Abstract
BACKGROUND AND PURPOSE: Correctly diagnosing metastatic nodes is important for the follow-up of patients with clinical N0 stage neck disease and oral cancer. A combination of helical CT and Doppler sonography may facilitate the accurate detection of lymph node metastasis in patients with clinical N0 stage neck disease.
METHODS: A combination of contrast-enhanced helical CT and Doppler sonography was performed to monitor the necks of 58 patients with initial clinical N0 stage neck disease. Of these patients, 17 underwent surgery; nodal metastasis in the neck was histopathologically confirmed. A node was diagnosed as metastatic if it fulfilled the CT criteria for metastatic nodes (short-axis diameter equal to or greater than the cutoff points for each level of the neck or central nodal necrosis) and if, additionally, it did not exhibit sonographic features for nonmetastatic nodes (normal hilar echogenicity and hilar flows). The presence of metastasis was confirmed histopathologically.
RESULTS: During the follow-up periods, metastatic nodes were histologically confirmed in 17 (29%) patients. Of 30 metastatic nodes from the 17 patients with metastatic nodes, 22 (73%) appeared within the first year and 28 (93%) within the first 2 years; 20 developed from nonmetastatic nodes, and 10 were newly detectable. The combined criteria were effective in revealing 26 (87%) nodes, yielding 87% sensitivity, 100% specificity, and 100% positive and 99% negative predictive values. The independent use of one of these techniques alone resulted in low (67%) or moderate (87%) positive predictive values for sonography and CT, respectively. Seven hundred forty-one (97%) of 761 nodes that were nonmetastatic at initial examination remained nonmetastatic (737 nodes) or had disappeared (four nodes). As a result, a combination of CT and sonography was effective in revealing all 17 cases of metastatic nodes.
CONCLUSION: A combination of contrast-enhanced helical CT and Doppler sonography is useful for the follow-up study of clinical N0 stage neck disease.
Accurate detection of metastatic nodes during early stages of the disease in patients with head and neck cancer is critical for case management strategy and for prognosis (1). In particular, management of the clinically negative neck (clinical N0 stage neck disease) has been one of the major controversial issues regarding head and neck cancer, and the assessment of clinical N0 stage neck disease by different radiologic techniques has been widely studied. If a patient with head and neck cancer is to be treated by neck dissection or radiation therapy on the basis of some radiologic technique with 60% accuracy for the detection of metastatic nodes, this means that he or she is undergoing possibly unnecessary neck dissection or radiation therapy at the risk of 40% misdiagnosis. Clinical N0 stage neck disease may be treated by some type of selective neck dissection or radiation therapy; however, both prophylactic radiation therapy and elective neck dissection in cases of clinical N0 stage neck disease are subject to debate (2, 3).
CT and MR imaging have been widely used to diagnose cervical metastatic nodes in patients with head and neck cancer (1, 4). The diagnostic ability of these techniques was reported to be moderate (1, 5, 6). For example, when a minimal axial diameter of 10 mm was used for a size criterion, CT depicted metastatic nodes with 42% sensitivity and 99% specificity for assessment per node and 89% sensitivity and 73% specificity for assessment per neck (5). Receiver operating characteristic analysis also showed that varying combinations of size and internal architectural criteria by CT yielded Az values (which express the diagnostic performance and are determined by calculating the area under each observer-specific receiver operating characteristic curve) of 0.80 to 0.87, associated with positive and negative predictive values of 0.83 and 0.50, respectively. MR imaging was reported to perform less efficiently than CT (5). To facilitate accurate diagnosis of metastatic nodes in association with clinical N0 stage neck disease, different technologies other than CT and MR imaging have been applied to this field. One of the most promising techniques may be sonography, which performed comparably with CT, mainly because of its greater potential to detect the intranodal architectural changes (6–8). Moreover, sonography-guided fine needle aspiration cytology was reported to decrease the risk of missing occult metastasis in patients with clinical N0 stage neck disease (9, 10). We evaluated the combination of helical CT and Doppler sonography for monitoring patients with clinical N0 stage neck disease and oral cancer during follow-up of the neck after treatment of the primary tumor.
