Abstract
BACKGROUND AND PURPOSE: Recent reports describe a high rate of thromboembolic events related to Guglielmi detachable coil (GDC) use in the treatment of cerebral aneurysms. The purpose of this study was to investigate electrolysis-related changes of blood as a potential cause of thromboembolic complications associated with GDC use.
METHODS: For in vitro observations, 15 GDCs (10 conventional coils and five insulated coils) were experimentally detached under microscopic observation. Three coils were detached in normal saline, five in human serum, and seven in heparinized human blood. For animal experiments, two coils were detached in two canine normal common carotid arteries with systemic heparinization. Immediately after detachment, the arteries were exposed, and clot formations were observed.
RESULTS: Significant amounts of gas bubbles were observed in all in vitro observations; more were seen in conventional coils, which required longer detachment times, than in insulated coils. Gas generation started with the growth of tiny bubbles into larger ones. In insulated coils, gas was generated only at the detachment zone, and no difference between saline, serum, and blood environments was observed. During detachment within heparinized blood, clot formations of 2–3-mm diameter were observed at the detachment zones of insulated coils. Animal experiments showed clot formation at the detachment zone, and bubble entrapments around the clots were also found.
CONCLUSION: The electrolytic detachment mechanism of the platinum coil can generate gas bubbles during the application of electric current. In association with electrothrombosis, this phenomenon may be a potential cause of thromboembolic complications during the treatment of cerebral aneurysms by use of GDCs.
The advent of the Guglielmi detachable coil (GDC) system has revolutionized the treatment of intracranial saccular aneurysms, and the safety and efficacy of endosaccular occlusion by use of the GDC has already been well documented (1–3). The advantage of the endovascular technique is relatively less invasive than surgical clipping that had been the mainstay of treatment for both ruptured and unruptured cerebral aneurysms. The GDC allows for precisely controlled endosaccular placement of coils, and its softness and spiral memory of various diameters allow the aneurysmal sac to be occluded with minimal injury to the wall. Recent advances in GDC technologies, including a wide variety of coil diameters, softer coils, 3D coils (4), and balloon protection of the parent artery (5, 6) or stent (7, 8) have markedly expanded its indication for treating aneurysms. In some institutions, endovascular treatment is now proposed as the primary technique of aneurysm treatment (2).
Although the endovascular approach with the GDC has been proved to be a safe and effective alternative to surgery, several complications can occur during or after the procedure, including procedure-related rupture of the aneurysm, compromise of the parent artery by protruded coils, or thromboembolic problems related with the delivery system or coil (9–12). Among these complications, the most frequent are cerebral ischemic problems due to thrombosis of the artery and distal embolic phenomena from embolized aneurysms or related devices (9, 10, 12–14). In their series of cerebral aneurysms, Pelz et al (9) reported that the rate of strokes related to endovascular treatment reached 28%.
Rordorf and colleagues (13) in their diffusion-weighted imaging study described a 61% rate of silent thromboembolic events related with GDC use. Klötzsch et al (14), using transcranial Doppler ultrasonography, detected asymptomatic microemboli in 31% of patients. Potential sources of thromboembolic events are catheters, preexisting thrombi within the aneurysmal sac, a partially occluded aneurysmal sac, and coil mass surface. In this study, we tested the hypothesis that the electrolytic detachment mechanism of the GDC system might be another source of thromboembolic complication.
Methods
Seventeen GDCs (Target Therapeutics, Fremont, CA) were experimentally detached under microscopic observation. Fifteen coils were detached during an in vitro experiment and two in animal arteries with active flow. Twelve coils were conventional (third-generation GDC, Target Therapeutics, Fremont, CA) and five were insulated (SynerG, fourth-generation GDC). The sizes and types of the coils used in this study are summarized in the Table.
