Abstract
BACKGROUND AND PURPOSE: Children with hemoglobin SS sickle cell disease are known to suffer cognitive impairment if they have silent infarct, but recent evidence suggests that patients with hemoglobin SS sickle cell disease may be impaired even if they are free of infarction. We test a hypothesis that cognitive impairment in children with hemoglobin SS sickle cell disease is associated with low hematocrit and MR imaging abnormalities.
METHODS: A cohort of 49 patients was examined, all of whom had hemoglobin SS sickle cell disease but no history of clinical stroke. The Wechsler scales, which are standardized and age-adjusted, were used to assess cognitive function. Patients also underwent MR imaging examination of the brain, and hematocrit was measured in a subset of 45 patients. MR images were evaluated by at least two readers, and abnormal imaging findings were evaluated by at least three readers. Any lesion was sufficient to be classified as abnormal, with lesions defined to include lacunar infarction, encephalomalacia, or leukoencephalopathy. Hematocrit data were used if obtained within 3 months of psychometric testing and if there were no confounding events in the patients’ charts. Wechsler test scores were then evaluated in relation to imaging findings and hematocrit values.
RESULTS: Patients with imaging abnormalities had more cognitive impairment than did patients with normal imaging findings in verbal intelligence quotient (P < .02) and verbal comprehension (P < .01). Patients with low hematocrit had cognitive impairment shown by many performance measures, including full-scale intelligence quotient (P < .006), verbal comprehension (P < .006), and freedom from distractibility (P < .02). Multivariate analysis showed that MR imaging and hematocrit were independent predictors of full-scale intelligence quotient.
CONCLUSION: Focal brain injury, revealed by MR imaging, is associated with cognitive impairment, but our data suggest that diffuse brain injury may also contribute to impairment. These findings show that impairment is multifactorial and suggest that chronic brain hypoxia is part of the pathophysiology of sickle cell disease.
Silent infarction is defined as an ischemic change in brain tissue that is visible on MR images of patients with no clinical history of stroke. Silent infarction is relatively common in children with sickle cell disease and is associated with impaired cognitive function (1). However, new findings suggest that cognitive impairment can also be present in patients with sickle cell disease who are free of focal brain damage (2). The incidence of mild mental deficiency was elevated at least 11-fold in a small sample of patients with sickle cell disease with no clinical history of stroke, and the full-scale intelligence quotient of these patients correlated with hematocrit (2). This suggests that cognitive impairment in children with sickle cell disease may be a function of chronic hypoxia of the brain (2, 3). Because this hypothesis has been controversial (4), we sought to elucidate the relationship between full-scale intelligence quotient, MR imaging, and hematocrit in a cohort of patients with hemoglobin SS sickle cell disease, the most severe form of sickle cell disease.
Methods
Patients
A cohort of 49 patients with hemoglobin SS sickle cell disease was examined, all of whom had no history of clinical stroke and were seen at our institution. Most patients were tested as part of an ongoing, prospective study of sickle cell disease (2), and these patients were probably representative of patients with hemoglobin SS sickle cell disease at St. Jude. There were 27 boys and 22 girls in the study population, and the average age of the patients was 9.5 years (SD = 3.9 years; range, 4–19.7 years) at the time of study participation.
Psychometric Testing
Patients were examined by using the Wechsler scales (5), a standard psychometric test of intellectual development. The Wechsler Intelligence Scales for Children-Revised (WISC-R) was used for 15 patients, and 34 patients who were examined more recently underwent testing with the Wechsler Intelligence Scales for Children-Version III (WISC-III). If multiple examinations were available for a patient, we used the most recent available scores, because the test is more accurate in older patients (5) and because this strategy results in more patient exposure to disease effects.
Patients were tested by a state-licensed psychologist who was blinded to clinical findings and who used standard testing materials (5). The primary outcome measures were the age-adjusted scores: full-scale intelligence quotient, verbal intelligence quotient, and performance intelligence quotient. The composite factor scores derived from the full-scale intelligence quotient were verbal comprehension, perceptual organization, freedom from distractibility, and processing speed.
MR Imaging
Patients underwent MR imaging of the brain at 1.5 T (Siemens Medical Systems, Iselin, NJ) with a standard quadrature head coil. A conventional T1-weighted image set was acquired in the transverse plane with parameters as follows: 266/6 (TR/TE), 23-cm field of view, 90-degree flip angle, 256 × 256 matrix, and three acquisitions in an imaging time of 4 min 35 s. A conventional T2-weighted image set was acquired in the same orientation with parameters as follows: 3.5/19, 93; 23-cm field of view; 256 × 256 matrix; and one acquisition in an imaging time of 5 min 7 s. In March of 2000, we also added fluid-attenuated inversion recovery imaging in the same orientation, with parameters as follows: 9000/119, 2470-ms inversion time, 23-cm field of view, 180-degree flip angle, 154 × 256 matrix, and one acquisition in an imaging time of 3 min 27 s. MR angiography was also included in the examination, but no patient was determined to have an abnormality solely on the basis of MR angiography findings.
