Abstract
Internal aeration is crucial for root growth under waterlogged conditions. Many wetland plants have a structural barrier that impedes oxygen leakage from the basal part of roots called a radial oxygen loss (ROL) barrier. ROL barriers reduce the loss of oxygen transported via the aerenchyma to the root tips, enabling long-distance oxygen transport for cell respiration at the root tip. Because the root tip does not have an ROL barrier, some of the transferred oxygen is released into the waterlogged soil, where it oxidizes and detoxifies toxic substances (e.g., sulfate and Fe2+) around the root tip. ROL barriers are located at the outer part of roots (OPRs). Their main component is thought to be suberin. Suberin deposits may block the entry of potentially toxic compounds in highly reduced soils. The amount of ROL from the roots depends on the strength of the ROL barrier, the length of the roots, and environmental conditions, which causes spatiotemporal changes in the root system’s oxidization pattern. We summarize recent achievements in understanding how ROL barrier formation is regulated and discuss opportunities for breeding waterlogging-tolerant crops.
Keywords: aerenchyma, apoplastic barrier, flooding, planar O2 optode, rhizosphere oxidation, root system, suberin
1. Introduction
Plants can suffer from hypoxia or even anoxia when soils become waterlogged (i.e., only the root system is immersed in water). Well-drained soil is porous and normally filled with gas; excess water fills the pores, preventing the entry of atmospheric oxygen, as the diffusivity of oxygen in water is approximately 10,000 times slower than it is in air (Jackson et al. 1985). Other problems associated with waterlogging are the accumulation of phytotoxic compounds in the soil (Ernst 1990, Kreuzwieser et al. 2004, Lamers et al. 1998, Ponnamperuma 1984) and a decline in the availability of some nutrients (e.g., NO3– and Fe3+) (Laanbroek 1990). Waterlogging damages many crops, including soybean, wheat, maize, oats, and barley (Bertholdsson 2013, Setter and Waters 2003). Moreover, the number of flooding events is increasing worldwide due to climate change (Hirabayashi et al. 2013), causing severe damage to crop yields (Fukao et al. 2019, Pedersen et al. 2017).
Plants can adapt to waterlogging-induced hypoxia by shifting metabolic pathways (Bailey-Serres and Voesenek 2008, Fukao et al. 2019) and reducing energy consumption (Bailey-Serres and Voesenek 2010, Fukao et al. 2019). When waterlogging is prolonged, some plants can survive by internal oxygen transport via aerenchyma, which is an interior gas space connected between the shoot and the root tip (Colmer 2003a, Colmer and Voesenek 2009, Pedersen et al. 2021b, Seago et al. 2005, Shiono et al. 2008, Yamauchi et al. 2018). A radial oxygen loss (ROL) barrier, together with aerenchyma, contribute to long-distance oxygen transport and waterlogging tolerance. This review examines how ROL barriers affect root growth, how their formation is regulated, and how they might be used to breed waterlogging-tolerant crops.
2. Adaptive significance of an ROL barrier for roots in waterlogged soil
2-1. Long-distance oxygen transport for respiration at the root tip
Under waterlogged conditions, oxygen molecules diffusing longitudinally through aerenchyma toward the root apex may be either consumed by respiration of root cells or diffused radially to the rhizosphere (Armstrong 1979, Colmer 2003a, Nishiuchi et al. 2012). Oxygen moving towards the root apex via the aerenchyma can leak into the surrounding soil (Armstrong 1979, Colmer and Voesenek 2009) in a process called radial oxygen loss (ROL). The amount of ROL is determined by the oxygen concentration gradient, the physical resistance to radial oxygen diffusion between aerenchyma and soil, and the consumption of oxygen by cells along the radial diffusion path (Fig. 1A) (Armstrong 1979, Armstrong and Beckett 1987, Colmer 2003a, Nishiuchi et al. 2012). ROL aerates the rhizosphere (Armstrong 1979), where it nourishes the oxidative microbial community (Martin et al. 2019) and detoxifies toxic reduced substances in the waterlogged soil (Blossfeld et al. 2011, Colmer 2003a, Martin et al. 2019, Neubauer et al. 2007, Nishiuchi et al. 2012). However, ROL reduces the oxygen supply to the root apex, preventing the roots from growing deeper into anaerobic soil (Armstrong 1979, Pedersen et al. 2021b, Sorrell et al. 2000).
Fig. 1.
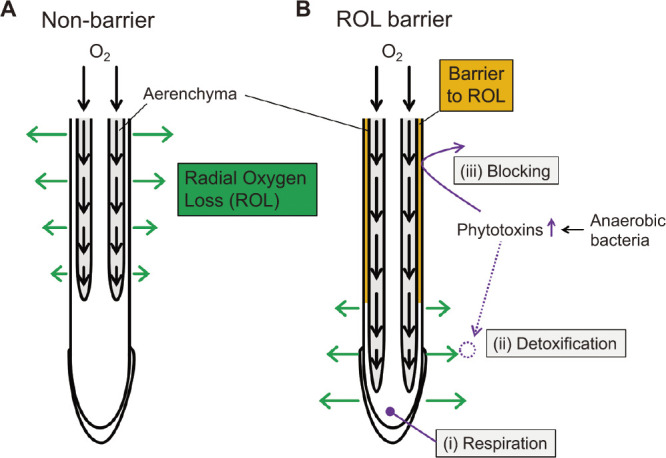
Adaptive significance of an ROL barrier for roots in waterlogged soil. (A) Oxygen molecules diffusing longitudinally through aerenchyma toward the root apex may either be consumed by respiration of root cells or diffuse radially to the rhizosphere (called radial oxygen loss, ROL). Although ROL aerates the rhizosphere, it also reduces the supply of oxygen to the root apex. (B) Many wetland species form an ROL barrier in the basal parts of roots. ROL barrier promotes longitudinal oxygen diffusion by preventing losses to anaerobic soils. (i) In anaerobic soils, enhanced movement of oxygen toward the apex enables active respiration of the cells at the root tips. Because the root tip does not have an ROL barrier, (ii) the ROL around the root tip detoxifies toxic reduced substances in the waterlogged soil. (iii) The barrier also blocks the entry of potentially toxic compounds in highly reduced soils.
Some wetland species form an ROL barrier in the outer cell layers exterior to the aerenchyma (Fig. 1B) (Nishiuchi et al. 2012, Yamauchi et al. 2018). Radial oxygen profiles across root tissues obtained with an oxygen microelectrode show that the ROL barrier is located in the outer cell layers, including sclerenchyma, exodermis/hypodermis, and epidermis (Armstrong et al. 2000, De Simone et al. 2003, Soukup et al. 2007). An ROL barrier along the basal part of roots enhances longitudinal oxygen diffusion to the root apex. This enables cells at the root tips that are surrounded by anaerobic soil to continue respiring [Fig. 1B (i)] (Armstrong 1979, Colmer 2003a, Nishiuchi et al. 2012, Pedersen et al. 2021b). Multiple mathematical models support the idea that an ROL barrier leads to increased root length (Armstrong 1979, Pedersen et al. 2021b, Sorrell et al. 2000). For instance, the maximum root length in roots with an ROL barrier was about twice that of roots without an ROL barrier when the cortex-to-stele area ratio was set at 10 (Pedersen et al. 2021b).
2-2. Detoxification of reduced substances around the root tip
In waterlogged soil, some soil phytotoxins are produced by anaerobic bacteria (Fig. 1B). Because the root tip does not have an ROL barrier, the ROL around the root tip can neutralize (oxidize) toxic reduced substances such as sulfate and Fe2+ [Fig. 1B (ii)] (Armstrong 1979, Colmer 2003a, Yamauchi et al. 2018). Recent studies using a planar O2 optode, an instrument that measures 2D in situ oxygen levels in vertical planes in the soil and rhizosphere, have shown that light conditions dynamically change oxygen levels in the rhizosphere (Larsen et al. 2015, Lenzewski et al. 2018, Martin et al. 2019) and that Fe2+ oxidization was spatiotemporally associated with ROL (Maisch et al. 2019). The use of diffusive gradients in thin films (DGTs) is a technique for measuring 2D metal flux and concentrations at the surface of sediments (Davison and Zhang 1994, Davison et al. 1997). A study using a planar O2 optode combined with sulfide DGTs in the seagrasses Halophila ocalis and Zostera muelleri grown in marine sediments showed that ROL from the root tips reduces sulfate concentrations in the rhizosphere (Martin et al. 2019). This detoxification of reduced toxic substances around the root tip enables the roots to elongate into anaerobic soils (Armstrong 1979, Pedersen et al. 2021b).
