Abstract
BACKGROUND AND PURPOSE: Identification of the intracranial collaterals assists in identifying patients with severe occlusive disease of the internal carotid arteries who are at lower risk of transient ischemic attacks (TIAs) and stroke. We investigated the usefulness of MR angiography in identifying functional collaterals of the circle of Willis.
METHODS: MR angiography of the circle of Willis was performed in 50 healthy volunteers. Visibility was used as the criterion to define the intracranial collaterals as being functional. Two observers independently assessed the MR angiograms. Results were compared with those of transcranial color duplex sonography (TCCD), and results of carotid compression tests were the standard of reference for the identification of functional intracranial collaterals.
RESULTS: With MR angiograms, reviewer 1 achieved a sensitivity of 85%, a specificity of 81%, a positive predictive value of 95%, and a negative predictive value of 55%. Reviewer 2 achieved a sensitivity of 87%, a specificity of 67%, a positive predictive value of 92%, and a negative predictive value of 53%. Interobserver agreement on MR angiograms was moderate (κ = 0.57, 95% confidence interval: 0.42, 0.72).
CONCLUSION: Visible collaterals of the circle of Willis on MR angiograms are able to supply collateral flow in the presence of carotid artery obstruction. However, the low negative predictive value of MR angiography indicates that, if collaterals are not visible, supplementary TCCD investigation is required.
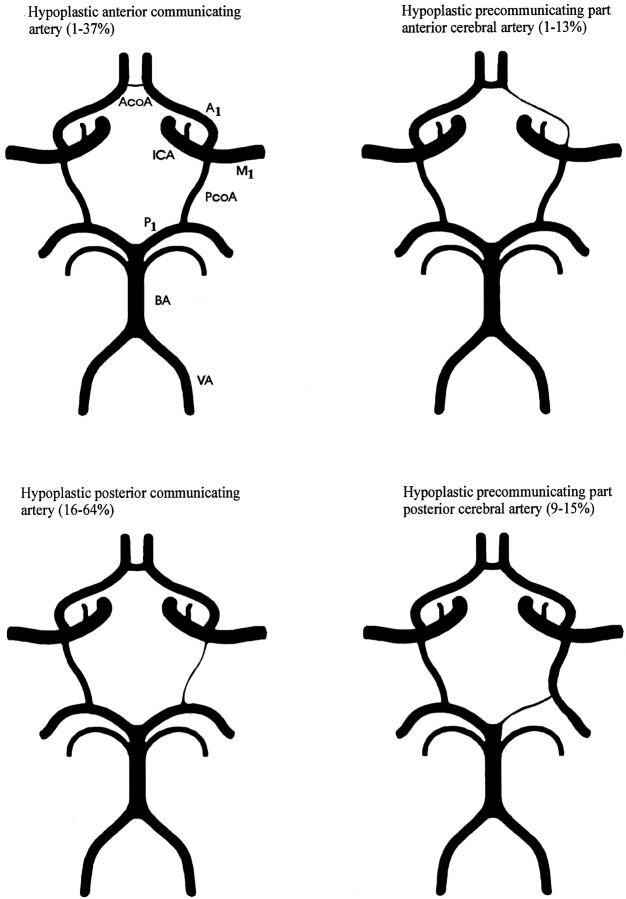
The circle of Willis, located at the base of the brain, provides the main route for collateral blood flow in severe occlusive disease of the internal carotid artery (ICA). Patients with an anterior communicating artery (AcoA) and a posterior communicating artery (PcoA) that supply the hemisphere distal to a severe ICA stenosis have a risk of transient ischemic attack (TIA) and stroke that is lower than that of patients without such collaterals (1). Furthermore, patients undergoing carotid endarterectomy who have collaterals supplying the operative side are less likely to have a perioperative stroke (1). Unfortunately, hypoplasia of vessel segments frequently hampers the collateral function of the circle of Willis (Fig 1). Identification of intracranial collaterals assists in identifying patients with severe ICA occlusive disease at lower risk of TIA and stroke (1). The prognostic value of functioning intracranial collaterals in patients with occlusive disease of the carotid artery is the subject of ongoing studies (2). Knowledge of the collateral ability of the circle of Willis is important for neurosurgeons, vascular surgeons, and interventional radiologists when a procedure in the intracranial or extracranial cerebral arteries is to be attempted.
Fig 1.
Examples and prevalence of circle of Willis anomalies that hamper collateral function. Prevalence is derived from studies by using an external diameter of 1 mm as a threshold for hypoplasia of collateral arteries. A1 indicates the precommunicating part of the anterior cerebral artery; M1, main trunk of the middle cerebral artery; P1, precommunicating part of the posterior cerebral artery; BA, basilar artery; and VA, vertebral artery.