All images were read and dictation made by a neuroradiologist, often working with a neuroradiology fellow. All images were reviewed by an experienced reader (R.G.S.) who had 8 years of experience in evaluating pediatric sickle cell disease and who sought to verify the dictation. For those patients whose dictation indicated abnormalities and for those patients for whom some question arose, the images were evaluated by another neuroradiologist (K.J.H.) who reconciled each questionable case, usually in consultation with the first radiologist. All MR images were therefore evaluated by at least two readers, and all abnormal findings were evaluated by at least three readers.
MR imaging findings were scored as normal or abnormal, relying on a combination of T1-weighted, T2-weighted, and fluid-attenuated inversion recovery images when available (6, 7). Small unifocal lesions (<1 cm) or large multifocal lesions were sufficient to classify MR imaging findings, with lesions broadly defined to include lacunar infarction, encephalomalacia, or leukoencephalopathy. Lacunar infarction was defined as a shelled-out volume, usually in white matter, visible on both T1- and T2-weighted MR images. Encephalomalacia was defined as any other infarctive change, including atrophy. Leukoencephalopathy was defined as degeneration or demyelination of white matter, usually seen as high signal intensity on T2-weighted MR images.
Hematocrit
Hematocrit data were used if obtained within 3 months of psychometric testing and if there were no significant confounding events in the patients’ chart (such as a recent transfusion). In no case was more than one hematocrit measurement available for a given imaging date. We were unable to fully analyze the cases of four patients for whom no concurrent hematocrit data were available.
Statistical Tests
Statistical analysis was conducted with DataDesk statistical software (Version 6; Data Description, Inc., Ithaca, NY), running on a Power Mac G4 (Apple Computer, Inc., Cupertino, CA). Pooled t tests were used for all group comparisons. Because of a clear prediction that patients with abnormal MR imaging findings or low hematocrit would suffer more cognitive impairment than would patients with normal MR imaging findings or normal hematocrit, we used a one-tailed probability function.
Results
Psychometric Testing
The mean full-scale intelligence quotient of the patients was 80.3 (±12.8 SD), which is significantly (P < .001) below the mean of normative data supplied with the test (5). Scores for verbal intelligence quotient, performance intelligence quotient, and the various factor scores were also significantly (P < .001) below the mean of normative data (5). The WISC-III scores were not significantly lower than WISC-R scores (78.0 ± 13.5 SD versus 85.4 ± 9.2 SD, not significant). Full-scale intelligence quotient measured with the WISC-III tended to decrease significantly with age (r = −0.29, P = .04), which is not the case in healthy children (5).
MR Imaging
Of the 49 patients with hemoglobin SS sickle cell disease who were evaluated, 21 (43%) had abnormal MR imaging findings (Table 1, Fig 1). No significant difference existed in age between patients whose MR imaging findings were normal (9.3 years ± 4.0 SD) and those whose MR imaging findings were abnormal (10.1 years ± 3.9 SD). The rate of clinically silent MR imaging abnormalities for our patients was somewhat higher than has been reported in some early studies (6,8,13) but was consistent with the incidence of abnormalities in other imaging studies (2,3,7). Lacunae were present in 52% of patients with any MR imaging abnormality and 22% of patients overall.
TABLE 1:
Summary of abnormal MR imaging findings for patients with hemoglobin SS sickle cell disease
| Finding | No. of Patients Affected | Proportion (%) |
|---|---|---|
| Tortuous intracranial vessels by MR angiography | 16 | 33 |
| Any lacunar infarction | 11 | 22 |
| Tortuous intracranial vessels by MR angiography with no other abnormality | 10 | 20 |
| Multiple, bilateral frontal white matter lacunae | 8 | 16 |
| Mild-to-moderate arterial stenosis by MR angiography | 7 | 14 |
| Single frontal white matter lacune | 4 | 8 |
| Lacunar infarction in corona radiata/centrum semiovale | 4 | 8 |
| Encephalopathy/leukoencephalopathy | 3 | 6 |
Note.—A total of 57% (28 of 49 patients) had abnormalities shown by either MR imaging or MR angiography, whereas 43% (21 of 49 patients) had abnormalities shown by MR imaging alone. The proportion of patients with any abnormal finding was calculated from a total of 49 patients evaluated. Because findings are not mutually exclusive, many patients are tabulated under more than one finding. Lacunar infarction was seen in 22% of all patients, and the majority of all lacunar infarctions were in white matter.
