Abstract
Summary: An 11-month-old boy was discovered to have a cleft palate, club foot, hypospadias, and myoclonic seizures. No in utero exposure to teratogens was identified. Brain MR imaging revealed a middle interhemispheric fusion variant of holoprosencephaly, diffuse polymicrogyria, and a hypoplastic brain stem; this was a distinctly unusual association of findings. We hypothesize that an unknown genetic factor causes disturbances of cleavage of the prosencephalon as well as neuronal migration and organization.
Holoprosencephaly (HPE) is characterized by a failure of induction and patterning of the rostral neural tube (basal forebrain). As a result, hypoplasia occurs, with a lack of separation of the telencephalon into two cerebral hemispheres. Deep brain structures, such as the basal ganglia, thalamic nuclei, hypothalamic nuclei, and mesencephalon are also often affected in HPE. HPE is caused by teratogens, such as those related to maternal diabetes, as well as genetic factors, such as trisomies and mutations (1). The developmental processes that are affected in this disorder involve dorsal and ventral patterning in the prosencephalon; these are in progress by day 35 of gestation (2). The middle interhemispheric (MIH) variant of HPE consists of an abnormal midline connection of the cerebral hemispheres in the posterior frontal and parietal regions, with normal interhemispheric separation of the basal forebrain, anterior frontal lobes, and occipital regions (2). The pattern of involvement differs from that of classical HPE, in which the rostrobasal portions of the prosencephalon are most severely affected (3). Recently, a case of MIH was described in a patient with a mutation of ZIC2 (4), a dorsalizing gene; a mutation of this gene can also cause classic HPE (5). This finding supports the classification of MIH as a type of HPE and also the concept of HPE being the result of defects in dorsoventral patterning.
Polymicrogyria (PMG) is a malformation of cortical development characterized by many small gyri separated by shallow sulci. It is believed to result from a developmental disorder or injury that occurs between 17 and 25 or 26 weeks’ gestation (6). This time is toward the end of the period of neuronal migration and the early phase of cortical organization (7) and much later than the time of the events that cause HPE. PMG is thought to be heterogeneous, the result of many different genetic and environmental causes (7–9). We present a patient with imaging findings of both MIH and diffuse PMG. To our knowledge, the association of these observations has not been reported previously. We also comment on possible causes of this finding.
Case Report
This now 11-month-old boy was born to a 23-year-old healthy mother with no previous pregnancies. No history of in utero exposure to infections, drugs, or other teratogens was elicited. The patient had no family history of brain malformations, developmental delays, facial abnormalities, or chromosomal abnormalities. The mother had no history of diabetes mellitus and did not have gestational diabetes. The pregnancy was complicated by eclampsia in the 8th month of pregnancy, and labor was induced at 36 weeks because of maternal hypertension. The baby was delivered by means of cesarean section because of a decreased fetal heart rate after the induction. Initial examination revealed a cleft palate, hypospadias, and a left clubfoot. Hypertelorism and epicanthal folds were also noted. Neurologic examination revealed normal pupillary light reflex, normal deep tendon reflexes, mildly increased muscle tone in four extremities, and a diminished Moro response. Chromosomal analysis showed a normal male karyotype. Electrolyte levels were normal.
