Abstract
BACKGROUND AND PURPOSE: Edema-like change along the optic tract commonly occurs in association with craniopharyngiomas. The aim of this study was to clarify whether it occurs in association with other common pituitary region tumors and to elucidate its mechanism as seen on MR images.
METHODS: Fifty patients with pituitary region tumors that were touching or compressing the optic pathway underwent heavily T2-weighted MR imaging before and after treatment.
RESULTS: Edema-like change along the optic tract was visible on the images of four of 25 pituitary adenomas, eight of 11 craniopharyngiomas, one germ cell tumor, and one malignant lymphoma and was not visible on the images of seven meningiomas and five Rathke’s cleft cysts. After therapeutic decompression of the optic pathway, the edema-like change disappeared and large Virchow-Robin spaces, present under normal conditions, became visible along the optic tract. Comparison of pre- and post-treatment coronal and axial view MR images revealed that the edema-like change had been located at, along, and/or around the large Virchow-Robin spaces along the optic tract.
CONCLUSION: Edema-like change occurs in association with pituitary region tumors other than craniopharyngiomas. It is related with distension of normally present large Virchow-Robin spaces adjacent to the optic tract. Because Virchow-Robin spaces are speculated to be a drainage route of interstitial fluid into the subarachnoid space, their distension may be related to the fluid retention in and along the Virchow-Robin spaces, the outlet of which into the subpial and/or subarachnoid space(s) is blocked by pituitary region tumors.
Because craniopharyngiomas are often associated with edema-like change in the adjacent brain parenchyma along the optic tract, such an MR imaging finding has been considered to be useful in the diagnosis of craniopharyngiomas (1–3). Accordingly, the mechanism of edema-like change was speculated to be regional inflammation due to microscopic leakage of the cyst contents, which is known to cause chemical meningitis, and the evocation of adjacent edema (1, 2). However, case reports of parasellar meningioma and metastases have been published with a similar edema-like change occurring along the optic pathway (4, 5). Therefore, this unique phenomenon is considered to be common in association with but nonspecific to craniopharyngiomas (5, 6). Reconsideration regarding how often it occurs in association with common pituitary region tumors and further studies of its mechanism are necessary.
Heavily T2-weighted images have been known to detail fine structures in and around the CSF space (7) and are considered to be sensitive in determining locations and distributions of brain edema. In this study, heavily T2-weighted MR imaging was performed of patients with sellar or parasellar tumors to clarify whether edema-like change occurs along the optic tract in association with common pituitary region tumors and to clarify its mechanism.
Methods
Patients
This study included 50 patients with pituitary region tumors that were treated at Chiba University Hospital and its affiliated hospital during a period of 2 years. Tumors included 25 pituitary adenomas, 11 craniopharyngiomas, seven parasellar meningiomas, five Rathke’s cleft cysts, one germ cell tumor, and one malignant lymphoma (Table 1). All the tumors were observed to be touching or compressing the optic pathways. Visual signs (visual field defect and/or decreased visual acuity), which were confirmed by Goldman’s perimetry, were positive in 41 patients. Of the nine patients without visual signs, four had functioning adenomas, one had a nonfunctioning adenoma, three had craniopharyngiomas, and one had a germ cell tumor. All patients underwent surgical procedures, and histologic evidence was obtained to prove each diagnosis. Informed consent was obtained from all patients, and the protocol for this study was approved by our institutional review boards.