Methods
Patients
To determine optimal size and Doppler sonographic criteria for metastatic cervical node, we first retrospectively studied 191 histopathologically proved metastatic and 1343 histopathologically proved nonmetastatic cervical nodes from 111 patients (37 women and 74 men) with oral squamous cell cancer. Of these patients, 70 were positive for metastatic cervical nodes. The primary cancers of the patient cohort included oral floor (n=22), tongue (n=36), palate (n=3), lower gingival (n=23), upper gingival (n=16), and buccal (n=11) squamous cell carcinomas.
Next, we prospectively studied a separate cohort of 58 patients (21 women and 37 men) with oral squamous cell carcinoma. The primary cancers of these patients included oral floor (n=17), tongue (n=13), palate (n=9), lower gingival (n=8), upper gingival (n=8), and buccal (n=3) squamous cell carcinomas. These patients were negative for cervical lymph node metastasis as assessed by CT and Doppler sonography at the initial examinations, so they were treated by excision of the primary tumors and chemotherapy. These patients were followed up by using helical CT and Doppler sonography. The time span between the two consecutive examinations in each of the patients ranged from 1 to 6 months; during the first year of the follow-up period, the span was approximately 1 or 2 months. After the first year of the follow-up period, it ranged from 3 to 6 months. However, the time span between the two consecutive examinations was dependent on the clinical status of the patient. For example, if a node(s) was suggestive of metastasis on palpation, CT and sonographic examination was performed as soon as possible. When metastasis to the cervical lymph nodes in these patients was depicted by CT and sonographic examinations, neck dissections were performed and then combined chemo- and radiation therapy was administered. Excised nodes were correlated to those on CT scans and sonograms and were histologically examined for metastasis.
In the present study, we extended the definition of clinical N0 stage neck disease so that it referred to that which is negative for cervical lymph node metastasis as assessed by CT and Doppler sonography at examination just after surgical excision of the primary tumor. The decision of clinical N0 stage neck disease was made by a single radiologist, and the follow-up study was performed by four radiologists other than the aforementioned one.
Of the 58 patients in the prospective study, 17 had become positive for nodal metastasis in their necks. We continued follow-up of the remaining 41 patients for at least 2 years.
Helical CT
The patients underwent scanning with a HiSpeed Advantage SG CT system (General Electric Medical Systems, Milwaukee, WI). The scanning orientation was parallel to the Frankfort horizontal line, which includes the inferior margin of the orbit and superior margin of the external auditory meatus. Scanning was performed with a collimation of 3 mm, a pitch of 1:1, a 512 × 512 matrix, a display field view of 23 cm, 120 kVp, and 200 mA. CT examination was performed after the administration of an IV bolus injection of approximately 100 mL (2 mL/kg body weight) iopamidol (Iopamiron 300; Schering, Berlin, Germany) at a rate of 1.0 mL/s. We obtained reformatted axial images of 3-mm thickness from these data.
All metastatic and nonmetastatic nodes were classified according to the criteria proposed by Som et al (11). Briefly, level I included all nodes above the hyoid bone, below the mylohyoid muscle, and anterior to the posterior edge of the submandibular gland. The upper and lower limits of level II were the skull base and the lower body of the hyoid bone, respectively. Level III nodes lay between the level of the lower body of the hyoid bone and the level of the lower margin of the cricoid cartilage arch. Level IV nodes were located between the lower margin of the cricoid cartilage arch and the level of the clavicle. Level V nodes lay between the skull base and the clavicle and also the posterior border of the sternocleidomastoid muscle. Level VI nodes lay inferior to the lower body of the hyoid bone, superior to the top of the sternum, and between the medial margin of the left and right common carotid arteries or the internal carotid arteries. We used this image-based nodal classification, because this method facilitated identification of the nodes during the follow-up study.
