significantly more cases of BMS in the RT group than in the OP group in multivariate analysis (P = 0.013). On the other hand, there were significantly more cases of dysuria, leg edema, hot flush/sweating, constipation and pelvic lymphocele in the OP group than in the RT group in univariate analysis, and leg edema was significantly more common in the OP group than in the RT group in multivariate analysis (P = 0.006) (Table 4). In Table 4, all patients were evaluated for any adverse events other than urinary stenosis, which was not seen in any patient in the RT or OP group.
Table 4.
Adverse events based on the physicians’ questionnairea
| RT (n = 50) | OP (n = 50) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | P-value | AOR | 95% CI | P-value | |||
| BM suppression | 39 | 21 | 0.2 | 0.1–0.5 | <0.001 | 0.3 | 0.1–0.7 | 0.012 |
| Diarrhea | 36 | 23 | 0.3 | 0.1–0.8 | 0.008 | 0.4 | 0.1–1.1 | 0.064 |
| Cystitis | 9 | 6 | 0.6 | 0.2–1.9 | 0.400 | |||
| Dysuria | 0 | 17 | − | <0.001 | ||||
| Constipation | 0 | 4 | − | 0.041 | ||||
| Pelvic lymphocele | 0 | − | 0.041 | |||||
| Hot flush/sweating | 2 | 10 | 6.0 | 1.2–29.0 | 0.014 | 3.7 | 0.6–21.2 | 0.147 |
| Rectum | 4 | 1 | 0.2 | 0.03–2.2 | 0.169 | 0.0 | 0.01–1.26 | 0.073 |
| Bladder | 4 | 1 | 0.2 | 0.03–2.2 | 0.169 | |||
| Small intestine | 3 | 3 | 1.0 | 0.2–5.2 | 1.000 | |||
| Large intestine | 1 | 1 | 1.0 | 0.1–16.4 | 1.000 | |||
| Leg edema | 1 | 15 | 4.9 | 1.5–16.2 | 0.005 | 6.4 | 1.7–24.0 | 0.006 |
| Bone | 2 | 1 | 0.5 | 0.04–5.6 | 0.558 | |||
aOR = odds ratio (side effect occurred easily in the OP group if >1, and in the RT group if <1), AOR = adjusted odds ratio, CI = confidence interval, BM = bone marrow.
Self-reported QOL according to the patients’ questionnaire
In total, 92% (92/100) of patients responded to the questionnaire (94% [47/50] and 90% [45/50] of patients in the RT and OP groups, respectively). As the questions were printed on both sides of the paper, some patients did not complete the form because they may have not noticed the back side.
In terms of subjective symptoms, there were significantly more patients with dysuria, constipation, leg edema, lower abdominal edema and hot flushes/sweating in the OP group than in the RT group in univariate analysis. In multivariate analysis, there were significantly more patients with leg edema and dysuria in the OP than in the RT group (Table 4).
Dysuria and subsequent changes
Based on the physicians’ questionnaire, no cases of dysuria were reported in the RT group, while 34% of patients in the OP group experienced dysuria (Table 4, 0% [0/50] vs 34% [17/50], univariate analysis, P < 0.001). Based on the patients’ questionnaire, significantly more patients complained of dysuria in the OP group (44% [22/50]) than in the RT group (8.5% [4/47]) (Table 5, multivariate analysis, P = 0.022). Further, based on the responses provided in the patients’ questionnaire, patients who developed dysuria sometimes, quite a bit, or very much, in either group, improved quite a bit, almost fully, or completely recovered, in 68% (15/22: OP) and 50% (2/4: RT) of cases, with no significant differences between the patients in the OP or RT groups.
Table 5.