TABLE 1:
Clinical profiles of 50 pituitary tumors
| Histology | No. of Patients | Sex (M:F) | Age (yr) | Edema-like Change Shown by MR Imaging (+:−) | Tumor Size (Height) (mm) | Visual Sign (+:−) |
|---|---|---|---|---|---|---|
| Pituitary adenoma | 25 | 11:14 | 23–73 | 4:21 | 21–52 | 20:5 |
| Craniopharyngioma | 11 | 5:6 | 12–68 | 8:3 | 22–39 | 8:3 |
| Meningioma | 7 | 1:6 | 31–72 | 0:7 | 19–58 | 7:0 |
| Rathke’s cleft cyst | 5 | 3:2 | 34–63 | 0:5 | 19–27 | 5:0 |
| Germ cell tumor | 1 | 1:0 | 11 | 1:0 | 34 | 0:1 |
| Malignant lymphoma | 1 | 1:0 | 62 | 1:0 | 29 | 1:0 |
MR Imaging
All patients underwent pre- and postoperative T1- and heavily T2-weighted MR imaging in three planes. The post-treatment MR imaging was performed 2 weeks to 4 months after treatment. T1-weighted MR imaging was performed with a 1.5-T MR imaging unit (Signa; GE Medical Systems, Milwaukee, WI). A standard head coil with a receive-transmit birdcage design was used. T1-weighted fast spin-echo imaging was performed before and after the IV administration of 10 mL of gadopentetate dimeglumine (Magnevist; Japan-Schering, Osaka, Japan). Imaging parameters for the T1-weighted images were as follows: 500/9 (TR/TE); field of view, 16 × 16 cm; matrix, 256 × 224; section thickness, 3 mm; section gap, 0.5 mm; number of signals acquired, two.
Heavily T2-weighted MR imaging was performed with a 1.5-T superconducting magnet (Philips Gyroscan ACS-NT; Philips, Best, The Netherlands) and a standard head coil. A heavily T2-weighted turbo spin-echo sequence was obtained to display images with the following parameters: 5800/200 (TR/TE); field of view, 20 × 20 cm; matrix, 256 × 224; section thickness, 3 mm; section gap, 0.5 mm; number of signals acquired, four.
All MR images were studied by three physicians (N.S., Y.U., T.U.). Determination of the pre- and postoperative presence or absence of edema-like change was evaluated, and the final decision was made by consensus. Edema-like change was considered to be present when high signal intensity along the optic tract was visible in one or more sections along the optic tract in axial and coronal planes on heavily T2-weighted images.
Statistics
Welch’s t test and Fisher’s exact probability were used for statistical analysis of the relations of the edema-like change to both tumor size and visual signs. Numerical value was expressed as means ± SD.
Results
Clinical data regarding the patients are summarized in Table 1.
MR Imaging Study before Treatment
Edema-like intensity along the optic tract was shown on the images of eight of 11 craniopharyngiomas, four of 25 pituitary adenomas, one mixed germ cell tumor, and one malignant lymphoma (Table 1, Figs 1-4). No images of meningiomas or Rathke’s cleft cysts showed edema-like change. When edema-like change was present, axial planes showed the change spreading posteriorly along the optic tract and surrounding brain parenchyma. In coronal planes, when the edema-like change was slight or minimal in its degree, it was located on the optic tract where a normally present large Virchow-Robin space was seen (Fig 1). The optic tract was usually difficult to differentiate from the edema-like change, because it became high signaled (Figs 1–4). A curved-linear high signal intensity, which seemed to be a dilated linear Virchow-Robin space, originated from the edema-like change (Fig 2). The edema-like change spread as far as the internal capsule and the basal ganglia in a case of malignant lymphoma (Fig 4).
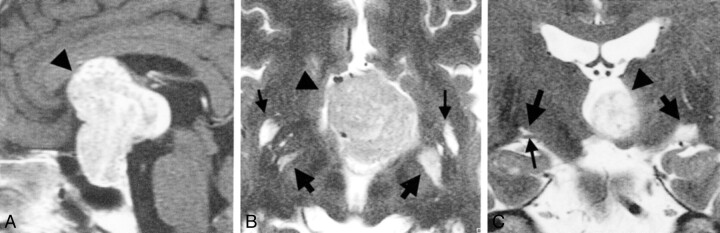
Fig 1.
Images from the case of a 59-year-old man with a giant pituitary adenoma.
A, Midsagittal contrast-enhanced T1-weighted MR image obtained before treatment. A huge tumor (arrowhead) is visible in and on the sella turcica.
B, Axial heavily T2-weighted MR image obtained one section above the level of the optic tract before treatment. Tumor (arrowhead) is visible in the third ventricle. Edema-like changes are visible bilaterally and are more prominent on the left side of the brain (right side of the figure) (large arrows). Large Virchow-Robin spaces related to anterior perforated substance are also visible (small arrows).
C, Coronal heavily T2-weighted MR image obtained before treatment. Large Virchow-Robin space (large arrow) is located on the right optic tract (small arrow). Medium sized arrow and arrowhead indicate the edema-like change on the left side and the tumor, respectively.