MR angiography is an attractive, noninvasive technique for assessing the anatomy of the circle of Willis (3). MR angiography, when compared with conventional angiography (Table 1), has had a moderate to high level of sensitivity and specificity in the identification of intracranial collaterals (4, 5). However, it is not clear whether opacification of intracranial collaterals on conventional angiograms or MR angiograms proves that they can act as functional collaterals when carotid arteries become stenotic or occluded. Furthermore, the results of these studies (4, 5) were obtained from a heterogeneous group of patients with intracranial aneurysms, arteriovenous malformations, tumors, subarachnoid or intracerebral hemorrhages, steno-occlusive disease, or strokes; this variety impedes unambiguous conclusions.
TABLE 1:
MR angiography compared with conventional angiography for the detection of intracranial collaterals
| Author* | No. of Subjects | AcoA |
PcoA |
||
|---|---|---|---|---|---|
| Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % | ||
| Patrux et al (4) | 54 | 89 | 100 | 81 | 100 |
| Stock et al (5) | 62 | 67 | 73 | 75 | 93 |
Numbers in parentheses are reference citations.
The aim of our study was to assess whether MR angiography is useful in identifying functional intracranial collaterals. We performed MR angiography of the circle of Willis in healthy subjects, and the results were compared with those of transcranial color duplex sonography (TCCD). This was combined with carotid compression tests, the results of which were considered the standard of reference for the assessment of the collateral function of the circle of Willis (6–8). In this study, collateral vessels were defined as functional when they showed the potential to act as collaterals. This definition did not imply that these vascular pathways would or would not serve as adequate collaterals to maintain normal tissue perfusion in cases of obstruction of the carotid arteries.
Methods
Subjects
After the study was approved by the local ethics committee, subjects were recruited through an advertisement in a regional newspaper. Fifty-six healthy volunteers, 28 men and 28 women with no known history of cerebrovascular disease, were included the study. Written informed consent was obtained from each volunteer in accordance with the requirements of the local ethics committee.
TCCD Examination
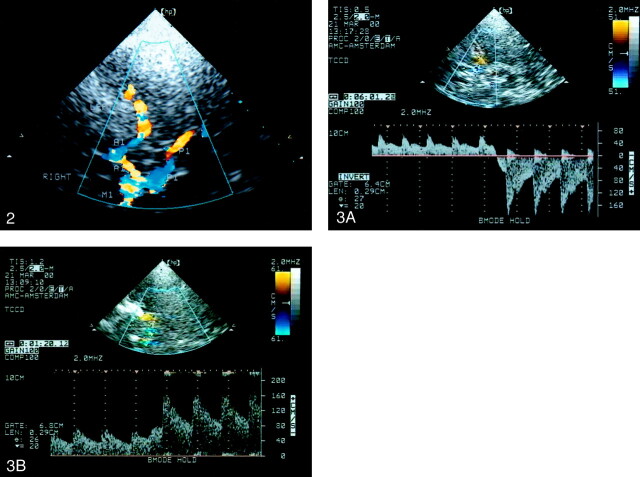
Duplex scanning of the extracranial arteries supplying the brain (4.5–5.5-MHz transducer, Sonos 2000; Hewlett Packard, Andover, MA) preceded transcranial investigation to exclude those subjects with possible extracranial arterial occlusive disease or carotid plaques preventing carotid compression. TCCD was performed with a low-frequency (2.0–2.5-MHz) transducer. Insonation of the main trunk of the middle cerebral artery, the precommunicating parts (A1) of both anterior cerebral arteries, and the precommunicating parts (P1) of both posterior cerebral arteries was performed in the standard manner via the temporal window (Fig 2). The details are reported elsewhere (9, 10).
Fig 2.
TCCD sonogram of the circle of Willis in the left temporal window and axial scanning plane at the level of the mesencephalon. The ipsilateral (left) M1, P1 (red), and A1 (blue) are shown. The contralateral (right) M1 and P1 (blue) and A1 (red) are also shown.
In each patient, the collateral function of the three primary collateral pathways—the AcoA and the right and left PcoAs—was determined. Because of its minimal size and low-flow state, the AcoA usually cannot be visualized by means of TCCD. In most cases, direct insonation of the PcoA is impeded by its low-flow state, the high frequency of hypoplasia (11), and its unfavorable position in relation to the sonography beam. For reliable assessment of the functional patency of both collaterals, carotid compression tests are required (12, 13). Collateral function of the AcoA was proved when blood flow in the A1 reversed during ipsilateral common carotid artery compression (Fig 3A). If blood flow reversal in the A1 could not be provoked with compression of the carotid artery, the anterior collateral pathway was defined as nonfunctional. Both A1 segments were routinely investigated by using carotid compression tests. Functional patency of the PcoA was defined by a peak systolic velocity (PSV) increase of more than 20% in the P1 segment of the posterior cerebral artery during ipsilateral carotid compression (Fig 3B), with this value being twice that expected from normal variation and measurement error (13). If the PSV increase in P1 was less than 20%, the posterior collateral pathway was defined as nonfunctional. In the case of a fetal-type posterior cerebral artery, a common anatomic variation in the circle of Willis, the PcoA is enlarged and accompanied by a thin or hypoplastic P1 (Fig 1). Such a large PcoA can be detected by using TCCD, enabling direct velocity measurements. In this type of posterior circle configuration, the flow direction in the PcoA is directed from the ICA to the posterior cerebral artery. If ipsilateral carotid compression caused a flow decrease instead of flow reversal in the PcoA, the posterior collateral pathway was defined as nonfunctional. To avoid artifacts due to turbulence near the origins of the communicating arteries when provoking collateral flow, velocity measurements were taken proximally in the A1 and P1, with the sample volume set as narrowly as possible.
