Abstract
BACKGROUND AND PURPOSE: Previous authors have shown that conventional myelography is superior to plain CT in the assessment of root compression in the lateral recess, but this question has never been evaluated with respect to MR imaging of the lumbar level. Our purpose was to assess the accuracy of MR imaging, conventional myelography, and postmyelography CT (CT myelography) of the lumbar level in identifying degenerative lateral recess root compression with surgical confirmation.
METHODS: MR imaging, conventional myelography, and CT myelography of the lumbar level were assessed in the imaging of 58 lateral recesses at 38 lumbar levels in 26 patients who underwent surgery for radiculopathy with degenerative lateral recess abnormality. Each lateral recess was graded as normal, small without root compression, small with root compression, or severe root compression.
RESULTS: MR imaging underestimated root compression in 28% to 29% of the cases in which root impingement was surgically confirmed. Conventional myelography underestimated root compression in only 5% to 7% of the cases and correctly predicted impingement in 93% to 95%. CT myelography underestimated root compression in 38% of the surgically confirmed cases.
CONCLUSION: MR imaging significantly underestimated root compression caused by degenerative changes in the lateral recess. Although MR imaging is a superb study when used in the search for degenerative disk disease and disk protrusion, conventional myelography is a crucial supplemental study that is necessary to confirm degenerative root impingement in the lateral recess as the cause of radiculopathy.
The causes of radiating leg pain are not fully understood. Attention has recently focused on root inflammation due to irritating substances expressed from the nucleus pulposus in patients with disk protrusion (1–6). Root compression is more controversial, because root impingement frequently occurs in the absence of symptoms (7–11). Most spine surgeons consider root compression to be a contributing cause of radiculopathy in cases of degenerative spinal stenosis and lateral recess stenosis (12, 13). Documentation of root impingement is an important factor in the preoperative assessment in such cases. Surgical intervention is aimed at decompressing the affected and clinically significant roots. In addition, spine interventional and injection techniques are being used more frequently, either as a provocative test to establish the origin of a patient’s pain (discography, nerve block, facet block) or as a direct treatment of back pain and sciatica (facet block, epidural steroids) (14–20). Accurate accounting of the locations of the degenerative changes or the presence of nerve root compression or inflammation is important in understanding the efficacy of these treatments and provocative tests.
The MR imaging features of lumbar degenerative disease have been described, but less is known regarding the accuracy of MR imaging in the detection of root compression that results from these degenerative changes (21). Wilmink (22) compared the abilities of plain CT and conventional myelography to predict root compression. In his study, plain CT frequently underestimated root compression that was revealed by conventional myelography. Correlation was weakest in the supra-axillary region or lateral recess. Wilmink concluded that cross-sectional imaging had limitations in predicting root compression revealed by conventional myelography.
It was our concern that root compression in the lateral recess might also be difficult to identify when interpreting MR images obtained at the lumbar level. The purpose of our study was to compare the identification of root compression in the lateral recess as revealed by MR imaging (the traditional imaging standard), conventional myelography, and postmyelography CT (CT myelography) and to correlate the findings of these studies with the surgical findings at the time of operative assessment.
Methods
During a 3-year period, we identified 210 patients at our institution who had undergone both MR imaging and conventional myelography, with CT myelography being performed within a 3-month interval. Choosing a time interval of 3 months was considered adequate to eliminate any significant change in degenerative disease between the studies performed. Reports of the studies were reviewed, and 50 patients were identified who had significant degenerative changes or root compression in the lateral recess region identified by either MR imaging or conventional myelography combined with CT myelography. The MR imaging, conventional myelography, and CT myelography studies of these 50 patients were graded for the presence of root impingement in the lateral recess, as described below. Of the 50 initial patients whose imaging studies were evaluated and graded, 26 went on to undergo operative decompression at one or more levels for symptom relief. This report focuses on the 26 patients who underwent surgical decompression.
Lumbar surgery (laminectomy or laminotomy) was performed on 58 lateral recesses at 38 lumbar levels in 26 patients. Only patients with degenerative changes causing root compression within the lateral recess were included in this review. Levels that had been previously operated on were not considered, and patients with root impingement caused by disk protrusion were excluded.
For 24 of the original 50 patients for whom degenerative root compression was identified, surgery was not performed. For those 24 patients, the clinical symptoms and pain patterns did not match the levels of root compression as noted on MR images, conventional myelograms, or CT myelograms.
Patient age ranged from 37 to 84 years, with an average age of 66 years. Sixteen patients were male and 11 were female.
Imaging Studies
MR imaging was performed on a 1.5-T system (General Electric, Milwaukee, WI). Sagittal T1-weighted (500–600/10–19/2–4 [TR/TE/NEX], 24-cm field of view, 3-mm-thick sections, 256 × 128 matrix) and fast spin-echo T2-weighted (4000+/85/2 [TR/TEeff/NEX], 24-cm field of view, 3-mm-thick sections, 256 × 128 matrix, echo train length of 16) images were obtained. An axial double-echo fast spin-echo T2-weighted sequence (4000+/17, 85/2;18-cm field of view; 5-mm-thick sections; 1-mm section gap; 256 × 192 matrix; echo train length of 8) was acquired to cover from S1 to L1. An oblique axial fast spin-echo T1-weighted sequence (500–600/17/4, 18-cm field of view, 4-mm-thick sections, 0- to 1-mm section gap, 256 × 192 matrix, echo train length of 4) was obtained from L4 to S1, with the best single angle parallel to both the L4−L5 and L5−S1 disk spaces.
Conventional myelograms were obtained in a standard fashion. For routine studies of the lumbar level, 12 to 17 mL of 180 mg I/cc iohexol (Nycomed, Princeton, NJ) was injected with 10 to 12 mL of 240 mg I/cc iohexol used when more than one spine region was studied. Standard anteroposterior, lateral, and oblique films were obtained in all patients. All three projections were available for review, but assessment of root impingement was determined primarily from the frontal and oblique images on which the individual nerve roots were clearly seen.
CT myelography was performed in a routine fashion after conventional myelography. Axial 3- to 5-mm images were obtained angled parallel to the disk space, scanning from pedicle to pedicle with complete interspace coverage. A standard 17-cm field of view was used, with 120 kv, 2-second scanning time, and 120 to 300 mA adjusted for patients’ size and body habitus.