Sizes and types of Guglielmi detachable coils used for in vitro and animal experiments
| Experiments | Conventional Coils | Insulated Coils (SynerG) |
|---|---|---|
| Saline | GDC-10 (4 mm × 10 cm), GDC-10 (5 mm × 15 cm) | GDC-10 (2 mm × 8 cm) |
| Heparinized serum | GDC-10 (5 mm × 15 cm), GDC-10 (6 mm × 6 cm), GDC-10 (6 mm × 6 cm) | GDC-10 (5 mm × 8 cm), GDC-10 (10 mm × 30 cm) |
| Heparinized blood | GDC-10 (2 mm × 2 cm, soft), GDC-10 (3 mm × 8 cm), GDC-10 (4 mm × 10 cm), GDC-10 (6 mm × 20 cm), GDC-18(8 mm × 30 cm) | GDC-10 (2 mm × 1 cm, Ultrasoft SR), GDC-10 (10 mm × 30 cm) |
| Canine carotid artery | GDC-10 (5 mm × 15 cm), GDC-18 (8 mm × 20 cm) | Not used in animal experiment |
For in vitro observations, 15 GDCs, including nine conventional (non-insulated, third-generation GDC) GDC-10 coils, one conventional GDC-18, and five SynerG (insulated, fourth-generation GDC) GDC-10 coils were experimentally detached. We used the same power supply unit (Target Therapeutics) that is usually used in clinical applications for all coils. Three coils were detached in normal saline, five in heparinized human serum, and seven in heparinized human whole blood. All in vitro observations were performed in transparent dishes of 10-cm diameter. As in clinical use, the positive terminal of the cable was connected to the proximal end of the wire part of the coil, and the negative ground terminal was connected to a needle, the tip of which was dipped into the liquid sample at the edge of the dish.
In every experiment, the entire part of the coil and terminal portion of the pusher wire, including the detachment zone, were totally dipped in the liquid sample before starting the electric current for detachment. Microcatheters were not used in the experiments with saline and serum but were used with blood (Excel-14, Target Therapeutics), and the tip of the catheter was also dipped in the blood during electrolytic detachment.
For the experiments with serum and whole blood, human blood samples were obtained from healthy volunteers and the samples were heparinized (500 U/10cc) immediately after sampling. A standard current of 1 mA was used, and the entire procedure of detachment was video-recorded and photographed. The entire in vitro experiments were performed at room temperature. Temperature change of saline or blood around the coil or ground electrode was not measured.
Under a protocol approved by the Clinical Research Institute of our institution, two adult mongrel dogs of 25–30 kg body weight were used for the animal experiments of this study. All procedures, including angiography, ultrasonography, and coil embolization were performed using sterile techniques, with animals under general anesthesia by endotracheal intubations. All angiographic and embolization procedures were performed via the transfemoral route. Both animals were heparinized by intravenous bolus administration of 2000 U just before the procedure. We checked the level of anticoagulation by measuring aPTT, and it was 29 seconds before and 110 seconds after heparinization.
Two of the conventional GDC-10 coils were placed using microcatheters with two markers (Excel-14) and detached in two canine normal common carotid arteries. Fluoroscopic and angiographic observation of the arterial blood flow during and after the detachment process showed slight decrease of blood flow without significant stasis or obstruction of the arterial lumen. Immediately after detachment, the arterial segment was ligated and opened by longitudinal incision. After gentle irrigation of the arterial lumen, the detached coil was examined with a surgical microscope.
Results
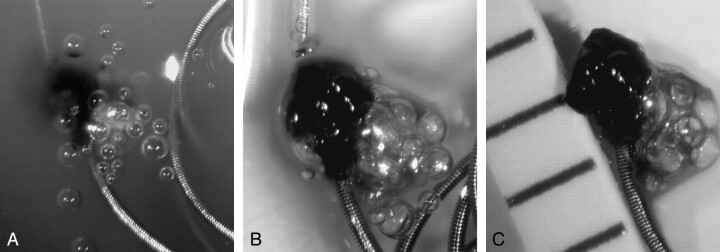
In all in vitro experiments using saline and serum, the generation of a significant amount of gas bubbles was clearly observed during electrolytic detachment. The amount of gas generated was much larger in the conventional coil, which required a longer time for detachment (Fig 1). Gas bubble generation was only observed during the electrolytic detachment process, starting with the growth of tiny microbubbles into larger ones. In all conventional coils, gas bubbles were generated from the entire surface of the platinum coil (Fig 1), whereas in SynerG coils, gas was generated only at the detachment zone (Fig 2A-C). In serum, localized brownish discolorations formed around the detachment zone during electrolytic detachment, which were not observed in the saline environment, and bubbles collected at the area of this discoloration even after detachment of the coil (Fig 2D).
Fig 1.
Depiction of the detachment process of a conventional coil (GDC-10, 4 mm × 10 cm) in saline. Many gas bubbles were generated from the coil and attached around the entire coil part. The bubbles varied in size.
Fig 2.