Fig 3.
Doppler spectra.
A, Spectrum shows blood flow reversal in the A1 during ipsilateral carotid compression indicating functional patency of the AcoA.
B, Spectrum shows blood flow velocity increase of more than 20% in the P1 during ipsilateral carotid compression indicating functional patency of the PcoA.
Compressions of the common carotid artery were applied for three to five cardiac cycles, low in the neck just proximal to the sternal head of the clavicle, to avoid a systemic cardiovascular reaction. To ensure the effectiveness of the compression, a photoplethysmograph that generated pulse tracings on a separate monitor was attached to the earlobe on the side of the compressed artery. Flattening of this pulse wave indicated cessation of blood flow through the common carotid artery and, thus, adequate compression.
MR Angiography
Three-dimensional (3D) time-of-flight (TOF) MR angiography of the circle of Willis was performed by using a 1.5-T MR system (Magnetom Vision; Siemens, Erlangen, Germany) within approximately 30 minutes of the TCCD examination. MR angiography was performed only in those subjects in whom TCCD examination could be performed successfully. The following imaging parameters were used: 39/6.5 (TR/TE), 20° flip angle, 200 × 150-mm field of view, 225 × 512 matrix, 40 sections with an 0.8-mm effective thickness that resulted in the coverage of a volume of 32 mm in the craniocaudal direction, and 0.67 × 0.39-mm pixel resolution (0.26-mm2 pixel area). Maximum intensity projection (MIP) images were produced at 12 equally spaced intervals perpendicular to the left-right axis that covered a total of 180° of rotation (15° per interval). The 3D TOF stack was positioned by using a three-plane, two-dimensional phase-contrast survey image. The total imaging time, including acquisition of the survey image and positioning, was approximately 10 minutes.
Interpretation was based on examination of the MR images, the single partitions of the volume of MR angiographic sequences, and the targeted MIPs of the vasculature. Two neuroradiologists (C.B.L.M., F-J.H.H.) independently reviewed the images from the individual examinations in a blinded fashion. The seven arteries evaluated included the AcoA, the A1 segments, the P1 segments, and the left and right PcoAs. A forced-choice method (visible or invisible) was used in the decision analysis. The signal intensity and caliber of the patent collateral arteries were variable, and identifying their whole length to ensure that their lumen was patent was not necessary. Special care was taken to differentiate the PcoAs from the anterior choroidal arteries by scrolling through the sections and sequentially determining the course of the arteries. The communication of the PcoA with the posterior cerebral artery had to be visualized to define the PcoA.
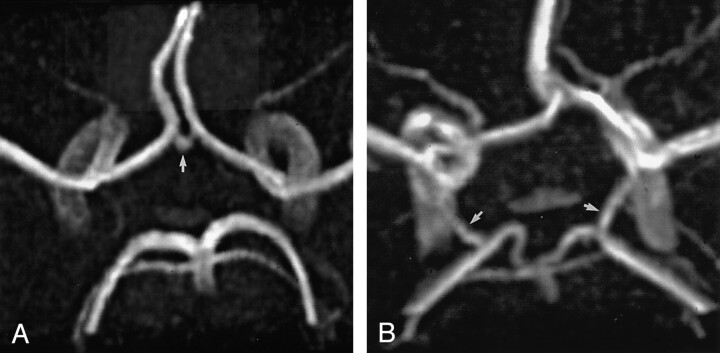
We hypothesized that visible collateral arteries on MR angiograms were patent and potentially capable of supplying collateral flow. Arteries that could not be visualized were defined as hypoplastic. Collateral flow through the anterior collateral pathway of the circle of Willis was deemed possible if both A1 segments connected by an AcoA were visible (Fig 4A). In cases of fusion of the anterior cerebral arteries, the anterior collateral pathway was also defined as functional. The posterior collateral pathway was defined as functional if the P1 segment and its accompanying PcoA were visible (Fig 4G). A vessel stem from the ICA that showed a diameter larger than the diameter of the ipsilateral P1 and that continued distally as the posterior cerebral artery was classified as fetal-type posterior cerebral artery (Fig 1D). Vessel stems from the ICA that showed diameters equal to or smaller than the diameter of the ipsilateral P1 were classified as PcoA. Length and diameter measurements of the collateral arteries were not taken.
Fig 4.
3D TOF MR angiograms.