Lesion Grading
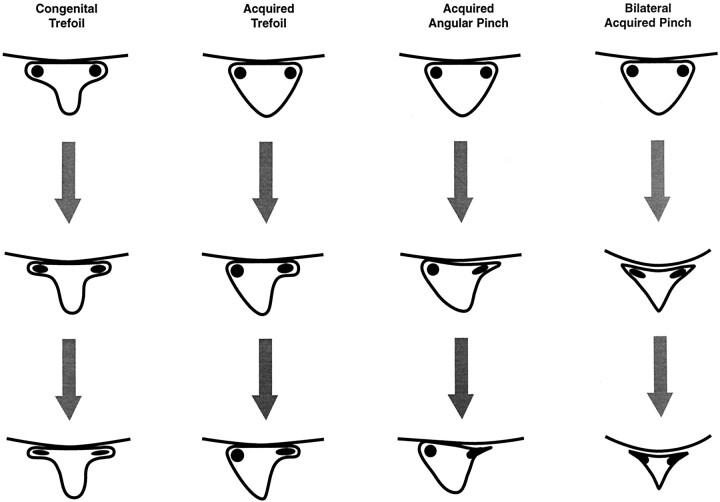
The purpose of this study was to determine the accuracy in identification of root compression in the lateral recess caused by lumbar degenerative changes. To compare lateral recess root compression by MR imaging, conventional myelography, and CT myelography, a grading system was devised that emphasized similar objective features of impingement and could be applied to all three imaging modalities. Root compression in the lateral recess typically occurs in two morphologic forms (Fig 1): trefoil narrowing of the lateral recess (congenital or acquired) secondary to diminished anteroposterior dimension of the lateral canal (Fig 1, columns A and B) or angular pinch-like encroachment of the lateral margin of the canal with subsequent pinch of the nerve root (Fig 1, columns C and D). The grading system emphasized recognition of the compressed nerve root and thecal sac along with identification of partial or complete exclusion of CSF from the corner of the canal and lateral recess.
Fig 1.
Illustrations show development of lateral recess stenosis.
Column 1, congenital trefoil canal. The lateral recess region becomes progressively narrowed because of either facet or endplate-disk margin degenerative changes. Column 2, acquired trefoil canal. Early facet degenerative changes and hypertrophy in a triangular canal develops a trefoil shape with the root positioned in a lateral recess niche. Progressive disk margin, endplate, or further facet degenerative changes leads to compression of the trapped root. Column 3, acquired angular pinch of the lateral recess. Simultaneous near equal facet, endplate, and disk margin degenerative changes lead to acute angle formation in the corner of the canal and lateral recess region. The root becomes progressively compressed in the lateral recess and may be medially deflected. Column 4, bilateral acquired angular pinch of the lateral recess. Bilateral facet, disk margin, and endplate degenerative changes can narrow the central spinal canal and the lateral recess region. This can produce both central spinal stenosis with cauda equina compression and individual nerve root compression within the abnormal lateral recess.
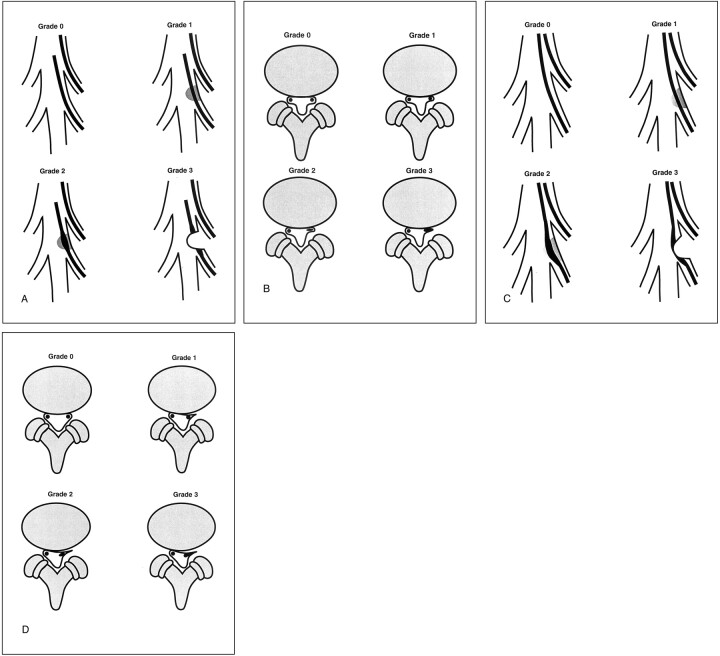
Root compression was graded on a 0- to 3-point scale, as summarized in Table 1 and Figure 2. The grading scale uses typical imaging features of lateral recess compression as identified by MR imaging, conventional myelography, or CT myelography. Grade definition was designed to characterize the same degree of root compression for MR imaging, conventional myelography, and CT myelography by using similar objective characteristics. This approach also separated features of degenerative disease from degenerative changes that clearly compressed the nerve root. Grades were defined as follows: grade 0, no lateral recess narrowing or root compression; grade 1, evidence of lateral recess narrowing but no objective evidence of root compression; grade 2, more significant lateral recess narrowing (angular or trefoil) with the nerve root judged to be flattened or widened but with preservation of CSF around the root in the recess; grade 3, severe nerve root compression within the lateral recess with obliteration of CSF from the recess.
TABLE 1:
Grading system for lateral recess root compression
| Grade | Myelography | CT Myelography or MR Imaging |
|---|---|---|
| 0 | Normal | Normal |
| 1 | Reduced contrast material within the lateral recess, indentation on the dural sac, nerve root is normal and unaffected | Reduced size of the corner of the lateral canal or recess; trefoil shape to the lateral recess, either congenital or acquired; early acute angular narrowing of the corner of the canal and thecal sac; nerve root is visualized and not widened, flattened, or altered |
| 2 | Reduced contrast material within the lateral recess (not obliterated); indentation on the dural sac; nerve root is flattened, widened, or laterally pinched and medially displaced due to canal corner changes | Reduced size of the corner of the lateral canal or lateral recess, trefoil shape and narrowing of the lateral recess, angular pinch-like shape and narrowing of the lateral canal and thecal sac, nerve root judged compressed in the small trefoil recess or angled pinch but recess judged not totally obliterated, nerve root may be deviated medially |
| 3 | Complete obliteration of contrast material from the lateral recess or corner of the canal; thumbprint-like obliteration of contrast material in the lateral recess; severe lateral pinch of the corner of the canal with obliteration of contrast material at the lateral margin; nerve root is trapped in the thumbprint compression, widened-flattened on the thumbprint, displaced medially and widened and flattened laterally due to lateral canal corner changes | Severe facet hypertrophy and disc/end plate changes, no CSF or space identified in the lateral recess or corner of the canal, severe angular pinch of the lateral corner of the canal, root may or may not be clearly visible, root may be seen coursing through the compressed lateral recess, root may be seen as medially displaced |
Fig 2.
Illustrations shows grading system for lateral recess stenosis as revealed by myelography and MR imaging.
A, Grading system for trefoil lateral recess stenosis as revealed by myelography. Grade 0, appearance is normal. Grade 1, some narrowing of the lateral recess, with slight diminution of contrast material in the recess but no nerve root compression. Grade 2, further reduction in the size of the lateral recess, with some objectively identified nerve root flattening and reduced contrast material in the recess. Grade 3, complete obliteration of the lateral recess with a typical thumbprint-like appearance and complete obliteration of contrast material. The nerve root is compressed with visual widening and flattening.
