Abstract
BACKGROUND AND PURPOSE: Whether misery perfusion (MP) commonly accompanies brain borderzones (BZs) in patients with major cerebral artery occlusion remains unclear. We elucidated topographic patterns of chronic hemodynamic failure in such patients.
METHODS: Twenty-four patients with unilateral occlusion or severe stenosis (>75% in diameter) of the internal carotid artery (ICA) or middle cerebral arterial (MCA) trunk with minimal or no infarct underwent PET with 15O-labeled gas inhalation. Mean cerebral blood flow (CBF), cerebral blood volume (CBV), cerebral metabolic rate of oxygen, oxygen extraction fraction (OEF), and CBV/CBF ratio were determined in the superficial BZs, internal BZ, and MCA territory excluding BZs. Values in BZs were standardized and compared with those in controls. Topographic distributions of regions with OEF greater than that in controls were determined.
RESULTS: Values in patients and controls were not significantly different. Topographic distributions included matched perfusion in 10 patients, MP in only the ipsilateral internal BZ in four, MP in both ipsilateral internal and superficial BZs in two, MP in the ipsilateral MCA territory including BZs in one, MP in the ipsilateral MCA territory including BZs and contralateral BZs in two, and MP in the ipsilateral MCA territories including BZs in five.
CONCLUSION: Only 25% of the patients had MP localized in affected BZs Although localized MP more frequently accompanied the internal BZ than other regions, no patient had elevated OEF in the superficial BZ alone. These results are inconsistent with clinical observations that 80% of BZ infarctions develop superficially. Thus, hemodynamic mechanisms may not cause most superficial BZ infarctions.
Borderzone (BZ) infarcts or watershed infarcts are ischemic lesions that occur in the junction between two main arterial territories. These account for approximately 10% of all brain infarcts (1). Supratentorial BZs consist of three regions: 1) the anterior BZ, the superficial area between the territories of the middle cerebral artery (MCA) and anterior cerebral artery (ACA); 2) the posterior BZ, the superficial area between the territories of the MCA and the posterior cerebral artery (PCA); and 3) the internal BZ, the deep area between the territories of the cortical branches and the penetrators of the MCA. Hemodynamic theory has been emphasized as the most predominant mechanism of BZ infarction; it states that mild-to-moderate hypotension selectively diminishes perfusion in the BZs in patients with major cerebral artery occlusive disease, (2–4). Recently, several investigators have reported that many BZ infarcts cannot be explained by the hemodynamic theory, or they are supposed to be embolic; that is, emboli of a certain small size occlude terminal portions of the cortical branches and cause superficial watershed infarction (5–14). These conflicting observations indicate the need to reexamine the pathogenesis of BZ infarcts.
By using positron emission tomography (PET), cerebral perfusion and metabolism have been studied in patients with occlusive diseases of the internal carotid artery (ICA), but whether hemodynamic impairments localized to the BZ areas actually exist remains controversial. Leblanc et al (15) reported that, in seven patients with severe ICA stenosis, cerebral blood flow (CBF) and the CBF-cerebral blood volume (CBV) ratio was significantly decreased in the anterior BZ. Yamauchi et al (16) reported that, in nine patients with ICA occlusion and good collateral circulation through the anterior communicating (Acom) artery, CBF was significantly decreased, whereas the oxygen extraction fraction (OEF) was significantly increased in the territory of MCA and the surrounding BZs, especially in the posterior BZ. Both groups concluded that BZs might be selectively vulnerable to ICA occlusive diseases. On the other hand, Carpenter et al reported that, in 32 patients with either severe ICA stenosis or occlusion, CBV/CBF ratios and OEF in the BZ did not significantly differ from those in control subjects; these results indicated no evidence for selective hemodynamic impairments in BZs (17). Carpenter et al used BZ/MCA ratios to study whether patients with abnormal hemodynamics in the MCA territory have further selective abnormalities localized to the BZs. Again, their results are conflicting, although the patterns of collateral supply of the patients might be different among the studies, and variability in the arterial distributions of the brain might have influenced their results (18). Furthermore, the hemodynamics in the internal BZ was not examined in the previous PET studies.
The purpose of this study was to evaluate the topographic patterns of chronic hemodynamic failure in patients with occlusive disease of the major cerebral arteries, with special interest in the internal and superficial BZs. We also sought to elucidate the mechanisms and hemodynamic implications of BZ infarction.
Methods
Subjects
In the patients who were admitted to our hospital between January 1, 1992, and May 31, 1994, we performed PET studies in 24 patients. These patients had either unilateral occlusion or severe stenosis (>75% in diameter) of the ICA or the trunk of the MCA, with minimal or no infarction according to the CT and MR imaging findings. (Minimal infarction was defined as lacunar infarction or cortical/subcortical infarction smaller than 15 mm in diameter.) Excluded were patients with a cardiac source of emboli and those who had ischemic lesions in the BZ areas, as shown on CT and MR images. The study group included 19 men and five women, with a mean age of 66.0 years (range, 52–81 years). They comprised three patients with no ischemic episodes, four with transient ischemic attacks (TIAs) and 17 with minor completed strokes. In the patients with completed stroke or TIA, the potential mechanism of stroke or TIA was clinically judged and classified into the following categories: artery-to-artery (A-to-A) embolic, hemodynamic, and unclassified; these categories were modified from the NINDS classification of cerebrovascular disease III (19). The diagnostic criteria of each category have been described previously (20). A-to-A embolism was diagnosed in patients without a cardiac source of emboli when 1) intraluminal filling defects suggesting emboli or recanalization of the previously occluded arteries distal to the proximal arterial lesions (occlusion, severe stenosis, or ulceration) were confirmed angiographically or when 2) a hemorrhagic infarction was detected with CT or MR imaging. Hemodynamic TIA or stroke was diagnosed when 1) hemodynamic episodes such as orthostatic hypotension and excessive antihypertensive medication were evident immediately before stroke onset or when 2) fluctuation of neurologic signs and symptoms was accompanied by orthostatic hypotension or decrease in blood pressure. Patients whose conditions did not fit these criteria were grouped as unclassified.
Both CT and MR imaging were performed in all patients. In patients with TIA or minor completed stroke, the examinations were performed after their neurologic status became stable. MR imaging evaluation was performed by using a 1.5-T unit (MAGNETOM; Siemens, Erlangen, Germany). T1-weighted and T2-weighted images were obtained.