Fig 4.
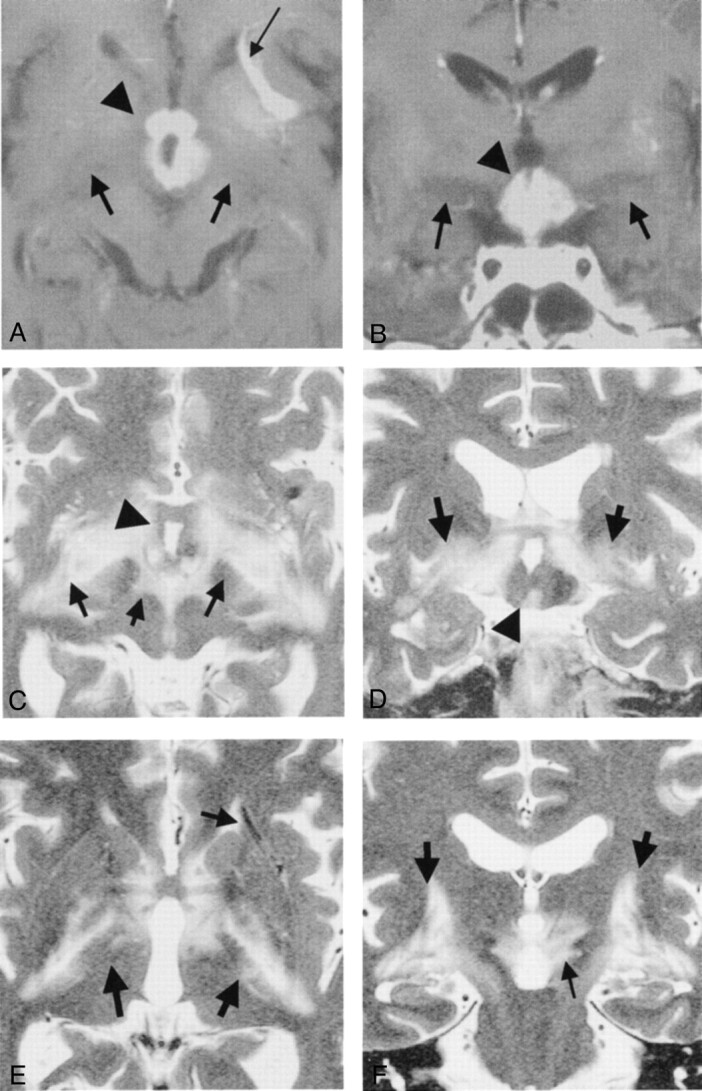
Images from the case of a 62-year-old man with malignant lymphoma.
A, Axial contrast-enhanced T1-weighted MR image obtained before treatment. Thick arrows, thin arrow, and arrowhead indicate edema-like change, incidentally found venous angioma, and tumor, respectively.
B, Coronal contrast-enhanced T1-weighted MR image obtained before treatment. High and low signal mass (arrowhead) is visible in the third ventricle. Low signal edema-like changes (arrows) are noted along the optic tracts. A venous angioma was incidentally found in the left anterior basal ganglia, as in A.
C, Axial heavily T2-weighted MR image obtained before treatment. Tumor (arrowhead) is visible. Marked edema-like changes are visible in the hypothalamus and midbrain and along the optic tracts (arrows).
D, Coronal heavily T2-weighted MR image obtained before treatment. Tumor (arrowhead) is visible. Marked edema-like changes are visible in the hypothalamus and midbrain and along the optic tracts (arrows).
E, Axial heavily T2-weighted MR image obtained before treatment. Edema-like changes (large arrows) extend as far as the basal ganglia and the internal capsule. The venous angioma (small arrow) is visible in the left anterior basal ganglia.
F, Coronal heavily T2-weighted MR image obtained before treatment. Edema-like changes (large arrows) extend as far as the basal ganglia and the internal capsule. Marked edema-like change is visible in the hypothalamus and midbrain (small arrow).
Fig 2.