Univariate and multivariate analysis of variable factors between the radiotherapy and operation group on the patients’ questionnaire
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scale | Y/Na | RT | OP | ORd | 95% CI | P-value | AOR | 95% CI | P-value |
| Clinical symptoms | |||||||||
| Genitourinary symptoms | |||||||||
| Difficulty emptying the bladder | Y/N | 4/43 | 22/28 | 8.4 | 2.6–27.1 | < 0.001 | 4.6 | 1.3–19.1 | 0.022 |
| Leaking of urine | Y/N | 7/41 | 14/36 | 2.3 | 0.8–6.3 | 0.106 | 1.0 | 0.3–4.0 | 0.975 |
| Pain when urinating | Y/N | 4/46 | 1/48 | 0.2 | 0.02–2.2 | 0.176 | |||
| Increased frequency of urination | Y/N | 12/38 | 8/39 | 0.6 | 0.2–1.77 | 0.396 | |||
| Hematuria | Y/N | 2/48 | 2/46 | 1.0 | 0.1–7.7 | 0.967 | |||
| Vaginal discharge | Y/N | 8/42 | 4/45 | 0.5 | 0.1–1.7 | 0.232 | |||
| Vaginal hemorrhage | Y/N | 1/49 | 0/49 | 0.0 | 0.320 | ||||
| Gastrointestinal symptoms | |||||||||
| Diarrhea | Y/N | 17/33 | 18/30 | 1.6 | 0.5–2.7 | 0.718 | 0.6 | 0.2–2.0 | 0.446 |
| Constipation | Y/N | 15/35 | 25/24 | 2.4 | 1.1–5.5 | 0.033 | |||
| Blood in stools | Y/N | 1/49 | 1/48 | 1.0 | 0.06–16.8 | 0.989 | |||
| Pain | |||||||||
| Lumbago | Y/N | 14/36 | 15/34 | 1.0 | 0.4–2.7 | 0.775 | |||
| Pain of the inguinal region | Y/N | 11/38 | 19/30 | 2.2 | 0.9–5.3 | 0.795 | |||
| Lower abdominal pain | Y/N | 7/43 | 11/38 | 1.8 | 0.6–5.0 | 0.276 | 1.3 | 0.3–4.9 | 0.717 |
| Leg or lower abdominal lymphedema | |||||||||
| Swelling in one or both legs | Y/N | 3/47 | 17/32 | 8.3 | 2.3–30.8 | <0.001 | 4.9 | 1.2–25.2 | 0.033 |
| Lower abdominal edema | Y/N | 1/40 | 9/40 | 11.0 | 1.3–90.7 | 0.007 | |||
| Peripheral neuropathy | |||||||||
| Tingling or numbness in feet | Y/N | 9/39 | 11/38 | 1.3 | 0.5–3.4 | 0.653 | |||
| Menopausal symptoms | |||||||||
| Hot flushes and/or sweats | Y/N | 10/37 | 19/26 | 2.7 | 1.1–6.8 | 0.031 | |||
| Body image after suffering from a cancer | |||||||||
| Feel less feminine as a result of disease or treatment | Y/N | 7/39 | 8/37 | 1.2 | 0.4–3.7 | 0.742 | |||
| Sexual partner existence | Y/N | 30/14 | 38/7 | 2.5 | 0.9–7.0 | 0.071 | |||
| Sexual vaginal functioning | |||||||||
| Vaginal dryness during sexual activity | Y/N | 5/8 | 8/17 | 0.7 | 0.2–3.0 | 0.690 | |||
| Vaginal shortness | Y/N | 1/12 | 6/19 | 3.8 | 0.4–35.5 | 0.219 | |||
| Vaginal tightness | Y/N | 2/11 | 6/19 | 1.7 | 0.3–10.1 | 0.537 | |||
| Pain during sexual intercourse | Y/N | 6/8 | 8/17 | 0.6 | 0.2–2.4 | 0.498 | |||
| Sexual worry | |||||||||
| Worry about pain during sexual intercourse | Y/N | 10/27 | 15/28 | 1.4 | 0.6–3.8 | 0.450 | |||
| Worry about post-coital genital bleeding | Y/N | 6/31 | 11/33 | 1.8 | 0.6–5.2 | 0.334 | |||
| Sexual activity, enjoyment and change | |||||||||
| Enjoying sexual activity | N/Y | 9/2 | 20/4 | 1.1 | 0.2–10 | 0.912 | |||
| Using something during sexual intercourse | Y/N | 2/11 | 8/16 | 2.8 | 0.5–15.5 | 0.241 | |||
| Frequency of sex before the treatmentb | Y/N | 10/25 | 20/26 | 1.9 | 0.8–4.9 | 0.169 | |||
| Frequency of sex after the treatmentb | Y/N | 2/33 | 8/38 | 3.5 | 0.7–17.5 | 0.114 | |||
| The time you started having sex after treatmenctc | Y/N | 7/26 | 17/27 | 2.3 | 0.8–6.6 | 0.102 | |||
| No change in the feelings and/or the condition of the body | Y/N | 26/6 | 33/11 | 0.7 | 0.2–2.1 | 0.519 | |||
| I did not gradually do sex because of my feeling and age | Y/N | 22/3 | 30/11 | 0.4 | 0.09–1.5 | 0.372 | |||
| I did not gradually do sex because of partner’s feeling and age | Y/N | 20/4 | 30/12 | 0.5 | 0.1–1.8 | 0.278 | |||
| Because my physical condition is not good, I do not bring myself to do sex | Y/N | 9/15 | 20/23 | 1.4 | 0.5–4.0 | 0.475 | |||
| Hate sexual intercourse as a result of cervical cancer. | Y/N | 13/11 | 23/18 | 1.1 | 0.4–3.0 | 0.880 | |||
| I became more interested in sexual intercourse than previously | Y/N | 4/20 | 7/36 | 1.0 | 0.3–3.7 | 0.967 | |||
| Partner declines or hates sexual intercourse after my treatment | Y/N | 9/13 | 20/20 | 1.4 | 0.5–4.1 | 0.492 | |||
| Partner became more interested in having sexual intercourse | Y/N | 4/17 | 6/34 | 0.8 | 0.2–3.0 | 0.685 | |||
| I do not want to have sexual inter-course, but I endure it for my partner | Y/N | 6/10 | 14/29 | 0.8 | 0.2–2.7 | 0.721 | |||
| After treatment, insertion became difficult | Y/N | 9/10 | 17/20 | 0.9 | 0.3–2.9 | 0.920 | |||
| No pleasant feeling at the time of sexual intercourse | Y/N | 11/8 | 20/16 | 0.9 | 0.3–2.8 | 0.868 | |||
aY/N: Yes/No.