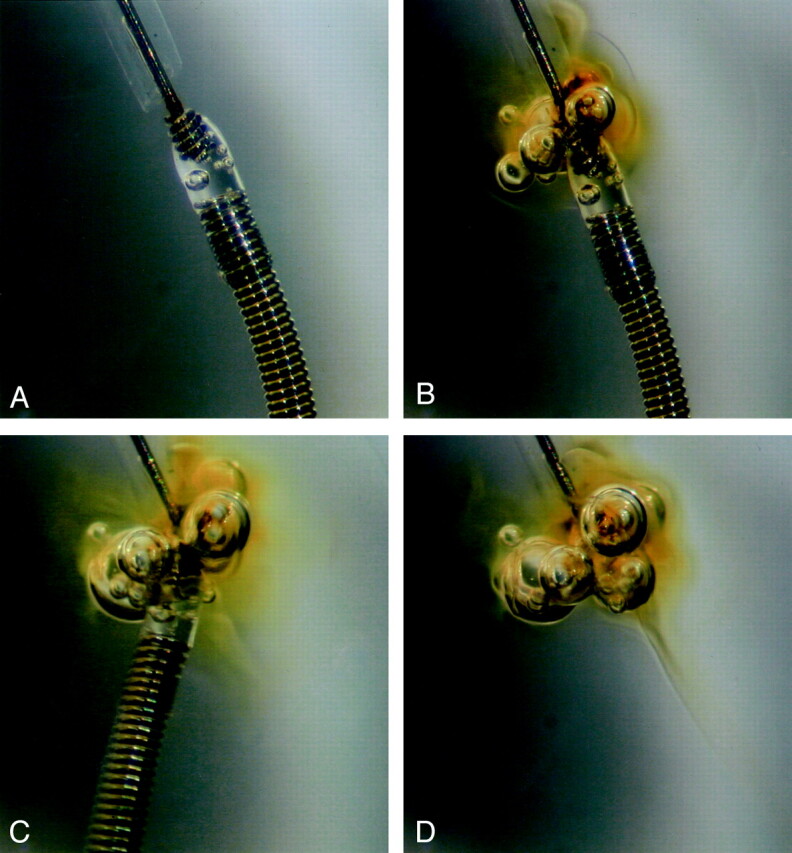
Depiction of detachment process of an insulated coil (GDC-10 SynerG, 6 mm × 6 cm) in heparinized human serum.
A, Before applying electric current, no gas bubbles surround the coil, and the detachment zone of the device is clearly seen with a transparent insulating plug.
B, After starting the electrolytic detachment process, gas bubbles are generated from the detachment zone, and a localized brownish discoloration is seen around the detachment zone.
C, The coil has just detached, and the bubbles enlarge.
D, After detachment, the coil moves out of the field, and the bubbles remain attached at the tip of the pusher wire and collected in the area of brownish discoloration. Because discoloration and collection of gas bubbles around this area were absent in the saline experiment, this brownish discoloration may be related to an electrolytically induced protein coagulation.
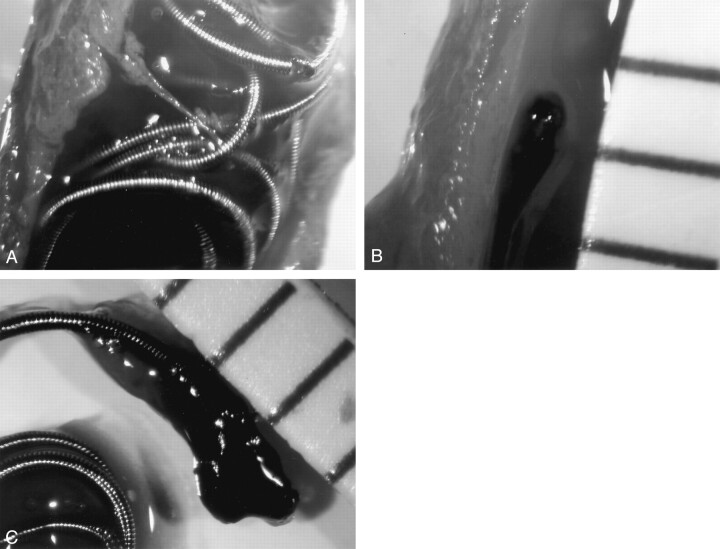
In all experimental detachments of coils within heparinized whole blood, gas bubble generation was clearly observed at the detachment zone of the SynerG coil and from the entire surface of the coil part of the conventional coils during electrolytic detachment (Fig 3). Dark blood clot formations of 2–3-mm diameter were also observed around the detachment zones of the SynerG coil (Fig 3). In the conventional coils, blood clots formed at the detachment zone and at the coil crossing zones. During detachment, the blood clot formed at the detachment zone gradually enlarged, became multi-lobulated in shape, and mixed with gas bubbles. After detachment and when the pusher wire of the coil was withdrawn, the 1–3-mm diameter blood clot was densely attached to the tip of the microcatheter (Fig 3).