In 19 patients, arterial lesions of the neck and brain vessels were evaluated by means of conventional angiography or intra-arterial digital subtraction angiography. For the remaining five patients, intravenous digital subtraction angiography was performed in two patients with ICA stenosis. Both MR angiography and carotid sonography was performed in two patients with ICA occlusion and in one with MCA trunk occlusion. For the symptomatic patients, the evaluation of the cerebral artery was performed within 1 week of their admission to the hospital, and the results were confirmed in a repeat evaluation before the PET study to ensure that the proximal arterial lesion did not change. The extent of stenosis was obtained as the ratio of the diameter at the narrowest portion to the diameter at the distal portion that seemed normal. For the 19 patients who underwent conventional angiography or intra-arterial digital subtraction angiography, the most predominant route of blood flow to the affected MCA territory was evaluated and classified as blood flow through the stenotic lesion, the Acom artery, the posterior communicating (Pcom) artery, the ophthalmic artery, or the leptomeningeal anastomosis.
The cerebrovascular risk factors were hypertension in 21 patients, diabetes mellitus in eight, and hyperlipidemia in nine. Severe stenosis of the ICA were present in 10 patients; occlusion of ICA, in six; and severe stenosis or occlusion of MCA trunk, in eight. Infarction in the MCA territory was not evident on CT and MR images in two patients. Twelve patients had lacunes, whereas the other 10 patients had small cortical/subcortical infarcts in the MCA territory. In 21 symptomatic patients, neurologic deficits at onset were hemiparesis in 19, hemisensory disturbance in three, aphasia in five, and anosognosia or unilateral spatial neglect in two. The stroke or TIA mechanism was A-to-A embolic in five, hemodynamic in six, and unclassified in 10. Clinical and neuroradiologic data for the patients are summarized in Table 1.
TABLE 1:
Patient characteristics
| Case No. | Age (y)/Sex | Associated Conditions | Type of Ischemia | Mechanism | Neurologic Deficits* | CT/MRI Findings | Angiographic Findings | Main Flow to Affected MCA† | Other Collateral‡ | PET Findings, Distribution of MP§ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66/M | HT, HLP | Completed stroke | Hemodynamic | L sensory deficit | Bil lacunar infarcts | R ICS | Antegrade flow | None | Matched perfusion (G1) |
| 2 | 71/M | HT | Completed stroke | A-to-A embolism | L hemiparesis, L hemianopsia | R small cortical infact | R ICS | Antegrade flow | None | Matched perfusion (G1) |
| 3 | 62/M | DM, HT, HLP, MI, ASO | Completed stroke | Unclassified | R hemiparesis, motor aphasia | L lacunar infarcts | L ICS | None | None | Affected IBZ and SBZ (G3) |
| 4 | 66/M | DM, HT, AP, ASO | Completed stroke | Unclassified | R hemiparesis | L lacunar infarct | L ICS | Antegrade flow | Acom→L ACA | Matched perfusion (G1) |
| 5 | 78/M | HT | Completed stroke | Unclassified | L hemiparesis, L sensory deficit | R small cortical infact | R ICS | Antegrade flow | None | Matched perfusion (G1) |
| 6 | 81/F | HT, ASO, af | Completed stroke | Hemodynamic | R hemiparesis, R sensory deficit | L lacunar infarct | L ICS | None | None | Bil MCAt including BZ (G6) |
| 7 | 66/F | HT | Asymptomatic | None | R ICS | None | None | Affected IBZ alone (G2) | ||
| 8 | 63/F | HT, HLP | TIA | A-to-A embolism | R hemiparesis, motor aphasia | Bil lacunar infarcts | L ICS | Antegrade flow | Acom→L ACA | Matched perfusion (G1) |
| 9 | 68/M | HT, HLP | TIA | Hemodynamic | L hemiparesis | Bil lacunar infarcts + R small cortical infact | R ICS | R opthalmic A→ | Acom→R ACA | Affected MCAt including BZ + non-affected BZ (G5) |
| 10 | 68/M | DM, HT, HLP | Completed stroke | A-to-A embolism | R hemiparesis | Bil lacunar infarcts + L small cortical infact | L ICS | Antegrade flow | None | Matched perfusion (G1) |
| 11 | 62/M | DM, HT, HLP | Completed stroke | Unclassified | R hemiparesis | L lacunar infarct | L ICO | Acom→ | None | Affected IBZ alone (G2) |
| 12 | 52/F | DM, HT | Completed stroke | Unclassified | R hemiparesis, motor aphasia | L lacunar infarct | L ICO | Acom→ | L PCA→LM | Affected IBZ and SBZ (G3) |
| 13 | 70/M | HT | Completed stroke | A-to-A embolism | L hemiplegia, USN, anosgnosia | R small cortical infact | R ICO | R ACA→LM | A com→R ACA | Affected MCAt including BZ + non-affected BZ (G5) |
| 14 | 69/M | HT | TIA | Hemodynamic | L hemiparesis | R small cortical infact | R ICO | R opthalmic A→ | A com→R ACA | Bil MCAt including BZ (G6) |
| 15 | 75/M | HT, AP | Completed stroke | A-to-A embolism | L hemiparesis | R small cortical infact | R ICO | None | None | Affected IBZ alone (G2) |
| 16 | 69/M | DM | Completed stroke | Unclassified | Global aphasia | L small cortical infact | L ICO | Pcom→ | None | Bil MCAt including BZ (G6) |
| 17 | 58/M | DM, ASO | TIA | Hemodynamic | R hemiparesis, motor aphasia | L lacunar infarct | L MCS | L ACA, PCA→LM | A com→L ACA | Bil MCAt including BZ (G6) |
| 18 | 60/M | DM, HT | Completed stroke | Unclassified | R hemiparesis | L lacunar infarct | L MCO | L ACA, PCA→LM | None | Affected MCAt including BZ (G4) |
| 19 | 64/M | HT | Completed stroke | Hemodynamic | R hemiparesis | L lacunar infarct | L MCO | None | None | Matched perfusion (G1) |
| 20 | 56/F | HT, HLP | Completed stroke | Unclassified | L hemiparesis | R lacunar infarcts | R MCO | R ACA→LM | None | Affected MCAt including BZ + non-affected BZ (G5) |
| 21 | 67/M | HT, HLP | Completed stroke | Unclassified | R hemiparesis, R hemianopsia, anosognosia | L small cortical infacts | L MCO | L ACA, PCA→LM | None | Matched perfusion (G1) |
| 22 | 71/F | HT, HLP | Completed stroke | Unclassified | R hemiparesis | None | L MCO | L ACA→LM | A com→L ACA | Affected IBZ alone (G2) |
| 23 | 54/M | DM, HT | Asymptomatic | Bil lacunar infarcts | R MCO | R ACA, PCA→LM | None | Matched perfusion (G1) | ||
| 24 | 69/M | Asymptomatic | L small cortical infacts | L MCO | L ACA→LM | None | Matched perfusion (G1) |
Note.—Abbreviations are as follows: af indicates atrial fibrillation; AP, angina pectoris; ASO, arteriosclerosis obliterans; DM, diabetes mellitus; HLP, hyperlipidemia; IBZ, internal BZ; ICO, occlusion of the ICA; ICS, severe stenosis of the ICA; MCAt, MCA territory; MCO, occlusion of the M1 portion; MCS, severe stenosis of the M1 portion; HT, hypertension; MI, old myocardial infarction; SBZ, superficial BZ; USN, unilateral spatial neglect; Bil, bilateral.