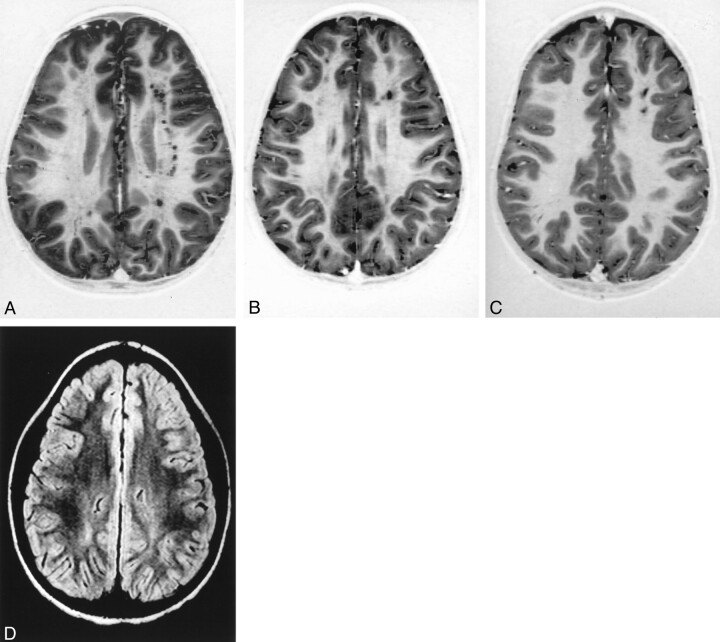
Fig 1.
Range of abnormalities shown by MR images of young patients with hemoglobin SS sickle cell disease.
A, T1-weighted MR image of a 4.3-year-old boy with bilateral lacunae in the centrum semiovale and paraventricular white matter. Image section is 3 cm thick. Image shows multiple lacunae, the largest of which is approximately 7 mm in diameter.
B, T1-weighted MR image of a 5.0-year-old girl with multiple small bilateral white matter lacunae in the centrum semiovale and the corona radiata. Image section is 3 cm thick. Image shows several lacunae, the largest of which is approximately 7 mm in diameter.
C, T1-weighted MR image of a 5.4-year-old boy with bilateral lacunar infarcts involving both frontal lobes and the centrum semiovale on both sides. Image section is 3 cm thick. Image shows two lacunae, the largest of which is approximately 6 mm in diameter.
D, Fluid-attenuated inversion recovery image of an 8.4-year-old boy with bilateral leukoencephalopathy in the centrum semiovale. Image section is 3 cm thick. Image shows abnormally high signal intensity. Because fluid-attenuated inversion recovery images null signal intensity from CSF, this is not a partial volume effect from the ventricle.
When MR imaging findings were used to stratify the cases (Table 2), few significant differences were observed in psychometric test results between patients with normal MR imaging findings versus those with abnormal MR imaging findings. Of the nine test score comparisons presented in Table 2, the only comparisons that reached significance were those for verbal intelligence quotient (P < .02) and verbal comprehension factor of the WISC-III (P < .01). No significant difference in hematocrit between patients with and without MR imaging abnormality was found (Table 2).
TABLE 2:
Psychometric test scores of patients with sickle cell disease stratified by MR imaging findings
| Comparison | Normal MR Imaging Findings |
Abnormal MRI |
Δ | Pooled t Test P < | ||
|---|---|---|---|---|---|---|
| No. | Mean ± SD | No. | Mean ± SD | |||
| Mean hematocrit | 30 | 25.0 ± 4.8 | 15 | 23.0 ± 5.8 | −8.0% | NS |
| Full-scale IQ | 33 | 81.1 ± 11.0 | 16 | 78.6 ± 16.1 | −3.1% | NS |
| WISC-R FSIQ | 10 | 83.0 ± 8.0 | 5 | 90.2 ± 10.5 | +8.6% | NS |
| WISC-III FSIQ | 23 | 80.2 ± 12.1 | 11 | 73.4 ± 15.7 | −8.5% | NS |
| Verbal IQ | 23 | 84.6 ± 12.3 | 11 | 74.1 ± 15.2 | −12.4% | 0.02 |
| Performance IQ | 23 | 78.9 ± 12.5 | 11 | 77.5 ± 16.3 | −1.8% | NS |
| WISC-III factor scores | ||||||
| VC factor | 22 | 86.0 ± 12.9 | 11 | 74.4 ± 13.5 | −13.5% | 0.01 |
| PO factor | 23 | 80.0 ± 13.2 | 11 | 75.7 ± 16.4 | −5.4% | NS |
| FD factor | 22 | 89.0 ± 11.9 | 11 | 86.2 ± 17.2 | −3.1% | NS |
| PS factor | 20 | 85.3 ± 14.0 | 11 | 93.7 ± 19.9 | +9.9% | NS |
Note.—IQ indicates intelligence quotient; WISC-R (FSIQ), Wechsler Intelligence Scales for Children-Revised full-scale intelligence quotient; WISC-III FSIQ, Wechsler Intelligence Scales for Children-Version III full-scale intelligence quotient; VC, verbal comprehension; PO, perceptual organization; FD, freedom from distractibility; PS, processing speed; NS, not significant. When MR imaging findings were used to partition patients into those with normal MR imaging findings and those with abnormal MR imaging findings; few significant differences in psychometric test scores were observed. The Δ value was calculated as the percent reduction in psychometric test scores in the abnormal MR imaging group, as compared with test scores in the normal MR imaging group. A one-way pooled t test was used to test for significance because of a prediction that patients with abnormal MR imaging findings would have lower psychometric test scores. Patients with normal MR imaging findings were not significantly younger than patients with abnormal MR imaging findings, nor was there a significant difference in hematocrit.