In this study, we used varying cutoff points of nodal short-axis diameter as size criteria for metastatic nodes in each level of the neck (Table 1). As aforementioned, we obtained these cutoff points from a separate cohort of 111 patients with oral cancer. Varying cutoff points for short-axis diameter in level I of the neck yielded sensitivity, specificity, and accuracy, respectively, of 90%, 76%, and 83% (7 mm), 82%, 90%, and 86% (8 mm), and 70%, 90%, and 86% (9 mm); in level II, 92%, 89%, and 91% (8 mm), 90%, 95%, and 92% (9 mm), and 82%, 98%, and 90% (10 mm); in level III, 100%, 91%, and 96% (6 mm), 79%, 94%, and 87% (7 mm), and 63%, 97%, and 80% (8 mm); in level IV or lower levels, 100%, 91%, and 96% (6 mm), 100%, 97%, and 99% (7 mm), and 88%, 99%, and 94% (8 mm). Thus, the best cutoff points for the short-axis diameter of the node in levels I through IV or lower were 8, 9, 6, and 7 mm, respectively. These values were 1 to 2 mm longer than those reported by van den Brekel et al (12). The other size criteria tested, such as long-axis diameter and volume of the node, did not perform well, compared with the short-axis diameter. The use of central nodal necrosis as an additional criterion to short-axis diameter improved the sensitivity. Short-axis diameters were determined from a single axial CT scan of a lymph node, perpendicular to the long axis of the node. The short-axis diameter recorded was the longest of the diameters that crossed perpendicular to the long axis of the node. We also classified a node as metastatic, irrespective of its nodal size, when it exhibited central nodal necrosis on CT scans.
TABLE 1:
Combined criteria of CT and sonography for metastatic cervical nodes
| CT criteria | |
| Size criteria | |
| Level of the neck | Short-axis diameter cut-off point |
| I | ≥8 mm |
| II | ≥9 mm |
| III | ≥6 mm |
| IV | ≥7 mm |
| Internal architecture | |
| Central nodal necrosis | |
| Sonographic criteria | |
| Internal architecture | |
| No hilar echo or hilar blood flow | |
| A node is metastatic if it fulfills the CT criteria (size or internal architecture or both) plus the sonographic criteria | |
Sonography
Gray scale sonography and power Doppler sonography were performed by using a Logiq 700 unit (General Electric Yokogawa Medical Systems, Tokyo, Japan) equipped with a wide bandwidth (range, 6–13 MHz) transducer. Gray scale sonography was performed at 10 MHz. Power Doppler sonography was performed at 8 MHz, and standard Doppler settings were chosen for optimal detection of the signals from the lymph node vessels, which had low velocity flow. Common settings of pulse repetition frequency (500 Hz) and wall filter (75 or 62 Hz) were used.
The sonographic criteria for nonmetastatic nodes used in this study were the presence of normal hilar echoes and the presence of normal hilar blood flow (Table 1) (7). The hilum is identified as a highly echogenic structure in the central part of the node. Metastatic tumor cells frequently invade into this part of the node, replacing the echogenicity of the normal hilum. Multivariate feature analysis showed that the presence of hilar echoes and the presence of normal hilar flow were the sonographic features that were predictive of nonmetastatic lymph nodes (13).
Diagnosis of the Node
A node was diagnosed as metastatic when it fulfilled the CT criteria for metastatic nodes (short-axis diameter equal to or greater than the cutoff points for each level of the neck or the presence of central nodal necrosis) and, additionally, it did not exhibit Doppler sonographic features for nonmetastatic nodes (the presence of normal hilar echogenicity and hilar flows) (Table 1). Thus, the nonmetastatic nodes indicate those having either or neither of the CT and sonographic criteria. All images were read by two radiologists (M.S., K.Y.) in consensus. We also compared sizes and Doppler sonographic features of nodes between pre- and post-treatment studies. No interval change was observed in any patient.