bClassified as Yes/No. Frequency greater than once a month: Yes (Y); frequency, less than once a month: No (N).
cUnder 1 year: Y; interval of more than 1 year or never: N.
dOR = odds ratio (side effect occurred easily in the OP group if >1, and in the RT group if <1), CI = confidence interval, AOR = adjusted odds ratio.
On the other hand, postoperative dysuria was significantly more prevalent in patients in the postoperative RT group (48.3% [14/29]) than in patients in the OP alone group (14.3% [3/21]) (P = 0.009) based on the responses collected from the physicians’ questionnaire, while postoperative dysuria was not significantly different between the two groups (postoperative RT group (44.8% [13/29]) vs OP alone group (42.9% [9/21]) (P = 0.890)) based on the responses provided in the patient’ questionnaire.
Leg edema in the OP group
Assessment of leg edema differs between the physician and the patient. As assessed by the physician, postoperative RT was not associated with a severe risk of leg edema (P = 0.123: Fig. 1a). However in the patients’ questionnaire, irreversible severe (Score 4–5) leg edema was significantly more observed in the postoperative RT group than in the RT only group (37% [10/27] vs 0% [0/22]; P = 0.002: Fig. 1b). There was a discrepancy between the physicians’ evaluation and the patient-reported outcome.
Fig. 1.
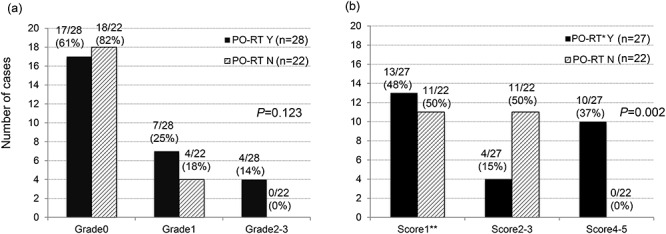
Degree of leg edema in those complaining of leg edema according to the presence or absence of postoperative RT (PO-RT) in the OP group on (a) the physicians’ and (b) the patients’ questionnaire. * One patient with PO-RT did not answer the question. Y: yes, N: no. Score1**, not at all; 2, a little; 3, sometimes; 4, almost always; 5, always.
Change in sexual activity
In total, 88% of the patients answered detailed questions on sexual activity (Q32–45). There were no significant differences in sexual activity between the RT and the OP groups (Table 4). In total, 85% (85/100) of the patients responded to the question regarding the frequency and start time of sexual intercourse (Q32–34). Of these, 62% (53/85: RT, 52% [20/38]; OP, 70% [33/47]) were engaged in sexual activity within the past 1 year before treatment, while only 39% (33/85: RT, 26% [10/38]; OP, 47% [22/47]) were engaged in sexual activity within 1 year after treatment. This showed that the frequency of sexual intercourse at 1 year after treatment decreased significantly as compared to 1 year before treatment in both groups (overall: P = 0.001; RT group: P = 0.019; OP group: P = 0.021, Fig. 2).
Fig. 2.
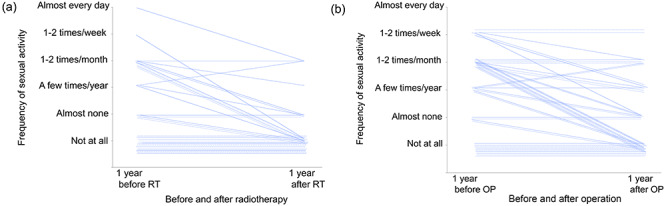
Change in frequency of sexual activity 1 year before or after (a) radiotherapy or (b) operation in patients with uterine cervical cancer.