Defects at the onset in patients with TIA and at the time of hemodynamic studies in other patients.
In patients who underwent conventional angiography or intra-arterial digital subtraction angiography.
Number shows the type of the MP distribution.
PET studies
Regional CBF, OEF, cerebral metabolic rate of oxygen (CMRO2), and CBV were measured by using a Headtome IV PET scanner (Shimadzu, Kyoto, Japan) with a spatial resolution of 4.5 mm at full width half maximum and the 15O-labeled gas-inhalation technique (21, 22). An emission scan with an external 68Ge-68Ga ring source was corrected for the effects of tissue attenuation by using corresponding transmission scans. Transmission scanning was performing when a patient was in a supine position with his or her eyes closed. Two separate scans were performed during the continuous inhalation of 15O-labeled carbon dioxide (C15O2) and molecular oxygen (15O2) for measurement of CBF and OEF, respectively. The third scan was obtained after a 2-minute inhalation of 15O -labeled carbon monoxide (C15O) for the measurement of CBV. During the scans, serial blood samples were obtained through a fine-gauge radial (or brachial) arterial catheter for measuring the arterial isotope activity, arterial oxygen content (O2C), and arterial pCO2. A value of CMRO2 was calculated with the following equation: CMRO2 = CBF × OEF × O2C.
In the patients with stroke or TIA, PET was performed at least 3 weeks after the latest ischemic event, when their neurologic conditions became stable. In asymptomatic patients, PET was performed at least 4 weeks after the first CT examination. Four healthy volunteers with a mean age of 47.5 years (range, 30–63 years) underwent PET studies to determine the normal values. These patients consisted of three men and one woman who were free of any cerebrovascular risk factors and neurologic deficits. No focal brain lesions were detected with CT and MR imaging in these subjects.
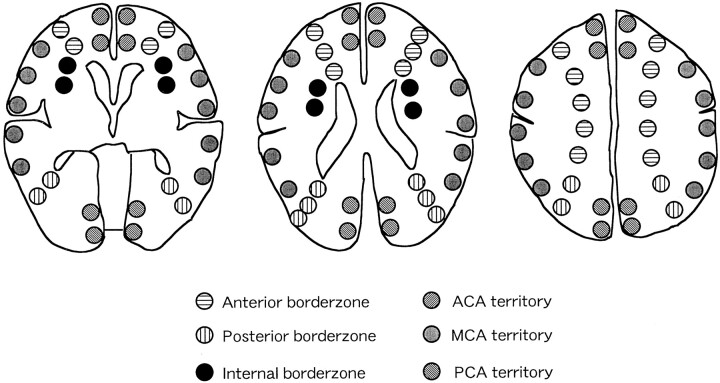
As shown in Figure 1, we analyzed the PET images in three tomographic planes parallel to the orbitomeatal line: 1) the levels of the basal ganglia and thalamus, 2) the body of the lateral ventricle, and 3) the centrum semiovale. Each image was examined by placing 18–20 circular regions of interest (ROIs) that were 9 mm in diameter. According to the atlas of Damasio (23), ROIs were placed at the territories of the ACA, MCA, PCA, anterior BZ, posterior BZ, and internal BZ. The mean values of each PET parameter in these vascular territories were obtained. To detect a selective BZ hemodynamic impairment, we calculated the ratios of the PET parameter in each BZ to that in the ipsilateral MCA territory and used them as standardized values. According to their arterial lesions, the patients were classified into the following three groups: 1) patients with severe stenosis of the ICA (ICA stenosis group), 2) those with occlusion of the ICA (ICA occlusion group), and 3) those with severe stenosis or occlusion of MCA trunk (MCA group). The averages of absolute and standardized PET values obtained in each group were compared with those in the healthy volunteers. We also calculated the 95% confidence interval of OEF in each vascular territory of the control group and defined misery perfusion (MP) as the legions with OEF above this interval. We determined the topographic distribution of the MP in the patient groups.
Fig 1.
Location of regions of interest. These are divided into six areas on the basis of the idealized standard concept of arterial distributions of the brain, as follows: territories of the ACA, MCA, PCA, anterior BZ (between the territories of the ACA and MCA), posterior BZ (between the territories of the MCA and PCA), and internal BZ (between the territories of the cortical branches and penetrators of the MCA).
Statistical Analysis
Statistical analyses were performed by using an analysis of variance (ANOVA) with Scheffé F correction. A P value of < .05 was considered to indicate a significant difference.
Results
Comparison of the Absolute and Standardized Values
Table 2 shows the mean and SD values for regional CBF, CBV, CMRO2, OEF, and CBF/CBV ratio for the patient and control groups. In the hemisphere ipsilateral to the vascular lesion, all of the patient groups had a significantly decreased regional CBF and CMRO2, both in the BZs and MCA territory. All patient groups tended to have an elevated OEF and a prolonged CBF/CBV ratio, both in the BZs and MCA territory. The ICA occlusion group alone had a significantly increased OEF in all vascular territories, except for anterior BZ. This group also had a significantly prolonged CBF/CBV ratio in those areas, except for the posterior BZ, as compared with values in the control group. In the hemisphere contralateral to the vascular lesion, the CBF tended to decrease, while the OEF tended to increase both in the BZs and the MCA territory. There were no significant differences in each PET parameter in either hemisphere among the patient groups.
