Abstract
BACKGROUND AND PURPOSE: Proton MR spectroscopy has revealed impaired neuronal integrity in the motor cortex of patients with amyotrophic lateral sclerosis (ALS). We hypothesized that the N-acetylaspartate (NAA)-creatine (Cr) ratios in the motor cortex and adjacent brain could reflect the therapeutic effectiveness of gabapentin (GBP) treatment in ALS.
METHODS: Eight patients with ALS underwent MR spectroscopy before and 26.5 days ± 8.8 after starting GBP. In 10 patients with ALS who were not treated with GBP, paired spectra were obtained 21.4 days ± 7.2 apart. Fourteen healthy subjects underwent a single MR spectroscopic examination. The NAA/Cr ratio was measured in the precentral gyrus, the postcentral gyrus, the superior parietal lobule, the supplementary motor area, and the premotor cortex.
RESULTS: The NAA/Cr ratio was decreased in the precentral and postcentral gyri of patients with ALS compared with healthy controls. In those with ALS, the change in the NAA/Cr ratio was not different between treated patients and untreated patients in any of the regions studied.
CONCLUSION: No improvement in neuronal integrity was detected in motor and nonmotor cerebral regions after GBP treatment. This result agrees with that of prior investigations showing the equivocal clinical effectiveness of GBP for ALS and supports the validity of the NAA/Cr ratio as a surrogate of therapeutic effectiveness.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder of unknown cause. Several lines of evidence implicate excessive glutamate-mediated excitotoxicity as being an important pathogenetic mechanism (1). Gabapentin (GBP) is a structural analog of the neurotransmitter gamma-aminobutyric acid. Although the mechanism of action of this drug is unclear, it is known to modulate gamma-aminobutyric acid and glutamate neurotransmitter systems by reducing the synthesis of glutamate and hastening its degradation (2).
The antiglutamatergic properties of GBP were demonstrated by its ability to protect against glutamate-mediated excitotoxicity in rat organotypic spinal cord cultures (3). Hyperexcitability of the cortex in patients with ALS (4) can be reversed with GBP treatment (5). A potential protective effect in ALS was demonstrated by its ability to prolong survival in a transgenic mouse model of familial ALS (6). Results of preliminary human studies suggested the benefits of GBP in patients with ALS, including the reduction of fasciculations (7) and muscular cramps (8). Investigators in a placebo-controlled trial (9) and an open-label study (10) reported a trend toward slowing the progression of limb weakness, although this was not statistically significant. A follow-up phase III trial revealed that GBP did not have any clinical effectiveness (11). Before this definitive trial was completed, many physicians, including ourselves, used GBP for the symptomatic management of ALS, in the hopes of achieving a disease-modifying benefit. During this period, we used proton MR spectroscopy to evaluate the effect of GBP on perirolandic cortical neuronal integrity in patients with ALS.
MR spectroscopy allows for the in vivo assessment of brain metabolites. The largest peak in MR spectra of brain comes from N-acetylaspartate (NAA), a compound localized exclusively to neurons in the adult brain (12, 13). Another major peak representing total creatine (Cr) content arises from creatine and phosphocreatine, two tightly coupled compounds that are localized in both neurons and glia and distributed relatively homogeneously throughout the brain. Changes in NAA/Cr ratio have been used as a marker of neuronal integrity in ALS, in which it has been found to be decreased in the primary motor cortex (14 –19) and surrounding perirolandic region (14). These findings imply neuronal loss or dysfunction of their upper motor neurons.
We have previously shown an increase in NAA/Cr in the precentral gyrus of patients with ALS after treatment with the antiglutamatergic drug riluzole (20); this presumably reflects improved corticoneuronal integrity due to a reversal of glutamate excitotoxicity. The present study was undertaken to look for similar changes in the NAA/Cr ratio in association with GBP therapy in ALS. If GBP has a beneficial effect on injured cortical neurons in ALS, we hypothesized that this effect is reflected by an increase in NAA/Cr ratio. In addition, we expected drug-related changes to be region specific; that is, they would occur in those areas of the brain where ALS is found. Thus, our previous approach was expanded upon by evaluating multiple regions in the perirolandic region.