The pattern of intelligence quotient scores was different, depending on whether MR imaging findings were abnormal. Verbal intelligence quotient was significantly higher than performance intelligence quotient among patients with normal MR imaging findings (P < .008) but not among patients with abnormal MR imaging findings. In the group of patients with abnormal imaging findings, verbal and performance intelligence quotient scores were equally reduced.
Hematocrit
The mean hematocrit in the 45 patients for whom we had appropriate data was 24.4 (±5.2 SD). We used a mean hematocrit value of 27 as a cutoff point to stratify patients (2). Patients with low hematocrit performed significantly more poorly (Table 3) on six of the nine measures of cognitive performance, including full-scale intelligence quotient (P < .006), verbal intelligence quotient (P < .02), verbal comprehension (P < .006), and freedom from distractibility (P < .02). The largest difference in factor scores for patients with low hematocrit was for verbal comprehension and freedom from distractibility scores, which were approximately 12% lower in the severely anemic patients. Among patients with more normal hematocrit, there was no significant difference between verbal intelligence quotient and performance intelligence quotient (P = .11).
TABLE 3:
Psychometric test scores of patients with sickle cell disease stratified by hematocrit
| Comparison | Hematocrit ≥ 27 | Hematocrit < 27 | Δ | Pooled t test P < | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean ± SD | No. | Mean ± SD | |||||
| Mean hematocrit | 17 | 29.2 ± 3.2 | 28 | 21.4 ± 3.7 | −26.7% | 0.0001 | ||
| Full-scale IQ | 17 | 86.0 ± 13.8 | 28 | 76.9 ± 11.3 | −10.6% | 0.006 | ||
| WISC-R FSIQ | 6 | 90.5 ± 9.0 | 9 | 82.0 ± 8.1 | −9.4% | 0.04 | ||
| WISC-III FSIQ | 11 | 83.5 ± 12.4 | 19 | 74.5 ± 12.0 | −10.8% | 0.03 | ||
| Verbal IQ | 11 | 87.1 ± 12.5 | 19 | 77.1 ± 12.9 | −11.5% | 0.02 | ||
| Performance IQ | 11 | 82.7 ± 12.6 | 19 | 76.2 ± 12.2 | −7.9% | NS | ||
| WISC-III factor scores | ||||||||
| VC factor | 10 | 89.9 ± 13.4 | 19 | 77.0 ± 11.7 | −14.3% | 0.006 | ||
| PO factor | 11 | 84.0 ± 13.7 | 19 | 75.8 ± 12.9 | −9.8% | NS | ||
| FD factor | 10 | 96.2 ± 12.1 | 19 | 85.3 ± 13.3 | −11.3% | 0.02 | ||
| PS factor | 10 | 88.6 ± 11.7 | 18 | 87.7 ± 18.5 | −1.0% | NS | ||
Note.—IQ indicates intelligence quotient; WISC-R (FSIQ), Wechsler Intelligence Scales for Children-Revised full-scale intelligence quotient; WISC-III FSIQ, Wechsler Intelligence Scales for Children-Version III full-scale intelligence quotient; VC, verbal comprehension; PO, perceptual organization; FD, freedom from distractibility; PS, processing speed; NS, not significant. When hematocrit was used to partition patients into those with low hematocrit values (<27) and those with more normal hematocrit values (≥27), significant differences in psychometric test scores were observed: The Δ value was calculated as the percent reduction in the comparison value in the low hematocrit group, compared with the value in the more normal hematocrit group. A one-way pooled t test was used to test for significance because of a prediction that patients with low hematocrit would have lower psychometric test scores. Patients with low hematocrit were not significantly younger than patients with more normal hematocrit (9.6 versus 9.4 years, P = .43).
Regression analysis was used to further explore the relationship between hematocrit and cognitive impairment. We found that hematocrit was significantly correlated with full-scale intelligence quotient, verbal intelligence quotient, performance intelligence quotient, and the factors for verbal comprehension, perceptual organization, and freedom from distractibility (Table 4). Overall, hematocrit was able to explain approximately 23% of the variance in full-scale intelligence quotient and an average of 13% of the variance in factor scores. These findings argue that although a significant relationship exists between hematocrit and patient cognitive impairment, other variables are also important in accounting for variance in full-scale intelligence quotient.