A, Angiogram of the circle of Willis with the complete anterior configuration shows the AcoA (arrow).
B, Angiogram of the circle of Willis with the complete posterior configuration shows the right and left PcoAs (arrows)
Data Analysis
Two neuroradiologists (C.B.L.M., F-J.H.H.) who were unaware of the TCCD findings independently judged the visibility of the vessels forming the anterior and two posterior collateral pathways on the MR angiograms. MR results were then compared with the findings of TCCD examinations. We determined the sensitivity, specificity, positive and negative predictive values, and the interobserver agreement for the identification of functional intracranial collateral pathways with MR angiography. Agreement was considered poor if κ was 0.40 or lower, moderate if κ was between 0.41 and 0.60, and good if κ was 0.61 or higher. At the end of the study, the reasons for disagreement were analyzed by re-reviewing the data in a consensus meeting.
Results
Subjects
One of the 56 volunteers was excluded because of >50% stenosis of both ICAs. In four subjects, TCCD examination could not be performed because of bilateral impenetrable temporal windows. In one case, the MR examination had to be stopped because the volunteer had claustrophobia. In a total of 50 subjects, 25 men and 25 women with a mean age of 51 years (range, 28–74 years), both MR angiography and TCCD was successfully performed.
TCCD Examination
In three (6%) subjects, carotid compression did not result in cross-flow through the anterior collateral pathway. Two subjects had a nonfunctional AcoA and one subject had a nonfunctional right A1 segment. In 24 (24%) posterior collateral pathways, collateral flow could not be provoked by carotid compression. This was due to a nonfunctional PcoA in 17 (17%) cases and a nonfunctional P1 segment in seven (7%) cases. Twenty-five (25%) PcoAs could be visualized with TCCD, enabling direct velocity measurements. In 24 of these PcoAs, the blood flow direction in the resting state was toward the basilar artery. Blood flow in 17 visualized PcoAs reversed on ipsilateral carotid compression; this observation indicated a functional posterior collateral pathway. In the other seven PcoAs that could be visualized, blood-flow velocity decreased on ipsilateral carotid compression; this indicated a nonfunctional posterior collateral pathway. In one PcoA that showed a blood-flow direction toward the ICA, carotid compression caused a marked velocity enhancement; this indicated a functional posterior collateral pathway.
MR Angiography
Circle Anomalies
In all subjects, scrolling through the axial source images was required to correctly identify vessel segments and to determine the course and communication of the collateral arteries. In two subjects, a fusion of the anterior cerebral arteries over a short distance was found, and in one subject, the anterior cerebral arteries formed a common trunk and split distally into two A2 (postcommunicating) segments. One subject had a double AcoA, and in another, a third anterior cerebral artery arising from the AcoA was found. One subject had duplication of the left A1 segment, and in another subject, the right A1 segment was missing. In 17 (17%) cases, a fetal posterior cerebral artery was found, showing a PcoA with a caliber larger than that of the ipsilateral P1. In one case, calibers of the PcoA and P1 were approximately similar. In the remaining 82 (82%) cases, the P1 had a diameter greater than that of the ipsilateral PcoA. In two subjects, a duplication of the left superior cerebellar artery was found.
Collateral Pathways
The anterior collateral pathway was judged nonfunctional in nine (18%) subjects by reviewer 1 and in six (12%) subjects by reviewer 2. The posterior collateral pathway was judged nonfunctional in 31 (31%) cases by reviewer 1 and in 28 (28%) cases by reviewer 2. The sensitivity, specificity, and positive and negative predictive values of MR angiography for the identification of functional collateral pathways are shown in Table 2. There were no significant differences between the two observers. Table 3 shows the interobserver agreement. After consensus review of the data, the anterior and posterior collateral pathway were judged nonfunctional in six (12%) and 29 (29%) cases, respectively.
TABLE 2:
Detection of collateral pathways (n = 150) in 50 healthy subjects
|
Number of findings |
|||
|---|---|---|---|
| Detection with MR Angiography* | Detection with TCCD* |
Total | |
| Yes | No | ||
| Observer 1 | |||
| Yes | 105 | 5 | 110 |
| No | 18 | 22 | 40 |
| Total | 123 | 27 | 150 |
| Observer 2 | |||
| Yes | 107 | 9 | 116 |
| No | 16 | 18 | 34 |
| Total | 123 | 27 | 150 |
|
Diagnostic discrimination |
|||
|---|---|---|---|
| Measure | Observer 1† | Observer 2† | |
| Sensitivity, % | 85 (79, 92) | 87 (81, 93) | |
| Specificity, % | 81 (62, 94) | 67 (46, 84) | |
| Positive predictive value, % | 95 (90, 99) | 92 (86, 96) | |
| Negative predictive value, % | 55 (39, 71) | 53 (35, 70) |
Yes indicates functional. No indicates nonfunctional.
Numbers in parentheses are the 95% confidence intervals.
TABLE 3:
Interobserver agreement with MR angiography for the detection of collateral pathways (n = 150)*
κ = 0.57 (95% confidence interval: 0.42, 0.72).