Methods
Subjects
We examined 14 patients (Table 1) with clinically definite or probable ALS as defined by the El Escorial criteria (21). Ten of these patients underwent two MR spectroscopic examinations separated by 21.4 days ± 7.2 (mean ± SD); they were designated the untreated group. After undergoing the second examination, four of these patients started GBP at an escalating dose over 3 days to a target dose of 800 mg TID, and they subsequently underwent follow-up imaging during treatment. Four other patients underwent only a baseline examination; they started GBP in a similar fashion, and then underwent a follow-up examination. As a result, eight patients were included in the treated group, with treatment duration of 26.5 days ± 8.8 between their paired examinations. Fourteen healthy control subjects underwent MR spectroscopy. All subjects gave their informed consent.
TABLE 1:
Subject characteristics
| Characteristic | ALS-S Group* | ALS-U Group | ALS-T Group | HC Group |
|---|---|---|---|---|
| Number | 14 | 10 | 8 | 14 |
| Male-to-female ratio | 9:5 | 7:3 | 5:3 | 8:6 |
| Age, y* | 64.0 ± 11.8 | 63.9 ± 12.7 | 68.4 ± 9.8 | 58.0 ± 15.9 |
| Disease duration, mo* | 25.8 ± 16.6 | 26.4 ± 19.6 | 28.4 ± 14.7 | Not applicable |
| Baseline NAA/Cr ratio* | ||||
| PrCG | 2.29 ± 0.21† | 2.29 ± 0.21‡ | 2.23 ± 0.19† | 2.52 ± 0.19 |
| PoCG | 2.30 ± 0.25 | 2.33 ± 0.26 | 2.24 ± 0.21‡ | 2.42 ± 0.17 |
| Par | 2.38 ± 0.27 | 2.30 ± 0.26 | 2.44 ± 0.24 | 2.45 ± 0.23 |
| SMA | 2.26 ± 0.27 | 2.26 ± 0.29 | 2.24 ± 0.21 | 2.37 ± 0.20 |
| PMC | 2.50 ± 0.20 | 2.54 ± 0.20 | 2.51 ± 0.16 | 2.59 ± 0.24 |
Note.—ALS-S indicates all subjects with ALS; ALS-T, treated subjects with ALS subjects; ALS-U, untreated subjects with ALS; HC, healthy control subjects; Par, superior parietal lobule; PoCG, postcentral gyrus; PMC, premotor cortex; PrCG, precentral gyrus; and SMA, supplementary motor area.
Values are expressed as the mean ± SD.
P < .01 compared with the value in the HC group.
P < .05 compared with the value in the HC group.
MR Spectroscopy
MR studies were performed by using a 1.5-T Gyroscan system (Philips Medical Systems, Eindhoven, the Netherlands). Sagittal and axial T1-weighted locator images were used to plan a volume of interest placed high in the cranium and centered on the central sulcus. The volume of interest was 20-mm thick in the craniocaudal axis and approximately 75 mm in the anteroposterior and left-right dimensions, depending on the subject’s skull size and shape. MR spectroscopy was performed by using point-resolved spectroscopy (TR/TE, 1750/272; 250 × 250-mm field of view; 32 × 32 phase-encoding steps) with water suppression by means of selective excitation. Data postprocessing was performed on a SunSparc workstation (Sun Microsystems, Santa Clara, CA). Software developed in house was used to calculate Cr and NAA intensities by integration of the area under each peak. The mean NAA/Cr ratio for the precentral gyrus was determined by averaging the NAA/Cr ratios for all voxels that included at least 50% of the precentral gyrus in both left and right hemispheres. Voxels were excluded if the choline and Cr peaks were not resolved at the half-peak height. In a similar fashion, the mean NAA/Cr ratio was determined for the postcentral gyrus, superior parietal lobule, supplementary motor area, and premotor cortex (Fig). Of these, all except the superior parietal lobule make significant contributions to the corticospinal tract (22), whereas ALS is most evident in the precentral gyrus and postcentral gyrus (23). The change in the NAA/Cr ratio (ΔNAA/Cr ratio) over time was calculated between paired images from each subject by subtracting the mean NAA/Cr ratio for a region on the first image from that of the region on the second image. The volume of interest was repositioned in the identical location as the first image by following anatomic landmarks on the planning images from the first examination.