TABLE 4:
Relationship of hematocrit to WISC-III scores in children with sickle cell disease
| Test Measure or Factor Score | Sample Size | Correlation r = | ANOVA P < | Adjusted R2 |
|---|---|---|---|---|
| WISC-III FSIQ | 30 | 0.50 | 0.005 | 22.7 |
| Verbal IQ | 30 | 0.42 | 0.02 | 14.8 |
| Performance IQ | 30 | 0.46 | 0.01 | 18.8 |
| WISC-III factor scores | ||||
| VC factor | 19 | 0.50 | 0.006 | 22.2 |
| PO factor | 20 | 0.46 | 0.01 | 18.8 |
| FD factor | 19 | 0.39 | 0.04 | 12.2 |
| PS factor | 18 | 0.13 | NS | 0.0 |
Note.—WISC-III indicates Wechsler Intelligence Scales for Children-Version III; ANOVA, analysis of variance; FSIQ, full-scale intelligence quotient; IQ, intelligence quotient; VC, verbal comprehension; PO, perceptual organization; FD, freedom from distractibility; PS, processing speed; NS, not significant. Regression of hematocrit with full-scale intelligence quotient and with various sub-tests or factor scores of the WISC-III. The correlation (r) is reported, together with the level of significance calculated by analysis of variance. The proportion of variance in psychometric measures, explained by hematocrit (R2), is expressed as a percentage.
MR Imaging Findings and Hematocrit as Predictors of Full-Scale Intelligence Quotient
We used a multivariate approach to examine the subset of 30 patients for whom we had WISC-III data, MR imaging findings, and hematocrit values (Table 5). Multivariate analysis showed a significant effect of imaging findings on full-scale intelligence quotient (one-tailed, P = .016) and of hematocrit on full-scale intelligence quotient (one-tailed, P = .038). There was no significant interaction between MR imaging and hematocrit (P = .717), suggesting that MR imaging findings and hematocrit are independent predictors of full-scale intelligence quotient.
TABLE 5:
Full-scale intelligence quotient as a function of MR imaging findings and hematocrit
| Hematocrit ≥ 27 | Hematocrit < 27 | Average FSIQ | |
|---|---|---|---|
| Normal MR imaging findings | 86.8 ± 9.9 | 77.5 ± 9.8 | 81.2 ± 10.7 |
| n = 8 | n = 12 | n = 20 | |
| Abnormal MR imaging findings | 75.0 ± 16.5 | 69.3 ± 14.3 | 71.0 ± 14.3 |
| n = 3 | n = 7 | n = 10 | |
| Average FSIQ | 83.5 ± 12.4 | 74.5 ± 12.0 | 77.8 ± 12.7 |
| n = 11 | n = 19 | n = 30 |
Note.—FSIQ indicates full-scale intelligence quotient, as measured by the WISC-III in children cross-stratified by hematocrit and MR imaging findings. Multivariate analysis showed a significant effect of MR imaging findings on full-scale intelligence quotient (one-tailed, P = .016) and of hematocrit on full-scale intelligence quotient (one-tailed, P = .038). There was no significant interaction between MR imaging and hematocrit (P = .717), suggesting these factors are independent predictors of full-scale intelligence quotient.
Discussion
Children with sickle cell disease suffer impairment of cognitive function (1–3, 8–18). Although some studies report little or no cognitive impairment among patients with no history of clinical stroke (9,16,18), a general consensus exists that patients with clinical stroke do show cognitive impairment (1,8,13,15). We herein confirm that patients with silent infarct have cognitive impairment, compared with patients with normal MR imaging findings (Tables 2 and 5). However, we observed that patients who have completely normal MR imaging findings can still show significant cognitive impairment with respect to normative data (Tables 2 and 5), suggesting that MR imaging is not sensitive to some types of damage that can produce cognitive impairment. The degree of impairment is greater in children with low hematocrit (Table 3), and hematocrit alone is able to explain approximately 23% of the variance in full-scale intelligence quotient (Table 4). Furthermore, abnormal MR imaging and low hematocrit are independent predictors of full-scale intelligence quotient in patients with sickle cell disease (Table 5). These findings show that cognitive impairment is associated with focal brain damage (MR imaging abnormality), but they also show that cognitive impairment can be associated with diffuse damage that is not revealed by conventional MR imaging.
We report a high incidence of MR imaging abnormality among patients with hemoglobin SS sickle cell disease (Table 1). Our findings differ from those of the Cooperative Study of Sickle Cell Disease, a multi-institutional, longitudinal study of children with sickle cell disease. The Cooperative Study of Sickle Cell Disease found that approximately 26% of patients with hemoglobin SS sickle cell disease with no history of stroke had abnormal MR imaging findings (6,8), whereas we report that 43% of such patients had abnormal MR imaging findings. One reason why we report a higher incidence of imaging abnormality could be that MR imaging itself has improved; the first patients accrued to our study were enrolled in 1994, whereas the first patients accrued to the Cooperative Study of Sickle Cell Disease (8) were enrolled in 1989. Furthermore, some of the Cooperative Study of Sickle Cell Disease participant institutions used MR imaging units that had a field strength of only 0.6 or 1.0 T (6, 8), whereas all our imaging was performed at 1.5 T. Finally, we used 3-mm-thick sections in some of the patient examinations, whereas 5-mm-thick sections were used in the earlier studies (6, 8). The higher field strength and thinner sections we used would make leukoencephalopathy, for example, more apparent (Fig 1D). It seems likely that recent improvements in MR imaging methodology are sufficient to explain why we report a higher prevalence of lesions in our study.