Yes indicates functional. No indicates nonfunctional.
Discussion
The results of this study show that, despite the fact that MR angiography lacks the potential of carotid compression tests, its sensitivity in identifying nonrecruited but potentially functional intracranial collaterals is relatively high (Table 2). However, the technique failed in identifying potential functional collaterals in a relatively high number of cases, resulting in a low negative predictive value. The interobserver agreement (Table 3) was moderate. Disagreement between the observers was mainly caused by the often small collaterals. In cases in which the observers were uncertain about the visibility of collaterals, the strictly applied forced-choice method often resulted in disagreement. However, after consensus review of the MR angiograms, some pitfalls also arose. The anterior choroidal artery (an intracranial branch of the ICA) runs very close, and in several sections of the MR angiographic source images parallel, to the PcoA. In some sections, the anterior choroidal artery can run so close to the posterior cerebral artery that it appears to be connected to it. When the PcoA is hypoplastic or aplastic, such anterior choroidal arteries can easily be misinterpreted as the PcoA. In two subjects who had a duplication of the superior cerebellar artery and a hypoplastic ipsilateral P1, one of the reviewers wrongly identified the most superiorly located branch of the duplicated superior cerebellar artery as P1.
An important advantage of MR angiography compared with TCCD is that the collateral anatomy of the circle of Willis can be assessed in most subjects. In this series, the failure rate was only 2% because of claustrophobia of one volunteer. In contrast, the success of TCCD depends on the quality of the temporal window, which is determined by the patient’s age, sex, and race. The failure rate of TCCD in identifying the individual intracranial arteries in white men older than 60 years is less than 10%; however, in elderly white women, the failure rate might be as high as 30–40% (14). In this series, the failure rate of TCCD was 7%. The main limitation of MR angiography is its limited resolution, which hampers the detection of small but potentially functional collaterals. The identification of arteries with a diameter less than 0.8 mm is difficult, because their diameter is smaller than the currently used pixel size of 0.79 × 0.79 mm. However, approximately 40% of the PcoAs have a diameter less than 0.8 mm (15), and the threshold diameter for supplying collateral flow is even smaller, namely between 0.4 and 0.6 mm (16–18) This implies that a number of functional collaterals with a diameter less than 0.8 mm might not be detected by using MR angiography. It could also explain the relatively high number of false-negative functional collaterals in our study (Table 2). On the other hand, the low number of false-positive functional collaterals (Table 2) indicates that communicating arteries that can be visualized, even those that are barely visible, are capable of supplying collateral flow. With the continuous improvement of MR techniques, difficulties in the identification of very small intracranial vessels may soon diminish.
Detection of the AcoA and PcoA with MR angiography may be hindered for a number of reasons other than limited resolution. The AcoA has a mean length of 2.6 mm, but it can be as short as 0.3 mm (19). In such cases, the proximal parts of the anterior cerebral arteries are close to each other. Furthermore, blood flow in the anterior cerebral arteries is higher than in the AcoA, resulting in higher signal intensity. This combines to make detection of the AcoA impossible. One method that might improve detection of the AcoA is to apply a saturation band across the contralateral cavernous carotid artery. This might improve visualization of the AcoA, and it may also provide information regarding the contribution of flow from the ipsilateral carotid artery to the anterior cerebral arteries. The PcoA has a mean outer diameter (1.3 mm) smaller than that of the AcoA (1.5 mm); however, it is on average five times longer than the AcoA, ie, 12.6 versus 2.6 mm (11, 19). Furthermore, the PcoA does not have vessels with a higher signal intensity running alongside it; this situation makes its identification less difficult.
TCCD might also fail to reveal a functional PcoA. The proximal part of the postcommunicating segment (P2) of the posterior cerebral artery has the same color and flow direction as P1 and can easily be mistaken for it. Exact positioning of the TCCD sample volume in the P1 is difficult, particularly if it is relatively short (mean length, 7.0 mm; range, 3.0–20.0 mm) (11). Erroneous positioning of the TCCD sample volume in P2 leads to an incorrect diagnosis of nonfunctionality of the PcoA because PSV in P2 usually does not increase during carotid compression. In one subject who had a nonfunctional PcoA during TCCD examination, MR angiography showed a visible PcoA and an ipsilateral, visible P1 with a length of only 3–4 mm.
It can be argued that, in patients with steno-occlusive disease of the carotid arteries, the identification of intracranial collaterals with MR angiography is less difficult because of the potentially enhanced flow through them. However, the AcoA and PcoAs are not recruited until stenosis in the ICA has reduced the lumen by 80% or more (1, 16, 20). In cases of lesser stenosis, intracranial collateralization is absent, and flow through the communicating arteries is low or negligible (21, 22).