Fig 3.
Depiction of the detachment process of an insulated coil (GDC-10 SynerG, 6 mm × 20 cm) in heparinized human whole blood.
A, During detachment, with the detachment zone dipped in the blood, a dark area of thrombus formation is clearly seen with many gas bubbles generated and floating to the surface level.
B, After detachment, the blood was gently irrigated by saline, and a lobulated thrombus is attached at the proximal tail of the coil part. Many gas bubbles remain in the thrombus.
C, After removal of the fluid, the bubble remains attached in the thrombus; the thrombus is 1.5 mm in diameter.
During coil detachment in the normal arteries of the animal experiments, ultrasonographic monitoring with a 7.5-MHz linear transducer failed to detect intraluminal gas bubble generation. Microscopic observations of the arterial lumen immediately after detachment and exposure of the detached coils showed a blood clot densely attached at the proximal end of the coil, with micro-bubbles attached around the surface of the coil (Fig 4). There was no clot found around the coil surface or other area of the ligated arterial lumen.
Fig 4.
Depiction of the detachment process of a conventional coil (GDC-18, 8 mm × 20 cm) in the canine carotid artery.
A, Immediately after detachment of the coil, the segment of the artery is ligated and opened by use of a longitudinal arteriotomy. Without arteriotomy, multiple and tiny gas bubbles surround the coil.
B, A thrombus with elongated appearance is seen at the proximal tail of the detached coil.
C, After removal of the coil from the artery, the thrombus measures 3 mm in diameter.
In this study, we did not measure the volume of the gas generation, analyze the chemical components of the gas, or measure the weight or volume of the thrombus formed.
Discussion
Endosaccular occlusion with a detachable coil has become popular in the treatment of intracranial aneurysms. For safe occlusion with minimal injury to the aneurysmal wall, the softness of the coil and ease of retrieval of the device are important. Since its introduction in 1990, the GDC system with electrolytic detachment mechanism has been recognized as a safe and effective device for endosaccular occlusion of ruptured or unruptured intracranial aneurysms (1–3, 15, 16). The motionless detachment of the coil by electrolytic detachment mechanism has seemed the ideal solution for minimizing injury to the aneurysmal wall. There are several different kinds of coil detachment mechanisms, including screw-type mechanical detachment, thermal electrolytic detachment, and hydraulic mechanical detachment. The advantage of the mechanical detachment system is faster detachment time than for the electrolytic mechanism, although its detachment mechanism potentially causes motion of the coil during detachment.
Electrothrombosis induced by attraction of negatively charged blood cells to the positively charged electrode (coil) has been one of the basic principles in the development of the GDC system (15). Recently, Padolecchia et al (17) reported the result of their in vitro investigation that clearly demonstrated the effect of electrothrombosis. However, the advantage of the electrolytic detachment mechanism of the GDC system for the induction of thrombosis is very speculative. Electrothrombosis is not thought to be an advantage of the GDC system for several reasons. In the initial treatment, the sac is packed as densely as possible, and the procedure is usually stopped when the last coil cannot be inserted to prevent delayed recurrence (2). Although thrombus formation may play a role in the acute protective effect in cases of ruptured aneurysm, the most important treatment factor should be the mechanical occlusion by the coil mass. One cannot expect such electrothrombosis around the coil with the new, currently used GDC design. In this fourth-generation GDC, the SynerG, the entire coil is insulated from the electric current by a polymer plug between the detachment zone and the platinum coil. With this modification, the electric current applied can be concentrated on the detachment. The detachment time can be shortened, and the electrothrombosis can occur only at the detachment zone because the coil is no longer a part of the electrode.