A key question is whether patients with sickle cell disease show cognitive impairment specifically because of their disease. Perhaps the best way to address this question is to compare patients with their healthy siblings (Table 6). Normative data supplied with the Wechsler scales (5) do not constitute a fair comparison for our patients, because normative data are drawn from a control population selected to represent a broad range of income levels, races, and environments. When patients with sickle cell disease, who tend to be poor (11), urban, and black, are compared with normative persons, who are usually more affluent, suburban, and white, significant differences can arise for many reasons other than intelligence (2,3,19). Apparent intellectual deficits in children who live in inner cities can be associated with low socioeconomic status (20, 21) or cultural bias could exist in the test instruments (22). Furthermore, children of low socioeconomic status usually suffer more than do children of high socioeconomic status from problems that impair performance on cognitive tests, including inadequate prenatal medical care (23), prenatal drug or alcohol exposure (24), birth complications (25, 26), inadequate nutrition (27), iron-deficiency anemia (28), poor general health status (21), untreated attention deficit disorder (29), environmental exposure to lead (30), educational aridity (31), poor school attendance (32), homelessness (33), a deficient social environment (22), and the withering effects of racism (19). We think it is not possible to make a meaningful comparison between the published normative data and a sample of children who are chronically ill, impoverished, and educationally deficient. Therefore, the critical comparison is between patients and their healthy siblings. A compilation of recent data (Table 6) suggests that patients do show significant cognitive impairment when compared with the best available control group. Statistical analysis of these data shows that the full-scale intelligence quotient difference between patients and control participants is both robust and statistically significant (Table 6).
TABLE 6:
Mean full-scale intelligence quotient in children with sickle cell disease
| Test Instrument | Patient Score | No. | Control Score | No. | Reference |
|---|---|---|---|---|---|
| WISC-R | 88.8 | 126 | Armstrong et al., 1996 | ||
| 77.7 | 21 | 94.3 | 21 | Swift et al., 1989 |
|
| 82.7 | 43 | 88.0 | 30 | Wasserman et al., 1991 |
|
| 72.3 | 28 | 73.5 | 19 | Knight et al., 1995 | |
| 85.7 | 31 | 92.0 | 31 | Noll et al., 2001 | |
| 85.4 | 15 | Present study | |||
| WISC-III | 82.8 | 165 | Wang et al., 2001 | ||
| 83.9 | 156 | 90.3 | 76 | Bernaudin et al., 2000 |
|
| 81.8 | 41 | Brown et al., 2000 | |||
| 86.0 | 30 | 92.1 | 15 | Watkins et al., 1998 |
|
| 78.0 | 34 | Present study | |||
| Weighted mean | 83.6 | 89.1 |
Note.—Mean full-scale intelligence quotient for 690 patients with sickle cell disease, as compared with 192 control participants. Data are from testing of patients and age- and race-similar control participants with the WISC-R or WISC-III. The weighted mean is an average of data from all studies, weighted for the number of study participants in each of the studies. If a conservative assumption is made that the SD of patient and control scores is ± 15, as in Wechsler normative data, then the difference in weighted mean between patients and control participants is significant (P < .000003). To be even more conservative, we then excluded studies that did not include control data collected concurrent with patient data or that derived control data from non-siblings, and we assumed that the sample size of patients was equivalent to the sample size of control participants. This left four studies (underlined), with a sample size of 142 patients and 142 control participants; the full-scale intelligence quotient difference between patients and control participants was still significant (P < .00001).
The cause of cognitive impairment in patients with sickle cell disease is not well understood. It has been hypothesized that all patients with sickle cell disease with cognitive deficit have suffered an undiagnosed clinical stroke or a subclinical ischemic event (1). However, our results (Table 2) and the results of other recent research (2,8,13,18) suggest that cognitive impairment can be present in patients with sickle cell disease who have never suffered an ischemic event. In our study, a relatively high proportion of patients had abnormal MR imaging findings, suggesting that the ability of MR imaging to detect occult ischemic lesions may have increased. Nevertheless, we still found cognitive impairment in the group of children with normal MR imaging findings. The WISC-III full-scale intelligence quotient of children in our study who had normal MR imaging findings (Table 2) was significantly less than the weighted full-scale intelligence quotient of control participants (Table 6), even if we assume that the SD was ±15 in both groups (P < .005). Furthermore, both the present study and the Cooperative Study of Sickle Cell Disease (8) found that cognitive ability tends to decrease as patients age, which is not seen in healthy children and which is presumably a reflection of the cumulative effect of disease on the brain.