Correlation of Dissected Lymph Nodes to CT Scans and Sonograms
Topographical correlation between dissected nodes and CT scans or sonograms was performed by node was performed by using a reporting system as previously described (7). The report includes data concerning the approximate location relative to the surrounding anatomic structures, such as vessels and muscles, and the sizes of the enlarged nodes as shown on CT scans and sonograms. At surgery, the lymph nodes were excised en bloc along with the adjacent reference structures to more easily ascertain the spatial relationship between the excised nodes and surrounding structures, such as muscles, salivary glands, and veins. The size of the node was also used to compare with the results of CT and sonography. Surgeons and at least one of the radiologists who performed CT and sonographic examinations together compared the excised nodes with the CT scans and sonograms. Final decisions were reached by consensus among them. The excised nodes that matched those on CT scans and sonograms were then examined histopathologically. These data were associated with a map illustrating metastatic or nonmetastatic nodes evaluated by CT and sonography. These procedures enabled the surgeons to correlate the dissected nodes to the nodes evaluated by CT and sonography.
Data Analysis
We calculated sensitivity (the number of metastatic nodes at imaging and histology and the number of metastatic nodes at histology) and specificity (the number of nonmetastatic nodes at imaging and histology and the number of nonmetastatic nodes at histology). Positive predictive value (the number of metastatic nodes at imaging and histology and the number of metastatic nodes at imaging) and negative predictive value (the number of nonmetastatic nodes at imaging and histology and the number of nonmetastatic nodes at imaging) were also calculated.
Results
Two hundred fifty-five nodes were obtained from the 17 patients who underwent surgery. All 17 patients were positive for metastatic nodes, and 30 metastatic nodes were histopathologically confirmed (Table 2). Metastases occurred in six patients with oral floor cancer, three with tongue cancer, one with palate cancer, five with lower gingival cancer, one with upper gingival cancer, and one with buccal mucosa cancer. Twenty-two (73%) of 30 metastatic nodes appeared within the first year after negative results were obtained at initial examination, and 28 (93%) metastatic nodes were diagnosed within the first 2 years. We also obtained 225 histopathologically proved nonmetastatic nodes from the 17 patients who underwent surgery (Table 2). An additional 516 nodes, which were categorized as nonmetastatic on the basis of CT and sonography findings, were included in the present study. Of the 58 patients, 41 remained negative for metastatic cervical nodes as judged on the basis of CT and sonography findings.
TABLE 2:
Performance of combined criteria of helical CT and Doppler sonography for 17 patients with recurrent cancer in the cervical lymph nodes
| Lymph nodes (n=255) | |||
| No. of metastasis | 30 | ||
| No. of reactive nodes | 225 | ||
| Combination | CT | Sonography | |
| No. of false positive nodes | 0 | 5 | 13 |
| No. of false negative nodes | 2 | 3 | 2 |
| Sensitivity (%) | 87 | 83 | 87 |
| Specificity (%) | 100 | 97 | 94 |
| Positive predictive value (%) | 100 | 87 | 67 |
| Negative predictive value (%) | 99 | 99 | 99 |
A combination of helical CT and Doppler sonography correctly depicted 26 (87%) of the 30 metastatic nodes. The combined criteria yielded 87% sensitivity, 100% specificity, and 100% positive and 99% negative predictive values (Table 2). The single use of CT or sonography provided comparable sensitivities and negative predictive values; however, the single use of either of these two techniques provided lower (87% for CT and 67% for sonography) positive predictive values, compared with their combination. Of the 26 metastatic nodes, 18 were confirmed in nodes that were diagnosed as nonmetastatic at the initial examinations that produced negative results and then diagnosed as metastatic on the basis of helical CT and Doppler sonography findings after varying follow-up periods (Fig 1). Eight metastatic nodes were identified on the basis of CT and Doppler sonography criteria during varying follow-up periods. CT and Doppler sonography did not correctly depict four (13%) metastatic nodes, two of which were not detected by this combined technique and two that were detected but did not fulfill the diagnostic criteria for metastatic nodes.