Medical guidance for patients regarding sexual activity
Following treatment, only 31% (31/100) of all patients received medical guidance from a physician or nurse. These included: 24% (12/50) and 38% (19/50) in the RT and the OP groups, respectively. Physicians or nurses provided direct guidance on sexual life to 4 and 17 patients in the RT and OP groups, respectively. Of the 21 physicians who participated in this study, 11 were males and 10 were females. However, the physician who filled out the questionnaire and the physician who gave the guidance regarding sexual intercourse after treatment to the patient were different for the OP alone group. For this reason, we researched the gender of the physicians who gave sexual advice. As shown in Table 2, the guidance items regarding sexual intercourse from medical staff to patients is: 1. Instructed, 2. Not instructed, 3. Pamphlet, 4. Others. We considered responses that were 1, 3, or 4 as instructed. Guidance rates for male doctors was 28.6% (18/63) and for female doctors 35.1% (13/37) (P = 0.493). There was no relationship between providing sexual activity guidance and the gender of the physician.
DISCUSSION
Given the difference in treatment policies for RT and the surgical methods for uterine cervical cancer between Western countries and Japan, the post-RT or post-surgery QOL in Japan should be compared cautiously with that of Western countries.
In this study, we found significant differences in BMS in the RT group and leg edema in the OP group as assessed according to the physicians’ questionnaire. This finding was attributed to the significantly higher rate of combined chemotherapy in the RT group than in the OP group.
Postoperative lymphedema of the legs is a considerable problem due to RH, which includes complete pelvic lymph node dissection [12]. Many studies reported postoperative lymphedema of the legs [12–19], with the rate of lymphedema being higher in patients treated with OP and RT combined than in those treated with OP alone [19]. Further, one study reported that although emotional distress and QOL issues improved during the first 2 years after cervical cancer diagnosis, lymphedema and menopausal symptoms persisted [17]. In our study, leg edema was significantly more common in the OP group than in the RT group, according to both the physicians’ and the patients’ questionnaires. Meanwhile, the physician’s questionnaire showed no significant difference in the severity of postoperative lymphedema of the leg between the post-operative RT group and the OP only group. However, based on the patients’ subjective symptoms self-assessment, the degree of lymphedema of the leg in the OP group was significantly higher in patients with post-operative RT than that in the OP only group (Fig. 1).
In line with a previous study, we also observed a large gap between the patients’ subjective assessment of symptoms and physicians’ objective evaluation of lymphedema [5]. Despite the physicians’ assurance that the leg edema would cause minimal discomfort, most patients tend to consider leg edema as a major problem in performing the activities of daily living. Therefore, the self-reported patients’ QOL questionnaires in our study were considered very important.
Bladder, ano-rectal and sexual complications are common following RH for cervical cancer. In general, the incidence of temporary voiding dysfunction is higher following RH, while the incidence of urine storage dysfunction is higher following CCRT [20–22].
The major adverse event following RH for invasive cancer of the cervix is postoperative bladder dysfunction. Bladder dysfunction is a direct result of injury to the sensory and motor nerve supply to the detrusor muscle of the bladder [13]. Post-operative RT was associated with significantly more contracted and unstable bladder [13]. Butler-Manuel et al. compared QOL before and after OP and reported that urinary incontinence, particularly of urge incontinence, and voiding difficulties as well as tenesmus increased significantly after OP (P < 0.05 and P < 0.05, respectively) [20]. Like the findings of Katepratoom et al. [21], the incidence of difficulty in bladder emptying was significantly higher after OP as compared to RT in our study by both physicians’ (P < 0.001: univariate analysis) and patients’ (P = 0.022: multivariate analysis) questionnaires. In our study, the incidence of post-operative dysuria in the post-operative RT group was higher than in the OP alone group in the physicians’ questionnaire, but there was no difference between the two groups in the patients’ questionnaire. This was the opposite difference in assessment between physicians’ questionnaire and patients’ questionnaire to that for leg edema.
Rectal bleeding is a late adverse effect of RT [23,24]. Chronic adverse effects of intestinal RT can cause telangiectasis of the rectum and changes to the blood vessels of the rectal tissues [23]. However, we found no significant difference in rectal bleeding between the RT and OP groups in both the physicians’ (RT group: 8% [4/50], OP group: 2% [1/50], P = 0.073: multivariate analysis) and the patients’ (RT group: 2.0% [1/49], OP group: 2.1% [1/48], P = 0.989: univariate analysis) questionnaire in our study (Table 4 and 5). Diarrhea is a chronic symptom after RT [22, 25]. In our study, univariate analysis of the physicians’ questionnaire showed that diarrhea was significantly more frequent in the RT group (RT group: 72% [36/50], OP group: 46% [23/50], P = 0.008); however, there was no significant difference between the two groups as per the patients’ questionnaire (RT group: 39.4% [13/33], OP group: 60% [18/30], P = 0. 718). Hu et al. reported only minor differences in long-term QOL at least 2 years post-treatment between OP and RT patients, where pelvic neural dysfunction was significantly higher in the OP group, while intestinal dysfunction was higher in the RT group [24].