Fig 1.
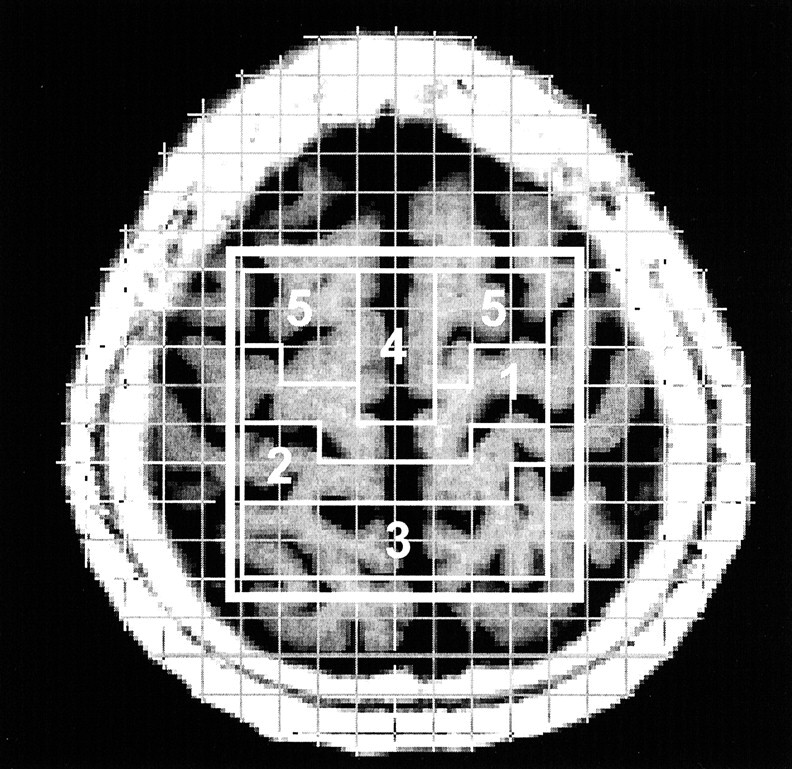
Axial T1-weighted MR image with a superimposed phase-encoding grid and volumes of interest. Voxels selected for the five cortical-subcortical regions are outlined: 1 indicates the precentral gyrus; 2, the postcentral gyrus; 3, the superior parietal lobule; 4, the supplementary motor area; and 5, the premotor cortex.
Statistics
The Mann-Whitney test was used to compare the means between groups, and the Wilcoxon signed rank test was used to compare the means within groups. The level of significance was set to P = .05.
Results
NAA/Cr ratios were lower in all five regions of the 14 subjects with ALS compared with those of 14 healthy control subjects (Table 1). The difference was most marked in the precentral gyrus for all subjects with ALS (P = .007), as well as for the baseline images of the subgroup treated with GBP (P = .01) and the untreated subgroup (P = .006). The NAA/Cr ratio in the postcentral gyrus was lower in the treated ALS subgroup (P = .04), with an overall trend for all patients with ALS (P = .1). The NAA/Cr ratio was not correlated with age or sex. The treated and untreated groups were not different with respect to age, disease duration, or baseline NAA/Cr across the five regions.
The forced vital capacity (available in 13 of the 14 patients with ALS) was 89.9% ± 26.3. The score on the ALS functional rating scale (available in 11 of the 14 patients with ALS) was 29.0 ± 5.6.
The NAA/Cr ratio did not change significantly in any of the regions of the treated or untreated patients between paired images. A significant net difference in ΔNAA/Cr was not found between the treated group and the untreated group, although trends existed for a decrease in the superior parietal lobule and supplementary motor area (Table 2). We observed a trend toward a correlation between ΔNAA/Cr in the superior parietal lobule and the duration of treatment (r = 0.68, P = .07). No other correlations were found between ΔNAA/Cr in any region for age, sex, disease duration, or treatment duration.
