Abstract
Summary: We report a case of solitary fibrous tumor (SFT) causing isolated hypoglossal nerve palsy. The neuroimaging appearance of the tumor was indistinguishable from that of schwannoma or meningioma. Immunohistochemical tests demonstrated strong reactivity for CD34 but an absence of staining for S100 and epithelial membrane antigen; this profile is indicative of an SFT. SFTs are mesenchymal tumors that can affect the dura-covered segments of cranial nerves. They may be considered in the differential diagnosis of an isolated cranial nerve palsy.
Isolated hypoglossal nerve (cranial nerve XII) palsy is an uncommon clinical presentation, and tumors of cranial nerve XII are rare. We describe a solitary fibrous tumor (SFT) of the hypoglossal nerves. This is a rare mesenchymal, nonmeningothelial tumor that may occur in the CNS. To our knowledge, this is the first report of an SFT causing a cranial nerve palsy.
Case Report
A 47-year-old woman noticed that she was having increasing difficulty in phonation for a year. It was gradually affecting her job and causing notable dysarthria. On examination, the muscles of the left half of her tongue were atrophied. With the sole exception of an inability of the tongue to reach the right inner cheek, no other cranial nerve deficits were noted. Results of a neurologic examination were normal, with no evidence of long-tract signs. No visible or palpable abnormality was present in the neck, nasopharyngeal, oropharyngeal and sublingual spaces.
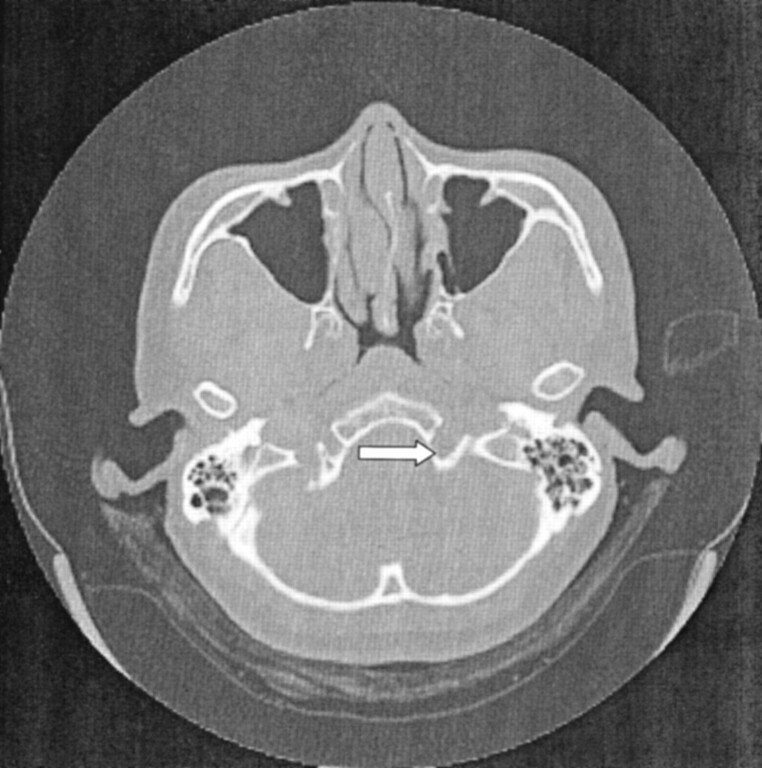
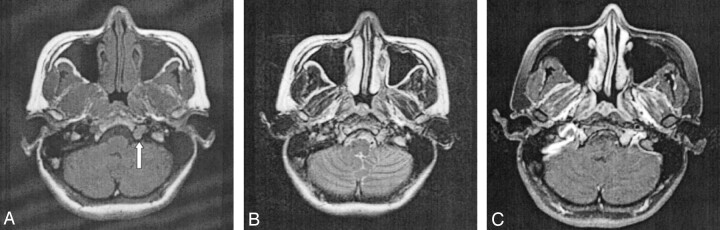
CT of the brain and skull base demonstrated a smooth widening of the left hypoglossal foramen with preservation of the cortical bone (Fig 1). No bony destruction was present in the rest of the skull base or in other cranial nerve foramina. MR imaging revealed the presence of a well-circumscribed, extra-axial tubular mass; this measured 1.5 × 0.7 × 1.5 cm and occupied the widened left hypoglossal canal. The tumor was homogeneously isointense relative to brain parenchyma on both T1- and T2-weighted images and showed uniform enhancement after the intravenous administration of contrast material (Fig 2). It did not extend into the cisternal or root entry-zone portions of cranial nerve XII. A schwannoma of the hypoglossal nerve was diagnosed with a differential diagnosis of meningioma. At surgery, a soft, fleshy, well-encapsulated tumor was found adherent to the left hypoglossal nerve along the lateral aspect. Complete surgical excision was achieved.