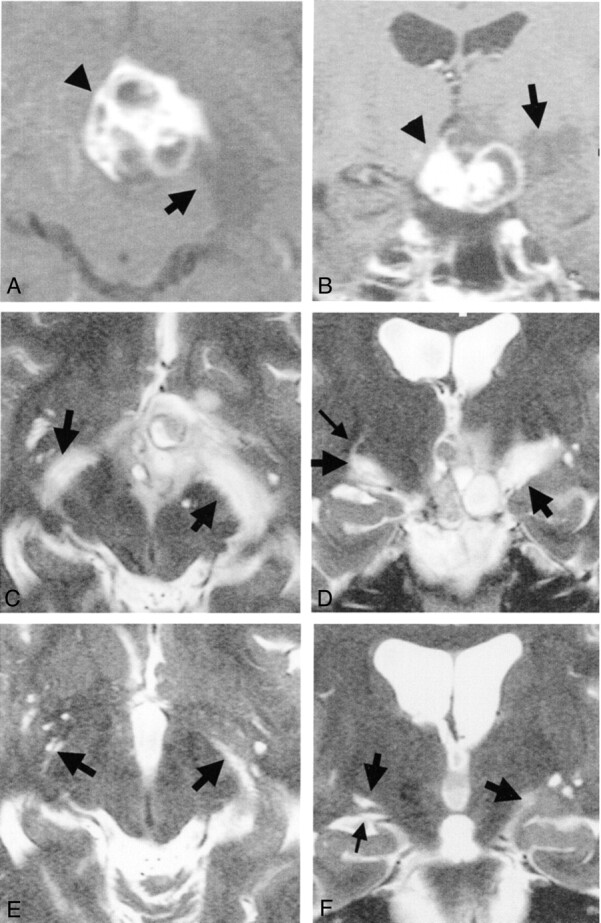
Images from the case of a 33-year-old woman with craniopharyngioma.
A, Axial contrast-enhanced T1-weighted MR image obtained before treatment. Arrow and arrowhead indicate edema-like change and tumor, respectively.
B, Coronal contrast-enhanced T1-weighted MR image obtained before treatment. High and low signal mass (arrowhead) is visible at the suprasellar cistern. Low signal edema-like change (arrow) is noted along the left optic tract.
C, Axial heavily T2-weighted MR image obtained before treatment. Arrows indicate edema-like change.
D, Coronal heavily T2-weighted MR image obtained before treatment. Edema-like changes (large arrows) are visible bilaterally along the optic tract and are more prominent on the left side of the brain (right side of the figure). Optic tracts are difficult to differentiate from edema-like changes. On the right side, a curvilinear high signal intensity (small arrow), originating from the edema-like change, is visible.
E, Axial heavily T2-weighted MR image obtained one section above the level of the optic tract 3 months after surgery (at level similar to that shown in C). Edema-like change has disappeared on the right side of the brain. Large Virchow-Robin spaces (arrow, right side of brain), which are present under normal conditions, are visible on the same side. Edema-like change (arrow, left side of brain) seems to remain on the left side.
F, Coronal heavily T2-weighted MR image obtained 3 months after surgery (at level similar to that shown in D). Large Virchow-Robin space (large arrow, right side of brain) is visible on the right optic tract (small arrow). Edema-like change (large arrow, left side of brain) remains on the left side.
Relation of Edema-like Change to Tumor Size or Visual Signs in Cases of Pituitary Adenomas and Craniopharyngiomas
In association with pituitary adenomas, edema-like change was present in four cases (three cases with and one case without visual signs), with tumor height of 35.5 mm ± 14.2. Twenty-one cases without edema-like change (17 cases with and four cases without visual signs), revealed a height of 28.9 mm ± 6.2. In pituitary adenomas, edema-like change was unrelated to tumor size and presence or absence of visual signs (height, P = .42, Welch’s t test; visual signs, P = .83, Fisher’s exact probability) (Table 1).
In association with craniopharyngiomas, edema-like change was present in eight cases (seven cases with and one case without visual signs), with tumor height of 32.8 mm ± 5.2. Three cases without edema-like change (one case with and two cases without visual signs) revealed a height of 29 mm ± 7. In association with craniopharyngiomas, as well aspituitary adenomas, edema-like change was independent of tumor height and presence or absence of visual signs (height, P = .45, Welch’s t test; visual signs, P = .15, Fisher’s exact probability). In association with both tumors, the edema-like change was independent of tumor size as well as presence or absence of visual signs.