TABLE 2:
Results
| ΔNAA/Cr* | ALS-U Group† | ALS-T Group† | Net Change‡ |
|---|---|---|---|
| PrCG | −0.033 ± 0.14 | 0.020 ± 0.11 | 0.053 ± 0.06 |
| PoCG | −0.094 ± 0.18 | −0.043 ± 0.18 | 0.051 ± 0.09 |
| Par | 0.016 ± 0.27 | −0.230 ± 0.34 | −0.246 ± 0.15§ |
| SMA | 0.077 ± 0.23 | −0.073 ± 0.13 | −0.150 ± 0.09§ |
| PMC | −0.047 ± 0.21 | −0.120 ± 0.22 | −0.077 ± 0.10 |
ΔNAA/Cr = NAA/Cr on the second image—NAA/Cr on the first image.
Data are the mean ± SD.
Net change = ΔNAA/Cr(ALS-T) − Δ NAA/Cr(ALS-U). Data are the mean ± SE.
P < 0.1.
Discussion
In this study, we used MR spectroscopy to examine the effects of GBP treatment on the motor and nonmotor cortices in ALS. The usefulness of MR spectroscopy in evaluating upper motor neuron integrity in ALS has been previously demonstrated (14 –17, 19). Here, we confirm the previous findings of a decreased NAA/Cr, which reflects impaired neuronal integrity in the motor cortex of patients with ALS. Like Pioro et al (14), we found the greatest diminution of the NAA/Cr ratio in the precentral gyrus, followed by the postcentral gyrus, with normal levels in the supplementary motor area, premotor cortex, and superior parietal lobule. This finding is concurs with the pathology of ALS, which is most evident in these two regions and more severe in the precentral gyrus than in the postcentral gyrus (23). By MR spectroscopic measures, our group of patients had impaired corticoneuronal integrity and, thus, the possibility for an increase in NAA/Cr ratio. An increase would not be anticipated if corticoneuronal integrity ratios were normal.
MR spectroscopy has been used to assess the therapeutic response in various clinical disorders (24–28). We previously showed that MR spectroscopy can detect an increase in NAA/Cr ratio in response to riluzole treatment in the precentral gyrus of patients with ALS; this finding suggests improvement of upper motor neuron integrity (20). NAA/Cr increased in 11 patients at 24 days ± 8 after the initiation of riluzole therapy, from 2.14 ± 0.26 to 2.27 ± 0.24 (mean ± SD, P = .044). In 12 patients not receiving medication, NAA/Cr ratio decreased from 2.17 ± 0.20 to 2.08 ± 0.20 (mean ± SD, P = .099) between paired images separated by 21 days ± 6. Thus, the change in the NAA/Cr ratio for the treated group with respect to the untreated group was an increase of 0.22 ± 0.095 (mean ± SE, P = .008). It is postulated that riluzole, a drug that prolongs tracheostomy-free survival (29), reduces glutamate-mediated excitotoxicity in a population of sublethally injured neurons, resulting in increased NAA production. With a similar duration (1 month) of oral Cr supplementation, MR spectroscopy demonstrated a decline in the NAA/Cr ratio in healthy control subjects but no change in the NAA/Cr ratio in patients with ALS. This result was interpreted as a relative increase in NAA in the subjects with ALS on the basis of improved mitochondrial respiration (30). Bowen et al (31) evaluated the spectroscopic indices with riluzole and GBP treatment in a small number of patients with ALS and did not find an increase in NAA. In distinction to our protocol, a stimulated-echo acquisition mode sequence was used with a short TE of 20 ms and with quantitation of metabolites sampled from a single 8-mL voxel. Our multivoxel approach allows for sampling of more parenchyma, improving the signal-to-noise ratio, and the long TE used in our studies facilitates the quantitation by eliminating the underlying signal intensity from other resonances with short T2s. Their patients were already taking riluzole or GBP and had discontinued treatment 2 weeks before the first imaging session. The proportion of patients taking each drug and if combination therapy was used are unknown. This washout period could have been insufficient washout. The follow-up image was obtained at variable time points: at 2 weeks for five patients and at 4, 10, and 18 weeks each for three others. Thus, notable differences in methods could account for the discrepancy in their results and ours. Notably, current methods for the absolute quantitation of spectroscopic resonance intensities are subject to a propagation of errors because of the assumptions necessary; thus, they are likely to be able to detect drug-related effects than methods in which ratios (eg, NAA/Cr) are reported. The quantitation of NAA with respect to Cr in the same voxel corrects for multiple potential sources of error in the quantitation of NAA levels in vivo and requires only the assumption that brain Cr levels do not substantially change between the two imaging sessions.