Fig 1.
Axial CT scan of the skull base shows smooth, well-defined widening of the left hypoglossal canal (arrow). No bony destruction is demonstrated.
Fig 2.
Axial MR images.
A, T1-weighted image reveals a well-circumscribed, elongated tumor occupying the hypoglossal canal (arrow). The T1 signal intensity is homogeneously isointense relative to gray matter.
B, On the corresponding T2-weighted image, the mass is also uniformly isointense relative to gray matter.
C, After the intravenous injection of contrast material, homogeneous enhancement of the tumor is observed.
Microscopic examination showed a moderately cellular tumor composed of uniform, fibroblastlike spindle cells arranged in intersecting fascicles. Mitoses were rare, and necrosis was absent. A background of small, hyalinized blood vessels was noted. No staghorn vessels were evident (Fig 3). Immunohistochemical studies demonstrated strong diffuse positive staining of the tumor cells for CD34 (Fig. 4), but negative staining results for S100, epithelial membrane antigen (EMA), smooth-muscle actin, and desmin. This profile was indicative of SFT and excluded meningioma, nerve sheath tumor and smooth muscle tumor. An MIB-1 immunostaining assay was performed, and a labeling index of 2% was found. One year after surgery, the patient’s deficit stabilized with mild difficulty of phonation and left hemiatrophy of the tongue.
Fig 3.
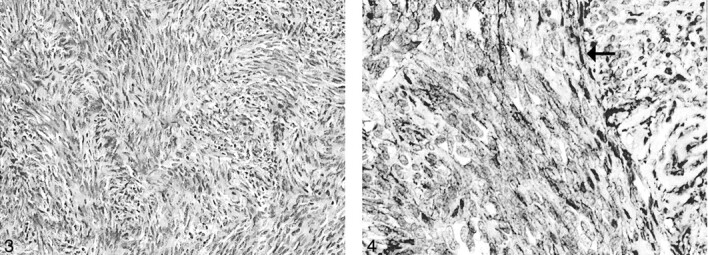
Uniform spindle cells are arranged in fascicles with thin bands of intercellular collagen (hematoxylin-eosin stain, original magnification ×100).
Fig 4.

Tumor cells (arrow) stain positively for CD34. (original magnification ×200).
Discussion
The hypoglossal nerve is a pure motor nerve that innervates both the intrinsic and extrinsic muscles of the tongue. It is divided into five segments: medullary, cisternal, skull base, nasopharyngeal and oropharyngeal, carotid space, and sublingual. Different disorders affect each segment, and localizing a lesion to a particular segment allows the radiologist to narrow the differential diagnosis to palsy of cranial nerve XII (1).
Supranuclear hypoglossal nerve lesions do not result in denervation atrophy of the tongue muscles: Characteristic atrophy of the tongue is only seen when the nuclear or peripheral segments of the hypoglossal nerve are involved (2). The left and right hypoglossal nuclei are closely located together within the paramedian medulla and beneath the hypoglossal trigone of the fourth ventricle. Therefore, medullary lesions such as multiple sclerosis, syringobulbia, amyotrophic lateral sclerosis, and bulbar poliomyelitis often result in bilateral lower motor neuron palsy of the tongue. For lesions involving the cisternal course of cranial nerve XII, cranial nerve IX, X, and XI nerves are often also affected because they are in close spatial relationship to the hypoglossal nerve roots. In a series of 32 patients with paralysis of the hypoglossal nerve and tongue atrophy, Tommasi-Davenas et al (3) found only eight cases of isolated cranial nerve XII palsy without neurologic involvement of other the cranial nerves or the long tracts. In most cases, the cause was tumor, especially bony metastasis. In another study of nine cases of unilateral isolated hypoglossal nerve palsy, researchers found metastatic disease in three patients (4).