After Treatment
After surgery, chemotherapy, and/or radiation, the tumor disappeared or shrank remarkably and was detached from the optic pathway and/or the third ventricle floor. MR images after such successful treatment evidenced the disappearance or decrease of the edema-like change, showing its capability to undergo a reversible process (Figs 2 and 3). After disappearance or decrease of the edema-like change along the optic tract, normally present large Virchow-Robin spaces became visible in the area at which the edema-like change had been present (Figs 2 and 3).
Fig 3.
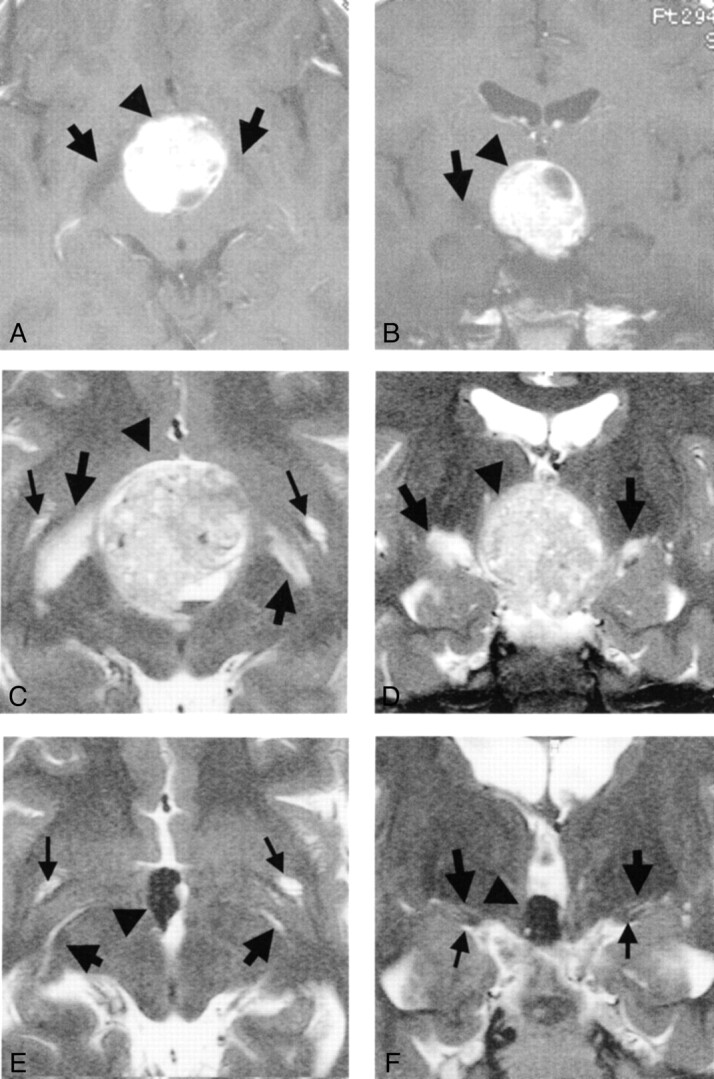
Images from the case of an 11-year-old boy with a germ cell tumor.
A, Axial contrast-enhanced T1-weighted MR image obtained before treatment. Edema-like change along the optic tract is visible bilaterally (arrows). Arrowhead indicates slightly hyperintense mass.
B, Coronal contrast-enhanced T1-weighted MR image obtained before treatment. Round and almost high signal mass (arrowhead) is visible at the suprasellar region. Edema-like change along the optic tract is visible on the right side of the brain (left side of the figure) (arrow).
C, Axial heavily T2-weighted MR image obtained before treatment. Large arrows, small arrows, and arrowheadindicate edema-like changes, Virchow-Robin spaces, and tumor, respectively. Findings concur with those in D.
D, Coronal heavily T2-weighted MR image obtained before treatment. Edema-like changes (large arrows), more prominent on the right side of the brain (left side of the figure), are visible along the optic tracts. Tumor (arrowhead) is visible. Large Virchow-Robin spaces associated with anterior perforated substance are visible.
E, Axial heavily T2-weighted MR image obtained 2 months after treatment (at level similar to that shown in C). Large Virchow-Robin spaces associated with anterior perforated substance (small arrows) are visible.