GBP, like riluzole, is believed to have anti-gluatamatergic properties (2, 3). We proposed that, in ALS, a population of sublethally injured neurons exists secondary to glutamate-mediated excitotoxicity and that the function of these neurons can be improved with GBP treatment, with a restoration of NAA production toward normal. An approach similar to that applied in our riluzole study was used in a treated group compared with an untreated group, since progressive neuronal degeneration would be expected to blunt any increase in NAA due to a drug response. In both protocols, the treatment duration and period between imaging sessions in the untreated group were approximately 3 weeks. A similar number of patients were examined, although each group in this study included fewer subjects. In addition to the region examined in the first study, we examined not only the precentral gyrus but also other relevant perirolandic regions that are conveniently accessible with this volume-of-interest placement. The pathologic changes of ALS are most profound in the precentral gyrus and postcentral gyrus (23), and four of the five regions substantially contribute to the corticospinal tract (precentral gyrus, postcentral gyrus, supplementary motor area, premotor cortex) (22). The superior parietal lobule functions as a control. Preliminary results of this study have been previously presented (32).
The ΔNAA/Cr in the treated and untreated subjects was not significant, nor was the difference in ΔNAA/Cr between these groups significantly different. This observation is in keeping with the lack of effectiveness of GBP in the treatment of ALS (11). Trends were observed for decreased ΔNAA/Cr in superior parietal lobule and supplementary motor area. This finding is possibly a statistical artifact, as the SD of ΔNAA/Cr between the treated patients and untreated patients were more discrepant in these regions than in the other regions. Conversely, it may represent a real effect on impaired upper motor neurons that arise from the supplementary motor area; however, this would be surprising in the absence of a response from the other regions sampled that give rise to the upper motor neurons (precentral gyrus, postcentral gyrus, premotor cortex), and the response here is seemingly a negative one. In addition to the mentioned trend in the superior parietal lobule, treatment duration and ΔNAA/Cr were correlated in this region. One may speculate that these observations are related to the utility of GBP in the treatment of pain. Exactly how GBP acts to decrease or modify pain is not known (33); however, evidence from positron emission tomography and functional MR imaging demonstrates that the parietal lobe (among other brain regions) is involved in pain processing (34). Further MR spectroscopic studies are needed to substantiate these findings.
A change could possibly have been detected after a different interval of drug treatment, or the sample size in this study may have been too small to reveal an effect. However, this protocol was adequate to reveal improvements in the NAA/Cr ratio with another anti-glutamatergic drug (riluzole) when the sample size was not substantially larger. Furthermore, the treatment duration was similar to that used in the previously mentioned studies (20, 30). Patients selected for more severe neuronal impairment on the basis of a lower NAA/Cr ratio may have shown a more prominent response, as the baseline NAA/Cr ratio in the riluzole study was lower that that of the patients in this study. Finally, an effect on the NAA/Cr ratio could have been missed if both NAA and Cr quantities changed in the same direction. Although one report states that Cr is decreased in ALS (35), other groups have found no change (36, 37), and it is unlikely that Cr would change over 3 weeks and unlikely that GBP would affect the metabolism of Cr and phosphocreatine.
MR spectroscopy has many potential roles in the evaluation of ALS. It may help in ascertaining upper motor neuron involvement when it is not obvious during clinical examination. MR spectroscopy could also be used to follow disease progression. The most exciting and useful role that MR spectroscopy could play is in providing a surrogate marker of therapeutic effectiveness. In this respect, it could be used to screen novel potential treatments and applied to evaluate drug responses in individual patients. Thus, it is useful to know that an MR spectroscopy response is seen with a drug such as riluzole, which has been shown to be effective in ALS, and that no response is observed with an ineffective drug like GBP.