We did not measure the volume of the clots formed in this study, and the size of the clots observed in multiple experimental detachments were usually small; the methods of our experiment may differ from clinical situation. However, the result of our study clearly showed thrombus formation at the detachment zone even in the animal experiment in which arterial blood flow was preserved. If this phenomenon occurs in a clinical context, we believe that the small electrolytically induced blood clots may have some clinical implications in several areas. In the later part of the usual procedure, the GDC detachment zone could become exposed to the parent arterial blood flow. The clot formed during the detachment process can be distally embolized, attached at the distal end of the coil, or attached at the tip of the microcatheter after withdrawal of the pusher part. If it is embolized distally, the clot might be a causative factor for procedure-related silent thromboembolic problems that have been documented in some investigations (13, 18, 19). If the clot remains attached at the tail of the coil or at the tip of the microcatheter, it may grow larger during repeated coil detachment procedures and may eventually compromise the parent arterial lumen or be distally embolized to occlude larger arteries. Although the sizes of the clots observed in this study were not large enough to occlude a major artery, they may act as a nidus for subsequent thrombus formation and thereby cause thromboembolic complication.
Gas generation around the electrodes during electrolysis of liquid material is a well-known phenomenon, and hydrogen and oxygen gas can be generated during electrolysis of pure water. Nonetheless, we cannot presume what kind of gas can be generated when electric current is applied in blood because of the complexity of blood components. In this study, we confirmed that a significant amount of gas is generated during electrolytic detachment of the GDC. Because the amount of gas generated in electrolysis is directly proportional to the amplitude of electric current and time, the amount should be larger in conventional than in insulated coils, as indeed was the case in our results.
The size of each bubble, usually less than 1 mm in diameter, was very small. Therefore, we do not think that the gas bubbles generated during the detachment process played a major role in thromboembolic problems related with GDC placement. However, the thrombi formed around the detachment zone were quite lobulated in shape, probably because of the entrapment of gas bubbles within the thrombus during generation. This synchronous generation of gas bubbles and thrombus may enable the gas bubble entrapment to make the clot bigger.
In the experiments using serum, we noticed a localized brownish discoloration around the detachment zone, which was not observed in the saline experiments (Fig 2). Owing to the lack of this discoloration in saline, we presumed that this discoloration was a feature of electrolytically related protein coagulation. However, we could not confirm this presumption. This phenomenon was possibly caused by some temperature change related with the electrolytic detachment process. In clinical situations, this may not occur with active circulation of arterial blood. In this study, gas bubbles that were generated and mixed with this discoloration grew bigger during detachment, collected at the area of discoloration, and remained there even after detachment of the coil (Fig 2D). This finding supports our explanation of bubble entrapment within the clot during generation.
We tried to confirm by experimental coil detachment in the normal canine carotid arteries whether clot formation and gas bubble generation might occur in an environment with active blood flow. The arterial segment was already exposed surgically before the commencement of coil detachment to ligate the arterial segment immediately after electrolytic detachment. The arterial wall was opened by longitudinal arteriotomy after ligation, and small amounts of gas bubbles were found without the performance of any procedure other than arteriotomy (Fig 4A). The amount of gas appeared to be much smaller than that of the in vitro experiments. Considering that the detachment process occurred in an environment with active arterial blood flow, weak bubble formation is quite natural. Clot formation at the proximal tail of the coil was clearly demonstrated after detachment of the coil (Fig 4), as was the case in the in vitro experiments.
The results of this study confirm that the electrolytic detachment mechanism of GDC can generate gas and thrombi from the detachment zone of a coil. Comparison of the findings of in vitro experiments with those of animal experiments suggests that most gas bubbles will be embolized distally by the active blood flow during detachment. Considering the small size of the bubbles initially generated, it is probable that they do not cause arterial occlusion that can be depicted at angiography. However, the sizes of the thrombi generated and attached to the tip of the catheter or the proximal tail of the coil are much larger than those of bubbles, and these thrombi are large enough to occlude arteries of significant size. It remains to be confirmed whether this phenomenon actually causes symptomatic complications.
There are many theoretical thromboembolic sources other than the use of GDC in procedures for endovascular treatment of cerebral aneurysms. During selection of the parent artery, a small atheromatous plaque might be dislodged and embolized, a fresh thrombus might be formed at the surface of the guiding catheter or microcatheter, tiny air bubbles might be infused by injection of heparinized saline or contrast media, preexisting thrombi might be dislodged by a coil and embolized, or a fresh thrombus might be formed at the surface of the coil mass. Small areas of high signal intensity can be found on diffusion-weighted MR images even after diagnostic angiographic procedures (18). Considering the well-documented clinical results of using GDCs in aneurysm treatment, we do not think that the possible incidence of this phenomenon is a critical drawback for clinical GDC use, and we still use this device at our institution. However, it is clear that this phenomenon is one of the many causative factors of procedure-related thromboembolic problems in the endovascular treatment of cerebral aneurysms by use of GDC.