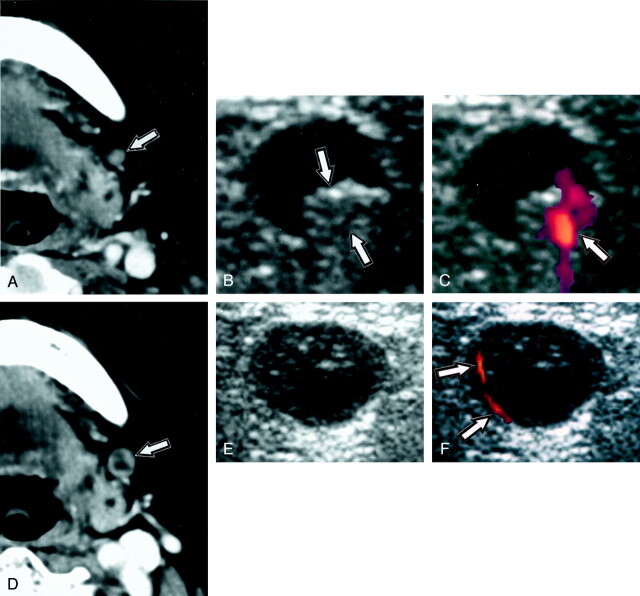
Fig 1.
Images from the case of an 83-year-old woman with upper gingival cancer.
A, Contrast-enhanced axial CT scan shows reactive node (arrow; short-axis diameter, 4 mm) in level I.
B, Gray scale sonogram of the same node as that shown in A shows reactive node exhibiting normal hilar echogenicity (arrows).
C, Doppler sonogram shows blood flow signals (arrow) overlapping hilar echogenicity.
D, Contrast-enhanced axial CT scan, obtained 10 months after the examinations that produced negative results (shown in A–C), shows metastatic node (arrow; short-axis diameter, 8 mm) exhibiting central nodal necrosis.
E, Gray scale sonogram of the same node as that shown in D shows metastatic node without hilar echogenicity.
F, Doppler sonogram shows abnormal blood flow signals in periphery (arrows). No hilar blood flow signal intensity is seen.
The surgical specimens contained two metastatic nodes that were not detectable by CT or Doppler sonography during the follow-up period. One of these nodes exhibited a short-axis diameter of 3 mm and was located in level I of a patient with upper gingival cancer. The other node was 4 mm in short-axis diameter and was located in level I of a patient with buccal mucosa cancer. In these patients, however, another metastatic node was depicted correctly by CT and Doppler sonography in the same neck levels. In a patient with oral floor cancer, two metastatic nodes were judged negative on the basis of CT size criteria. These nodes were 5 and 6 mm in short-axis diameter and were located in levels II and III, respectively. The same patient had another metastatic node with central nodal necrosis in level I.
A total of 761 nonmetastatic nodes were detected in the 58 patients at the initial examinations that produced negative results. Of these, 71 nodes were larger than the corresponding short-axis diameter cutoff points, but they displayed sonographic features characteristic of nonmetastatic nodes (hilar echogenicity or hilar flows or both). Seven hundred forty-one nonmetastatic nodes that were depicted at the initial examinations, including the 71 nodes, remained nonmetastatic (737 nodes) or became undetectable (four nodes) during the follow-up periods. At surgery, one metastatic node with a short-axis diameter of 3 mm was found in level I of a patient with lower gingival cancer, where a metastatic node with central nodal necrosis and a short-axis diameter of 8 mm was also present in the same neck level. As a result, a combination of helical CT and Doppler sonography correctly depicted all 17 patients with metastatic adenopathy.