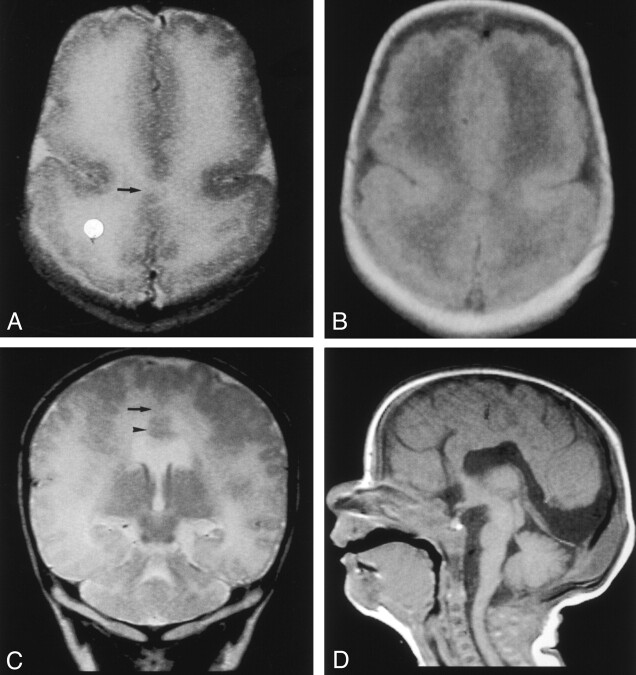
MR imaging was performed with a 1.5-T superconducting magnet when the patient was aged 3 weeks. Axial T1- and T2-weighted images showed interhemispheric fusion of the gray matter and white matter and an absence of the interhemispheric fissure (IHF) in the posterior frontal and parietal lobes (Figure, 1A–C). The gyral pattern was abnormal, showing an increased cortical thickness as much as 7 mm and extensive irregularity of the cortical-white matter junction in the bilateral frontal, parietal, occipital, and lateral temporal lobes. An IHF was identified frontally and occipitally. The coronal fast spin-echo T2-weighted image (Figure, 1C) also showed subcortical heterotopia. The sagittal T1-weighted image (Figure, 1D) showed a hypoplastic brain stem, especially in the basis pontis. From these findings, the diagnosis of MIH, diffuse PMG, and a hypoplastic brain stem was established.
Fig 1.
MR images obtained when the patient was aged 3 weeks.
A and B, Fast spin-echo T2-weighted (TR/TE/NEX, 5460/106/2) (A) and spin-echo T1-weighted (540/14/1) (B) images show interhemispheric fusion of the gray matter and white matter (arrow in A) and an absence of the IHF in the posterior frontal and parietal lobes. The gyral pattern is abnormal, showing an increased cortical thickness and extensive irregularity of the cortical–white matter junction in the bilateral frontal and parietal lobes.
C, Coronal fast spin-echo T2-weighted image shows interhemispheric fusion of the gray and white matter (arrow) and subcortical heterotopia (arrowhead).
D, Sagittal spin-echo T1-weighted image shows a hypoplastic basis pontis.
At 7 months of age, the patient started having myoclonic seizures with 4–5 clusters each day upon awakening. The EEG revealed background slowing and disorganization, with multifocal epileptiform discharges. Without medication, the patient has had no further seizures to date. His head circumference was 40.5 cm at 8 months (−2.5 SD). At 9 months, he was able to maintain good head control. He could also roll over, sit momentarily without support, and crawl for short distances. Although he had a normal pupillary light reflex, he had no visual tracking and a poor response to light. No visual evoked response could be elicited. He babbled and smiled. His cleft palate was repaired at the age of 10 months.
Discussion
We present a patient with a cleft palate; lower extremity abnormalities; genital abnormalities; myoclonic seizures; and imaging findings of MIH, diffuse PMG, and brain stem hypoplasia. Two observations suggest that environmental factors are unlikely to cause these central nervous system abnormalities: 1) the absence of a known history of in utero exposure to infections, drugs, maternal diabetes mellitus, or other teratogens, and 2) the fact that the cleavage of the prosencephalon takes place at the 5th gestational week, whereas cortical organization occurs between 17–26 weeks gestation.
Although MIH is considered a variant of HPE, the sites of involvement in this malformation differ from those seen in classic HPE (2). Instead of the most severe involvement being in the most rostral basal midline, patients with MIH have relative sparing of the basal forebrain, with a well-formed anterior IHF and normal or nearly normal caudates, hypothalami, and basal ganglia. However, they have more severe involvement of the thalami and the IHF in the region of the posterior frontal lobes and parietal lobes. In addition, facial dysmorphism is mild, if it is present (2). ZIC2, located on human chromosome 13q32, is an orthologue of mouse Zic2, and it is one of four genes identified as causes of human HPE (5). Zic2 is expressed in the dorsal region of the embryonic roof plate, and it plays important roles in neural tube closure and in the differentiation of the roof plate of the developing embryo. In mice, mutation of Zic2 results in defective neural tube closure and HPE. In a study of more than 150 human fetal brains with HPE, all five fetuses with MIH were found to have a deletion of 13q, which includes ZIC2 (5). These findings strongly suggest that MIH could be specifically linked to hemizygous loss of function of the human gene ZIC2. In another study of 509 unrelated individuals with various types of HPE, 16 had a ZIC2 mutation, but only one of these (with a small in-frame deletion) had MIH (4). This finding of MIH in a patient with a small, in-frame deletion raises the possibility that this deletion results in a partially functional allele and that the severity of the malformation, ranging from MIH to alobar HPE, depends on the level of function of the protein.