Conclusion
Our results brought up possible problems related to electrolytic detachment of the GDC system. The electrolytic detachment mechanism of the platinum coil in the GDC system may generate gas bubbles and possibly lead to thrombus formation at the detachment zone of the GDC during the application of electric current. Although our experimental setup does not mirror clinical circumstances and we did not elucidate the chemical profiles or amount of gas generated, this phenomenon, in association with thrombus formation, may lead to thromboembolic complications in the treatment of cerebral aneurysms by use of GDC.
Footnotes
Presented at the 40th annual meeting of the American Society of Neuroradiology, May 11–17, 2002, Vancouver, Canada.
References
- 1.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracerebral aneurysms: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 2.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–356 [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Viñuela F, Duckwiler G, Gobin YP, Guglielmi G. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg 1999;90:207–214 [DOI] [PubMed] [Google Scholar]
- 4.Cloft HJ, Joseph GJ, Tong FC, Goldstein JH, Dion JE. Use of three-dimensional detachable coils in the treatment of wide-necked cerebral aneurysms AJNR Am J Neuroradiol 2000;21:1312–1314 [PMC free article] [PubMed] [Google Scholar]
- 5.Malek AM, Halbach VV, Phatouros CC, et al. Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery 2000;46:1397–1406 [DOI] [PubMed] [Google Scholar]
- 6.Aletich VA, Debrun GM, Misra M, Charbel F, Ausman JI. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg 2000;93:388–396 [DOI] [PubMed] [Google Scholar]
- 7.Wilms G, van Calenbergh F, Stockx L, Demaerel P, van Loon J, Goffin J. Endovascular treatment of a ruptured paraclinoid aneurysm of the carotid syphon achieved using endovascular stent and endosaccular coil placement. AJNR Am J Neuroradiol 2000;21:753–756 [PMC free article] [PubMed] [Google Scholar]
- 8.Pride GL Jr, Horowitz MB, Purdy PD. Endovascular problem solving with intravascular stents. AJNR Am J Neuroradiol 2000;21:532–540 [PMC free article] [PubMed] [Google Scholar]
- 9.Pelz DM, Lowne SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1541–1547 [PMC free article] [PubMed] [Google Scholar]
- 10.Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol 1998;19:157–165 [PMC free article] [PubMed] [Google Scholar]
- 11.McDougall CG, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB. Causes and management of aneurysmal hemorrhage occurring during embolization with GDC. J Neurosurg 1998;89:87–92 [DOI] [PubMed] [Google Scholar]
- 12.Brilstra EH, Rinkel GJE, van der Graaf Y, van Rooij WJJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 1999;30:470–476 [DOI] [PubMed] [Google Scholar]
- 13.Rordorf G, Bellon RJ, Budzik RF, et al. Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol 2001;22:5–10 [PMC free article] [PubMed] [Google Scholar]
- 14.Klötzsch C, Nahser HC, Henkes H, Kühne D, Berlit P. Detection of microemboli distal to cerebral aneurysms before and after therapeutic embolization. AJNR Am J Neuroradiol 1998;19:1315–1318 [PMC free article] [PubMed] [Google Scholar]
- 15.Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach: part 2, preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 16.Kallmes D, Kallmes MH., Cloft HJ, Dion JE. Guglielmi detachable coil embolization for unruptured aneurysms on non-surgical candidates: a cost-effectiveness exploration. AJNR Am J Neuroradiol 1998;19:167–176 [PMC free article] [PubMed] [Google Scholar]
- 17.Padolecchia R, Guglielmi G, Puglioli M, et al. Role of electrothrombosis in aneurysm treatment with Guglielmi detachable coils: an in vitro scanning electron microscopic study. AJNR Am J Neuroradiol 2001;22:1757–1760 [PMC free article] [PubMed] [Google Scholar]
- 18.Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999;354:1594–1597 [DOI] [PubMed] [Google Scholar]
- 19.Biondi A, Oppenheim C, Vivas E, et al. Cerebral aneurysms treated by Guglielmi detachable coils: evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2000;21:957–963 [PMC free article] [PubMed] [Google Scholar]