F, Coronal heavily T2-weighted MR image obtained 2 months after treatment (at level similar to that shown in D). Edema-like change has disappeared bilaterally, although the tumor (arrowhead) remains in the third ventricle. Normally present large Virchow-Robin spaces (large arrows) are visible. The optic tracts (small arrows) are also visible. Large arrows and arrowhead indicate Virchow-Robin spaces and tumor, respectively.
Discussion
Edema-like Change Associated with Sellar and Parasellar Tumors
Several reports concerning edema-like change along the optic pathway in association with craniopharyngiomas are presented in the literature (1–3). In 1998, Nagahata et al (2) reported findings of this edema-like change along the optic pathway in five of eight patients with craniopharyngiomas and none with large pituitary adenomas or tuberculum sellae meningiomas, thereby revealing its diagnostic significance. On the basis of our study, although edema-like change was much more common in association with craniopharyngiomas, it was not neuroradiologically specific to them; heavily T2-weighted MRimages revealed its occurrence in association with pituitary adenomas, a germ cell tumor, and a malignant lymphoma (4, 5).
MR Imaging Study of the Mechanism of Edema-like Change
This study revealed that edema-like change occurring along the optic tract is related to distension of Virchow-Robin spaces as shown by the following three MR imaging findings. First, after disappearance or decrease of the edema-like intensity after successful treatment, normally present large Virchow-Robin spaces along the optic tract became visible in the area at which the edema-like change had been present. Second, a curvilinear high signal intensity originating from the edema-like change seemed to be a physiologically present linear Virchow-Robin space, which became dilated under the pathologic condition. Third, an almost normal large Virchow-Robin space and the optic tract were visible when the edema-like change was minimal. These MR imaging findings provided evidence of edema-like formation related to distension of large Virchow-Robin spaces along the optic tract.
It is well known that the Virchow-Robin space surrounds each cerebral vessel (8–10). The space is filled with CSF and was thought to be directly communicated with the subarachnoid space (11). However, it is now considered that the Virchow-Robin spaces are extensions of the subpial spaces (12, 13) and that the pia mater separates the Virchow-Robin space from the subarachnoid space. Anatomically, the Virchow-Robin spaces communicate with the subarachnoid spaces through holes in the pia mater (11, 14). Physiological studies of the movement of tracers injected into brain parenchyma in rats and rabbits have revealed their appearance in Virchow-Robin spaces and subarachnoid spaces (12, 14). Based on such anatomic and physiological studies, outward pathways from extracellular, to Virchow-Robin, to subpial, to subarachnoid spaces have been proved to be present (11, 13), and the Virchow-Robin spaces are considered to be a drainage route of interstitial fluid into the subarachnoid space (8, 15). Such interstitial fluid sources account for at least 10% of CSF production in humans (16).
Regarding a mechanism of edema-like change, based on our MR imaging findings and a review of literature, it is speculated that the sellar and parasellar tumors block the outward pathway of the Virchow-Robin spaces and the subarachnoid and subpial spaces at the suprasellar area. The blockage of the outlet of the Virchow-Robin spaces may induce distention of the Virchow-Robin spaces and local interstitial fluid retention. Thus, MR imaging findings of edema-like change along the optic tract may be due to interstitial fluid retention and distended Virchow-Robin spaces in patients with pituitary region tumors. Thus, the change may be associated with CSF formation other than by the choroid plexus and possibly a new pattern of brain edema (14, 16). Therefore, the clinical application of this study is expected to be potentially high, and MR imaging systems with higher magnetic fields may succeed in showing the detailed pathologic anatomy of edema-like change.
Clinical Significance
Our study shows that the presence of edema-like change has little relation to visual signs despite its occurring at or around the optic tract. This is in accordance with a previous report of craniopharyngiomas (1). Thus, the low symptomatic significance of edema-like change in cases of common pituitary tumors may be why it remained clinically unrecognized until the availability of superconductive MR imaging apparatus capable of depicting fine structures and sensitive for determining water location. However, it has been reported that in patients with pituitary metastasis, the appearance of edema-like change along the optic tract well corresponded to the initiation of visual complaint (5). Because it also has been reported to evoke vasogenic edema around surrounding neural tissue (6), anatomic distinction of the edema-like change from the edema of vasogenic origin seems to be difficult. Nevertheless, these reports show that edema-like change is possibly different between cases of benign tumors and cases of malignant tumors. Its clinical significance needs to be investigated in the future.