In its course distal to the base of skull, the nasopharyngeal, oropharyngeal, carotid, and sublingual segments of cranial nerve XII may be affected by vascular aneurysm; local infections; rheumatoid arthritis; surgical (eg, carotid endarterectomy) or accidental trauma; birth injuries; or tumors of the neck, salivary glands, or base of the tongue (2). In such situations, the abnormality is often apparent on clinical history taking or physical examination.
Our patient presented with clinical signs that clearly localized the lesion to the skull base segment of cranial nerve XII. Neuroimaging revealed a uniformly enhancing tumor of the hypoglossal nerve with expansion of the hypoglossal canal. The tumor was isointense relative to brain parenchyma on both T1- and T2-weighted images, and it showed fairly homogeneous contrast enhancement. These findings are nonspecific and may be due to a variety of neoplastic or inflammatory conditions.
In 1931, Klemperer and Rabin first described SFT in the pleura as a localized form of mesothelioma (5). Since then, SFT has been detected in different locations, including the pericardium, mediastinum, nasopharyngeal sinuses, thyroid, liver, mesentery, orbit, and nervous system. A review of the English-language literature revealed 16 cases of SFT affecting the CNS: nine cases were intracranial and seven were spinal (6–13). Of the intracranial tumors, all were meningeal based and grew to considerable size before causes symptoms of mass effect. To our knowledge, no case affecting the cranial or spinal nerves in isolation has been reported.
The histogenesis of SFT has been a matter of debate, but it is thought to arise from mesenchymal elements (14). This uncommon neoplasm consists of monomorphous sheets of spindle cells embedded in a collagenous matrix. It has no distinctive features on light microscopy; this feature makes it difficult to differentiate SFT from other spindle cell tumors such as fibrous meningioma, schwannoma, and neurofibroma. The histopathologic differential diagnosis also includes dura-based mesenchymal tumors such as hemangiopericytoma (HPC), fibroma, fibrosarcoma, and malignant fibrous histiocytoma (15–17).
The immunohistochemical features of SFT are, however, characteristic and essential in distinguishing SFT. Most important is the observation that more than 90% of SFTs contain cells that express an antigen recognized by CD34 (18). SFTs are S100- and EMA-negative, whereas meningiomas are EMA-positive and CD34-negative, and nerve sheath tumors are S100-positive and CD34-negative. In our case, the tumor was negative for EMA and S100 but strongly and diffusely positive for CD34m, consistent with SFT. A recent group (14) has suggested that SFTs are derived from CD34-positive dura-based fibroblasts. This may explain why the tumor in our patient affected only the dura-covered segment of the nerve in the hypoglossal canal; the cisternal segment, which lacks a dural covering, was spared.
Distinguishing SFT from HPC is especially important. HPC is the most common dura-based neoplasm of mesenchymal origin and accounts for 0.4% of all intracranial tumors (15). HPC is an aggressive tumor that tends to recur after resection, and it occasionally metastasizes extracranially (19). On the contrary, meningeal SFTs are slow growing, and to our knowledge, malignant meningeal SFTs have not been described (20). With the exception of hypervascularity and bone erosion, HPC may be indistinguishable from meningioma on neuroimages (8, 19). However, although HPCs may contain CD34-positive cells, distinguishing HPC from SFT is not difficult on the basis of the histopathologic findings: Unlike SFT, HPCs seldom show a fascicular arrangement of cells or the characteristic bands of intercellular collagen. Thus, the accurate diagnosis of SFT and its differentiation of SFT from HPC are important because it has implications for further treatment and the prognosis.