As sexual life differs between Japanese and Western people, a simple comparison is difficult. Japanese people are generally more reluctant to perform sexual activity [8] as compared to Westerners. Despite this, various reports have investigated post-treatment sexual activity in uterine cervical cancer patients. These reports show varying patterns of deterioration, compromise and improvement in sexual activity before and after various treatment modalities. Some authors have reported that cervical cancer survivors treated with RT had worse sexual function than the control group and those treated with RH and lymph node dissection [12, 18, 25–29]. Irradiated women faced more difficulty in becoming sexually aroused, attaining vaginal lubrication and achieving sexual satisfaction, and experienced significantly more pain during intercourse than those in the RH or control group [18, 30]. Meanwhile, Butler-Manuel et al. reported that 55% of patients considered that their sex life was worse after the surgery and 13% ceased having sexual activity [20]. Chronic fibrotic changes in pelvic tissue after RT create vaginal atrophy, which leads to persistent sexual and vaginal problems, such as dyspareunia and a lack of lubrication in cervical cancer patients [18, 27, 29]. These problems compromised their sexual activity and satisfaction. On the other hand, early diagnosis and treatment could facilitate a gradual return to a normal life and even an improvement in sexual activity in both those who undergo OP and CCRT + OP [16]. Compared to surgery alone, intracavitary RT, external RT, or both, in addition to or instead of surgery, had a small effect on the risk of reduced vaginal lubrication, shortness or inelasticity [31]. Kobayashi et al. [6] reported no significant differences in anxiety and depression scores among the three treatment modalities (RT, CCRT and OP + RT).
With respect to sexual activity, we found no statistically significant differences between the RT and OP groups based on the patients’ questionnaire. However, the frequency of sexual activity in both groups also decreased significantly after treatment as compared to before treatment, which was consistent with other reports [18,20].
In our study, only 31% of all patients (RT: 24%; OP: 38%) received guidance about post-treatment sexual activity from the medical staff; this low rate was attributed to busy outpatient clinics and lack of professional knowledge on sexual activities. Guidance rates of male doctors was 28.6% (18/63) and that of female doctors was 35.1% (13/37) (P = 0.493). There was no relationship between providing sexual activity guidance and the gender of the physician. Physicians are generally focused on monitoring relapse and late adverse events and do not have adequate time for consultations on the patients’ sexuality. Although sexuality is an important element of QOL, in Japan, there is insufficient support from healthcare workers on discussing sexuality. Further, it is difficult for patients to consult healthcare workers regarding sex [32]. Sexuality-related information in the context of adverse events after treatment should be provided to all patients, regardless of age or type of treatment [33]. Educating the medical staff on patient sexuality, providing information to patients and establishing a consultation desk is needed.
One of the causes of pain during sexual intercourse or during pelvic examination is dryness, vaginal adhesion due to RT and ovarian deficit symptoms due to oophorectomy. Vaginal pain can be improved by use of a vaginal dilator to prevent adhesion after RT or with jelly or mousse [34]. Preventing vaginal adhesion may lead to the early detection of cervical recurrence. The proportion of patients using a dilator after the initiation of RT was reported to decrease with time [35]. Training the medical support staff is also important. Books, pamphlets and lectures can be given to patients, partners and physicians to effectively provide more information on sexuality [32].
Our study has several limitations. First, this observational questionnaire survey study was not a prospective survey, and was conducted only once. However, there was no difference between the two groups with regard to the timing of the survey, so we evaluate that it is meaningful, even once. Second, since the questions were designed for a small number of patients in Japan, detailed analysis of every item was limited in between-group comparison. Third, post-operative irradiation was performed in 58% of the surgery group in this study. Since 67% (14/21) of the physicians who recruited patients were radiation oncologists, which was more than the 33% (7/21) of gynecologists, in this study more postoperative RT patients seemed to be recruited. Therefore, if we compare RT and OP alone, it seems to be biased. Fourth, the original QOL questionnaire used for this survey was developed by us. This QOL questionnaire, comprising a total of 45 items with disease-specific questions based on clinical practice in Japan, has not yet been validated. However, it is also being referenced in an ongoing Japanese clinical trial (JGOG1082). The usefulness of this QOL questionnaire should be further validated in prospective large-scale studies from now on. Future research requires a prospective design with long-term follow-up of QOL after treatment. To further develop our research, we are planning to release a pamphlet about the treatment of uterine cervical cancer.