Several studies of MR angiography for the evaluation of the collateral anatomy of the circle of Willis in healthy volunteers (3) and in patients with cerebrovascular disease (4, 5, 23, 24) have been published (Table 4). The higher number of hypoplastic communicating arteries found in cerebrovascular patients corresponds with the results of autopsy studies performed in the 1960s (25, 26) The reports of these morphologic studies were among the first to point out the possible relationship between ischemic brain infarction and hypoplasia of collaterals of the circle of Willis. The contrasting results of Hartkamp et al (23) (Table 4) are probably due to the fact that they studied a subgroup of survivors of ICA occlusion with only minor disabling neurologic deficits; this approach resulted in a relatively more favorable outcome. More recently, the presence of functional intracranial collaterals in patients with severe ICA occlusive disease was shown to be negatively correlated with the severity of symptoms (27, 28). Furthermore, the risk of TIA and hemispheric stroke in patients with symptomatic ICA stenosis is reduced when functional collaterals are present (1). In patients with severe ICA occlusive disease, an effective collateral function of the circle of Willis might protect the brain not only from ischemia due to a critical reduction in cerebral perfusion pressure (29–31) but also from the harmful effects of thromboembolism (28, 32, 33). The AcoA is generally considered the most important route for collateral flow in cases of severe ICA occlusive disease (17, 29, 34–38), but collateral flow via the PcoA has also been shown to be of clinical importance (30, 39).
TABLE 4:
Percentage of nonvisualized collaterals in 3D time-of-flight MR angiography studies
| Study* | No. of Subjects | Age, y | AcoA, % | A1, % | PcoA, % | P1, % |
|---|---|---|---|---|---|---|
| Healthy volunteers | ||||||
| Krabbe-Hartkamp et al (3) | 150 | 55 | 19 | 3 | 28 | 2 |
| Current study† | 50 | 51 | 10 | 1 | 22 | 7 |
| Cerebrovascular patients | ||||||
| Patrux et al (4) | 54 | 44 | 54 | 5 | 56 | 8 |
| Stock et al (5) | 62 | 52 | 40 | 4 | 39 | 4 |
| Hartkamp et al (23) | 75 | 62 | 7 | 3 | 21 | 2 |
| Brunereau et al (24) | 109 | 21–82 | 45 | 2 | 44 | 12 |
Numbers in parentheses are reference citations.
Data were derived from the consensus review.
Direction of blood flow can be assessed with phase-contrast MR angiography. Previous studies have indicated that phase-contrast MR angiography is a reliable method for assessing the direction of flow in the circle of Willis (36, 40). With this technique, the presence of retrograde flow in the A1 segments (which indicates collateral flow via the AcoA), the presence of posteroanterior flow in the PcoAs, and reversed flow in the ophthalmic arteries can be assessed. Potentially more important, phase-contrast techniques can be used to quantify velocities within a vessel. If the cross-sectional area is measured, flow can be calculated. This technique enables volume-flow measurements in the extracranial vessels (41) and also in the major intracranial arteries (42). Nevertheless, for the AcoA and PcoAs, the diameter is too small to permit reliable quantitative measurements of flow. The development of perfusion MR imaging has made the rapid assessment of cerebral hemodynamics possible. This technique produces hemodynamic information such as the relative regional cerebral blood volume and relative regional cerebral blood flow. In patients with symptomatic carotid occlusive disease, it is a promising technique for investigating the cerebral hemodynamics (43, 44). Perfusion MR imaging provides direct information related to a reduction in blood flow, which reflects the primary underlying pathophysiology of acute ischemia. In patients with cerebrovascular disease, coupling of the previously mentioned MR techniques can be used to investigate the distribution or redistribution of blood flow through various cerebral arteries, providing a comprehensive evaluation of flow condition.
Conclusion
3D TOF MR angiography can provide valuable information regarding the collateral anatomy and function of the circle of Willis. Using visibility as sole criterion, its sensitivity in the identification of functional collateral pathways is relatively high. If the vessel segments that form the anterior and posterior collateral pathway of the circle of Willis can be visualized by using MR angiography, it can be assumed with a high level of confidence that collateral flow is possible. However, the high false-negative rate indicates that if MR angiography fails to depict intracranial collaterals, supplementary TCCD examination is required. The moderate interobserver agreement is most probably a reflection of the difficulty in identifying very small communicating arteries with MR angiography.
Acknowledgments
The authors wish to thank Ruud Smit for his practical assistance during the MR examinations.