2-3. Spatiotemporal dynamics of rhizosphere oxidization
Effect of root length on ROL barrier formation
ROL barrier formation depends on the degree of soil waterlogging and the root developmental stage. In long roots of rice (ca. 110 mm), ROL barrier formation begins within nine hours of stagnant treatment and is completed within 24 h, while in short roots (ca. 60 mm), it takes 72–120 h to form a barrier (Shiono et al. 2011). Tissue age appears to affect ROL barrier formation because basal tissues of long roots are older than those of short roots, and suberin accumulation can be influenced by the developmental stage (Kotula et al. 2009, Schreiber et al. 1999). The length distribution of adventitious roots with an ROL barrier in rice (cv. Nipponbare) grown under stagnant conditions is shown in Supplemental Fig. 1. Roots that leaked oxygen only in the root tips were considered to have an ROL barrier. The figure shows the percentages of roots of a given length that formed an ROL barrier. In agreement with these results, Colmer et al. (2006) observed that an ROL barrier was formed along both long (>110 mm-long) and short (ca. 60 mm) adventitious roots of rice grown in stagnant conditions for 3–4 weeks. However, only less than half of very short (<40 mm-long) adventitious roots had an ROL barrier (Supplemental Fig. 1). In the very short roots, an ROL barrier might not be essential for supplying sufficient oxygen for root tip respiration because the distance for oxygen diffusion from root-shoot junction to tips of the very short roots is much shorter than it is for long loots. Curiously, some of the very short roots formed an ROL barrier (Supplemental Fig. 1). After five days of incubation at 26°C without plants, the agar solution at a depth of 5 mm was anoxic (0 mg/L) (Shiono, unpublished). We speculate that emergence of roots into the anoxic and reduced solution induces barrier formation even in very young adventitious roots.
In rice and common reed (Phragmites australis), short lateral roots along adventitious roots leak oxygen due to the lack of an ROL barrier (Armstrong 1970, Armstrong et al. 1996, Colmer 2003a). Thus, the soil around the lateral roots and the very short adventitious roots was thought to be oxidized. Recently, oxygen levels imaged with a planar O2 optode indicated that the soil around the plant basal-region where many short lateral roots and very short roots are located had high oxygen levels even though waterlogged soil without plants was anoxic (Larsen et al. 2015, Maisch et al. 2019). Fig. 2 illustrates how an ROL barrier mediates oxygen distribution in the root-system in soil-grown rice. Rice plants have many very short (<40 mm-long) roots around the root-shoot junction, where new roots emerge (Fig. 2A). Although most short roots (between 40 and 80 mm) have an ROL barrier, very short roots (<40 mm) tend to lack an ROL barrier (Fig. 2B, Supplemental Fig. 1), thereby leading to oxygen leakage and detoxification of reduced toxic substances to promote new root growth. On the other hand, most long roots form a strong ROL barrier along the subapical-to-basal regions, resulting in oxygen leakage only around the root tip (Fig. 2A, 2C). This allows long roots to serve as pioneers that detoxify reduced substances around the root tips, contributing to their active growth under waterlogging.
Fig. 2.
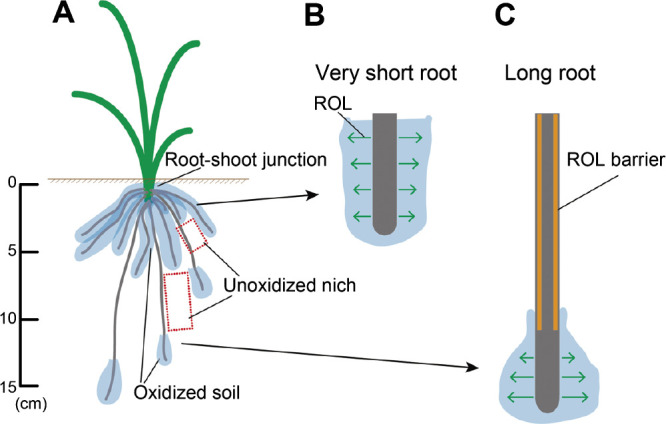
Predicted locations of oxidized rhizosphere and ROL barrier formation along short and long roots. (A) Predicted locations of oxidized soil (blue) in the rhizosphere. (B, C) Radial oxygen loss (green arrows) in very short (<50 mm) roots (B) and long roots (>100 mm) (C).
ROL barrier formation in long lateral roots
In rice and P. australis, short lateral roots do not form an ROL barrier (Armstrong 1970, Armstrong et al. 1996, Colmer 2003a). However, it was not known whether long lateral roots generate the barrier or not. Pedersen et al. (2021a) recently reported that Zea nicaraguensis, a wild relative of maize that grows in flood plains, forms an ROL barrier along the long lateral roots under stagnant conditions. The grass species has a sparse root system: Abiko et al. (2012) reported that 25-d-old Z. nicaraguensis grown under waterlogged soil for 21 d had only 15 adventitious roots. We speculate that the rhizosphere of Z. nicaraguensis has large unoxidized niches (rectangles in Fig. 2A) due to its sparse root system, and that long lateral roots elongate into these niches. Like the ROL barrier of adventitious roots (Fig. 1), the ROL barrier of long lateral roots may help the roots to grow into the reduced soils in the unoxidized niches. Monitoring the oxygen status in the niches during lateral roots elongation with a planar O2 optode would clarify whether long lateral roots help to adapt to waterlogged conditions.
Effect of photosynthetic activity on ROL
The photosynthetic activity of the shoot dynamically changes the oxygen level in the rhizosphere (Larsen et al. 2015, Lenzewski et al. 2018, Martin et al. 2019). Under light conditions, oxygen release is pronounced, resulting in a larger oxygenated zone in waterlogged soils. During the subsequent dark period, the oxygen concentration in the oxygenated region declined continuously, reaching the anoxic background level (Larsen et al. 2015, Lenzewski et al. 2018, Martin et al. 2019). Under field conditions, oxygen levels in the rhizosphere must be affected by light conditions and other environmental factors. For the evaluation or screening of crop waterlogging tolerance, the effect of the light condition also need to be considered in the field experiments.
3. Distribution of plant species that have ROL barriers
For the past two decades, many studies have evaluated ROL barrier formation in mono- and dicotyledonous species grown in wetland, non-wetland, and brackish water regions (Table 1). In general, ROL barriers do not form in waterlogging-sensitive non-wetland species including major crops, e.g., maize (Z. mays ssp. mays) (Abiko et al. 2012), wheat (Triticum aestivum) (McDonald et al. 2001b), barley (Hordeum vulgare) (McDonald et al. 2001a), Brassica napus (Voesenek et al. 1999), Pisum sativum (Healy and Armstrong 1972), and Sorghum bicolor (McDonald et al. 2002). Most waterlogging-tolerant species that can form an ROL barrier are distributed in wetlands (Table 1). Typical examples are rice and wild Echinochloa weeds that grow in paddy fields (Colmer et al. 1998, Ejiri and Shiono 2019), P. australis growing on riversides (Armstrong and Armstrong 2001), and Z. nicaraguensis and Oryza glumaepatula growing in flood plains (Abiko et al. 2012, Ejiri et al. 2020). However, ROL barriers were not found in some wetland species, including Paspalidium geminatum (Manzur et al. 2015), Ranunculus sceleratus (Visser et al. 2000), Rumex crispus (Manzur et al. 2015), R. palustris (Visser et al. 2000), and Salix martiana (De Simone et al. 2002). This may be because these species live in areas where soil substrates are not highly reduced (e.g., low in organic matter; flowing water) and thus may not need an ROL barrier (Colmer 2003a, Smits et al. 1990). Alternatively, these species may possess genetic factor(s) related to tolerance to toxic soil constituents under flooded reducing soil conditions.