The dorsoventral gradient of ZIC2 expression differs from the ventrodorsal gradient of the other three genes linked to HPE, namely, SHH in HPE3, SIX3 in HPE2, and TGIF in HPE4 (10). Indeed, Zic2 is involved in the differentiation of the roof plate, whereas the others are involved in differentiation of the floor plate. This difference may explain the mildness or absence of craniofacial malformations (2, 4, 5) and the occurrence of MIH with a ZIC2 mutation or deletion at 13q (5). Because the dorsal structures are primarily affected, deformities secondary to the incomplete formation of ventrally derived structures, such as hypotelorism and the lack of formation of the medial aspects of the hypothalamus and caudate, are less likely in MIH.
To understand cortical abnormalities in HPE, it is important to understand the presence of a mediolateral gradient of genetic expression in addition to the vertical (dorsoventral or ventrodorsal) gradients. In HPE, the most severely disorganized cerebral cortex (lacking normal lamination) is found at or near the midline. More laterally a transitional cortex is present, while still further laterally the cortex usually becomes laminated with a normal complement of neurons (10). MR imaging in 96 patients with classic HPE showed diffuse gyral anomalies in eight and anomalies limited to the anteromedial cortex in four; the latter probably reflected the aforementioned neuropathologic findings (11). The thickness of the cortex was normal in all patients (11). Seen on MR images as irregularity of the cortical-white matter junction and increased cortical thickness (12), PMG was not identified in any of the 96 patients (11). Another study used MR imaging to examine 21 patients with MIH and demonstrated dysplastic cerebral cortex or heterotopic gray matter in 18 (86%). Some of these cases were limited to thickening of the cortex lining the anterior IHF (2). In another study (5), microscopic examination of the cortex in one of the five fetuses with MIH revealed a diffuse lack of cortical organization. Still another patient had normal cortical architecture in the cerebral cortex except for disorganization, which was distinct from PMG, of the fused portion (personal communication, M. Hayashi, Department of Clinical Neuropathology, Tokyo Metropolitan Institute for Neuroscience, Japan).
Our review of the literature pertaining to MIH and PMG revealed no previous mention of association of the two malformations. However, Sztriha et al (13) reported a patient who had a combination of lobar HPE and schizencephaly. One of the authors (J.T.) has examined a patient with lobar HPE and schizencephaly (unpublished data, T. Kibe, K. Yokochi, Department of Pediatrics, Seirei-Mikatabara General Hospital, Japan). Some patients with schizencephaly, including those with familial cases, are reported to have associated mutations of the EMX2 homeobox gene, a gene expressed in the germinal matrix of the developing cerebral cortex, and the EMX2 protein seems to be required for the proper formation of the human cerebral cortex (14, 15). Because schizencephaly is usually accompanied by PMG and because both PMG and schizencephaly have been reported in the same family, these two abnormalities are considered to have the same or similar etiology (which is due to abnormal, late neuronal migration and cortical organization, as in PMG-schizencephaly complex) (7). Cases of HPE associated with schizencephaly would suggest the possibility of an unknown genetic factor that affects different stages of neuronal development: cleavage of the prosencephalon (HPE and MIH) and neuronal migration and organization (PMG and schizencephaly). This hypothesis may explain the association of MIH and diffuse PMG, as observed in our patient. That is, one of the protein products of the genes that cause MIH may later be involved in cortical organization. Recently, a genetic locus for bilateral perisylvian PMG and bilateral frontoparietal PMG has been mapped to Xq28 and 16q12.2–21, respectively (8, 9). Although the molecular mechanisms involved in cortical organization (eg, neurite extension, synaptogenesis, and neuronal migration) are not completely elucidated, the future cloning of the gene for familial PMG could offer important biologic clues to understand the molecular basis of a possible relationship between MIH and diffuse PMG.