Conclusion
Edema-like change occurs in association with pituitary region tumors other than craniopharyngiomas. It is related to distension of normally present large Virchow-Robin spaces adjacent to the optic tract. Because Virchow-Robin spaces are speculated to be a drainage route of interstitial fluid, their distension may be related to the fluid retention in and along the Virchow-Robin spaces, the outlet of which into the subarachnoid space is blocked by pituitary region tumors.
Acknowledgments
We thank Drs. S. Sunada, S. Hoshi, and H. Tokunaga for medical assistance and S. Mizuno and M. Ishii for technical assistance at the Kawatetsu Chiba Hospital.
References
- 1.Higashi S, Yamashita J, Fujisawa H, Yamamoto Y, Kadoya M. Moustache appearance in craniopharyngiomas: unique magnetic resonance imaging and computed tomographic findings of perifocal edema. Neurosurgery 1990;27:993–996 [PubMed] [Google Scholar]
- 2.Nagahata M, Hosoya T, Kayama T, Yamaguchi K. Edema along the optic tract: useful MR finding for the diagnosis of craniopharyngiomas. AJNR Am J Neuroradiol 1998;19:1753–1757 [PMC free article] [PubMed] [Google Scholar]
- 3.Youl BD, Plant GT, Stevens JM, et al. Three cases of craniopharyngioma showing optic tract hypersignal on MRI. Neurology 1990;40:1416–1419 [DOI] [PubMed] [Google Scholar]
- 4.Sklar EM, Schaz NJ, Glaser JS, Sternau L, Seffo F. Optic tract edema in a meningioma of the tuberculum sellae. AJNR Am J Neuroradiol 2000;21:1661–1663 [PMC free article] [PubMed] [Google Scholar]
- 5.Saeki N, Murai H, Kubota M, Fujimoto N. Oedema along the optic tracts due to pituitary metastasis. Br J Neurosurg 2001;15:523–526 [DOI] [PubMed] [Google Scholar]
- 6.Miller JD, Ironside JW. Raised intracranial pressure oedema and hydrocephalus. In: Graham DI, Lantos PL, eds. Greenfield’s Neuropathology. vol 1, 6th ed. London: Arnold;1997. :157–195
- 7.Mamata Y, Muro I, Matsumae M, et al. Magnetic resonance cisternography for visualization of intracisternal fine structures. J Neurosurg 1998;88:670–678 [DOI] [PubMed] [Google Scholar]
- 8.Adachi M, Hosoya T, Haku T, Yamaguchi K. Dilated Virchow-Robin spaces: MRI pathological study. Neuroradiology 1998;40:27–31 [DOI] [PubMed] [Google Scholar]
- 9.Heier LA, Bauer CJ, Schwartz L, at al. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol 1989;10:929–936 [PMC free article] [PubMed] [Google Scholar]
- 10.Elster AD, Richardson DN. Focal high signal on MR scans of the midbrain caused by enlarged perivascular spaces: MR-pathologic correlation. AJNR Am J Neuroradiol 1991;11:1119–1122 [DOI] [PubMed] [Google Scholar]
- 11.Esiri MM, Gay D. Immunological and neuropathological significance of the Virchow-Robin space. J Neurol Sci 1990;100:3–8 [DOI] [PubMed] [Google Scholar]
- 12.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular spaces in the human cerebrum. J Anat 1990;170:111–123 [PMC free article] [PubMed] [Google Scholar]
- 13.Proesscholdt MG, Hutto B, Brady, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer[14C]inulin in rat. Neuroscience 2000;95:577–592 [DOI] [PubMed] [Google Scholar]
- 14.McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg 1983;59:369–383 [DOI] [PubMed] [Google Scholar]
- 15.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol 1981;240:F329–F336 [DOI] [PubMed] [Google Scholar]
- 16.Weller RO, Kida S, Zhang E. Pathways of fluid drainage from the brain: morphological aspects and immunological significance in rat and man. Brain Pathol 1992;2:227–294 [DOI] [PubMed] [Google Scholar]