It has been clearly shown that lacunar infarction is associated with a decrease in full-scale intelligence quotient (8,13), so ischemic brain injury can cause cognitive impairment. With the present study and with a previous study (2), we have shown that cognitive impairment in cases of sickle cell disease is associated with reduced hematocrit, even in the absence of ischemic damage. We herein report that MR imaging and hematocrit are independent predictors of full-scale intelligence quotient (Table 5), showing that cognitive impairment is multifactorial. That MR imaging findings were unable to identify all patients with cognitive impairment (Table 5) suggests that cognitive impairment may be associated with diffuse as well as focal brain injury (2). We also showed that there can be diffuse abnormality, specifically in the gray matter, and that such diffuse abnormality is present in a substantial fraction of children with normal MR imaging findings (2,7). If diffuse damage is responsible for cognitive impairment in some patients with sickle cell disease, this may explain why our study and several other studies report relatively poor cognitive ability among patients who have completely normal MR imaging findings (8,9,15). Some studies have reported no significant difference in cognitive ability between patients with normal MR imaging findings and those with abnormal MR imaging findings (15,35), suggesting that diffuse damage may be more important than focal damage in producing cognitive impairment.
Low hematocrit is a significant predictor of cognitive impairment in children with sickle cell disease (Tables 3–5), which suggests that cognitive impairment is associated with chronic hypoxia (2). Previously, we reported selective damage to gray matter in patients with sickle cell disease (7); because gray matter has a substantially higher metabolic rate that does white matter, the selectivity of damage to gray matter is consistent with the hypothesis that cognitive impairment is associated with chronic hypoxia (2,7). Furthermore, in young patients with sickle cell disease, the brain can show structural adaptations to chronic hypoxia (36). The mean basilar artery volume was 74% greater in patients with sickle cell disease than in control participants (P < .001), and basilar volume was inversely correlated with hematocrit (36). Thus, an increase in the volume of blood flow to the brain could be an adaptive response to anemia (36). Finally, there is an inverse relationship between basilar volume and full-scale intelligence quotient (r = −0.62, P < .005) in patients with sickle cell disease, such that patients with ectasia are more likely to show cognitive impairment (36). Assessed together, these results indicate that it is time to consider clinical trials of interventions to increase hematocrit, with the goal of preserving cognitive function.
Acknowledgments
We thank Rhonda Simmons and Leslie Music, who reviewed and collated the neuropsychometric data reported herein. We are grateful to our MR imaging technologists, Mary Fitchpatric, Mary Freeman, Crystal Manchester, and Mark Summers, who were responsible for the imaging.
Footnotes
This work was made possible by support from the National Heart, Lung, and Blood Institute (RO1 HL60022 to R.G.S.) and the American-Lebanese-Syrian Associated Charities.
References
- 1.DeBaun MR, Schatz J, Siegel MJ, et al. Cognitive screening examinations for silent cerebral infarctions in sickle cell disease. Neurology 1998;50:1678–1682 [DOI] [PubMed] [Google Scholar]
- 2.Steen RG, Xiong X, Mulhern RK, Langston JW, Wang WC. Subtle brain abnormalities in children with sickle cell disease: relationship to blood hematocrit. Ann Neurol 1999;45:279–286 [DOI] [PubMed] [Google Scholar]
- 3.Brown RT, Buchannan I, Doepke K, et al. Cognitive and academic functioning in children with sickle cell disease. J Clin Child Psychol 1993;22:207–218 [Google Scholar]
- 4.Steen RG, Xiong X, Mulhern RK, Langston JW, Wang WC. Reply to Letter to the Editor. Ann Neurol 2000;47:280 [Google Scholar]
- 5.Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. San Antonio: The Psychological Corp.; 1991
- 6.Moser FG, Miller ST, Bello JA, et al. The spectrum of brain MR abnormalities in sickle-cell disease: a report from the Cooperative Study of Sickle Cell Disease. AJNR Am J Neuroradiol 1996;17:965–972 [PMC free article] [PubMed] [Google Scholar]
- 7.Steen RG, Langston JW, Ogg RJ, Xiong X, Ye Z, Wang WC. Diffuse T1 reduction in gray matter of sickle cell disease patients: evidence of selective vulnerability to damage? Mag Reson Imag 1999;17:503–515 [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Enos L, Gallagher D, et al. Neuropsychometric performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr 2001;139:391–397 [DOI] [PubMed] [Google Scholar]