Table 1.
Plants species that have a tight barrier to ROL
| Species | Habitat | Induction | References |
|---|---|---|---|
| Dicotyledonous | |||
| Tabernaemontana juruana | W | Unknown | De Simone et al. (2002, 2003) |
| Caltha palustris | W | Inducible | Visser et al. (2000) |
| Bruguiera gymnorhiza | B | Unknown | Cheng et al. (2012) |
| Monocotyledonous | |||
| Echinochloa colona | N | Constitutive | Ejiri and Shiono (2019) |
| E. crus-galli var. praticola | N | Constitutive | Ejiri and Shiono (2019) |
| Colocasia esculenta | N/W | Unknown | Abiko and Miyasaka (2020) |
| E. crus-galli var. crus-galli | N/W | Constitutive | Ejiri and Shiono (2019) |
| Cyperus eragrostis | W | Constitutive | Manzur et al. (2015) |
| Critesion marinum | W | Inducible | McDonald et al. (2001a) |
| E. crus-galli var. formosensis | W | Constitutive | Ejiri and Shiono (2019) |
| E. crus-galli var. mitis | W | Constitutive | McDonald et al. (2001a, 2002) |
| E. oryzicola | W | Inducible | Ejiri and Shiono (2019) |
| Glyceria maxima | W | Unknown | Soukup et al. (2007) |
| Hordeum marinum ssp. marinum (Acc. H21) | W | Inducible | Garthwaite et al. (2003), Malik et al. (2011) |
| Juncus effusus | W | Constitutive | Visser et al. (2000) |
| Oryza glumaepatula | W | Constitutive | Ejiri et al. (2020) |
| O. rufipogon | W | Inducible | Ejiri et al. (2020) |
| O. sativa | W | Inducible | Colmer et al. (1998) |
| Phragmites australis | W | Unknown | Armstrong and Armstrong (2001) |
| Urochloa humidicola | W | Constitutive | Jiménez et al. (2019) |
| Zea nicaraguensis | W | Inducible | Abiko et al. (2012) |
| Halophila ovalis | B | Unknown | Connell et al. (1999) |
| Zostera muelleri | B | Unknown | Martin et al. (2019) |
W: Wetland, N: Non-wetland, B: Brackish water region, N/W: non-wetland or wetland.
Induction of an ROL barrier: Constitutive means an ROL barrier is formed under aerated (or well-drained) and stagnant (or waterlogged) conditions. Inducible means an ROL barrier is formed under stagnant (or waterlogged) conditions, but that is not formed under aerated (or well-drained) conditions. Unknown means an ROL barrier formed under stagnant (or waterlogged conditions), but that it was not evaluated under aerated (or well-drained) conditions. Thus, these species can form an ROL barrier, but it remains unclear whether it is constitutive.
ROL barriers are classified as inducible and constitutive (Table 1). In some wetland species, including rice (Colmer et al. 1998), H. marinum (Garthwaite et al. 2003), Z. nicaraguensis (Abiko et al. 2012), and Caltha palustris (Visser et al. 2000), an ROL barrier is induced in roots by growth in waterlogged soil or stagnant conditions, while roots remain leaky to oxygen under well-drained soil or aerated conditions. ROL barriers form constitutively (i.e., in the absence of waterlogging) in several wetland species, including Cyperus eragrostis (Manzur et al. 2015), Juncus effuses (Visser et al. 2000), wild Echinochloa species (Ejiri and Shiono 2019), O. glumaepatula (Ejiri et al. 2020), and Urochloa humidicola (Jiménez et al. 2019) (Table 1). The ability to form a constitutive or inducible barrier is considered an adaptation to waterlogging (Colmer 2003a). Recently, the patterns of ROL barrier formation were evaluated in annual wild Echinochloa species (Ejiri and Shiono 2019). All three Echinochloa crus-galli varieties including var. crus-galli, formosensis, and praticola formed a constitutive ROL barrier, whereas E. oryzicola, like rice (Colmer 2003b), had an inducible ROL barrier (Ejiri and Shiono 2019). Phylogenic analyses suggest that E. crus-galli (which is hexaploid) is derived from E. oryzicola (which is tetraploid) (Aoki and Yamaguchi 2008, 2009). E. oryzicola is limited to waterlogged paddies, while E. crus-galli adapts to wet and dry areas depending on the variety (Rao et al. 2007, Tanesaka et al. 2010, Yamasue 2001). It is expected that constitutive suberization in the outer root cell layers that form an ROL barrier also increases drought and salinity tolerance (Enstone et al. 2003, Kreszies et al. 2018, Ranathunge et al. 2011b). The ability of E. crus-galli to grow in a wide range of habitats may be due to its constitutive barrier that increases its tolerance for multiple stresses.
4. ROL barrier components that prevent radial oxygen diffusion
4-1. Suberin at the exodermis/hypodermis
Which compounds physically prevent oxygen loss through an ROL barrier? Lignin and suberin act as diffusion barriers in the extracellular spaces (apoplast) in the outer part of roots (OPRs) and endodermis. Lignin is a complex of polyphenolic polymers that provides mechanical strength and plant defense due to its resistance to degradation (Barros et al. 2015, Campbell and Sederoff 1996). Suberin is a hydrophobic macromolecule built from long-chain fatty acids, glycerol, and aromatic polymers (Graça 2015). Suberin lamellae are composed of suberin deposited on exodermal cell walls (Schreiber and Franke 2011).
When wetland plants, e.g., Phragmites australis (Soukup et al. 2007) and rice (Kotula et al. 2009), are grown under waterlogged or stagnant conditions for a long period (i.e., 2–3 weeks), both suberized and lignified cell walls are frequently observed in the OPRs at the basal part of roots. However, lignification in the OPRs is often unassociated with ROL barrier formation, depending on ecotypes, different developmental stages, or different genetic backgrounds (De Simone et al. 2003, Ejiri and Shiono 2019, Ejiri et al. 2020, Shiono et al. 2011, Watanabe et al. 2017). Four lines of evidence show that suberin in the OPRs is a major component of ROL barriers: 1) Z. nicaraguensis with ROL barrier develops suberin lamellae in the exodermis and lignin in the epidermis (Abiko et al. 2012, Watanabe et al. 2017). However, an introgression line of Z. nicaraguensis in a maize background (IL#468) with ROL barrier under stagnant conditions develops a well-suberized exodermis/hypodermis along the basal parts of adventitious roots, but the epidermis is not lignified as it is in maize (inbred line Mi29) (Watanabe et al. 2017). 2) Roots of five wild Echinochloa species that constitutively form an ROL barrier under aerated conditions also have a well-suberized exodermis/hypodermis (Ejiri and Shiono 2019). However, two of the five species (a wetland ecotype E. crus-galli var. crus-galli and a non-wetland ecotype E. colona) do not have lignified sclerenchyma (Ejiri and Shiono 2019). 3) A transcriptome analysis using laser-microdissected tissues of the OPRs in rice showed that many genes involved in suberin biosynthesis (but not in lignin biosynthesis) are upregulated during ROL barrier formation (Shiono et al. 2014b). 4) A metabolomic analysis in rice indicated that rice roots that form an ROL barrier accumulate malic acid and very-long-chain fatty acids, which are needed to synthesize suberin (Kulichikhin et al. 2014).