Discussion
We herein report that by using the size (short-axis diameter) and architectural (central nodal necrosis, nodal hilum, and hilar blood flows) criteria, the combination of helical CT and Doppler sonography accurately depicted the recurrence of cancer in the neck in a cohort of 58 patients with clinical N0 stage neck disease at initial examination. The combination of CT and Doppler sonography depicted metastatic nodes with an 87% sensitivity, 100% specificity, 100% positive and 99% negative predictive values in 17 patients who underwent surgery. These criteria were effective in identifying all 17 patients with histologically proved metastatic nodes.
Accuracy of sonography in diagnosing metastatic nodes in patients with head and neck cancer has been evidenced in recent studies (6–8, 14). Doppler sonography should be an adjunctive tool to CT for improved performance in diagnosing metastatic nodes in patients with head and neck cancer. The high (≥99%) negative predictive values of the single use of helical CT or Doppler sonography for metastatic cervical nodes can be used to advantage in the approach to patients with clinical N0 stage neck disease and oral cancer, because invasive procedures are probably not necessary in a patient with negative findings shown by either of these imaging techniques. However, our findings that the positive predictive values were relatively low (87% for CT and 67% for sonography) mean that patients with positive imaging findings will need to undergo fine-needle aspiration cytology (15). In contrast, the combination of CT and sonography resulted in high positive and negative predictive values (Table 2). In particular, our study population included 71 nonmetastatic nodes that had short-axis diameters greater than the threshold but displayed normal hila or hilar blood flows, or both, on Doppler sonograms, suggesting that the nodes were nonmetastatic. We did not examine histopathologically whether these nodes contained micrometastasis; however, the finding that these nodes did not enlarge during the 2-year follow-up period suggests that they were nonmetastatic.
Helical CT has an excellent ability to detect metastatic nodes in the necks of patients with head and neck cancer (16). However, the study population in the present study included substantial numbers of patients with relatively large lymph nodes, as evidenced by a high percentage of nodes with central necrosis. The average short-axis diameter of metastatic nodes was 11 mm. Of 15 metastatic nodes with central nodal necrosis, five (35%) were smaller than 10 mm in short-axis diameter. Thus, a substantial number of the metastatic nodes that appeared after varying lengths of follow-up periods in patients with clinical N0 stage neck disease were >10 mm in short-axis diameter and central nodal necrosis was detected in a number of nodes with short-axis diameter <10 mm. These findings were consistent with those of a previous study (17) in which central nodal necrosis occurred in 33% of nodes <10 mm in diameter.
We misdiagnosed four metastatic nodes. Of these, two undetected nodes were 3 and 4 mm in short-axis diameter on histologic specimens and were in the close vicinity of relatively large metastatic nodes; one metastatic node was 10 mm in short-axis diameter with central nodal necrosis, and the other metastatic node was 12 mm in short-axis diameter. It is likely that the presence of large metastatic nodes almost attaching to small ones led to the misinterpretation that these neighboring nodes were from a single metastatic node when viewed on sonograms. CT scan reconstruction in different planes may be beneficial for better detection of such small nodes located close to a larger, metastatic or nonmetastatic node(s) (16). The sizes of incorrectly diagnosed metastatic nodes were marginal to or smaller than the best cutoff points raised for the nodes in the corresponding levels of the neck. van den Brekel et al (9) appraised sonography-guided fine-needle aspiration biopsy as a follow-up technology for early detection of recurrence in the neck. A more recent study by Takes et al (10) showed that CT and sonography-guided fine-needle aspiration cytology were comparably predictive for metastasis in clinically negative necks. This sonography-aided technique is reliable as a diagnostic tool for metastatic nodes and may contribute to improving the diagnostic accuracy of the combined use of helical CT and Doppler sonography. van den Brekel et al (9) showed that the risk of missing occult metastasis with sonography-guided fine-needle aspiration cytology was lower than that expected after palpation only. We found in the present study that a combination of CT and sonography improved diagnostic performance over sonography alone. Therefore, it is expected that a combination of sonography and CT could depict metastatic nodes at an earlier stage than does palpation.