CONCLUSION
Post-treatment QOL change of RT and OP patients with early-stage cervical cancer were each characterized. Our findings will assist patients and physicians shared decision-making with respect to treatment choice. Healthcare professionals should provide patients with more guidance about coping with post-treatment changes in their QOL, including sexual life, irrespective of whether they undergo RT or OP.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank gynecologists and radiation oncologists at all 12 institutions who co-operated in the survey. We acknowledge Dr Kunihiko Kobayashi (Saitama Medical University International Medical Center), Dr Joseph Green (Tokyo University), and Mrs Nicola Biwaki who helped with the Japanese translation of EORTC QLQ–CX24. We also thank nurses and secretaries who checked the questionnaire we developed. Our greatest appreciation is extended to the patients who agreed to participate in this study. This work was presented at the following conferences: 19–21 July 2013 The 54th Annual Meeting of the Japan Gynecological Oncology Society, Tokyo, Japan; December 2014 The 28th Japanese Society for Radiation Oncology: Excellent Presentation Award, Yokohama, Japan; June 2015 The 15th International Congress of Radiation Research, Kyoto, Japan; August 2015 The 57th Annual Meeting of the Japan Gynecological Oncology Society, Morioka, Japan; 5–6 September 2015 The 68th Annual Meeting of the Chugoku Shikoku Society of Obstetrics and Gynecology, Kurashiki, Japan; October 2015 The 53rd Annual Meeting of Japan Society of Clinical Oncology, Kyoto, Japan; April 2016 The 75th Annual Meeting of the Japan Radiological Society: CyPos Award; Gold Medal, Yokohama, Japan; 13–16 April 2017 The 69th Annual Meeting of Japan Society of Obstetrics and Gynecology, Hiroshima, Japan; Newsletter article: June 2015 Japanese Society for Radiation Oncology (JASTRO) News Letter; Newspaper article: 17 September 2015 Medical Tribune (The 57th Annual Meeting of the Japan Gynecological Oncology Society).
Contributor Information
Yuko Kaneyasu, Department of Radiation Oncology, National Hospital Organization Fukuyama Medical Center, Hiroshima, Japan; Department of Radiation Oncology, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan.
Hisaya Fujiwara, Department of Obstetrics and Gynecology, Chugoku Rosai Hospital, Hiroshima, Japan; Department of Obstetrics and Gynecology, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan.
Tetsuo Nishimura, Radiation and Proton Therapy Center, Shizuoka Cancer Center, Shizuoka, Japan.
Hideyuki Sakurai, Department of Radiation Oncology, University of Tsukuba, Ibaraki, Japan.
Tomoko Kazumoto, Department of Radiation Oncology, Fukaya Red Cross Hospital, Saitama, Japan; Department of Radiation Oncology, Saitama Cancer Center, Saitama, Japan.
Hitoshi Ikushima, Department of Therapeutic Radiology, Tokushima University Graduate School, Tokushima, Japan.
Takashi Uno, Department of Diagnostic Radiology and Radiation Oncology, Chiba University Graduate School of Medicine, Chiba, Japan.
Sunao Tokumaru, Hyogo Ion Beam Medical Center, Hyogo, Japan; Department of Radiology, Saga University, Saga, Japan.
Yoko Harima, Department of Radiology, Kansai Medical University, Osaka, Japan.
Hiromichi Gomi, Radiation Oncology Center, St. Marianna University School of Medicine Hospital, Kanagawa, Japan.
Takafumi Toita, Radiation Therapy Center, Okinawa Chubu Hospital, Okinawa, Japan; Department of Radiology, Graduate School of Medical Science, University of the Ryukyus, Okinawa, Japan.
Midori Kita, Department of Radiology, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
Shin-ei Noda, Department of Radiation Oncology, Saitama Medical University International Medical Center, Saitama, Japan; Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma, Japan.
Takeo Takahashi, Department of Radiation Oncology, Saitama Medical University Saitama Medical Center, Saitama, Japan.
Shingo Kato, Department of Radiation Oncology, Saitama Medical University International Medical Center, Saitama, Japan.
Ayako Ohkawa, Department of Radiation Oncology, University of Tsukuba, Ibaraki, Japan; Department of Radiation Oncology, National Hospital Organization Mito Medical Center, Ibaraki, Japan.