The apoplast is a continuum of the extracellular space outside the plasma membrane (e.g., cell walls, xylem, and phloem), while the symplast is a continuum of intracellular space (e.g., cytosol and plasmodesmata). Roots take up water and ions via apoplastic and symplastic pathways. In general, suberin lamellae at the exodermis/hypodermis prevent water (Aloni et al. 1998, Kreszies et al. 2020), ions (Ranathunge et al. 2011a), and mycorrhizal fungi from moving through the apoplast (Enstone et al. 2003). In several grasses that form an ROL barrier with well suberized OPRs [e.g., rice (Ejiri et al. 2020), P. australis (Soukup et al. 2007), Z. nicaraguensis (Watanabe et al. 2017), wild Echinochloa species (Ejiri and Shiono 2019), and O. glumaepatula (W2165) (Ejiri et al. 2020)], tracers (e.g., periodic acids) were blocked from moving through the apoplast to the interior part of roots. In fact, rice roots that form an ROL barrier also have reduced permeability to NaCl (but remain permeable to water) (Ranathunge et al. 2011a). The difference in permeabilities may be due to different transport mechanisms and pathways (Ranathunge et al. 2011a). Generally, oxygen moves through the plasma membrane by diffusion, whereas water predominantly moves through the apoplast by bulk flow (Ranathunge et al. 2011a). Substances that are potentially toxic to plants (e.g., reduced metal ions) often accumulate in waterlogged soil (Colmer 2003a, Ernst 1990, Kreuzwieser et al. 2004, Lamers et al. 1998, Ponnamperuma 1984). Another benefit of ROL barriers in waterlogged soils is that their main component (suberin) can reduce the entry of such phytotoxins [Fig. 1B (iii)] (Kreszies et al. 2018, Ranathunge et al. 2011b, Watanabe et al. 2013, Yamauchi et al. 2018).
4-2. Oxygen permeable windows
In roots of several wetland species that form an ROL barrier, such as rice (Armstrong 1971), P. australis (Armstrong et al. 2000), H. marinum (H819) (Garthwaite et al. 2008), and wild Echinochloa species (Ejiri and Shiono 2019), the sites of the emergence of lateral roots contain passage cells that leak oxygen. Areas of passage cells where oxygen leaks and lateral roots emerge have been called “windows” (Armstrong et al. 2000). Armstrong et al. (2000), using a microelectrode, showed that oxygen levels in P. australis were higher at the surface of window sites than at non-window sites. Similarly, Ejiri and Shiono (2019), using methylene-blue staining, showed that oxygen was leaking in a spotty manner (probably from window sites) along the basal part of roots in Echinochloa accessions. Interestingly, in rice (Justin and Armstrong 1987), P. australis (Armstrong et al. 2000) and wild Echinochloa species (Ejiri and Shiono 2019, 2020), the exodermis at window sites lacks suberin lamellae, and the cortical cells are left intact without programmed cell death (i.e., without aerenchyma) (Fig. 3). However, the lateral root primordium does not reach the cortex and the exodermis. At window sites of E. crus-galli, lignin deposits at the sclerenchyma and the exodermis were also clearly reduced (Ejiri and Shiono 2020).
Fig. 3.
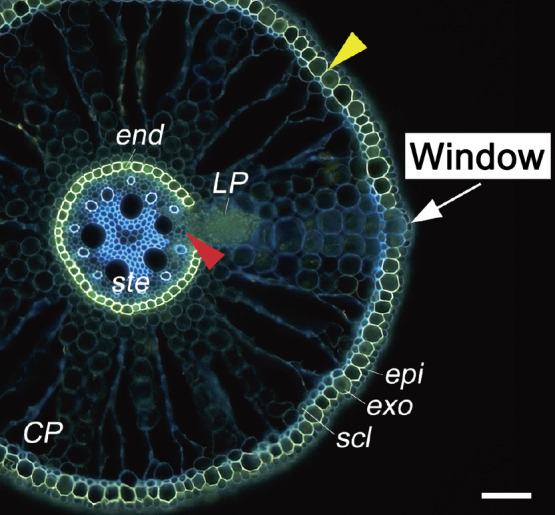
An oxygen-permeable window site in the adventitious roots of Echinochloa colona. Cross-section of an adventitious root showing the location of a window (white arrow) at a site in the exodermis where a lateral root is expected to emerge. The window is composed of passage cells that lack suberin lamellae. Suberin lamellae are indicated as yellow-green fluorescence with Fluorol Yellow 088 (yellow arrowhead). Red arrowhead indicates the pericycle from which lateral root primordia are predicted to emerge. Blue fluorescence indicates autofluorescence. Fluorol Yellow 088 staining was conducted as described in Ejiri and Shiono (2019). The plants were grown in an aerated nutrient solution for ten days and then transferred into a deoxygenated stagnant nutrient solution for 14 days. Abbreviations: CP, cortical parenchyma; end, endodermis; epi, epidermis; exo, exodermis; LP, lateral root primordia; scl, sclerenchyma; ste, stele. Scale bars: 100 μm.
Passage cells possess Casparian strips that are composed of lignin and small deposits of suberin (Schreiber and Franke 2011) but not suberin lamellae such as those that cover the plasma membrane of the exodermal cells (Peterson and Enstone 1996). The development of exodermal passage cells is well described in maize (Enstone and Peterson 1997, 2005), although the oxygen permeability of the passage cells has not been evaluated. In the younger part of maize root, the exodermis matured in a patchy manner with some unsuberized cells, some developing suberin lamellae, and some mature suberin lamellae (Enstone and Peterson 2005). Exodermal cells surrounding emerged lateral roots remained unsuberized longer than other cells in the layer, thus becoming exodermal passage cells (Enstone and Peterson 2005). Application of toxic sulfide to rice roots induced unusually high suberin and/or lignin accumulations in the OPRs, preventing lateral roots from penetrating the sclerenchyma and growing through the cortex of adventitious roots (Armstrong and Armstrong 2005). This suggests that well-suberized and/or well-lignified cells in the OPRs block the emergence of lateral roots.
4-3. Another potential component of an ROL barrier
Regions that histochemically stain for suberin do not always function as ROL barriers. In maize, well-developed suberin lamellae were histochemically detected at the exodermis under stagnant conditions, but the roots were leaky to oxygen (Watanabe et al. 2017). Suberin lamellae in the OPRs were not histochemically visualized in the roots of Halophila ovalis, in which an ROL barrier was observed (Martin et al. 2019). In rice, ROL barrier formation was induced by 48 h of stagnant conditions but suberin lamellae were not histochemically detected in the exodermis (Shiono et al. 2011). This failure to detect suberin may be due to a difference in suberin monomer composition and/or structure (Watanabe et al. 2017).
Rice appears to have another, unidentified component that impedes oxygen diffusion. It is an electron-dense material that is packed in the intercellular space between the exodermis and the epidermis. It is observed in rice roots after 48 h of stagnant treatment using transmission electron microscopy (Shiono et al. 2011). These roots do not have histochemically stained suberin lamellae. Similar electron-dense materials are found in the intercellular spaces in the outer part of soybean nodules (James et al. 1991, Parsons and Day 1990). The electron-dense material contains glycoproteins and forms an oxygen diffusion barrier (James et al. 1991, Parsons and Day 1990). This is necessary because the interior of the nodules must be anaerobic because nitrogenase activity that catalyzes the conversion of N2 to ammonia is inactivated by oxygen (Denison et al. 1992, Denison 1998). Further studies are needed to identify the electron-dense components that inhibit oxygen transport in the OPRs.