Surgeons and four radiologists who performed CT and sonography together compared the excised nodes. Although effort was taken to avoid bias or variation based on an observer, it is not completely denied that such a methodology may cause significant flaws in the present study. Another limitation in our study may be that there is obviously a bias considering that patients who undergo neck dissection on the basis of CT criteria are apt to have positive nodes. Similarly, smaller metastatic nodes will not be detected, because they do not fulfill CT criteria, and patients who undergo neck dissection on the basis of CT and sonography findings may harbor micrometastasis (18) that are not detected histopathologically. These limitations in our approach will alter the sensitivity and specificity data presented in the present study.
2-[18F]fluoro-2-deoxy-𝒹-glucose (FDG) positron emission tomography (PET) could be a functional imaging technique for the detection of metastatic nodes in the neck (19). Malignant nodes have higher glucose utilization than do normal nodes. Accordingly, FDG-PET might detect metastatic nodes that are negative with size criteria (20). However, metastatic nodes that contain large necrotic area are negative according to FDG-PET criteria because of the low glycolytic activity of the necrotic material. Further studies are necessary to facilitate clinical application of FDG-PET for imaging of metastatic cervical nodes.
In the present study, we found that recurrent cancer in the cervical node appeared as early as 14 days from a negative examination. Nodal metastases occurred within 1 year from the negative examinations in 71% (12 of 17 patients) and 94% (16 of 17 patients) within 2 years. These findings are consistent with those of a preceding report presented by van den Brekel et al (9), who reported that 14 patients with head and neck cancer displayed a similar profile of the time span between the negative results of fine-needle aspiration cytology and the tumor recurrence in the neck. Therefore, the first year of the follow-up period is critical for the recurrence of oral cancers in the neck, and the risk of the recurrence is very low beyond the second year of follow-up.
Conclusion
We showed that a combination of contrast-enhanced helical CT and Doppler sonography accurately depicted metastatic nodal recurrence in patients with clinical N0 stage neck disease and oral cancer. The combination yielded improved results compared with the single use of these techniques. The lymph node metastasis is a critical prognostic factor for the survival of patients with head and neck cancer, and to accurately diagnose cancer recurrence in the node is very important. The present study suggests that the combination of helical CT and Doppler sonography is very useful for routine examinations during follow-up of patients with clinical N0 stage neck disease and oral cancer. Ideally, the follow-up study should be at 1- to 2-month intervals for the first year and 3- to 6-month intervals for the ensuing 4 years. However, such intervals would heavily burden both patients and diagnostic equipment. Therefore, the intervals could be longer (eg, every 3 months for the first year of follow-up and every 6 months for the ensuing 4 years).