TABLE 2:
Values for regional CBF, CBV, CMRO2, OEF, and CBF/CBV ratio of patients and control subjects
| Value | Ipsilateral Side to the Vascular Lesion |
Contralateral Side to the Vascular Lesion |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | Anterior BZ | Posterior BZ | Internal BZ | MCA Territory | Hemisphere | Anterior BZ | Posterior BZ | Internal BZ | Non-BZ | |
| CBF (mL/100 g/min) | ||||||||||
| Control | 47.5 ± 9.4 | 44.0 ± 8.1 | 45.9 ± 9.2 | 43.1 ± 12.9 | 53.6 ± 11.9 | 47.5 ± 9.4 | 44.0 ± 8.1 | 45.9 ± 9.2 | 43.1 ± 12.9 | 53.6 ± 11.9 |
| ICA stenosis | 35.1 ± 6.1* | 29.7 ± 5.0* | 32.5 ± 6.1* | 28.6 ± 5.8* | 38.4 ± 8.0* | 37.8 ± 5.2* | 32.3 ± 4.6* | 34.9 ± 5.5* | 30.4 ± 3.4* | 43.2 ± 7.2 |
| ICA occlusion | 30.7 ± 5.5* | 26.5 ± 6.3* | 30.7 ± 4.5* | 26.9 ± 5.7* | 31.8 ± 7.0* | 34.3 ± 4.4* | 30.7 ± 4.7* | 32.6 ± 7.0* | 29.2 ± 3.1* | 37.7 ± 5.9* |
| MCA lesion | 35.4 ± 3.4* | 31.2 ± 4.0* | 33.1 ± 3.3* | 29.9 ± 4.8* | 36.6 ± 4.6* | 38.6 ± 4.6 | 33.5 ± 4.3* | 36.5 ± 5.9 | 33.2 ± 7.0 | 41.7 ± 4.9 |
| CBV (mL/100 g) | ||||||||||
| Control | 3.64 ± 0.41 | 3.06 ± 0.60 | 3.47 ± 0.40 | 2.45 ± 0.51 | 4.08 ± 0.53 | 3.64 ± 0.41 | 3.06 ± 0.60 | 3.47 ± 0.40 | 2.45 ± 0.51 | 4.08 ± 0.53 |
| ICA stenosis | 3.41 ± 0.46 | 2.67 ± 0.60 | 2.94 ± 0.76 | 2.51 ± 0.76 | 3.72 ± 0.62 | 3.35 ± 0.40 | 2.55 ± 0.42 | 3.16 ± 0.61 | 2.42 ± 0.55 | 3.79 ± 0.34 |
| ICA occlusion | 4.04 ± 0.91 | 3.24 ± 1.10 | 3.37 ± 0.70 | 3.34 ± 0.98 | 3.99 ± 0.88 | 3.37 ± 0.34 | 2.58 ± 0.71 | 3.31 ± 0.66 | 2.64 ± 0.66 | 3.32 ± 0.35* |
| MCA lesion | 3.65 ± 0.39 | 2.94 ± 0.52 | 3.33 ± 0.56 | 2.87 ± 1.28 | 3.93 ± 0.52 | 3.55 ± 0.65 | 3.00 ± 0.35 | 2.83 ± 0.95 | 2.78 ± 0.47 | 3.67 ± 0.39 |
| CMRO2 (mL/100 g/min) | ||||||||||
| Control | 34.0 ± 0.46 | 31.5 ± 5.1 | 35.8 ± 4.9 | 29.8 ± 5.5 | 37.7 ± 4.3 | 34.0 ± 0.46 | 31.5 ± 5.1 | 35.8 ± 4.9 | 29.8 ± 5.5 | 37.7 ± 4.3 |
| ICA stenosis | 25.9 ± 2.6* | 22.2 ± 2.9* | 25.6 ± 4.4* | 21.3 ± 3.3* | 28.2 ± 3.0* | 27.9 ± 3.3* | 24.1 ± 3.0* | 27.0 ± 4.4* | 23.4 ± 3.3* | 31.7 ± 4.6 |
| ICA occlusion | 27.3 ± 2.7* | 22.8 ± 3.2* | 29.0 ± 2.7* | 23.3 ± 4.3 | 28.9 ± 3.4* | 29.2 ± 1.8 | 26.3 ± 3.3 | 28.6 ± 3.4* | 24.8 ± 1.4 | 32.2 ± 3.2 |
| MCA lesion | 27.2 ± 2.2* | 23.5 ± 1.7* | 26.7 ± 3.3* | 22.4 ± 3.9* | 28.9 ± 4.2* | 29.2 ± 1.7* | 24.3 ± 2.5* | 28.8 ± 2.2* | 24.2 ± 2.7* | 33.4 ± 5.5 |
| OEF | ||||||||||
| Control | 41.9 ± 6.4 | 42.0 ± 7.5 | 45.2 ± 5.8 | 41.0 ± 6.3 | 41.4 ± 6.7 | 41.9 ± 6.4 | 42.0 ± 7.5 | 45.2 ± 5.8 | 41.0 ± 6.3 | 41.4 ± 6.7 |
| ICA stenosis | 47.1 ± 6.5 | 48.6 ± 8.0 | 49.8 ± 7.9 | 48.5 ± 8.1 | 46.2 ± 6.1 | 46.4 ± 5.7 | 47.5 ± 7.0 | 48.0 ± 7.1 | 47.6 ± 5.4 | 45.7 ± 5.4 |
| ICA occlusion | 53.7 ± 5.4* | 52.2 ± 4.8 | 56.5 ± 4.9* | 52.6 ± 5.7* | 54.4 ± 6.9* | 51.1 ± 2.8* | 51.1 ± 3.1 | 52.9 ± 4.7 | 50.3 ± 3.0 | 50.9 ± 2.9* |
| MCA lesion | 48.8 ± 5.1 | 49.0 ± 5.4 | 50.7 ± 5.7 | 48.7 ± 5.1 | 49.4 ± 5.4 | 47.3 ± 5.8 | 46.0 ± 6.3 | 50.8 ± 5.8 | 48.5 ± 7.4 | 46.8 ± 6.1 |
| CBF/CBV ratio (/min) | ||||||||||
| Control | 0.078 ± 0.016 | 0.071 ± 0.016 | 0.078 ± 0.013 | 0.060 ± 0.014 | 0.078 ± 0.013 | 0.078 ± 0.016 | 0.071 ± 0.016 | 0.078 ± 0.013 | 0.060 ± 0.014 | 0.078 ± 0.013 |
| ICA stenosis | 0.101 ± 0.019 | 0.093 ± 0.019 | 0.098 ± 0.036 | 0.090 ± 0.026 | 0.101 ± 0.024 | 0.093 ± 0.022 | 0.084 ± 0.018 | 0.096 ± 0.026 | 0.080 ± 0.024 | 0.093 ± 0.025 |
| ICA occlusion | 0.135 ± 0.039* | 0.130 ± 0.049* | 0.111 ± 0.037 | 0.121 ± 0.036* | 0.130 ± 0.032* | 0.101 ± 0.018 | 0.088 ± 0.028 | 0.108 ± 0.025 | 0.088 ± 0.023 | 0.090 ± 0.015 |
| MCA lesion | 0.106 ± 0.016 | 0.099 ± 0.023 | 0.102 ± 0.021 | 0.100 ± 0.039 | 0.111 ± 0.020 | 0.095 ± 0.014 | 0.092 ± 0.014 | 0.080 ± 0.031 | 0.090 ± 0.020 | 0.091 ± 0.013 |
Note.—Values are the mean ± SD.