Conclusion
Using MR spectroscopy, we found no significant effect of GBP treatment in the motor cortex of patients with ALS. This observation is concordant with the clinical ineffectiveness of GBP. More MR spectroscopy-based drug studies are needed to further develop the potential of spectroscopic markers as surrogates of therapeutic effectiveness in ALS.
Footnotes
Supported in part by the ALS Association.
Preliminary data from this study was presented at the 35th annual meeting of the Canadian Congress of Neurological Sciences, Ottawa, Canada, June, 2000.
References
- 1.Rothstein JD. Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv Neurol 1995;68:7–20 [PubMed] [Google Scholar]
- 2.Taylor CP, Gee NS, Su TZ, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 1998;29:233–249 [DOI] [PubMed] [Google Scholar]
- 3.Rothstein JD, Kuncl RW. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J Neurochem 1995;65:643–651 [DOI] [PubMed] [Google Scholar]
- 4.Eisen A. Corticospinal motor neurons: pathophysiology. In: Brown RHJ, Meininger V, Swash M, eds. Amyotrophic Lateral Sclerosis. London: Martin Dunitz, Ltd.2000;161–177
- 5.Caramia MD, Palmieri MG, Desiato MT, et al. Pharmacologic reversal of cortical hyperexcitability in patients with ALS. Neurology 2000;54:58–64 [DOI] [PubMed] [Google Scholar]
- 6.Gurney ME, Cutting FB, Zhai P, et al. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol 1996;39:147–157 [DOI] [PubMed] [Google Scholar]
- 7.Romano JG. Reduction of fasciculations in patients with amyotrophic lateral sclerosis with the use of gabapentin. Arch Neurol 1996;53:716. [DOI] [PubMed] [Google Scholar]
- 8.Serrao M, Rossi P, Cardinali P, Valente G, Parisi L, Pierelli F. Gabapentin treatment for muscle cramps: an open-label trial. Clin Neuropharmacol 2000;23:45–49 [DOI] [PubMed] [Google Scholar]
- 9.Miller RG, Moore D, Young LA, et al. Placebo-controlled trial of gabapentin in patients with amyotrophic lateral sclerosis: WALS Study Group—Western Amyotrophic Lateral Sclerosis Study Group. Neurology 1996;47:1383–1388 [DOI] [PubMed] [Google Scholar]
- 10.Mazzini L, Mora G, Balzarini C, et al. The natural history and the effects of gabapentin in amyotrophic lateral sclerosis. J Neurol Sci 1998;160(suppl)1:S57–S63 [DOI] [PubMed] [Google Scholar]
- 11.Miller RG, Moore DH, Gelinas DF, et al. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology 2001;56:843–848 [DOI] [PubMed] [Google Scholar]
- 12.Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 1991;45:37–45 [DOI] [PubMed] [Google Scholar]
- 13.Moffett JR, Namboodiri MA, Cangro CB, Neale JH. Immunohistochemical localization of N-acetylaspartate in rat brain. Neuroreport 1991;2:131–134 [DOI] [PubMed] [Google Scholar]
- 14.Pioro EP, Antel JP, Cashman NR, Arnold DL. Detection of cortical neuron loss in motor neuron disease by proton magnetic resonance spectroscopic imaging in vivo. Neurology 1994;44:1933–1938 [DOI] [PubMed] [Google Scholar]
- 15.Jones AP, Gunawardena WJ, Coutinho CM, Gatt JA, Shaw IC, Mitchell JD. Preliminary results of proton magnetic resonance spectroscopy in motor neuron disease (amyotrophic lateral sclerosis). J Neurol Sci 1995;129(suppl):85–89 [DOI] [PubMed] [Google Scholar]
- 16.Rooney WD, Miller RG, Gelinas D, Schuff N, Maudsley AA, Weiner MW. Decreased N-acetylaspartate in motor cortex and corticospinal tract in ALS. Neurology 1998;50:1800–1805 [DOI] [PubMed] [Google Scholar]
- 17.Block W, Karitzky J, Traber F, et al. Proton magnetic resonance spectroscopy of the primary motor cortex in patients with motor neuron disease: subgroup analysis and follow-up measurements. Arch Neurol 1998;55:931–936 [DOI] [PubMed] [Google Scholar]