Conclusion
An 11-month-old boy with a cleft palate, unilateral club foot, hypospadias, and myoclonic seizures was found to have MIH, diffuse PMG, and a hypoplastic brain stem; he had no known history of in utero exposure to teratogens. A review of HPE, MIH, and the PMG-schizencephaly complex, and of the rare association of HPE and schizencephaly, leads to the hypothesis that an unknown genetic factor causes disturbances of both cleavage of the prosencephalon (HPE and MIH) and late neuronal migration and organization (PMG and schizencephaly).
Acknowledgments
The authors wish to thank Dr Shinji Fujimoto, Department of Pediatrics, Nagoya City University, Aichi, Japan, for helpful advice.
References
- 1.Golden JA. Towards a greater understanding of the pathogenesis of holoprosencephaly. Brain Dev 1999;21:513–521 [DOI] [PubMed] [Google Scholar]
- 2.Simon EM, Hevner RF, Pinter JD, et al. The middle interhemispheric variant of holoprosencephaly. AJNR Am J Neuroradiol 2002;23:151–155 [PMC free article] [PubMed] [Google Scholar]
- 3.Simon EM, Hevner R, Pinter JD, Kinsman S, Hahn J, Barkovich AJ. Assessment of deep gray nuclei in holoprosencephaly. AJNR Am J Neuroradiol 2000;21:1955–1961 [PMC free article] [PubMed] [Google Scholar]
- 4.Brown LY, Odent S, David V, et al. Holoprosencephaly due to mutation in. ZIC2: alanine tract expansion mutations may be caused by parental somatic recombination. Hum Mol Genet 2001;10:791–796 [DOI] [PubMed] [Google Scholar]
- 5.Marcorelles P, Loget P, Fallet-Bianco C, et al. Unusual variant of holoprosencephaly in monosomy 13q. Pediatr Dev Pathol 2002;5:170–178 [DOI] [PubMed] [Google Scholar]
- 6.Golden JA. Cell migration and cerebral cortical development. Neuropathol Appl Neurobiol 2001;27:22–28 [DOI] [PubMed] [Google Scholar]
- 7.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development update. Neurology 2001;57:2168–2178 [DOI] [PubMed] [Google Scholar]
- 8.Piao X, Basel-Vanagaite L, Straussberg R, et al. An autosomal recessive form of bilateral frontoparietal polymicrogyria maps to chromosome 16q12.2–21. Am J Hum Genet 2001;70:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villard L, Nguyen K, Cardoso C, et al. A locus for bilateral perisylvian polymicrogyria maps to Xq28. Am J Hum Genet 2001;70:1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarnat HB, Flores-Sarnat L. Neuropathologic research strategies in holoprosencephaly. J Child Neurol 2001;16:918–931 [DOI] [PubMed] [Google Scholar]
- 11.Barkovich AJ, Simon EM, Clegg NJ, Kinsman SL, Hahn JS. Analysis of cerebral cortex in holoprosencephaly with attention to the sylvian fissures. AJNR Am J Neuroradiol 2002;23:143–150 [PMC free article] [PubMed] [Google Scholar]
- 12.Barkovich AJ, Hevner R, Guerrini R. Syndromes of bilateral polymicrogyria. AJNR Am J Neuroradiol 1999;20:1814–1821 [PMC free article] [PubMed] [Google Scholar]
- 13.Sztriha L, Varady E, Hetrtecant J, Nork M. Mediobasal and mantle defect of the prosencephalon: lobar holoprosencephaly, schizencephaly and diabetes insipidus. Neuropediatrics 1998;29:272–275 [DOI] [PubMed] [Google Scholar]
- 14.Brunelli S, Faiella A, Carra V, et al. Germline mutations in the homeobox gene EMX2 in patients with severe schizencephaly. Nature Gen 1996;12:94–96 [DOI] [PubMed] [Google Scholar]
- 15.Granata T, Farina L, Faiella A, et al. Familial schizencephaly associated with EMX2 mutation. Neurology 1997;48:1403–1406 [DOI] [PubMed] [Google Scholar]