P < .05, different from control as determined by means of ANOVA with the Scheffé F test.
Table 3 shows the standardized values for each PET parameter in the three patient groups and control subjects. There were no significant differences among the groups.
TABLE 3:
Standardized values for regional CBF, CBV, CMRO2, OEF, and CBF/CBV ratio of patients and control subjects
| Value | Ipsilateral Side to the Vascular Lesion |
Contralateral Side to the Vascular Lesion |
||||
|---|---|---|---|---|---|---|
| ABZ/MCA | PBZ/MCA | IBZ/MCA | ABZ/MCA | PBZ/MCA | IBZ/MCA | |
| CBF | ||||||
| Control | 0.83 ± 0.06 | 0.86 ± 0.09 | 0.80 ± 0.07 | 0.83 ± 0.06 | 0.86 ± 0.09 | 0.80 ± 0.07 |
| ICA stenosis | 0.78 ± 0.10 | 0.86 ± 0.11 | 0.75 ± 0.10 | 0.76 ± 0.07 | 0.82 ± 0.13 | 0.71 ± 0.08 |
| ICA occlusion | 0.83 ± 0.09 | 0.98 ± 0.09 | 0.83 ± 0.08 | 0.82 ± 0.05 | 0.86 ± 0.08 | 0.79 ± 0.12 |
| MCA lesion | 0.86 ± 0.11 | 0.91 ± 0.09 | 0.83 ± 0.15 | 0.80 ± 0.06 | 0.87 ± 0.08 | 0.79 ± 0.11 |
| CBV | ||||||
| Control | 0.75 ± 0.10 | 0.86 ± 0.12 | 0.60 ± 0.09 | 0.75 ± 0.10 | 0.86 ± 0.12 | 0.60 ± 0.09 |
| ICA stenosis | 0.73 ± 0.19 | 0.81 ± 0.22 | 0.70 ± 0.24 | 0.68 ± 0.12 | 0.84 ± 0.17 | 0.64 ± 0.15 |
| ICA occlusion | 0.81 ± 0.13 | 0.86 ± 0.19 | 0.81 ± 0.30 | 0.78 ± 0.22 | 1.04 ± 0.27 | 0.79 ± 0.18 |
| MCA lesion | 0.75 ± 0.13 | 0.85 ± 0.14 | 0.72 ± 0.30 | 0.82 ± 0.11 | 0.77 ± 0.25 | 0.76 ± 0.13 |
| CMRO2 | ||||||
| Control | 0.83 ± 0.06 | 0.95 ± 0.07 | 0.79 ± 0.10 | 0.83 ± 0.06 | 0.95 ± 0.07 | 0.79 ± 0.10 |
| ICA stenosis | 0.79 ± 0.09 | 0.91 ± 0.11 | 0.76 ± 0.09 | 0.77 ± 0.09 | 0.86 ± 0.13 | 0.74 ± 0.06 |
| ICA occlusion | 0.79 ± 0.07 | 1.01 ± 0.05 | 0.78 ± 0.09 | 0.82 ± 0.05 | 0.90 ± 0.09 | 0.78 ± 0.10 |
| MCA lesion | 0.83 ± 0.14 | 0.93 ± 0.08 | 0.78 ± 0.10 | 0.74 ± 0.11 | 0.88 ± 0.15 | 0.74 ± 0.13 |
| OEF | ||||||
| Control | 1.01 ± 0.03 | 1.10 ± 0.08 | 0.99 ± 0.07 | 1.01 ± 0.03 | 1.10 ± 0.08 | 0.99 ± 0.07 |
| ICA stenosis | 1.05 ± 0.09 | 1.08 ± 0.04 | 1.05 ± 0.09 | 1.04 ± 0.07 | 1.05 ± 0.06 | 1.04 ± 0.06 |
| ICA occlusion | 0.97 ± 0.05 | 1.05 ± 0.09 | 1.00 ± 0.13 | 1.00 ± 0.03 | 1.04 ± 0.06 | 0.99 ± 0.05 |
| MCA lesion | 0.99 ± 0.06 | 1.03 ± 0.04 | 0.99 ± 0.12 | 0.98 ± 0.03 | 1.09 ± 0.03 | 1.04 ± 0.06 |
| CBF/CBV ratio | ||||||
| Control | 0.91 ± 0.09 | 1.00 ± 0.07 | 0.76 ± 0.11 | 0.91 ± 0.09 | 1.00 ± 0.07 | 0.76 ± 0.11 |
| ICA stenosis | 0.96 ± 0.30 | 0.98 ± 0.33 | 0.94 ± 0.36 | 0.93 ± 0.19 | 1.08 ± 0.34 | 0.90 ± 0.36 |
| ICA occlusion | 0.98 ± 0.22 | 0.86 ± 0.15 | 0.95 ± 0.38 | 0.97 ± 0.28 | 1.20 ± 0.26 | 1.00 ± 0.37 |
| MCA lesion | 0.91 ± 0.21 | 0.92 ± 0.10 | 0.88 ± 0.26 | 1.02 ± 0.21 | 0.88 ± 0.35 | 0.99 ± 0.14 |
Note.—ABZ indicates the anterior BZ; IBZ, internal BZ; PBZ, posterior BZ. No significant differences were observed between the four groups (Kruskal-Wallis test).