References
- 1.Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJM. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke 2000;31:128–132 [DOI] [PubMed] [Google Scholar]
- 2.Nicolaides AN. Asymptomatic carotid stenosis and risk of stroke. Identification of a high risk group (ACSRS). A natural history study. Intl Angiol 1995;14:21–23 [PubMed] [Google Scholar]
- 3.Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, et al. Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR-angiograms. Radiology 1998;207:103–111 [DOI] [PubMed] [Google Scholar]
- 4.Patrux B, Laissy JP, Jouini S, Kawiecki W, Coty P, Thiébot J. Magnetic resonance angiography (MRA) of the circle of Willis: a prospective comparison with conventional angiography in 54 subjects. Neuroradiology 1994;36:193–197 [DOI] [PubMed] [Google Scholar]
- 5.Stock KW, Wetzel S, Kirsch E, Bongartz G, Steinbrich W, Radue EW. Anatomic evaluation of the circle of Willis: MR angiography versus intraarterial digital subtraction angiography. AJNR Am J Neuroradiol 1996;17:1495–1499 [PMC free article] [PubMed] [Google Scholar]
- 6.Müller M, Hermes M, Brückmann H, Schimrigk K. Transcranial Doppler ultrasound in the evaluation of collateral blood flow in patients with internal carotid artery occlusion: correlation with cerebral angiography. AJNR Am J Neuroradiol 1995;16:195–202 [PMC free article] [PubMed] [Google Scholar]
- 7.Lindegaard KF, Bakke SJ, Grolimund P, Aaslid R, Huber P, Nornes H. Assessment of intracranial hemodynamics in carotid artery disease by transcranial Doppler ultrasound. J Neurosurg 1985;63:890–898 [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner RW, Baumgartner I, Mattle HP, Schroth G. Transcranial color-coded duplex sonography in the evaluation of collateral flow through the circle of Willis. AJNR Am J Neuroradiol 1997;18:127–133 [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdahn U, Becker G, Winkler J, Greiner K, Perez J, Meurers B. Transcranial color-coded real-time sonography in adults. Stroke 1990;21:1680–1688 [DOI] [PubMed] [Google Scholar]
- 10.Tsuchiya T, Yasaka M, Yamaguchi T, Kimura K, Omae T. Imaging of the basal cerebral arteries and measurement of blood flow velocity in adults by using transcranial real-time color flow Doppler sonography. AJNR Am J Neuroradiol 1991;12:497–502 [PMC free article] [PubMed] [Google Scholar]
- 11.Saeki N, Rhoton AL. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg 1977;46:563–578 [DOI] [PubMed] [Google Scholar]
- 12.Aaslid R, Markwalder T, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982;57:769–774 [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri R, Padayachee TS, Lewis RR, Gosling RG, Cox TCS. Non-invasive assessment of the circle of Willis using transcranial pulsed Doppler ultrasound with angiographic correlation. Clin Radiol 1992;46:193–197 [DOI] [PubMed] [Google Scholar]
- 14.Hoksbergen AWJ, Legemate DA, Ubbink DTh, Jacobs MJHM. Success rate of transcranial color-coded duplex ultrasonography in visualizing the basal cerebral arteries in vascular patients over 60 years of age. Stroke 1999;30:1450–1455 [DOI] [PubMed] [Google Scholar]
- 15.Hillen B. The variability of the circle of Willis: univariate and bivariate analysis. Acta Morphol Neerl-Scand 1986;24:87–101 [PubMed] [Google Scholar]
- 16.Rosenkranz K, Langer R, Felix R. Transcranial Doppler sonography: collateral pathways in internal carotid artery obstructions. Angiology 1991;42:819–826 [DOI] [PubMed] [Google Scholar]
- 17.Cassot F, Vergeur V, Bossuet P, Hillen B, Zagzoule M, Marc-Vergnes JP. Effects of anterior communicating artery diameter on cerebral hemodynamics in internal carotid artery disease. Circulation 1995;92:3122–3131 [DOI] [PubMed] [Google Scholar]
- 18.Hoksbergen AWJ, Fülesdi B, Legemate DA, Csiba L. The collateral configuration of the circle of Willis: transcranial color-coded duplex ultrasonography and comparison with postmortem anatomy. Stroke 2000;31:1346–1351 [DOI] [PubMed] [Google Scholar]
- 19.Perlmutter D, Rhoton AL. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg 1976;45:259–272 [DOI] [PubMed] [Google Scholar]
- 20.Martin PJ, Smith JL, Gaunt ME, Naylor AR. Assessment of intracranial primary collaterals using transcranial color-coded real-time sonography. J Neuroimag 1995;5:199–205 [DOI] [PubMed] [Google Scholar]
- 21.Dickey PS, Kailasnath P, Bloomgarden G, Goodrich I, Chaloupa J. Computer modeling of cerebral blood flow following internal carotid artery occlusion. Neurol Res 1996;18:259–266 [DOI] [PubMed] [Google Scholar]
- 22.Viedma A, Jiménez-Ortiz C, Marco V. Extended circle of Willis model to explain clinical observations in periorbital arterial flow. J Biomech 1997;30:265–272 [DOI] [PubMed] [Google Scholar]