5. Mechanisms underlying ROL barrier formation
5-1. Environmental triggers for ROL barrier formation
ROL barriers are induced in some wetland species including rice by stagnant or waterlogged conditions (Colmer et al. 1998). How does waterlogging induce ROL barrier formation? Prolonged waterlogging causes the depletion of oxygen and the accumulation of other products, such as ethylene, CO2, and phytotoxins (e.g., Fe2+ and organic acids) in the soil, as well as pH changes in the soil (i.e., a decrease in alkaline soil and an increase in acidic soil) (Ponnamperuma 1984). Low oxygen and high ethylene initiate aerenchyma formation, promoting longitudinal diffusion of oxygen (Shiono et al. 2008, Voesenek and Sasidharan 2013, Yamauchi et al. 2018). Interestingly, in rice, ROL barrier formation is not triggered by low oxygen, elevated ethylene and CO2 (Colmer et al. 2006), or low pH (4.7 or 5.7) (Colmer et al. 2019). On the other hand, ROL barrier formation can be stimulated by some soil phytotoxins including Fe2+ (Mongon et al. 2014), sulfide (Armstrong and Armstrong 2005), and organic acids (Armstrong and Armstrong 2001, Kotula et al. 2014) that are produced by anaerobic bacteria in waterlogged soils. However, adding sulfide (0.174 mM) or organic acids (e.g., acetic acids 0.4–1.5 mM) to a hydroponic solution was found to inhibit root growth due to their toxicity and result in increased suberization and/or lignification of cell walls in the OPRs (Armstrong and Armstrong 2001, 2005, Kotula et al. 2014). Recently, Colmer et al. (2019) demonstrated that non-toxic levels (<0.05 mM) of organic acids such as acetic, propionic, butyric, and hexanoic acids initiate ROL barrier formation in rice roots, without affecting root tissue respiration. Mongon et al. (2014) also showed that non-toxic levels of Fe2+ (0.36–0.54 mM) induce an ROL barrier under aerated conditions, without inhibiting root growth. Thus, ROL barrier formation in rice is considered to be triggered by accumulations of soil phytotoxins in waterlogged soil (Pedersen et al. 2021b).
The sensing and signal mechanisms underlying phytotoxin-induced ROL barrier formation in rice remain unknown. However, ethylene, a crucial hormone in acclimation to waterlogging, does not appear to be involved as it did not induce ROL barrier formation (Colmer et al. 2006).
5-2. Molecular mechanisms of ROL barrier formation
In rice, an ROL barrier can be induced by waterlogging (Colmer et al. 1998), making rice a model plant for understanding the molecular regulation of ROL barrier formation. For example, analyses of gene expressions in rice forming an ROL barrier under stagnant nutrient solution (Kulichikhin et al. 2014, Shiono et al. 2014b), non-aerated nutrient solution with high Si levels (1.78 mM) (Fleck et al. 2011), or non-aerated nutrient solution with organic acids (Colmer et al. 2019) were demonstrated. A transcriptome analysis using laser-microdissected tissues of the OPRs identified 98 genes that were strongly upregulated during ROL barrier formation in rice (Shiono et al. 2014b). These included suberin biosynthesis genes such as CYTOCHROME P450 (OsCYP86B3, LOC_Os10g34480) and ABC TRANSPORTER (OsABCG5, LOC_Os03g17350), and transcription factor genes containing WRKY, AP2, and MYB domains (Shiono et al. 2014b). Many (65–78%) of the promoters of the upregulated transcription factors had putative cis-elements such as WRKY, AP2, NAC, and MYB (Shiono et al. 2014b). Application of low concentrations of organic acids also upregulated two suberin biosynthesis genes (OsABCG5 and OsCYP86B3) (Colmer et al. 2019).
Waßmann (2014) analyzed suberin formation in the OPRs using a rice mutant lacking a suberin biosynthesis gene, OsCYP86B3. In cyp86b3 mutant roots, C24 to C30 ω-OH fatty acids, which are major aliphatic suberin monomers, were hardly detectable. However, the level of other aliphatic suberin compounds (α-OH acids) and aromatic suberin compounds (coumaric acid and ferulic acid) were similar in the mutant and wild type. Additionally, in the mutant, suberin lamellae at the exodermis/hypodermis were detected by histochemical staining. ROL barrier formation in cyp86b3 was evaluated qualitatively with methylene blue staining and quantitatively with a cylindrical oxygen electrode. As in the wild type, in cyp86b3 grown under stagnant conditions, oxygen leaked from the root apex but not from the basal parts, indicating the presence of a tight ROL barrier. Unexpectedly, the lack of C24-C30 ω-OH fatty acids did not affect the oxygen permeability of rice roots.
Shiono et al. (2014a) examined another rice mutant of OsABCG5, called reduced culm number1 (rcn1). In this mutant, Fluorol Yellow 088 staining did not reveal any suberin lamellae in the exodermis/hypodermis, even under stagnant conditions. The amounts of the major aliphatic suberin monomers originating from C28 to C30 fatty acids and ω-OH fatty acids were significantly lower in rcn1 than in the wild type. Apoplastic tracers (periodic acid and berberine) rapidly passed through the OPRs and into the roots of rcn1, indicating that the apoplastic barrier at the exodermis/hypodermis was impaired (Shiono et al. 2014a). Another study found that rcn1 hyper-accumulated sodium under waterlogged conditions (Matsuda et al. 2014). Under saline waterlogged soil conditions, the sodium contents of rcn1 were about 2 times greater in the shoots and about 1.4 times greater in the roots than those in the wild type (Matsuda et al. 2014). Under these conditions, the growth of rcn1 was significantly inhibited, suggesting that the inhibited growth was due to salinity stress (Matsuda et al. 2014). However, ROL barrier formation in the rcn1 mutant has not been evaluated. Further studies on the ROL barrier formation of suberin mutants are required to reveal its molecular mechanisms.
6. Future perspectives for improving waterlogging tolerance in crops
Most waterlogging-sensitive crops cannot form an ROL barrier and/or a significant amount of aerenchyma. Some attempts have been made to improve waterlogging tolerance in crops by introducing genes or quantitative trait loci (QTLs) associated with ROL barrier formation from waterlogging-tolerant wild relatives. H. marinum, a waterlogging-tolerant wild relative of wheat, inducibly forms an ROL barrier under stagnant conditions (Garthwaite et al. 2003). To obtain wheat varieties with inducible ROL barriers in the roots, wheat cultivars [e.g., Chinese Spring (CS)] were hybridized with H. marinum accessions (e.g., H21), producing amphiploid (Malik et al. 2011) and disomic chromosome addition lines for H21 in CS wheat (Konnerup et al. 2017). Under stagnant conditions, four of the amphiploid lines, including the H21-CS amphiploid, had tight ROL barriers (Malik et al. 2011). On the other hand, Konnerup et al. (2017) did not detect ROL barriers in any of six disomic chromosome addition lines for chromosomes 1, 2, 4, 5, 6, or 7 of H21 in CS wheat. The gene involved in ROL barrier formation may be located on chromosome 3 of H21, but unfortunately a disomic chromosome addition line of chromosome 3 of H21 was not isolated and could not be tested (Konnerup et al. 2017). The wild relative of maize, Z. nicaraguensis, generates an inducible ROL barrier under stagnant conditions, while maize inbred line “Mi29” does not (Abiko et al. 2012). Analyses of Z. nicaraguensis introgression lines in the genetic background of domesticated maize identified a major locus associated with ROL barrier formation in a segment of the short arm of chromosome 3 of Z. nicaraguensis (Watanabe et al. 2017). Recently, we found that some accessions of a wild rice species, O. glumaepatula, have a constitutive ROL barrier under aerated conditions (Ejiri et al. 2020). O. glumaepatula has an AA-genome, making it possible to cross it with cultivated rice that does not form a constitutive ROL barrier. We are presently mapping ROL-related QTLs in introgression lines of O. glumaepatula to identify the genes that regulate constitutive ROL barrier formation.
Plants form an ROL barrier as a strategy for adapting to waterlogged soils, but an ROL barrier alone is not enough to survive. For adequate root aeration under waterlogging, well-developed aerenchyma formation is also essential. Additionally, soil-surface roots that develop in shallow soil with relatively higher oxygen levels even under waterlogging would escape the toxic reduced-substances in deeper soil. Several studies have attempted to identify QTLs that regulate aerenchyma formation (Mano and Omori 2008, 2009, Mano et al. 2016, Zhang et al. 2016, 2017) and soil-surface roots (Mano et al. 2005, Uga et al. 2012). A gene that regulates the development of soil-surface roots in rice was recently identified as qSOR1 (quantitative trait locus for SOIL SURFACE ROOTING1) and was found to involve gravitropic responses that act through negative regulation via auxin signaling (Kitomi et al. 2020). A combination of QTLs involved in various agronomic traits is useful for applied breeding, which is called QTL pyramiding (Ashikari and Matsuoka 2006, Nagai et al. 2012). A combination of QTLs regulates root aeration (i.e., an ROL barrier and aerenchyma formation) and a shallow root system by QTL pyramiding must be one of the promising approaches to breed waterlogging-tolerant crops.