Topographic Distribution of the MP
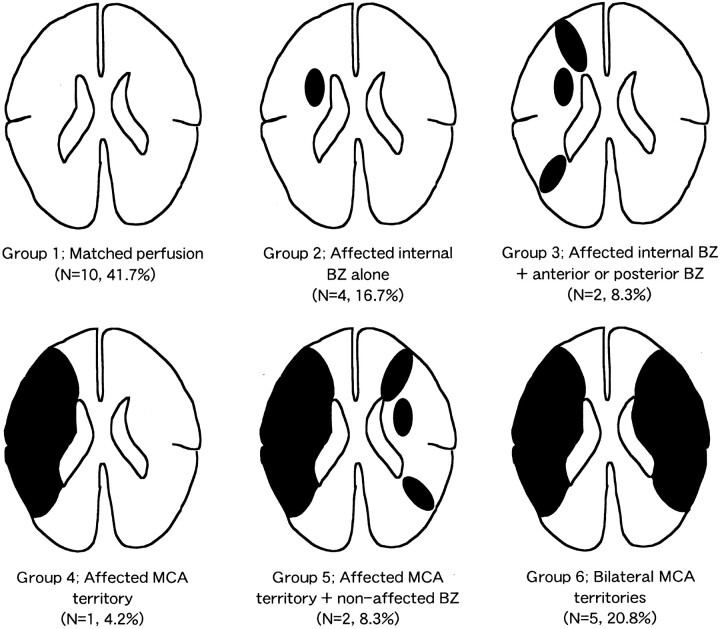
Topographic distributions of the areas with elevated OEF were classified into six patterns as shown in Fig 2. Matched perfusion throughout the brain was found in 10 patients (group 1), MP was found in only the ipsilateral internal BZ in four (group 2), MP was found in both the ipsilateral internal and superficial BZs in two (group 3), MP was found in the ipsilateral MCA territory including BZs in one (group 4), MP was found in the ipsilateral MCA territory including BZs and the contralateral BZs in two (group 5), and MP was found in the bilateral MCA territories including BZs in five (group 6). MP localized in the ipsilateral BZs (groups 2 and 3) was demonstrated in six of 24 patients. Although the internal BZ was most frequently accompanied with localized MP, as compared with the other regions, no patients had elevated OEF in the superficial BZs alone.
Fig 2.
Distribution of MP in unilateral occlusive disease of the major cerebral arteries.
Table 4 shows the topographic pattern of MP by patient group. MP in only the ipsilateral BZs was seen in all groups, 20% of the patients in the ICA stenosis group, 50% in the ICA occlusion group, and 12.5% in the MCA group. There was no significant difference regarding the distribution of the areas with elevated OEF among the groups (contingency table analysis, P = .42).
TABLE 4:
Distribution of MP in each patient group
| Group | ICA Stenosis | ICA Occlusion | MCA Lesion* | Total |
|---|---|---|---|---|
| 1 | 6 | 0 | 4 | 10 |
| 2 | 1 | 2 | 1 | 4 |
| 3 | 1 | 1 | 0 | 2 |
| 4 | 0 | 0 | 1 | 1 |
| 5 | 0 | 1 | 1 | 2 |
| 6 | 2 | 2 | 1 | 5 |
| Total | 10 | 6 | 8 | 24 |
MCA lesions include severe stenosis or occlusion of the trunk of the MCA.
Table 5 shows the relationship of the topography of MP to the most predominant routes of blood flow to the affected MCA territory. Patients with antegrade flow through the ICA had matched perfusion. Patients with collateral flow crossing through the Acom artery to the MCA had MP in only the BZ of the affected hemisphere. Patients whose affected MCA territory was perfused through only the leptomeningeal collateral channels had various topographies of the MP. Patients whose affected MCA territory was perfused through the ophthalmic or Pcom artery had the MP in both MCA territories. There was a significant difference in the topography of the area with an elevated OEF among the collateral flow patterns (contingency table analysis, P < .05).
TABLE 5:
Distribution of MP according to the collateral flow patterns
| Group | Main Route of Blood Flow to the Affected MCA Territory |
Total | |||
|---|---|---|---|---|---|
| Antegrade | Acom | Lept | OA/Pcom | ||
| 1 | 5 | 0 | 4 | 0 | 9 |
| 2 | 0 | 1 | 1 | 0 | 2 |
| 3 | 0 | 1 | 0 | 0 | 1 |
| 4 | 0 | 0 | 1 | 0 | 1 |
| 5 | 0 | 0 | 2 | 0 | 2 |
| 6 | 0 | 0 | 1 | 3 | 4 |
| Total | 5 | 2 | 9 | 3 | 19 |
Note.—Lept indicates leptomeningeal anastomosis; OA, ophthalmic artery.
Discussion
When we analyzed the standardized values of the PET parameters, we found no evidence that selective hemodynamic failure consistently occurs in the internal and superficial BZ areas. A small number of patients with ICA/MCA occlusive disease, 25% in the present series, had elevated OEF localized in the BZ areas, which always included the internal one. The internal BZ area was more frequently accompanied by localized MP than were the other regions. On the other hand, no patients had elevated OEF in only superficial BZ areas. These results were inconsistent with the clinical observation that approximately 80% of BZ infarctions develop superficially (4). Superficial BZ infarction may not be caused by a hemodynamic mechanism.