- 23.Hartkamp MJ, van der Grond J, van Everdigen KJ, Hillen B, Mali WPTM. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke 1999;30:2671–2678 [DOI] [PubMed] [Google Scholar]
- 24.Brunereau L, Lévy C, Arrivé L, et al. Anatomie du polygone de Willis en ARM 3D temps de vol avec analyse des partitions. J Radiol 1995;76:573–577 [PubMed] [Google Scholar]
- 25.Alpers BJ, Berry RG. Circle of Willis in cerebral vascular disorders. Arch Neurol 1963;8:398–402 [DOI] [PubMed] [Google Scholar]
- 26.Battacharji SK, Hutchinson EC, McCall AJ. The circle of Willis-The incidence of developmental abnormalities in normal and infarcted brains. Brain 1967;90:747–758 [DOI] [PubMed] [Google Scholar]
- 27.Hedera P, Bujdakova J, Traubner P. Effect of collateral flow patterns on outcome of carotid occlusion. Eur Neurol 1995;35:212–216 [DOI] [PubMed] [Google Scholar]
- 28.Silvestrini M, Vernieri F, Troisi E, et al. Cerebrovascular reactivity in carotid artery occlusion: Possible implications for surgical management of selected groups of patients. Acta Neurol Scand 1999;99:187–191 [DOI] [PubMed] [Google Scholar]
- 29.Ringelstein EB, Weiller C, Weckesser M, Weckesser S. Cerebral vasomotor reactivity is significantly reduced in low-flow as compared to thromboembolic infarctions: the key role of the circle of Willis. J Neurol Sci 1994;121:103–109 [DOI] [PubMed] [Google Scholar]
- 30.Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 1994;330:1565–1570 [DOI] [PubMed] [Google Scholar]
- 31.Mull M, Schwarz M, Thron A. Cerebral hemispheric low-flow infarcts in arterial occlusive disease. Lesion patterns and angiomorphological conditions. Stroke 1997;28:118–123 [DOI] [PubMed] [Google Scholar]
- 32.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism and ischemic stroke. Arch Neurol 1998;55:1475–1482 [DOI] [PubMed] [Google Scholar]
- 33.Omae T, Mayzel-Oreg O, Li F, Sotak CH, Fisher M. Inapparent hemodynamic insufficiency exacerbates ischemic damage in a rat microembolic stroke model. Stroke 2000;32:2494–2499 [DOI] [PubMed] [Google Scholar]
- 34.Hoksbergen AWJ, Legemate DA, Ubbink DT, de Vos HJ, Jacobs MJHM. Influence of the collateral function of the circle of Willis on hemispherical perfusion during carotid occlusion as assessed by transcranial colour-coded duplex ultrasonography. Eur J Vasc Endovasc Surg 1999;17:486–492 [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Bresnahan MV, Kearse LA, Yanez P, Young TI. Anterior communicating artery collateral flow protection against ischemic change during carotid endarterctomy. J Neurosurg 1993;79:379–382 [DOI] [PubMed] [Google Scholar]
- 36.Miralles M, Dolz JL, Cotillas J, et al. The role of the circle of Willis in carotid occlusion: Assessment with phase contrast MR angiography and transcranial duplex. Eur J Endovasc Surg 1995;10:424–430 [DOI] [PubMed] [Google Scholar]
- 37.Hedera P, Bujdakova J, Traubner P, Pancak J. Stroke risk factors and development of collateral flow in carotid occlusive disease. Acta Neurol Scand 1998;98:182–186 [DOI] [PubMed] [Google Scholar]
- 38.Kluytmans M, van der Grond J, van Everdingen KJ, Klijn CJM, Kappelle LJ, Viergever MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke 1999;30:1432–1439 [DOI] [PubMed] [Google Scholar]
- 39.Steinberg GK, Drake CG, Peerless SJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: Immediate results and long-term outcome in 201 patients. J Neurosurg 1993;79:161–173 [DOI] [PubMed] [Google Scholar]
- 40.Ross MR, Pelc NJ, Enzmann DR. Qualitative phase contrast MRA in the normal and abnormal circle of Willis. AJNR Am J Neuroradiol 1993;14:19–25 [PMC free article] [PubMed] [Google Scholar]
- 41.Marks MP, Pelc NJ, Ross MR, Enzmann DR. Determination of cerebral blood flow with a phase-contrast cine MR imaging technique: Evaluation of normal subjects and patients with arteriovenous malformations. Radiology 1992;182:467–476 [DOI] [PubMed] [Google Scholar]
- 42.Enzmann DR, Ross MR, Marks MP, Pelc NJ. Blood flow in major cerebral arteries measured by phase-contrast cine MR. AJNR Am J Neuroradiol 1994;15:123—129 [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda M, Yuh WTC, Ueda T, et al. Severe occlusive carotid artery disease: Hemodynamic assessment by MR perfusion imaging in symptomatic patients. AJNR Am J Neuroradiol 1999;20:43–51 [PubMed] [Google Scholar]
- 44.Apruzzese A, Silvestrini M, Floris R, et al. Cerebral hemodynamics in asymptomatic patients with internal carotid artery occlusion: a dynamic susceptibility contrast MR and transcranial Doppler study. AJNR Am J Neuroradiol 2001;22:1062–1067 [PMC free article] [PubMed] [Google Scholar]