Akiko Tozawa-Ono, Department of Gynecology, St. Marianna University School of Medicine, Toyoko Hospital, Tokyo, Japan.
Hiroki Ushijima, Department of Radiation Oncology, Saitama Cancer Center, Saitama, Japan.
Yoko Hasumi, Department of Obstetrics and Gynecology, Mitsui Memorial Hospital, Tokyo, Japan; Department of Gynecology, Saitama Cancer Center, Saitama, Japan.
Yasuyuki Hirashima, Division of Gynecology, Shizuoka Cancer Center, Shizuoka, Japan.
Yuzuru Niibe, Department of Public Health, Kurume University School of Medtioicine, Fukuoka, Japan.
Tomio Nakagawa, Department of Radiation Oncology, National Hospital Organization Fukuyama Medical Center, Hiroshima, Japan.
Tomoyuki Akita, Department of Epidemiology, Infectious Disease Control and Prevention, Graduate school of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan.
Junko Tanaka, Department of Epidemiology, Infectious Disease Control and Prevention, Graduate school of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan.
Tatsuya Ohno, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma, Japan.
FUNDING
This work was supported by research funding from the Japanese Society for Radiation Oncology (JASTRO).
CONFLICT OF INTEREST
None declared.
References
- 1. Landoni F, Maneo A, Colombo A et al. Randomized study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute: Clinical Announcement. Bethesda, MD: United States Department of Health and Human Services, Public Health Service; February, 1999. [Google Scholar]
- 3. Report of the gynecological tumor committee . Annual report of 2016 patient. Acta Obstet Gynecol Japonica 2018;70:1317–71. [Google Scholar]
- 4. National Cancer Center . Cancer Information Services. Japan. [Google Scholar]
- 5. Tanaka T, Ohki N, Kojima A et al. Radiotherapy negates the effect of retroperitoneal nonclosure for prevention of lymphedema of the legs following pelvic lymphadenectomy for gynecological malignancies: An analysis from a questionnaire survey. Int J Gynecol Cancer 2007;17:460–4. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi M, Ohno T, Noguchi W et al. Psychological distress and quality of life in cervical cancer survivors after radiotherapy. Do treatment modalities, disease stage, and self-esteem influence outcome. Int J Gynecol Cancer 2009;19:1264–8. [DOI] [PubMed] [Google Scholar]
- 7. Greimel ER, Vlasic KK, Waldenstrom SC et al. The European organization for research and treatment of cancer (EORTC) quality-of-life questionnaire cervical cancer module EORTC QLQ-CX24. Cancer 2006;107:1812–22. [DOI] [PubMed] [Google Scholar]
- 8. Araki C, Ishida M, Ohkawa R et al. Sexuality of middle and old age. Jpn J Sexol 2016; harunosora, Japan. [Google Scholar]
- 9. Sakurai H, Takahashi M, Suzuki Y et al. Changes of sexual activity in patients with uterine cervical carcinoma treated with radiation therapy. J Jpn Soc Ther Radiol Oncol 2003;15:187–91. [Google Scholar]
- 10. Kaneyasu Y, Fujiwara H, Nishimura T et al. Development of a Japanese QOL questionnaire for patients with uterine cervical cancer. Japanese J Gynecol Oncol 2018;36:682–91. [Google Scholar]
- 11. General Rules for Clinical and Pathological Study of Uterine Cervical Cancer in Japan . IN: Kudo R, Yakushiji M, editors. Japan Society of Obstetrics and Gynecology, The Japanese Society pf Pathplogy, Japan Radiologinal Society, editors group. 2 nd English ed. Tokyo Japan: Kanehara Shuppan; 1999. [Google Scholar]
- 12. Derks M, van Lonkhuijzen LR, Bakker RM et al. Long-term morbidity and quality of life in cervical cancer survivors: A Multicenter comparison between surgery and radiotherapy as primary treatment. Int J Gynecol Cancer 2017;27:350–6. [DOI] [PubMed] [Google Scholar]
- 13. Krishnansu ST, Bradley JM. Invasive Cervical Cancer. In: Di Saia PJ, Creasman WT (ed). Clinical Gynecologic Oncology. 8th ed. Philadelphia: Elsever, 2012, 69–90. [Google Scholar]
- 14. Fuller J, Guderian D, Kohler C et al. Lymph Edema of the lower extremities after adenectomy and radiotherapy for cervical cancer. Strahlenther Onkol 2008;206–11. [DOI] [PubMed] [Google Scholar]
- 15. Pieterse QD, Maas CP, Ter Kuile MM et al. An observational longitudinal study to evaluate mictin, defecation, and sexual function after radical hysterectomy with pelvic lymphadenectomy for early-stage cervical cancer. Int J Gynecol Cancer 2006;1119–29. [DOI] [PubMed] [Google Scholar]