B, Grading system for trefoil lateral recess stenosis as revealed by MR imaging. Grade 0, appearance is normal. Grade 1, narrowing of the lateral recess but no objective identification of root flattening or compression. Grade 2, further narrowing of the lateral recess with root flattening identified and some preservation of the space lateral to the root in the lateral recess. Grade 3, severe root compression with severe narrowing of the lateral recess and complete obliteration of any CSF space surrounding or lateral to the nerve root.
C, Grading system for acquired angular pinch lateral recess stenosis as revealed by myelography. Grade 0, appearance is normal. Grade 1, some narrowing of the lateral recess, with reduction of contrast material; some distortion of the anterior and posterolateral margin of the thecal sac due to facet or disk degenerative changes is usually seen. Grade 2, further narrowing of the corner of the canal, with reduction of contrast material in the lateral recess, some medial deflection of the nerve root, and some nerve root flattening due to compression; contrast material is still visualized lateral to the nerve root in the corner of the lateral recess. Grade 3, severe angular lateral recess compression with complete obliteration of contrast material lateral to the nerve root, root flattening and widening due to compression, and some medial root deflection.
D, Grading system for acquired angular pinch lateral recess stenosis as revealed by MR imaging. Grade 0, appearance is normal. Grade 1, early narrowing of the lateral recess due to anterior degenerative changes from disk bulge or endplate spur and posterolateral degenerative changes due to facet or ligament hypertrophy; nerve root is medially displaced, but no objective evidence of root flattening or compression is noted. Grade 2, further narrowing of the corner of the canal due to endplate, disk, and facet degenerative changes with early root compression identified; root is slightly widened or flattened and may be medially displaced and contrast material is still identified lateral to the nerve root. Grade 3, severe lateral recess impingement with definite root compression, no contrast material identified lateral to the root in the corner of the canal, and some medial root deflection.
Image Evaluation
Two neuroradiologists (W.S.B., L.L.) experienced in MR imaging, conventional myelography, and CT myelography independently reviewed the imaging studies. Readers were blinded to the clinical indication for the examination, which patients went on to receive surgical treatment, surgically treated levels, and surgical results. Levels L2–L3 through L5−S1 were evaluated on the basis of all imaging studies without knowledge of which levels elicited suspicion of degenerative root compression or which levels eventually underwent surgical treatment. Each lateral recess was evaluated and graded separately using the above-described 0- to 3-point scale.
Although all differences between observations could be considered significant, differences that reflected a call of root impingement (grades 2 and 3) on one study versus no root impingement (grades 0 and 1) on the comparison study were considered most relevant. We therefore focused on the relevant differences, which would suggest root compression by one technique or reader and no root compression by the other technique or reader.
Surgical Assessment
The surgical reports and charts were retrospectively reviewed to assess the operative findings at the time of surgery. In addition, initial presenting symptoms and clinical progress after surgery were recorded and tabulated.
Results
Surgical Results
In 10 patients, the preoperative symptoms were bilateral and bilateral lumbar decompression was performed. In 11 patients, the preoperative symptoms were unilateral and unilateral single or multilevel decompression was performed. In five patients, the primary leg pain was unilateral but imaging suggested a component of canal stenosis and bilateral decompression was performed. Surgical reports specifically described the canal appearance and presence or absence of root impingement at all surgically explored levels. Root compression was identified and documented for all 58 surgically explored lateral recesses at all 38 decompressed levels. All patients had complete or partial resolution of their preoperative pain by the time of discharge. In 10 (38%) patients, complete preoperative pain relief was reported, and in 15 (58%), moderate to significant pain improvement was documented. In one patient, preoperative pain was reported as improving, but slowly. In a second patient, preoperative leg pain had improved but a preoperative foot drop had not resolved by the time of discharge.
Imaging Results
MR imaging-, conventional myelography-, and CT myelography-based predictions of lateral recess root compression that was later surgically confirmed are summarized in Table 2. MR imaging predicted lateral recess root compression in 41 to 42 recesses (71% to 72%) but failed to identify root compression in 16 to 17 (28% to 29%) where impingement was documented surgically. (Figs 3–6) In eight instances, both readers failed to appreciate root compression at the same location. Both experienced readers had similar overall results of predicting root impingement by either technique.
TABLE 2:
Identification of root compression in 58 surgically confirmed lateral recesses
| Myelography |
CT Myelography |
MR Imaging |
||||
|---|---|---|---|---|---|---|
| Root Compression Grades 2 and 3 | No Root Compression Grades 0 and 1 | Root Compression Grades 2 and 3 | No Root Compression Grades 0 and 1 | Root Compression Grades 2 and 3 | No Root Compression Grades 0 and 1 | |
| Reader 1 | 54 | 4 | 36 | 22 | 41 | 17 |
| Reader 2 | 55 | 3 | 36 | 22 | 42 | 16 |
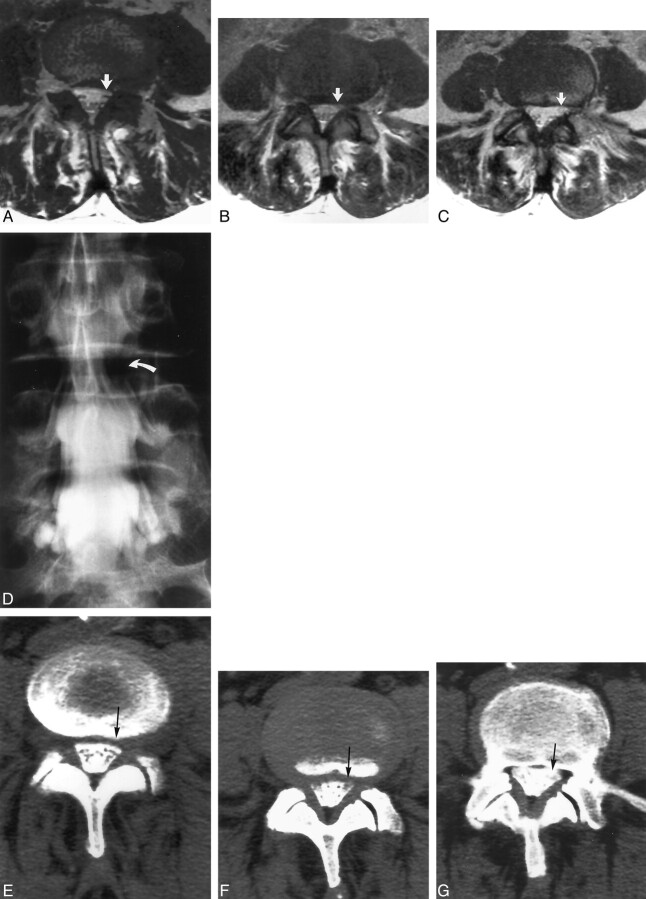
Fig 3.