Author Contribution Statement
M.E. drafted the manuscript with contributions of T.M., T.F., and K.S. K.S. wrote the article with contributions of M.E., T.M., and T.F. All authors edited the manuscript and approved the final manuscript.
Supplementary Material
Acknowledgments
A part of this work was partly supported by JSPS KAKENHI (JP16KK0173, JP17K15211, and JP19K05978 to K.S.).
Literature Cited
- Abiko, T., Kotula L., Shiono K., Malik A.I., Colmer T.D. and Nakazono M. (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 35: 1618–1630. [DOI] [PubMed] [Google Scholar]
- Abiko, T. and Miyasaka S.C. (2020) Aerenchyma and barrier to radial oxygen loss are formed in roots of Taro (Colocasia esculenta) propagules under flooded conditions. J. Plant Res. 133: 49–56. [DOI] [PubMed] [Google Scholar]
- Aloni, R., Enstone D.E. and Peterson C.A. (1998) Indirect evidence for bulk water flow in root cortical cell walls of three dicotyledonous species. Planta 207: 1–7. [Google Scholar]
- Aoki, D. and Yamaguchi H. (2008) Genetic relationship between Echinochloa crus-galli and Echinochloa oryzicola accessions inferred from internal transcribed spacer and chloroplast DNA sequences. Weed Biol. Manag. 8: 233–242. [Google Scholar]
- Aoki, D. and Yamaguchi H. (2009) Oryza sh4 gene homologue represents homoeologous genomic copies in polyploid Echinochloa. Weed Biol. Manag. 9: 225–233. [Google Scholar]
- Armstrong, W. (1970) Rhizosphere oxidation in rice and other species: a mathematical model based on the oxygen flux component. Physiol. Plant. 23: 623–630. [Google Scholar]
- Armstrong, W. (1971) Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging. Physiol. Plant. 25: 192–197. [Google Scholar]
- Armstrong, W. (1979) Aeration in higher plants. Adv. Bot. Res. 7: 225–332. [Google Scholar]
- Armstrong, W. and Beckett P.M. (1987) Internal aeration and the development of stelar anoxia in submerged roots—a multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytol. 105: 221–245. [Google Scholar]
- Armstrong, J., Armstrong W., Beckett P., Halder J., Lythe S., Holt R. and Sinclair A. (1996) Pathways of aeration and the mechanisms and beneficial effects of humidity- and Venturi-induced convections in Phragmites australis (Cav.) Trin. ex Steud. Aquat. Bot. 54: 177–197. [Google Scholar]
- Armstrong, W., Cousins D., Armstrong J., Turner D.W. and Beckett P.M. (2000) Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann. Bot. 86: 687–703. [Google Scholar]
- Armstrong, J. and Armstrong W. (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. Am. J. Bot. 88: 1359–1370. [PubMed] [Google Scholar]
- Armstrong, J. and Armstrong W. (2005) Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann. Bot. 96: 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari, M. and Matsuoka M. (2006) Identification, isolation and pyramiding of quantitative trait loci for rice breeding. Trends Plant Sci. 11: 344–350. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres, J. and Voesenek L.A.C.J. (2008) Flooding stress: acclimations and genetic diversity. Annu. Rev. Plant Biol. 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres, J. and Voesenek L.A.C.J. (2010) Life in the balance: a signaling network controlling survival of flooding. Curr. Opin. Plant Biol. 13: 489–494. [DOI] [PubMed] [Google Scholar]
- Barros, J., Serk H., Granlund I. and Pesquet E. (2015) The cell biology of lignification in higher plants. Ann. Bot. 115: 1053–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholdsson, N.-O. (2013) Screening for barley waterlogging tolerance in Nordic barley cultivars (Hordeum vulgare L.) using chlorophyll fluorescence on hydroponically-grown plants. Agronomy 3: 376–390. [Google Scholar]
- Blossfeld, S., Gansert D., Thiele B., Kuhn A.J. and Lösch R. (2011) The dynamics of oxygen concentration, pH value, and organic acids in the rhizosphere of Juncus spp. Soil Biol. Biochem. 43: 1186–1197. [Google Scholar]
- Campbell, M.M. and Sederoff R.R. (1996) Variation in lignin content and composition—Mechanisms of control and implications for the genetic improvement of plants. Plant Physiol. 110: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H., Chen D.-T., Tam N.F.-Y., Chen G.-Z., Li S.-Y. and Ye Z.-H. (2012) Interactions among Fe2+, S2–, and Zn2+ tolerance, root anatomy, and radial oxygen loss in mangrove plants. J. Exp. Bot. 63: 2619–2630. [DOI] [PubMed] [Google Scholar]
- Colmer, T.D., Gibberd M.R., Wiengweera A. and Tinh T.K. (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J. Exp. Bot. 49: 1431–1436. [Google Scholar]
- Colmer, T.D. (2003a) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 26: 17–36. [Google Scholar]
- Colmer, T.D. (2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann. Bot. 91: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer, T.D., Cox M.C.H. and Voesenek L.A.C.J. (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol. 170: 767–778. [DOI] [PubMed] [Google Scholar]
- Colmer, T.D. and Voesenek L.A.C.J. (2009) Flooding tolerance: suites of plant traits in variable environments. Funct. Plant Biol. 36: 665–681. [DOI] [PubMed] [Google Scholar]
- Colmer, T.D., Kotula L., Malik A.I., Takahashi H., Konnerup D., Nakazono M. and Pedersen O. (2019) Rice acclimation to soil flooding: low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant Cell Environ. 42: 2183–2197. [DOI] [PubMed] [Google Scholar]
- Connell, E.L., Colmer T.D. and Walker D.I. (1999) Radial oxygen loss from intact roots of Halophila ovalis as a function of distance behind the root tip and shoot illumination. Aquat. Bot. 63: 219–228. [Google Scholar]
- Davison, W. and Zhang H. (1994) In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 367: 546–548. [Google Scholar]
- Davison, W., Fones G.R. and Grime G.W. (1997) Dissolved metals in surface sediment and a microbial mat at 100-μm resolution. Nature 387: 885–888. [Google Scholar]
- De Simone, O., Haase K., Müller E., Junk W.J., Gonsior G. and Schmidt W. (2002) Impact of root morphology on metabolism and oxygen distribution in roots and rhizosphere from two Central Amazon floodplain tree species. Funct. Plant Biol. 29: 1025–1035. [DOI] [PubMed] [Google Scholar]
- De Simone, O., Haase K., Müller E., Junk W.J., Hartmann K., Schreiber L. and Schmidt W. (2003) Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four Amazonian tree species. Plant Physiol. 132: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison, R.F., Hunt S. and Layzell D.B. (1992) Nitrogenase activity, nodule respiration, and O2 permeability following detopping of alfalfa and birdsfoot trefoil. Plant Physiol. 98: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison, R.F. (1998) Decreased oxygen permeability: a universal stress response in legume root nodules. Bot. Acta 111: 191–192. [Google Scholar]
- Ejiri, M. and Shiono K. (2019) Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Front. Plant Sci. 10: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiri, M., Sawazaki Y. and Shiono K. (2020) Some accessions of Amazonian wild rice (Oryza glumaepatula) constitutively form a barrier to radial oxygen loss along adventitious roots under aerated conditions. Plants (Basel) 9: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiri, M. and Shiono K. (2020) Groups of multi-cellular passage cells in the root exodermis of Echinochloa crus-galli varieties lack not only suberin lamellae but also lignin deposits. Plant Signal. Behav. 15: 1719749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone, D.E. and Peterson C.A. (1997) Suberin deposition and band plasmolysis in the corn (Zea mays L.) root exodermis. Can. J. Bot. 75: 1188–1199. [Google Scholar]