A selection bias of the patients may possibly have influenced the present results. Our classification of stroke mechanisms was derived without knowledge of the distribution of infarcts on CT and MR images, and therefore, they not affected by the neuroimaging results. A potential stroke mechanism in the present patients was sometimes an A-to-A embolic one (five of 24 patients). Only six of the 24 patients had a clinical diagnosis of a condition caused by the hemodynamic mechanism. However, none of these six (patients 1, 6, 9, 14, 17, 19) had the localized MP in the BZ areas. On the other hand, of five patients whose stroke mechanism was A-to-A embolic, only one (patient 15) had elevated OEF in the internal BZ alone. These results suggest that patient selection, or the potential mechanism diagnosed before the PET studies, did not directly influence the PET results.
van der Zwan et al (18) reported anatomic variations in vascular territories on the basis of findings from an autopsy series. This raised the possibility that the location of ROIs might be sometimes inaccurate because individual arterial territories cannot be identified in vivo. This is an unavoidable problem in PET studies that focused on the regional hemodynamic status in watershed areas. Another problem was the inadequate matching of ages between our patients and control subjects. We examined four healthy controls to determine the normal values of the PET parameters. The control data obtained from the present series were fairly comparable with those determined with similar PET equipment (24). In the PET studies, our control subjects were younger than the patients (47.5 years ± 13.8 and 66.0 years ± 7.0, respectively). This difference could imply that the control values were not appropriate for comparisons with the patient data. However, we did not detect any age-related change in the PET parameters. Previous PET studies have demonstrated that gray matter CBF, CBV, and/or CMRO2 decrease with advancing age, but gray matter OEF and CBV/CBF (or CBF/CBV) and all PET parameters in the white matter remain fairly stable (21, 25–27). Therefore, at least for OEF and CBV/CBF, the values in control subjects can be used as the standard in evaluating the hemodynamic state in patients.
As shown in Table 5, the main route of blood flow to the affected MCA territory had a significant relationship to topographic pattern of the MP. All five patients who had an antegrade flow through the stenotic ICA also had matched perfusion throughout the brain. The two patients with collateral flow through the Acom artery to the affected MCA territory also had MP in only the affected BZ areas, which always included the internal one. Nine patients whose affected MCA territory was perfused through the leptomeningeal anastomosis alone have a variety of topographic patterns of the MP. The remaining three patients with an ophthalmic artery or a Pcom artery as a collateral channel to the affected MCA territory had MP of the bilateral MCA territory. In Table 5, six patients had MP in the contralateral MCA territory (group 5 or 6). In four of these patients, the Acom artery was also functioning as a collateral channel, but in the remaining two (patients 16 and 20), the affected hemisphere was not perfused from the contralateral ICA. Therefore, it was difficult to explain the MP of the contralateral MCA territory on the basis of the collateral circulation in these two cases. After all, in 17 of 19 patients in Table 5, the relationship between the distribution of the MP and the collateral circulation was appropriate. In two others (patients 16, 20), the distribution of the MP could not be explained by collateral circulation alone. When the perfusion pressure in the MCA declines, superficial BZ areas can be salvaged by the leptomeningeal collateral flow from the ACA and/or the PCA, provided this channel is functioning. This type of collateral flow is often insufficient to save the internal BZ because the perforators of MCA are terminal arteries. This reason is perhaps why the internal BZ was more vulnerable to ischemia than the superficial ones.
The present results do not support the PET observations by Leblanc et al (15) and Yamauchi et al (16) that the superficial BZ areas are hemodynamically vulnerable to ischemia in patients with ICA occlusive disease and good collateral flows. We found no significant differences in the superficial BZ/MCA ratio of CBV/CBF and OEF between the ICA stenosis/occlusion groups and the control group; this was consistent with the observation by Carpenter and colleagues (17). Several differences in methodology exist among these studies, and these may account for the differences in results. Leblanc et al selected patients with ICA stenosis, and Yamauchi et al selected those with ICA occlusion and good collateral circulation through the Acom artery. In contrast, Carpenter et al and we selected those with ICA/MCA occlusive disease, which has nothing to do with collateral circulation. As we mentioned earlier, the main route of blood flow to the affected MCA territory had a significant relationship to topographic pattern of the MP. It should be noted that, in our study, two patients with good collateral flow through the Acom artery also had MP in only the affected BZ areas. Furthermore, Carpenter et al and we used the BZ/MCA ratio to account for the hemodynamic status of the ipsilateral MCA territory. This approach allowed us to determine whether patients with abnormal MCA hemodynamics have a further selective abnormality localized to the BZs.
It has generally been thought that most BZ infarctions arise from hemodynamic events (2–4). However, some authors (5–14) have suggested that embolization may play an important role in the pathogenesis of these infarcts. Pollanen et al (7) reported three autopsy cases of the anterior BZ infarction caused by thromboemboli. In their autopsy series, Masuda et al (11) reported that atheromatous embolism in the brain frequently caused anterior and posterior BZ infarcts by occluding the terminal cortical branches. Thus, emboli of a certain small size might occlude terminal portions of the cortical branches and cause a so-called watershed infarction. Recently, Caplan et al (14) emphasized the interaction of hypoperfusion and embolization, that is, that decreased perfusion reduces the washout and clearance of emboli that enters the vascular bed of hypoperfused regions. In either theory, embolization may play an important role in the pathogenesis of superficial BZ infarctions. Our results provided further support for this concept.
A question remains as to why posterior BZ infarction occurs in ICA occlusive disease. The posterior BZ area can easily be supplied by a blood flow through the leptomeningeal collateral channels from the PCA. To take another example, superficial BZ infarctions often occur in MCA occlusive disease. The superficial BZ areas, however, are hemodynamically resistant to ischemia because of leptomeningeal collateral channels. Such BZ infarctions are not easily explained with the hemodynamic theory, and thus, they are more likely to be embolic in nature. Recently, some authors have reported that the patients with internal BZ infarction have hemodynamic impairment more severe than that of patients with superficial BZ infarctions (27–29). A hemodynamic mechanism may play an important role in the development of internal BZ infarction. We have to clinically observe the part of the brain that becomes necrotic in the case of certain hemodynamic infarctions associated with occlusive disease of a major cerebral artery. Furthermore, we also need to compare the present results obtained in patients without BZ infarcts with the PET findings in patients with BZ infarcts.
Conclusion
We found no evidence that selective hemodynamic failure consistently occurs in the internal and superficial BZ areas in patients with occlusive disease of the major cerebral arteries. A small number of patients, 25% in the present series, had elevated OEF localized in the BZ areas, which always included the internal one. Although the internal BZ area was more frequently accompanied by localized MP than were the other regions, no patients had elevated OEF in only superficial BZ areas. These results are inconsistent with the clinical observation that approximately 80% of BZ infarctions develop superficially. Thus, most superficial BZ infarctions may not be associated with the hemodynamic mechanism.