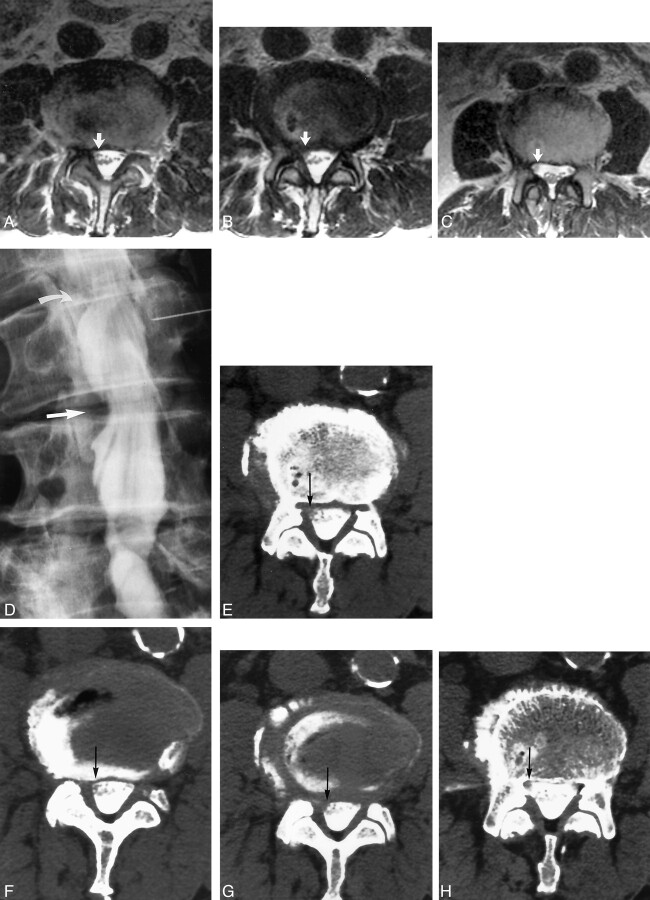
Images of a 66-year-old man with left leg pain and weakness.
A−C, Contiguous axial view T2-weighted MR images obtained at the L4–L5 level. Canal distortion is present on the right at the disk margin. Narrowing of the left lateral recess was judged to be root compression by one observer but only canal distortion by the other observer because of visualization of the roots free within the canal in the left lateral recess region (arrows).
D, Conventional myelogram shows left lateral recess root compression at L4–L5 (curved arrow). Root compression was confirmed at surgery. The patient achieved complete recovery from leg pain after decompression.
E−G, Contiguous axial view post-myelogram CT images obtained at the L4–L5 level show slight canal distortion on the right but a normal appearing left lateral recess (arrows), similar to the findings of the MR imaging study. Both observers labeled this left lateral recess as noncompressive (grade 1).
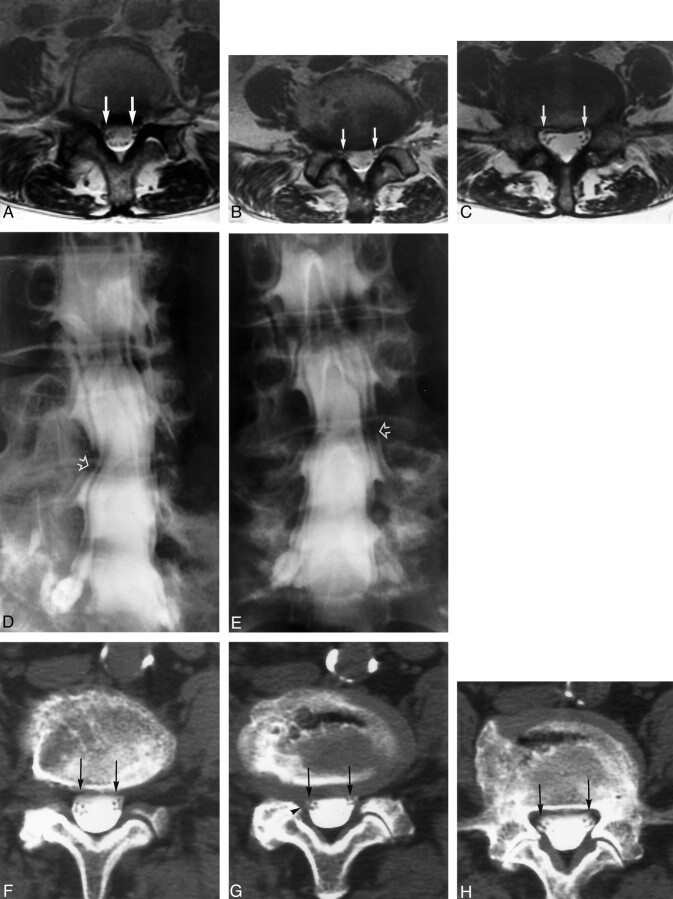
Fig 6.
Images of a 71-year-old female with left leg pain.
A−C, Contiguous axial view T2-weighted MR images obtained at the L4–L5 level were judged as normal bilaterally and assessed as grades 0 and 1 by both observers (arrows).
D and E, Conventional oblique and anteroposterior myelogram images were judged grade 2 bilaterally by both observers at L4–L5 (open arrows). Decompressive laminectomy at L4–L5 reported bilateral root compression in the lateral recess at L4–L5. The patient experienced significant improvement in leg pain at the time of discharge.
F−H, Contiguous axial post-myelogram CT images obtained at the L4–L5 level show some canal asymmetry in the lateral recesses (arrows), with slight distortion of the canal in the lateral recess on the right (G, arrowhead). One observer labeled this distortion as root compressive, whereas the other labeled this level as normal in the lateral recesses bilaterally.
Root compression was predicted by using conventional myelography in 54 to 55 (93% to 95%) lateral recesses for which impingement was confirmed surgically. Conventional myelography failed to predict root compression in three to four (5% to 7%) instances (Fig 7). In three instances, both readers failed to appreciate root compression at the same level.
Fig 7.