References
- 1.Anzai Y, Brunberg JA, Lufkin RB. Imaging of nodal metastases in the head and neck. J Magn Reson Imaging 1997;7:774–783 [DOI] [PubMed] [Google Scholar]
- 2.Paulino AC, Fisher SG, Marks JE. Is prophylactic neck irradiation indicated in patients with squamous cell carcinoma of the maxillary sinus? Int J Radiat Oncol Biol Phys 1997;39:283–289 [DOI] [PubMed] [Google Scholar]
- 3.Hicks WL Jr, North JH Jr, Loree TR, et al. Surgery as a single modality therapy for squamous cell carcinoma of the oral tongue. Am J Otolaryngol 1998;19:24–28 [DOI] [PubMed] [Google Scholar]
- 4.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol 1992;158:961–969 [DOI] [PubMed] [Google Scholar]
- 5.Curtin HD, Ishwaran H, Mancuso AA, Dalley RW, Caudry DJ, McNeil BJ. Comparison of CT and MR imaging in staging of neck metastases. Radiology 1998;207:123–130 [DOI] [PubMed] [Google Scholar]
- 6.Sumi M, Ohki M, Nakamura T. Comparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. AJR Am J Roentgenol 2000;176:1019–1024 [DOI] [PubMed] [Google Scholar]
- 7.Ariji Y, Kimura Y, Hayashi N, et al. Power Doppler sonography of cervical lymph nodes in patients with head and neck cancer. AJNR Am J Neuroradiol 1998;19:303–307 [PMC free article] [PubMed] [Google Scholar]
- 8.Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology 1992;183:215–220 [DOI] [PubMed] [Google Scholar]
- 9.van den Brekel MW, Reitsma LC, Quak JJ, et al. Sonographically guided aspiration cytology of neck nodes for selection of treatment and follow-up in patients with N0 head and neck cancer. AJNR Am J Neuroradiol 1999;20:1727–1731 [PMC free article] [PubMed] [Google Scholar]
- 10.Takes RP, Righi P, Meeuwis CA, et al. The value of ultrasound with ultrasound-guided fine-needle aspiration biopsy compared to computed tomography in the detection of regional metastases in the clinically negative neck. Int J Radiat Oncol Biol Phys 1998;40:1027–1032 [DOI] [PubMed] [Google Scholar]
- 11.Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol 2000174:837–844 [DOI] [PubMed] [Google Scholar]
- 12.van den Brekel MW, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: how reliable is it? AJNR Am J Neuroradiol 1998;19:695–700 [PMC free article] [PubMed] [Google Scholar]
- 13.Chikui T, Yonetsu K, Nakamura T. Multivariate analysis of sonographic findings of metastatic cervical lymph nodes: contribution of blood flow features revealed by power Doppler sonography for predicting metastasis. AJNR Am J Neuroradiol 2000;21:561–567 [PMC free article] [PubMed] [Google Scholar]
- 14.Tschammler A, Ott G, Schang T, Seelbach-Goebel B, Schwager K, Hahn D. Lymphadenopathy: differentiation of benign from malignant disease: color Doppler US assessment of intranodal angioarchitecture. Radiology 1998;208:117–123 [DOI] [PubMed] [Google Scholar]
- 15.van den Brekel MW, Castelijns JA, Stel HV, et al. Occult metastatic neck disease: detection with US and US-guided fine-needle aspiration cytology. Radiology 1992;180:457–461 [DOI] [PubMed] [Google Scholar]
- 16.Steinkamp HJ, Hosten N, Richter C, Schedel H, Felix R. Enlarged cervical lymph nodes at helical CT. Radiology 1994;191:795–798 [DOI] [PubMed] [Google Scholar]
- 17.Friedman M, Roberts N, Kirschenbaum GL, Colombo J. Nodal size of metastatic squamous cell carcinoma of the neck. Laryngoscope 1993;103:854–856 [DOI] [PubMed] [Google Scholar]
- 18.van den Brekel MW, van der Waal I, Meijer CJ, Freeman JL, Castelijns JA, Snow GB. The incidence of micrometastases in neck dissection specimens obtained from elective neck dissections. Laryngoscope 1996;106:987–991 [DOI] [PubMed] [Google Scholar]
- 19.Strauss LG. Sensitivity and specificity of positron emission tomography (PET) for the diagnosis of lymph node metastases. Recent Results Cancer Res 2000;157:12–19 [DOI] [PubMed] [Google Scholar]
- 20.Jabour BA, Choi Y, Hoh CK, Rege SD, Soong JC, Lufkin RB, et al. Extracranial head and neck: PET imaging with 2-[F-18]fluoro-2-deoxy-𝒹-glucose and MR imaging correlation. Radiology 1993;186:27–35 [DOI] [PubMed] [Google Scholar]