Footnotes
Supported in part by Special Coordination Funds for Promoting Science and Technology (Encouragement of COE and Strategic Promotion System for Brain Science) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; research grants for cardiovascular diseases 5A-5, 6A-1, 12A-2, and 12C-10 from the Ministry of Health, Labour and Welfare of Japan; and a research grant from the Japan Cardiovascular Research Foundation.
Presented at the 3rd World Stroke Congress, September 1–4, 1996, Munich, Germany.
References
- 1.Jorgensen L, Torvik A. Ischaemic cerebrovascular diseases in an autopsy series, II: prevalence, location, pathogenesis and clinical course of cerebral infarcts. J Neurol Sci 1969;9:285–320 [DOI] [PubMed] [Google Scholar]
- 2.Zülch KJ. Über die entstehung und lokalisation der hirninfarkte. Zentralbl Neurochir 1961;21:158–178 [PubMed] [Google Scholar]
- 3.Bogousslavsky J, Regli F. Unilateral watershed cerebral infarcts. Neurology 1986;36:373–377 [DOI] [PubMed] [Google Scholar]
- 4.Bogousslavsky J, Regli F. Borderzone infarctions distal to internal carotid artery occlusion: Prognostic implications. Ann Neurol 1986;20:346–350 [DOI] [PubMed] [Google Scholar]
- 5.Beal MF, Williams RS, Richardson EP Jr, Fisher CM. Cholesterol embolism as a cause of transient ischemic attacks and cerebral infarction. Neurology 1981;31:860–865 [DOI] [PubMed] [Google Scholar]
- 6.Torvik A. The pathogenesis of watershed infarcts in the brain. Stroke 1984;15:221–223 [DOI] [PubMed] [Google Scholar]
- 7.Pollanen MS, Deck JHN. Directed embolization is an alternate cause of cerebral watershed infarction. Arch Pathol Lab Med 1989;113:1139–1141 [PubMed] [Google Scholar]
- 8.Pollanen MS, Deck JHN. The mechanism of embolic watershed infarction: experimental studies. Can J Neurol Sci 1990;17:395–398 [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi T, Minematsu K. Pathogenetic mechanism of watershed infarction [Japanese]. J Jpn Coll Angiol 1991;31:107–110 [Google Scholar]
- 10.Graeber MC, Jordan JE, Mishra SK, Nadeau SE. Watershed infarction on computed tomographic scan: an unreliable sign of hemodynamic stroke. Arch Neurol 1992;49:311–313 [DOI] [PubMed] [Google Scholar]
- 11.Masuda J, Yutani C, Ogata J, Kuriyama Y, Yamaguchi T. Atheromatous embolism in the brain: a clinicopathologic analysis of 15 autopsy cases. Neurology 1994;44:1231–1237 [DOI] [PubMed] [Google Scholar]
- 12.Hennerici M, Daffertshofer M, Jakobs L. Failure to identify cerebral infarct mechanisms from topography of vascular territory lesions. AJNR Am J Neuroradiol 1998;19:1067–1074 [PMC free article] [PubMed] [Google Scholar]
- 13.Chaves CJ, Silver B, Schlaug G, Dashe J, Caplan LR, Warach S. Diffusion- and perfusion-weighted MRI patterns in borderzone infarcts. Stroke 2000;31:1090–1096 [DOI] [PubMed] [Google Scholar]
- 14.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1988;55:1475–1482 [DOI] [PubMed] [Google Scholar]
- 15.Leblanc R, Yamamoto YL, Tyler JL, Diksic M, Hakim A. Borderzone ischemia. Ann Neurol 1987;22:707–713 [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi H, Fukuyama H, Kimura J, Konishi J, Kameyama M. Hemodynamics in internal carotid artery occlusion examined by positron emission tomography. Stroke 1990;21:1400–1406 [DOI] [PubMed] [Google Scholar]
- 17.Carpenter DA, Grubb RL Jr, Powers WJ. Borderzone hemodynamics in cerebrovascular disease. Neurology 1990;40:1587–1592 [DOI] [PubMed] [Google Scholar]
- 18.van der Zwan A, Hillen B, Tulleken CA, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg 1992;77:927–940 [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Neurological Disorders and Stroke Ad Hoc Committee. Classification of cerebrovascular diseases III. Stroke 1990;21:637–676 [DOI] [PubMed] [Google Scholar]
- 20.Hirano T, Minematsu K, Hasegawa Y, Tanaka Y, Hayashida K, Yamaguchi T. Acetazolamide reactivity on 123I-IMP single photon emission computed tomography in patients with major cerebral artery occlusive disease: Correlation with positron emission tomography parameters. J Cereb Blood Flow Metab 1994;14:763–770 [DOI] [PubMed] [Google Scholar]
- 21.Frackowiak RSJ, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr 1980;4:727–736 [DOI] [PubMed] [Google Scholar]
- 22.Lammertsma AA, Jones T, Frackowiak RSJ, Lenzi GL. A theoretical study of the steady-state model for measuring regional cerebral blood flow and oxygen utilization using oxygen-15. J Comput Assist Tomogr 1981;5:544–550 [DOI] [PubMed] [Google Scholar]
- 23.Damasio H. A computed tomographic guide to the identification of cerebral vascular territories. Arch Neurol 1983;40:138–142 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi S, Fukuyama H, Yamauchi H, Kimura J. Hemodynamics in the cerebral cortex and basal ganglia - Observation on normal volunteers and patients with lacunas using PET [Japanese]. Clin Neurol (Tokyo) 1991;31:1070–1076 [PubMed] [Google Scholar]
- 25.Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 1984;15:635–641 [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K. Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 1986;17:1220–1228 [DOI] [PubMed] [Google Scholar]
- 27.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990;113:27–47 [DOI] [PubMed] [Google Scholar]
- 28.Moriwaki H, Matsumoto M, Hashikawa K, et al. Hemodynamic aspect of cerebral watershed infarction: assessment of perfusion reserve using iodine-123-iodoamphetamine SPECT. J Nucl Med 1997;38:1556–1562 [PubMed] [Google Scholar]
- 29.Derdeyn CP, Khosla A, Videen TO, et al. Severe hemodynamic impairment and border zone-region infarction. Radiology 2001;220:195–201 [DOI] [PubMed] [Google Scholar]