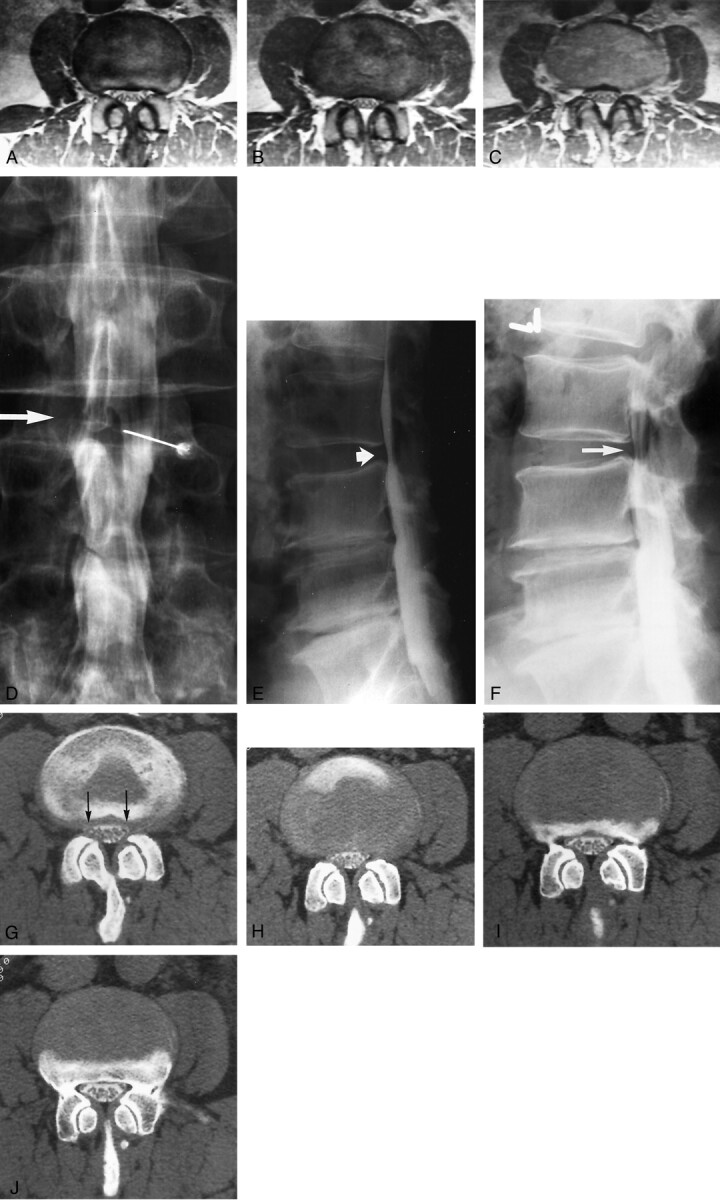
Images of a 56-year-old man with right leg pain and anterior thigh pain. Neurogenic claudication was not present. The patient had a history of surgery at L3–L4 and L4–L5.
A−C, Contiguous axial view proton density–weighted MR images show a small canal at L2–L3 but no overt evidence of root compression. Both observers labeled this as grades 0 and 1 bilaterally.
D, Anteroposterior view conventional myelogram obtained with the patient in the prone position shows a relatively normal canal at L2–L3 (arrow).
E, Lateral view conventional myelogram obtained with the patient in the prone position shows a normal canal at L2–L3 (short arrow).
F, Lateral view conventional myelogram obtained at the L2–L3 level with the patient in the standing extended position shows reduction in canal size with some posterior defect likely related to buckling of the ligamentum flavum while in extension, leading to some degree of spinal stenosis (arrow). Surgical findings documented lateral recess root compression bilaterally at L2–L3. Postoperatively, the patient achieved moderate recovery of strength and sensation, with improvement in right leg pain by the time of discharge.
G−J, Contiguous axial post-myelogram CT images obtained at the L2–L3 level show a small canal and slight lateral recess distortion bilaterally (G, arrows). One observer graded this level as small lateral recesses but no root compression (grade 1), and the other observer graded this as root compressive (grade 2).
Root compression was predicted by CT myelography in 36 (62%) lateral recesses by both readers, but the readers failed to identify root compression in 22 (38%) for which impingement was documented surgically. In 14 instances, both readers failed to appreciate root compression as shown by CT myelography at the same location.
Discussion
Ciric et al (13) and Mikhael et al (23) popularized lateral recess stenosis. Lee et al (24) further defined the anatomy of lateral canal stenosis and the lateral recess. The lateral recess is the region of the lumbar canal that is bordered laterally by the pedicle, posteriorly by the superior articular facet and ligamentum flavum, and anteriorly by the vertebral body, endplate margin, and disk margin. This region corresponds to the supra-axillary region as defined by Wilmink (22). Measurements of the bone margins of the lateral recess suggest narrowing with possible root compression when the anteroposterior dimension is below 4 mm (13, 23).
Lateral recess stenosis develops in two basic ways, as shown in Figure 1. If a congenital trefoil-shaped lateral recess is present initially, the recess becomes smaller and root compression occurs as the disk margin enlarges (because of endplate spur or disk bulge) or the superior articular facet hypertrophies.
In the triangular-shaped canal, progressive facet, endplate, and disk margin changes act together to alter the shape of the lateral recess region (Fig 1). If early facet hypertrophy occurs, a trefoil shape of the spinal canal ensues, resembling the congenital trefoil arrangement (Fig 1, column 2). The laterally positioned nerve root becomes compressed by further facet growth or disk margin change. This creates a degenerative trefoil arrangement.
If facet, endplate, and disk margin changes occur simultaneously (Fig 1, column 3), an acute angled shape to the lateral margin of the canal ensues and the nerve root becomes displaced, pinched, and compressed within this region. This creates root compression in the lateral recess region with a pinch-like arrangement.
The above description establishes two types of lateral recess impingement that can be identified. In the congenital or acquired trefoil canal, the nerve root lies in a lateral position and becomes compressed in an anteroposterior fashion within the lateral recess niche. This creates a thumbprint-like compression identified by conventional myelography and an anteroposterior narrowing of the lateral recess niche as shown on axial images. This is similar to the traditional description of lateral recess stenosis first described by Ciric et al (13) and Mikhael et al (23). For simplicity, we refer to this as trefoil lateral recess compression.
In the canal that maintains a more triangular shape but develops simultaneous facet, endplate, and disk margin degenerative changes, the lateral recess develops an acute angle. The nerve root may be compressed and deflected medially between the endplate or disk margin and the facet or ligamentum flavum in the acutely angled corner of the canal. We refer to this as angular lateral recess compression. This is similar to flattening of the ventrolateral angle in the supra-axillary region as described by Wilmink (22). Overlap between these two types of compression can occur.
The cause of radiculopathy is complex and controversial. The classic report presented by Mixter and Barr (25) initiated widespread focus on disk protrusion and root compression as an important cause. Spinal stenosis as a distinct syndrome was popularized by Verbiest (26). Radiculopathy associated with a stenotic spinal canal or lateral recess is well recognized (13, 27, 28). Degenerative facet disease is known to cause radiating pain in the absence of disk protrusion or root compression and is frequently relieved by joint injection (29, 30).
The significance of root compression in association with disk protrusion or degenerative disease has become less clear. Root compression in the absence of symptoms has been described by many authors (7–11). This includes compression due to disk protrusion as well as degenerative disease. Recently, epidural and root inflammation caused by components of the nucleus pulposus has gained great popularity as a primary cause of radiculopathy in degenerative spine disease (1–6).
The importance of a compressed or stretched nerve root should not be summarily dismissed. Several studies have shown that irritated nerve roots subjected to mechanical compression or stretch can reproduce the symptoms of radiculopathy (31, 32). Acute mechanical compression has been shown to result in root edema and inflammation (33–40). The extent of root edema and degree of root dysfunction increased with the applied compression force and duration (35, 36). The compression force required to induce these changes were typically in the range of 100 to 200 mm Hg. The rate of compression delivery was also important. Compression delivered with a rapid onset rate, such as 0.05 to 0.1 seconds, induced greater nerve dysfunction and edema (35, 36).