- 9.Armstrong FD, Thompson RJ, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics 1996;97:864–870 [PubMed] [Google Scholar]
- 10.Swift AV, Cohen MJ, Hynd GW, et al. Neuropsychologic impairment in children with sickle cell anemia. Pediatrics 1989;84:1077–1085 [PubMed] [Google Scholar]
- 11.Wasserman AL, Wilimas JA, Fairclough DL, Mulhern RK, Wang W. Subtle neuropsychological deficits in children with sickle cell disease. Am J Pediatr Hematol Oncol 1991;13:14–20 [DOI] [PubMed] [Google Scholar]
- 12.Knight S, Singhal A, Thomas P, Serjeant G. Factors associated with lowered intelligence in homozygous sickle cell disease. Arch Dis Child 1995;73:316–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernaudin F, Verlhac S, Freard F, et al. Multicenter prospective study of children with sickle cell disease: radiographic and psychometric correlation. J Child Neurol 2000;15:333–343 [DOI] [PubMed] [Google Scholar]
- 14.Watkins KE, Hewes DK, Connelly A, et al. Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Dev Med Child Neurol 1998;40:536–543 [DOI] [PubMed] [Google Scholar]
- 15.Brown RT, Davis PC, Lambert R, Hsu L, Hopkins K, Eckman J. Neurocognitive functioning and magnetic resonance imaging in children with sickle cell disease. J Pediatr Psychol 2000;25:503–513 [DOI] [PubMed] [Google Scholar]
- 16.Fowler MG, Whitt JK, Lallinger RR, et al. Neuropsychologic and academic functioning of children with sickle cell anemia. J Dev Behav Pediatr 1988;9:213–220 [PubMed] [Google Scholar]
- 17.Noll RB, Stith L, Gartstein MA, et al. Neuropsychological functioning of youths with sickle cell disease: comparison with non-chronically ill peers. J Pediatr Psychol 2001;26:69–78 [DOI] [PubMed] [Google Scholar]
- 18.Powars DR, Conti PS, Wong W-Y, et al. Cerebral vasculopathy in sickle cell anemia: diagnostic contribution of positron emission tomography. Blood 1999;93:71–79 [PubMed] [Google Scholar]
- 19.Sattler JM. Assessment of ethnic minority children. In: Assessment of Children. 3rd ed. San Diego: Jerome M. Sattler, Publisher, Inc.;1992. :563–596
- 20.Kaufman AS, Doppelt JE. Analysis of WISC-R standardization data in terms of the stratification variables. Child Dev 1976;47:165–171 [Google Scholar]
- 21.Kramer RA, Allen L, Gergen PJ. Health and social characteristics and children’s cognitive functioning: results from a national cohort. Am J Pub Health 1995;85:312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki LA, Valencia RR. Race-ethnicity and measured intelligence: educational implications. Am Psychol 1997;52:1103–1114 [Google Scholar]
- 23.Naeye RL, Peters EC. Antenatal hypoxia and low IQ values. Am J Dis Child 1987;141:50–54 [DOI] [PubMed] [Google Scholar]
- 24.Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 1997;21:150–161 [PubMed] [Google Scholar]
- 25.Wallace IF, Rose SA, McCarton CM, Kurtzberg D, Vaughan HG. Relations between infant neurobehavioral performance and cognitive outcome in very low birthweight preterm infants. J Dev Behav Pediatr 1995;16:309–317 [PubMed] [Google Scholar]
- 26.Nield TA, Langenbacher D, Poulsen MK, Platzker AC. Neurodevelopmental outcome at 3.5 years of age in children treated with extracorporeal life support: relationship to primary diagnosis. J Pediatr 2000;136:338–344 [DOI] [PubMed] [Google Scholar]
- 27.Gorman KS. Malnutrition and cognitive development: evidence from experimental/quasi-experimental studies among the mild-to-moderately malnourished. J Nutr 1995;125:2239S–2244S [DOI] [PubMed] [Google Scholar]
- 28.Pollitt E. Iron deficiency and cognitive function. Annu Rev Nutr 1993;13:521–537 [DOI] [PubMed] [Google Scholar]
- 29.Detterman DK, Thompson LA. What is so special about special education? Am Psychol 1997;52:1082–1090 [DOI] [PubMed] [Google Scholar]
- 30.Needleman HL, Shell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood: an 11-year follow-up report. N Engl J Med 1990;311:83–88 [DOI] [PubMed] [Google Scholar]
- 31.Brody N. Intelligence, schooling, and society. Am Psychol 1997;52:1046–1050 [Google Scholar]
- 32.Ceci SJ, Williams WM. Schooling, intelligence, and income. Am Psychol 1997;52:1051–1058 [Google Scholar]
- 33.Rubin DH, Erickson CJ, San Agustin M, Cleary SD, Allen JK, Cohen P. Cognitive and academic functioning of homeless children compared with housed children. Pediatrics 1996;97:289–294 [PubMed] [Google Scholar]
- 34.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998;91:288–294 [PubMed] [Google Scholar]
- 35.Kugler S, Anderson B, Cross D, et al. Abnormal cranial magnetic resonance imaging scans in sickle-cell disease: neurological correlates and clinical implications. Arch Neurol 1993;50:629–635 [DOI] [PubMed] [Google Scholar]
- 36.Steen RG, Langston JW, Ogg RJ, Manci E, Mulhern RK, Wang W. Ectasia of the basilar artery in children with sickle cell disease: relationship to hematocrit and psychometric measures. J Stroke Cerebrovasc Dis 1998;7:32–43 [DOI] [PubMed] [Google Scholar]