- Enstone, D.E., Peterson C.A. and Ma F. (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J. Plant Growth Regul. 21: 335–351. [Google Scholar]
- Enstone, D.E. and Peterson C.A. (2005) Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant Cell Environ. 28: 444–455. [Google Scholar]
- Ernst, W.H.O. (1990) Ecophysiology of plants in waterlogged and flooded environments. Aquat. Bot. 38: 73–90. [Google Scholar]
- Fleck, A.T., Nye T., Repenning C., Stahl F., Zahn M. and Schenk M.K. (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J. Exp. Bot. 62: 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao, T., Barrera-Figueroa B.E., Juntawong P. and Peña-Castro J.M. (2019) Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Front. Plant Sci. 10: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite, A.J., von Bothmer R. and Colmer T.D. (2003) Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct. Plant Biol. 30: 875–889. [DOI] [PubMed] [Google Scholar]
- Garthwaite, A.J., Armstrong W. and Colmer T.D. (2008) Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytol. 179: 405–416. [DOI] [PubMed] [Google Scholar]
- Graça, J. (2015) Suberin: the biopolyester at the frontier of plants. Front. Chem. 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, M.T. and Armstrong W. (1972) The effectiveness of internal oxygen transport in a mesophyte (Pisum sativum L.). Planta 103: 302–309. [DOI] [PubMed] [Google Scholar]
- Hirabayashi, Y., Mahendran R., Koirala S., Konoshima L., Yamazaki D., Watanabe S., Kim H. and Kanae S. (2013) Global flood risk under climate change. Nat. Clim. Change 3: 816–821. [Google Scholar]
- Jackson, M.B., Fenning T.M., Drew M.C. and Saker L.R. (1985) Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta 165: 486–492. [DOI] [PubMed] [Google Scholar]
- James, E.K., Sprent J.I., Minchin F.R. and Brewin N.J. (1991) Intercellular location of glycoprotein in soybean nodules: effect of altered rhizosphere oxygen concentration. Plant Cell Environ. 14: 467–476. [Google Scholar]
- Jiménez, J.C., Kotula L., Veneklaas E.J. and Colmer T.D. (2019) Root-zone hypoxia reduces growth of the tropical forage grass Urochloa humidicola in high-nutrient but not low-nutrient conditions. Ann. Bot. 124: 1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justin, S.H.F.W. and Armstrong W. (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytol. 106: 465–495. [Google Scholar]
- Kitomi, Y., Hanzawa E., Kuya N., Inoue H., Hara N., Kawai S., Kanno N., Endo M., Sugimoto K., Yamazaki T.et al. (2020) Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 117: 21242–21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerup, D., Malik A.I., Islam A.K.M.R. and Colmer T.D. (2017) Evaluation of root porosity and radial oxygen loss of disomic addition lines of Hordeum marinum in wheat. Funct. Plant Biol. 44: 400–409. [DOI] [PubMed] [Google Scholar]
- Kotula, L., Ranathunge K., Schreiber L. and Steudle E. (2009) Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J. Exp. Bot. 60: 2155–2167. [DOI] [PubMed] [Google Scholar]
- Kotula, L., Colmer T.D. and Nakazono M. (2014) Effects of organic acids on the formation of the barrier to radial oxygen loss in roots of Hordeum marinum. Funct. Plant Biol. 41: 187–202. [DOI] [PubMed] [Google Scholar]
- Kreszies, T., Schreiber L. and Ranathunge K. (2018) Suberized transport barriers in Arabidopsis, barley and rice roots: from the model plant to crop species. J. Plant Physiol. 227: 75–83. [DOI] [PubMed] [Google Scholar]
- Kreszies, T., Eggels S., Kreszies V., Osthoff A., Shellakkutti N., Baldauf J.A., Zeisler-Diehl V.V., Hochholdinger F., Ranathunge K. and Schreiber L. (2020) Seminal roots of wild and cultivated barley differentially respond to osmotic stress in gene expression, suberization, and hydraulic conductivity. Plant Cell Environ. 43: 344–357. [DOI] [PubMed] [Google Scholar]
- Kreuzwieser, J., Papadopoulou E. and Rennenberg H. (2004) Interaction of flooding with carbon metabolism of forest trees. Plant Biol. 6: 299–306. [DOI] [PubMed] [Google Scholar]
- Kulichikhin, K., Yamauchi T., Watanabe K. and Nakazono M. (2014) Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ. 37: 2406–2420. [DOI] [PubMed] [Google Scholar]
- Laanbroek, H.J. (1990) Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquat. Bot. 38: 109–125. [Google Scholar]
- Lamers, L.P.M., Tomassen H.B.M. and Roelofs J.G.M. (1998) Sulfate-induced entrophication and phytotoxicity in freshwater wetlands. Environ. Sci. Technol. 32: 199–205. [Google Scholar]
- Larsen, M., Santner J., Oburger E., Wenzel W.W. and Glud R.N. (2015) O2 dynamics in the rhizosphere of young rice plants (Oryza sativa L.) as studied by planar optodes. Plant Soil 390: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzewski, N., Mueller P., Meier R.J., Liebsch G., Jensen K. and Koop-Jakobsen K. (2018) Dynamics of oxygen and carbon dioxide in rhizospheres of Lobelia dortmanna—a planar optode study of belowground gas exchange between plants and sediment. New Phytol. 218: 131–141. [DOI] [PubMed] [Google Scholar]
- Maisch, M., Lueder U., Kappler A. and Schmidt C. (2019) Iron lung: how rice roots induce iron redox changes in the rhizosphere and create niches for microaerophilic Fe(II)-oxidizing bacteria. Environ. Sci. Tech. Let. 6: 600–605. [Google Scholar]
- Malik, A.I., Islam A.K.M.R. and Colmer T.D. (2011) Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum–wheat amphiploids. New Phytol. 190: 499–508. [DOI] [PubMed] [Google Scholar]
- Mano, Y., Omori F., Muraki M. and Takamizo T. (2005) QTL mapping of adventitious root formation under flooding conditions in tropical maize (Zea mays L.) seedlings. Breed. Sci. 55: 343–347. [Google Scholar]
- Mano, Y. and Omori F. (2008) Verification of QTL controlling root aerenchyma formation in a maize × teosinte “Zea nicaraguensis” advanced backcross population. Breed. Sci. 58: 217–223. [Google Scholar]
- Mano, Y. and Omori F. (2009) High-density linkage map around the root aerenchyma locus Qaer1.06 in the backcross populations of maize Mi29 × teosinte “Zea nicaraguensis”. Breed. Sci. 59: 427–433. [Google Scholar]
- Mano, Y., Omori F., Tamaki H., Mitsuhashi S. and Takahashi W. (2016) DNA marker-assisted selection approach for developing flooding-tolerant maize. Jpn. Agric. Res. Q. 50: 175–182. [Google Scholar]
- Manzur, M.E., Grimoldi A.A., Insausti P. and Striker G.G. (2015) Radial oxygen loss and physical barriers in relation to root tissue age in species with different types of aerenchyma. Funct. Plant Biol. 42: 9–17. [DOI] [PubMed] [Google Scholar]
- Martin, B.C., Bougoure J., Ryan M.H., Bennett W.W., Colmer T.D., Joyce N.K., Olsen Y.S. and Kendrick G.A. (2019) Oxygen loss from seagrass roots coincides with colonisation of sulphide-oxidising cable bacteria and reduces sulphide stress. ISME J. 13: 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, S., Nagasawa H., Yamashiro N., Yasuno N., Watanabe T., Kitazawa H., Takano S., Tokuji Y., Tani M., Takamure I.et al. (2014) Rice RCN1/OsABCG5 mutation alters accumulation of essential and nonessential minerals and causes a high Na/K ratio, resulting in a salt-sensitive phenotype. Plant Sci. 224: 103–111. [DOI] [PubMed] [Google Scholar]
- McDonald, M.P., Galwey N.W. and Colmer T.D. (2001a) Waterlogging tolerance in the tribe Triticeae: the adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ. 24: 585–596. [Google Scholar]