Biomechanical factors can dramatically alter the spine. Nerve root motion occurring with leg motion has been shown in several studies associated with straight leg raising (41–45). Experimental weight bearing has been shown to change the size of the disk and neural foramen (46). Flexion and extension can change the size of the spinal canal, lateral recess, and neural foramen, leading to changes in cauda equina or isolated root compression (47, 48). Spinal canal pressure measurements in patients with spinal stenosis reveal canal pressures in the range of 100 mm Hg, similar to the experimental pressures that induce root edema and dysfunction (49). It is not difficult to reason that a sudden mechanical change to the spine could lead to acute root compression resulting in root inflammation and edema. A chronically compressed and irritated root could experience difficulty in recovery.
Clinical factors also support the relevance of root compression as an important parameter. Spinal stenosis is a compressive process that results in radiating leg discomfort or neurogenic claudication (12). Surgical decompression relieves leg symptoms for these patients. Lateral recess stenosis frequently occurs in the absence of disk protrusion but clinically presents with similar radiculopathy (13, 50). Spine surgeons seek confirmation of root impingement as support for pursuing surgical decompression. Although MR imaging is considered to be a useful screening technique, obtaining conventional myelograms and CT myelograms is encouraged by spine surgeons to confirm root compression and identify all affected levels (12, 50).
The accuracy of cross-sectional imaging in predicting root impingement was questioned by Wilmink (22). In his comprehensive evaluation, the imaging features of root compression were defined and comparison was made between plain CT and conventional myelography. His study included a broad group of patients with both disk protrusion and degenerative causes of root compression. Conventional myelography was used as the criterion standard, and results were compared by several observers. Interobserver correlation for conventional myelography was found to be good but not perfect. Correlation between plain CT and conventional myelography in predicting root compression was surprisingly poor. Plain CT underestimated root impingement documented by conventional myelography for a significant number of compressed roots. This observation was consistent among several observers and included more than one observation opportunity. Wilmink noted problems predicting root compression with both disk protrusion and degenerative spine changes but noted the greatest degree of discordance with degenerative disease in the supra-axillary region or lateral recess.
Our results parallel the findings presented by Wilmink (22). Despite our being familiar with the data presented by Wilmink, we encountered considerable difficulty in predicting root compression in the lateral recess when using MR imaging. MR imaging failed to predict root compression in the lateral recess in 28% to 29% of the cases for which root impingement was documented at operative inspection. Although false-negative results still occur, conventional myelography was significantly more accurate at predicting lateral recess compression, with an accuracy rate of 93% to 95%.
Surprisingly, we also encountered great difficulty in identifying root compression in the lateral recess by using CT myelography. Root impingement in the lateral recess was not identified by CT myelography in 22 (38%) of the cases for which root compression was confirmed during surgical observation. The reason for suboptimal recognition of root compression by MR imaging and CT myelography is unclear. In most of these instances in which root compression was not predicted, degenerative changes were present but the root was visualized in the thecal sac, either free in the lateral recess or displaced medially but not overtly compressed.
The parallel between our results and the findings presented by Wilmink (22) are not surprising. Axial plain CT scans, CT myelograms, and MR images render a similar anatomic presentation with similar limitations displaying the features of root compression. The prediction of root compression in the lateral recess by plain CT, CT myelography, or MR imaging relies primarily on the degree of niche formation in the corner of the canal or angular pinch-like corner shape because the root is often not directly visualized. Root compression is more obvious when seen by using conventional myelography; identification follows from the compressed root appearance or exclusion of dye from an expected normal recess or niche.
Volume-acquired MR imaging or CT myelography sequences such as MR myelography or spiral CT with multi-planar reconstruction could potentially improve the identification of root compression in the lateral recess (51–54). The ability of these techniques to produce images of consistent quality along with their contribution to the prediction of root compression would require comprehensive and systematic assessment.
Additional factors may play a role in the differences in predicting root compression by MR imaging, conventional myelography, and CT myelography. A number of authors have indicated the changes in the size and shape of the spinal canal with variations in position (46–48). Flexion can open the canal, diminishing stenosis, and extension can worsen stenosis and root compression. Weight bearing is known to increase the dimensions of a disk bulge and to reduce corner sizes because of ligamentous buckling (46). These two features may accentuate myelographic representation of root compression, especially in the standing extended position. Flexion, such as caused by a protuberant abdomen, could also reduce the identification of root compression by myelography (Fig 7).
We chose to focus on degenerative root compression in the lateral recess for several reasons. Disk protrusion is defined when there is a focal bulge of the disk margin (7). The presence of this focal disk deformity by CT or MR imaging is the specific indicator of disk pathologic abnormality and alerts the radiologist to the possibility of root irritation. In addition, a clinician reading a report that identifies disk protrusion will naturally equate this finding with radiculopathy.
The features of degenerative disease create a different problem in image interpretation. Lumbar degenerative disease tends to be diffuse and to occur at multiple levels. Combinations of disk bulge or endplate spur along with facet and ligament hypertrophy usually coexist and leave an unreliable clue to possible root impingement. Itemizing these degenerative changes is nonspecific, and prediction of root impingement, considering this geometry, is unpredictable. Correct judgment of root compression may be critical to the ultimate management of these cases. Suspicion of lateral recess impingement by CT or MR imaging in the face of unexplained radiculopathy should result in further study, such as myelography, or referral to a neurosurgeon. Under-appreciated lateral recess impingement in a patient undergoing decompressive laminectomy could result in persistent radiculopathy and failed back syndrome. Seeing the degenerative changes but lacking objective identification of root impingement can create a significant case management problem. A legitimate root impingement problem may be missed under these circumstances.
Conclusion
In conclusion, although MR imaging is a superb screening study for the lumbar spine, lateral recess stenosis causing root compression may be missed by MR imaging. MR imaging underestimated lateral recess root compression in 28% to 29% of the cases for which the compression was documented at operative decompression, whereas conventional myelography failed to documented nerve impingement in only 5% to 7% of affected recesses. CT myelography was also inaccurate in the identification of lateral recess root impingement, underestimating root compression in 38% of affected recesses. In older patients in whom degenerative multilevel root impingement is typically present, MR imaging may miss root compression at a significant number of levels, especially within the lateral recesses. Even when MR imaging suggests lateral recess compression, conventional myelography may be a crucial preoperative study for comprehensive and reliable mapping of all compressed roots.