- 18.Ellis CM, Simmons A, Andrews C, Dawson JM, Williams SC, Leigh PN. A proton magnetic resonance spectroscopic study in ALS: correlation with clinical findings. Neurology 1998;51:1104–1109 [DOI] [PubMed] [Google Scholar]
- 19.Chan S, Shungu DC, Douglas-Akinwande A, Lange DJ, Rowland LP. Motor neuron diseases: comparison of single-voxel proton MR spectroscopy of the motor cortex with MR imaging of the brain. Radiology 1999;212:763–769 [DOI] [PubMed] [Google Scholar]
- 20.Kalra S, Cashman NR, Genge A, Arnold DL. Recovery of N-acetylaspartate in corticomotor neurons of patients with ALS after riluzole therapy. Neuroreport 1998;9:1757–1761 [DOI] [PubMed] [Google Scholar]
- 21.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis: Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994;124(suppl):96–107 [DOI] [PubMed] [Google Scholar]
- 22.Parent A. Carpenter’s Human Neuroanatomy. 9th ed. Baltimore: Lippincott Williams & Wilkins;1996. :864–936
- 23.Martin JE, Swash M. The pathology of motor neuron disease. In: Leigh PN, Swash M, eds. Motor Neuron Disease, Biology and Management. London: Springer-Verlag;1995. :93–118
- 24.De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med 1995;34:721–727 [DOI] [PubMed] [Google Scholar]
- 25.Uno M, Ueda S, Hondo H, Matsumoto K, Harada M. Effectiveness of revascularization surgery evaluated by proton magnetic resonance spectroscopy and single photon emission computed tomography. Neurologia Medico-Chirurgica 1996;36:560–566 [DOI] [PubMed] [Google Scholar]
- 26.Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy. Evidence from proton MR spectroscopic imaging. Neurology 1997;49:1525–1533 [DOI] [PubMed] [Google Scholar]
- 27.Vion-Dury J, Nicoli F, Salvan AM, Confort-Gouny S, Dhiver C, Cozzone PJ. Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet 1995;345:60–61 [DOI] [PubMed] [Google Scholar]
- 28.Moore GJ, Bebchuk JM, Hasanat K, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biological Psychiatry 2000;48:1–8 [DOI] [PubMed] [Google Scholar]
- 29.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis: Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996;347:1425–1431 [DOI] [PubMed] [Google Scholar]
- 30.Vielhaber S, Kaufmann J, Kanowski M, et al. Effect of creatine supplementation on metabolite levels in ALS motor cortices. Exp Neurol 2001;172:377–382 [DOI] [PubMed] [Google Scholar]
- 31.Bowen BC, Pattany PM, Bradley WG, et al. MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2000;21:647–658 [PMC free article] [PubMed] [Google Scholar]
- 32.Kalra S, Caramanos Z, Cashman N, Genge A, Arnold DL. MRSI demonstrates lack of improvement in corticoneuronal integrity with gabapentin therapy in ALS. CJNS 2000;27:S18 [Google Scholar]
- 33.Ross EL. The evolving role of antiepileptic drugs in treating neuropathic pain. Neurology 2000;55:S41–S46 [PubMed] [Google Scholar]
- 34.Hudson AJ. Pain perception and response: central nervous system mechanisms. Can J Neurol Sci 2000;27:2–16 [DOI] [PubMed] [Google Scholar]
- 35.Pohl C, Block W, Karitzky J, et al. Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch Neurol 2001;58:729–735 [DOI] [PubMed] [Google Scholar]
- 36.Gredal O, Rosenbaum S, Topp S, Karlsborg M, Strange P, Werdelin L. Quantification of brain metabolites in amyotrophic lateral sclerosis by localized proton magnetic resonance spectroscopy. Neurology 1997;48:878–881 [DOI] [PubMed] [Google Scholar]
- 37.Sarchielli P, Pelliccioli GP, Tarducci R, et al. Magnetic resonance imaging and 1H-magnetic resonance spectroscopy in amyotrophic lateral sclerosis. Neuroradiology 2001;43:189–197 [DOI] [PubMed] [Google Scholar]