- McDonald, M.P., Galwey N.W., Ellneskog-Staam P. and Colmer T.D. (2001b) Evaluation of Lophopyrum elongatum as a source of genetic diversity to increase the waterlogging tolerance of hexaploid wheat (Triticum aestivum). New Phytol. 151: 369–380. [Google Scholar]
- McDonald, M.P., Galwey N.W. and Colmer T.D. (2002) Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant Cell Environ. 25: 441–451. [Google Scholar]
- Mongon, J., Konnerup D., Colmer T.D. and Rerkasem B. (2014) Responses of rice to Fe2+ in aerated and stagnant conditions: growth, root porosity and radial oxygen loss barrier. Funct. Plant Biol. 41: 922–929. [DOI] [PubMed] [Google Scholar]
- Nagai, K., Kuroha T., Ayano M., Kurokawa Y., Angeles-Shim R.B., Shim J.-H., Yasui H., Yoshimura A. and Ashikari M. (2012) Two novel QTLs regulate internode elongation in deepwater rice during the early vegetative stage. Breed. Sci. 62: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer, S.C., Toledo-Durán G.E., Emerson D. and Megonigal J.P. (2007) Returning to their roots: iron-oxidizing bacteria enhance short-term plaque formation in the wetland-plant rhizosphere. Geomicrobiol. J. 24: 65–73. [Google Scholar]
- Nishiuchi, S., Yamauchi T., Takahashi H., Kotula L. and Nakazono M. (2012) Mechanisms for coping with submergence and waterlogging in rice. Rice (N Y) 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, R. and Day D.A. (1990) Mechanism of soybean nodule adaptation to different oxygen pressures. Plant Cell Environ. 13: 501–512. [Google Scholar]
- Pedersen, O., Perata P. and Voesenek L.A.C.J. (2017) Flooding and low oxygen responses in plants. Funct. Plant Biol. 44: iii–vi. [DOI] [PubMed] [Google Scholar]
- Pedersen, O., Nakayama Y., Yasue H., Kurokawa Y., Takahashi H., Floytrup A.H., Omori F., Mano Y., Colmer T. D. and Nakazono M. (2021a) Lateral roots, in addition to adventitious roots, form a barrier to radial oxygen loss in Zea nicaraguensis and a chromosome segment introgression line in maize. New Phytol. 229: 94–105. [DOI] [PubMed] [Google Scholar]
- Pedersen, O., Sauter M., Colmer T.D. and Nakazono M. (2021b) Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 229: 42–49. [DOI] [PubMed] [Google Scholar]
- Peterson, C.A. and Enstone D.E. (1996) Functions of passage cells in the endodermis and exodermis of roots. Physiol. Plant. 97: 592–598. [Google Scholar]
- Ponnamperuma, F.N. (1984) Effects of flooding on soils. In: Kozlowski, T.T. (ed.) Flooding and plant growth, New York. [Google Scholar]
- Ranathunge, K., Lin J., Steudle E. and Schreiber L. (2011a) Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant Cell Environ. 34: 1223–1240. [DOI] [PubMed] [Google Scholar]
- Ranathunge, K., Schreiber L. and Franke R. (2011b) Suberin research in the genomics era—new interest for an old polymer. Plant Sci. 180: 399–413. [DOI] [PubMed] [Google Scholar]
- Rao, A.N., Johnson D.E., Sivaprasad B., Ladha J.K. and Mortimer A.M. (2007) Weed management in direct-seeded rice. Adv. Agron. 93: 153–255. [Google Scholar]
- Schreiber, L., Hartmann K., Skrabs M. and Zeier J. (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J. Exp. Bot. 50: 1267–1280. [Google Scholar]
- Schreiber, L. and R.B. Franke (2011) Endodermis and exodermis in roots. In: eLS, John Wiley & Sons Ltd., Chichester. [Google Scholar]
- Seago, J.L., Marsh L.C., Stevens K.J., Soukup A., Votrubová O. and Enstone D.E. (2005) A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Ann. Bot. 96: 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter, T.L. and Waters I. (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253: 1–34. [Google Scholar]
- Shiono, K., Takahashi H., Colmer T.D. and Nakazono M. (2008) Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci. 175: 52–58. [Google Scholar]
- Shiono, K., Ogawa S., Yamazaki S., Isoda H., Fujimura T., Nakazono M. and Colmer T.D. (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann. Bot. 107: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono, K., Ando M., Nishiuchi S., Takahashi H., Watanabe K., Nakamura M., Matsuo Y., Yasuno N., Yamanouchi U., Fujimoto M.et al. (2014a) RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J. 80: 40–51. [DOI] [PubMed] [Google Scholar]
- Shiono, K., Yamauchi T., Yamazaki S., Mohanty B., Malik A.I., Nagamura Y., Nishizawa N.K., Tsutsumi N., Colmer T.D. and Nakazono M. (2014b) Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). J. Exp. Bot. 65: 4795–4806. [DOI] [PubMed] [Google Scholar]
- Smits, A.J.M., Laan P., Thier R.H. and van der Velde G. (1990) Root aerenchyma, oxygen leakage patterns and alcoholic fermentation ability of the roots of some nymphaeid and isoetid macrophytes in relation to the sediment type of their habitat. Aquat. Bot. 38: 3–17. [Google Scholar]
- Sorrell, B.K., Mendelssohn I.A., McKee K.L. and Woods R.A. (2000) Ecophysiology of wetland plant roots: a modelling comparison of aeration in relation to species distribution. Ann. Bot. 86: 675–685. [Google Scholar]
- Soukup, A., Armstrong W., Schreiber L., Franke R. and Votrubová O. (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytol. 173: 264–278. [DOI] [PubMed] [Google Scholar]
- Tanesaka, E., Ohno T. and Yamaguchi H. (2010) Species diversity of the genus Echinochloa (Poaceae), native to eastern Australia: a focus on their habitat and the threat of exotic species. J. Crop Res. 55: 13–17. [Google Scholar]
- Uga, Y., Hanzawa E., Nagai S., Sasaki K., Yano M. and Sato T. (2012) Identification of qSOR1, a major rice QTL involved in soil-surface rooting in paddy fields. Theor. Appl. Genet. 124: 75–86. [DOI] [PubMed] [Google Scholar]
- Visser, E.J.W., Colmer T.D., Blom C.W.P.M. and Voesenek L.A.C.J. (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ. 23: 1237–1245. [Google Scholar]
- Voesenek, L.A.C.J., Armstrong W., Bögemann G.M., McDonald M.P. and Colmer T.D. (1999) A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Aust. J. Plant Physiol. 26: 87–93. [Google Scholar]
- Voesenek, L.A.C.J. and Sasidharan R. (2013) Ethylene – and oxygen signalling – drive plant survival during flooding. Plant Biol. 15: 426–435. [DOI] [PubMed] [Google Scholar]
- Waßmann, F.F.M. (2014) Suberin biosynthesis in O. sativa: characterisation of a cytochrome P450 monooxygenase, Doctoral thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, p. 124. https://d-nb.info/1077289758/34.
- Watanabe, K., Nishiuchi S., Kulichikhin K. and Nakazono M. (2013) Does suberin accumulation in plant roots contribute to waterlogging tolerance? Front. Plant Sci. 4: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, K., Takahashi H., Sato S., Nishiuchi S., Omori F., Malik A.I., Colmer T.D., Mano Y. and Nakazono M. (2017) A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant Cell Environ. 40: 304–316. [DOI] [PubMed] [Google Scholar]
- Yamasue, Y. (2001) Strategy of Echinochloa oryzicola Vasing. for survival in flooded rice. Weed Biol. Manag. 1: 28–36. [Google Scholar]
- Yamauchi, T., Colmer T.D., Pedersen O. and Nakazono M. (2018) Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 176: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Zhou G., Shabala S., Koutoulis A., Shabala L., Johnson P., Li C. and Zhou M. (2016) Identification of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor. Appl. Genet. 129: 1167–1177. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Fan Y., Shabala S., Koutoulis A., Shabala L., Johnson P., Hu H. and Zhou M. (2017) A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theor. Appl. Genet. 130: 1559–1568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