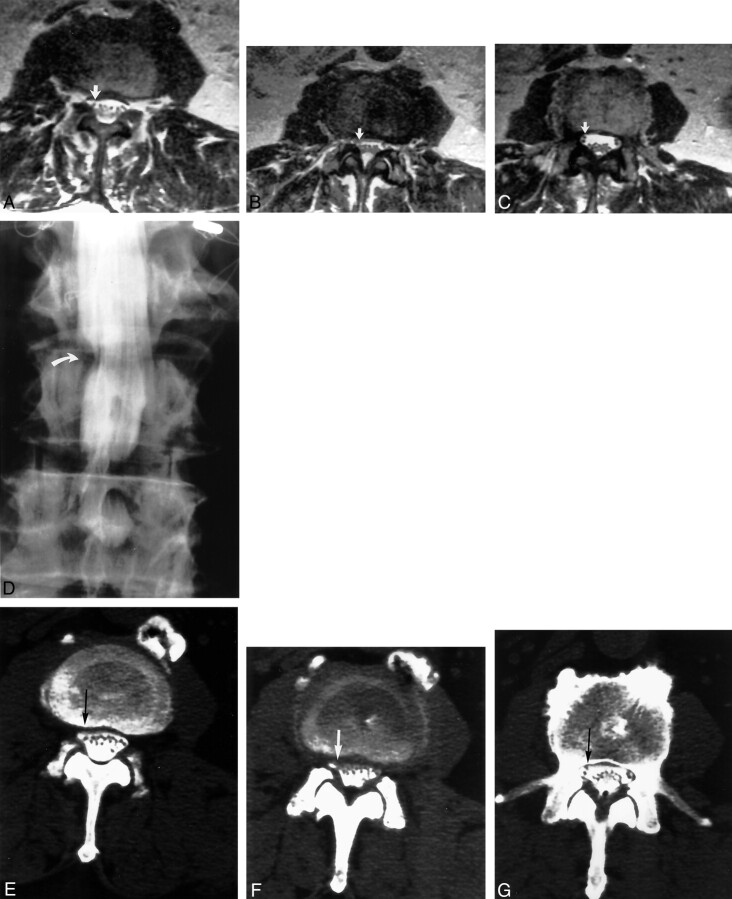
Fig 4.
Images of an 82-year-old man with right lower extremity weakness and pain, primarily in the upper leg and thigh. Electromyography suggested right L2, L3, and L4 abnormality.
A−C, Contiguous axial view T2-weighted MR images obtained at the L3–L4 level. Root compression was identified by one observer at L3–L4 on the right (arrows) but was labeled noncompressive (grade 1) by the second observer. Root compression was also correctly identified on the right by both observers at L2–L3 (grades 2 and 3).
D, Conventional myelogram shows right-sided root compression at L2–L3 (curved arrow) and L3–L4 (straight arrow), identified and assessed as grades 2 and 3 by both observers. The patient underwent right-sided keyhole decompression at L2–L3 and L3–L4. Severe root compression was surgically identified at both levels, and the patient achieved resolution of leg pain after surgical decompression.
E−H, Contiguous axial view post-myelogram CT images obtained at the L3–L4 level show slight angular distortion on the right lateral recess (arrows). The nerve roots within the canal are slightly more prominent at this level and may be somewhat edematous. Both observers labeled this lateral recess root compressive (grade 2).
Fig 5.
Images of a 70-year-old man with bilateral leg pain and weakness, with reduced sensation in both upper and lower legs.
A−C, Contiguous axial view T2-weighted MR images show a trefoil-shaped canal at L2–L3 that was judged to be root compression (grade 2) on the right by one observer because of the small recess size but was judged to be noncompressive (grade 0) by the other observer (arrows).
D, Conventional myelogram shows right-sided root compression at L2–L3 (curved arrow), assessed as grades 2 and 3 by both observers. Compression at L3–L4 was also identified by both observers by using MR imaging and conventional myelography. Surgical findings revealed evidence of root compression on the right at L2–L3 as well as at L3–L4. The patient was free of leg pain at the time of postoperative discharge.
E−G, Contiguous axial post-myelogram CT images obtained at the L2–L3 level show narrowing of the right lateral recess (arrows) with a normal appearance of the left lateral recess. One observer graded the right lateral recess as abnormal (grade 2), and the second observer graded this recess as narrow but not compressive (grade 1). Observer grading in this instance was reversed between MR imaging and CT myelography. One observer graded the MR imaging findings as root compressive but graded the CT myelography findings as not compressive. The other observer graded the MR imaging findings as compressive but graded the CT myelography findings as narrow but not root compressive.
References
- 1.McCarron RF, Wimpee MW, Hudkins PG, Laros GS. The inflammatory effect of nucleus pulposus: a possible element in the pathogenesis of low-back pain. Spine 1987;12:760–764 [DOI] [PubMed] [Google Scholar]
- 2.Franson RC, Saal JS, Saal JA. Human disc phospholipase A2 is inflammatory. Spine 1992[suppl 6] 17:S129–S132 [DOI] [PubMed] [Google Scholar]
- 3.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 1993;18:1425–1432 [PubMed] [Google Scholar]
- 4.Saal JS. The role of inflammation in lumbar pain. Spine 1995;20:1821–1827 [DOI] [PubMed] [Google Scholar]
- 5.Olmarker K, Blomquist J, Strömberg J, Nannmark U, Thomsen P, Rydevik B. Inflammatogenic properties of nucleus pulposus. Spine 1995;20:665–669 [DOI] [PubMed] [Google Scholar]
- 6.Kayama S, Konno S, Olmarker K, Yabuki S, Kikuchi S. Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes: an experimental study. Spine 1996;21:2539–2543 [DOI] [PubMed] [Google Scholar]
- 7.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med 1994;331:69–73 [DOI] [PubMed] [Google Scholar]
- 8.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am 1990;72:403–408 [PubMed] [Google Scholar]
- 9.Wiesel SW, Tsourmas N, Feffer HL, Citrin CM, Patronas N. A study of computer-assisted tomography: I. the incidence of positive CAT scans in an asymptomatic group of patients. Spine 1984;9:549–551 [PubMed] [Google Scholar]
- 10.Wilberger JE Jr, Pang D. Syndrome of the incidental herniated lumbar disc. J Neurosurg 1983;59:137–141 [DOI] [PubMed] [Google Scholar]
- 11.Hitselberger WE, Witten RM. Abnormal myelograms in asymptomatic patients. J Neurosurg 1968;28:204–206 [DOI] [PubMed] [Google Scholar]
- 12.Epstein JA, Epstein NE. Lumbar spondylosis and spinal stenosis. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGraw Hill;1996. :3831–3840
- 13.Ciric I, Mikhael MA, Tarkington JA, Vick NA. The lateral recess syndrome: a variant of spinal stenosis. J Neurosurg 1980;53:433–443 [DOI] [PubMed] [Google Scholar]
- 14.Tehranzadeh J. Discography 2000. Radiol Clin North Am 1998;36:463–495 [DOI] [PubMed] [Google Scholar]
- 15.Maldjian C, Mesgarzadeh M, Tehranzadeh J. Diagnostic and therapeutic features of facet and sacroiliac joint injection: anatomy, pathophysiology, and technique. Radiol Clin North Am 1998;36:497–508 [DOI] [PubMed] [Google Scholar]
- 16.Link SC, El-Khoury GY, Guilford WB. Percutaneous epidural and nerve root block and percutaneous lumbar sympatholysis. Radiol Clin North Am 1998;36:509–521 [DOI] [PubMed] [Google Scholar]
- 17.Murtagh R. The art and science of nerve root and facet blocks. Neuroimaging Clin North Am 1998;10:465–477 [PubMed] [Google Scholar]
- 18.Johnson BA. Image-guided epidural injections. Neuroimaging Clin North Am 1998;10:479–491 [PubMed] [Google Scholar]
- 19.Schellhas KP. Facet nerve blockade and radiofrequency neurotomy. Neuroimaging Clin North Am 1998;10:493–501 [PubMed] [Google Scholar]
- 20.Schellhas KP. Diskography. Neuroimaging Clin North Am 1998;10:579–596 [PubMed] [Google Scholar]