- 16. Ferrandina G, Mantegna G, Petrillo M et al. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: A prospective. longitudinal study. Gynecol Oncol 2012;124:389–94. [DOI] [PubMed] [Google Scholar]
- 17. Mantegna G, Petrillo M, Fuoco G et al. Long-term prospective longitudinal evaluation of emotional distress and quality of life in cervical cancer patients who remained disease-free 2-years from diagnosis. Bio Med Cancer 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frumovitz M, Sun CC, Schover LR et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol 2005;23:7428–36. [DOI] [PubMed] [Google Scholar]
- 19. Borgne GL, Mercier M, Woronoff AS et al. Quality of life in long-term cervical cancer survivors: A population-based study. Gynecol Oncol 2013;129:222–8. [DOI] [PubMed] [Google Scholar]
- 20. Butler-Manuel SA, Summerville K, Ford A et al. Self-assessment of morbidity following radical hysterectomy for cervical cancer. J Obstet Gynaecol 1999;19:180–3. [DOI] [PubMed] [Google Scholar]
- 21. Katepratoom C, Manchana T, Amornwichet N. Lower urinary tract dysfunction and quality of life in cervical cancer survivors after CCRT versus radical hysterectomy. Int Urogynecol J 2014;25:91–6. [DOI] [PubMed] [Google Scholar]
- 22. Klee M, Thranov I, Machin D. The patient’s perspective on physical symptoms after radiotherapy for cervical cancer. Gynecol Oncol 2000;76:14–23. [DOI] [PubMed] [Google Scholar]
- 23. Haboubi NY, Schofield PF, Rowland PL. The light and electron microscopic features of early and late phase radiation-induced proctitis. Am J Gastroenterol 1988;83:1140–4. [PubMed] [Google Scholar]
- 24. Hsu WC, Chung NN, Chen YC et al. Comparison of surgery or radiotherapy on complications and quality of life in patients with the stage IB and IIA uterine cervical cancer. Gynecol Oncol 2009;115:41–5. [DOI] [PubMed] [Google Scholar]
- 25. Kirchheiner K, Pötter R, Tanderup K et al. Health-related quality of life in locally advanced cervical cancer patients after definitive chemoradiation therapy including image guided adaptive brachytherapy: An analysis from the EMBRACE study. Int J Radiat Oncol Biol Phys 2016;94:1088–98. [DOI] [PubMed] [Google Scholar]
- 26. Jensen PT, Groenvold M, Klee MC et al. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2003;56:937–49. [DOI] [PubMed] [Google Scholar]
- 27. Greimel E, Winter R, Kapp KS et al. Quality of life and sexual functioning after cervical cancer treatment: A long-term follow-up study. Psycho-Oncology 2009;18:476–82. [DOI] [PubMed] [Google Scholar]
- 28. Bjelic-Radisic V, Jensen PT, Vlasic KK et al. Quality of life characteristics inpatients with cervical cancer. Euro J Cancer 2012;48:3009–18. [DOI] [PubMed] [Google Scholar]
- 29. Park SY, Bae DS, Nam JH et al. Quality of life and sexual problem in disease-free survivors of cervical cancer compared with the general population. Cancer 2007;110:2716–25. [DOI] [PubMed] [Google Scholar]
- 30. Cull A, Cowie VJ, Farquharson DIM et al. Early stage cervical cancer: Psychosocial and sexual outcomes of treatment. Br J Cancer 1993;68:1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergmark K, Avall-Lundqvist E, Dickman PW et al. Vaginal changes and sexuality in women with a history of cervicl cancer. New Eng J Med 1999;340:1383–9. [DOI] [PubMed] [Google Scholar]
- 32. Kiyoto S, Miyauchi K, Ikebe K et al. The healthcare workers’ awareness and support regarding the sexuality of cancer patients, their families and their partners. Palliat Care Res 2017;12:739–46. [Google Scholar]
- 33. Takahashi M, Ohno S, Inoue H et al. Impact of breast cancer diagnosis and treatment on women’s sexuality: A survey of Japanese patients. Psychooncology 2008;17:901–7. [DOI] [PubMed] [Google Scholar]
- 34. Takahashi M. The indication and use of vaginal dilators. Jpn J Sexol 2003;21:75–80. [Google Scholar]
- 35. Davidson SE, Burns MP, Routledge JA et al. The impact of radiotherapy for carcinoma of the cervix on sexual function assessed using the LENT SOMA scale. Radiother Oncol 2003;68:241–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