- 21.Grenier N, Kressel HY, Schiebler ML, Grossman RI, Dalinka MK. Normal and degenerative posterior spinal structures: MR imaging. Radiology 1987;165:517–525 [DOI] [PubMed] [Google Scholar]
- 22.Wilmink JT. CT morphology of intrathecal lumbosacral nerve-root compression. AJNR Am J Neuroradiol 1989;10:233–248 [PMC free article] [PubMed] [Google Scholar]
- 23.Mikhael MA, Ciric I, Tarkington JA, Vick NA. Neuroradiological evaluation of lateral recess syndrome. Radiology 1981;140:97–107 [DOI] [PubMed] [Google Scholar]
- 24.Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine 1989;13:313–320 [DOI] [PubMed] [Google Scholar]
- 25.Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 1934;211:210–215 [Google Scholar]
- 26.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 1954;36:230–237 [DOI] [PubMed] [Google Scholar]
- 27.Ehni G. Significance of the small lumbar spinal canal: cauda equina compression syndromes due to spondylosis. J Neurosurg 1969;31:490–494 [DOI] [PubMed] [Google Scholar]
- 28.Epstein JA, Epstein BS, Rosenthal AD, Carras R, Lavine LS. Sciatica caused by nerve root entrapment in the lateral recess: the superior facet syndrome. J Neurosurg 1972;36:584–589 [DOI] [PubMed] [Google Scholar]
- 29.Mooney V. Facet syndrome. In: Weinstein JN, Wiesel SW, eds. The Lumbar Spine: The International Society for the Study of the Lumbar Spine. Philadelphia: WB Saunders Company;1990. :422–441
- 30.Murtagh FR. Computed tomography guided anesthesia and steroid injection in the fact syndrome. In: Post JD, ed. Computed Tomography of the Spine. Baltimore: Williams & Wilkins;1984. :492–494
- 31.Smyth MJ, Wright V. Sciatica and the intervertebral disc: an experimental study. J Bone Joint Surg Am 1958;40:1401–1418 [PubMed] [Google Scholar]
- 32.Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am 1991;22:181–187 [PubMed] [Google Scholar]
- 33.Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded, experimental nerve compression. Scand J Plast Reconstr Surg 1977;11:179–187 [DOI] [PubMed] [Google Scholar]
- 34.Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984;9:7–15 [DOI] [PubMed] [Google Scholar]
- 35.Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression: an experimental study on the Pig Cauda Equina with special reference to differences in effects between rapid and slow onset of compression. Spine 1989;14:569–573 [PubMed] [Google Scholar]
- 36.Olmarker K, Holm S, Rydevik B. Importance of compression onset rate for the degree of impairment of impulse propagation in experimental compression injury of the porcine cauda equina. Spine 1990;15:416–419 [DOI] [PubMed] [Google Scholar]
- 37.Garfin SR, Rydevik BL, Brown RA. Compressive neuropathy of spinal nerve roots: a mechanical or biological problem? Spine 1991;16:162–166 [PubMed] [Google Scholar]
- 38.Rydevik B, Pedowitz RA, Hargens AR, Swenson MR, Myers RR, Garfin SR. Effects of acute, graded compression on spinal nerve root function and structure: an experimental study of the pig cauda equina. Spine 1991;16:487–493 [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi S, Yoshizawa H, Hachiya Y, Ukai T, Morita T. Vasogenic edema induced by compression injury to the spinal nerve root: distribution of intravenously injected protein tracers and gadolinium-enhanced magnetic resonance imaging. Spine 1993;18:1410–1424 [PubMed] [Google Scholar]
- 40.Garfin SR, Rydevik B, Lind B, Massie J. Spinal nerve root compression. Spine 1995;20:1810–1820 [DOI] [PubMed] [Google Scholar]
- 41.Smith SA, Massie JB, Chesnut R, Garfin SR. Straight leg raising. Anatomical effects on the spinal nerve root without and with fusion. Spine 1993;18:992–999 [PubMed] [Google Scholar]
- 42.Goddard MD, Reid JD. Movements induced by straight leg raising in the lumbo-sacral roots, nerves and plexus, and in the intrapelvic section of the sciatic nerve. J Neurol Neurosurg Psychiatry 1965;28:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charnley J, Manc MB. Orthopaedic signs in the diagnosis of disc protrusion with special reference to the straight-leg-raising test. Lancet 1951;1:186–192 [DOI] [PubMed] [Google Scholar]
- 44.Falconer MA, McGeorge M, Begg AC. Observations on the cause and mechanism of symptom-production in sciatica and low-back pain. J Neurol Neurosurg Psychiatr 1948;11:13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inman VT, Saunders JB. The clinico-anatomical aspects of the lumbosacral region. Radiology 1942;38:669–678 [Google Scholar]
- 46.Nowicki BH, Yu S, Reinartz J, Pintar F, Yoganandan N, Haughton VM. Effect of axial loading on neural foramina and nerve roots in the lumbar spine. Radiology 1990;176:433–437 [DOI] [PubMed] [Google Scholar]
- 47.Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine 1996;21:2412–2420 [DOI] [PubMed] [Google Scholar]
- 48.Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy: a dynamic CT-myelographic study. Spine 1987;12:488–500 [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Kagechika K, Takino T, Matsui T, Miyazaki T, Shima I. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine 1995;20:2746–2749 [DOI] [PubMed] [Google Scholar]
- 50.Ciric I, Mikhael MA. The spinal LR syndrome. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGraw Hill;1996. :3841–3845
- 51.Krudy AG. MR myelography using heavily T2-weighted fast spin-echo pulse sequences with fat presaturation. AJR Am J Roentgenol 1992;159:1315–1320 [DOI] [PubMed] [Google Scholar]
- 52.El Gammal T, Brooks BS, Freedy RM, Crews CE. MR myelography: imaging findings. AJR Am J Roentgenol 1995;164:173–177 [DOI] [PubMed] [Google Scholar]
- 53.Demaerel P, Bosmans H, Wilms G, et al. Rapid lumbar spine MR myelography using rapid acquisition with relaxation enhancement. AJR Am J Roentgenol 1997;168:377–378 [DOI] [PubMed] [Google Scholar]
- 54.El Gammal TA, Crews CE. MR myelography of the cervical spine. Radiographics 1996;16:77–88 [DOI] [PubMed] [Google Scholar]