Conclusion
This is the first report of SFT involving a cranial nerve. Although rare, SFT may be considered in the neuroimaging differential diagnosis of tumors affecting the segments of cranial nerves covered by dura mater. Immunohistochemistry is important in differentiating SFT from the more common meningioma and schwannoma, which may mimic SFT both radiologically and histologically.
References
- 1.Thompson EO, Smoker WR. Hypoglossal nerve palsy: a segmental approach. Radiographics 1994;4:939–958 [DOI] [PubMed] [Google Scholar]
- 2.Brazis PW, Masdeu JC, Biller J. Cranial nerve XII: the hypoglossal nerve. In: Localization in Clinical Neurology. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2001. :345–351
- 3.Tommasi-Davenas C, Vighetto A, Confavreux C, et al. Causes of paralysis of the hypoglossal nerve: apropos of 32 cases. Press Med 1990;19:864–868 [PubMed] [Google Scholar]
- 4.Combarros O, Alvarez de Arcaya A, Berciano J. Isolated unilateral hypoglossal nerve palsy: nine cases. J Neurol 1998;245:98–100 [DOI] [PubMed] [Google Scholar]
- 5.Klemperer P, Rabin CB. Primary neoplasms of the pleura. Arch Pathol 1931;11:385–412 [Google Scholar]
- 6.Ahn JY, Shim JY, Yang WI, et al. Meningeal solitary fibrous tumor as an unusual cause of exophthalmos: case report and review of literature. Neurosurgery 2001;48:1362–1367 [DOI] [PubMed] [Google Scholar]
- 7.Alston SR, Francel PC, Jane JA Jr. Solitary fibrous tumor of the spinal cord. Am J Surg Pathol 1997;21:477–483 [DOI] [PubMed] [Google Scholar]
- 8.Brunori A, Cerasoli S, Donati R, et al. Solitary fibrous tumor of the meninges: two new cases and review of the literature. Surg Neurol 1999;51:636–640 [DOI] [PubMed] [Google Scholar]
- 9.Carneiro SS, Scheithauer BW, Nascimento AG, et al. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol 1996;106:217–224 [DOI] [PubMed] [Google Scholar]
- 10.Kurtkaya O, Elmaci I, Sav A, et al. Solitary fibrous tumor: seventh reported case and review of literature. Spinal Cord 2001;39:57–60 [DOI] [PubMed] [Google Scholar]
- 11.Malek AM, Weller SJ, Price DL, et al. Solitary fibrous tumor presenting as a symptomatic intraspinal mass: case report. Neurosurgery 1997;40:844–847 [DOI] [PubMed] [Google Scholar]
- 12.Prayson RA, McMahon JT, Barnett GH. Solitary fibrous tumor of the meninges: case report and review of literature. J Neurosurg 1997;86:1049–1052 [DOI] [PubMed] [Google Scholar]
- 13.Vorster SJ, Prayson RA, Lee JH. Solitary fibrous tumor of the thoracic spine: case report and review of literature. J Neurosurg 2000;92:217–220 [DOI] [PubMed] [Google Scholar]
- 14.Cummings TJ, Burchette JL, McLendon RE. CD34 and dural fibroblasts: the relationship to solitary fibrous tumor and meningioma. Acta Neuropathol (Berl) 2001;102:349–354 [DOI] [PubMed] [Google Scholar]
- 15.Guthrie BL, Ebersold MJ, Scheithauer BJ, et al. Meningeal hemangiopericytoma: histopathological features, treatment and long-term follow-up of 44 cases. Neurosurgery 1989;25:514–522 [PubMed] [Google Scholar]
- 16.Okeda R, Mochizuki T, Terao E, et al. The origin of intracranial fibrosarcoma. Acta Neuropathol (Berl) 1980;52:223–230 [DOI] [PubMed] [Google Scholar]
- 17.Swamy KS, Shankar SK, Asha T, et al. Malignant fibrous histiocytoma arising from the meninges of the posterior fossa. Surg Neurol 1986;25:18–24 [DOI] [PubMed] [Google Scholar]
- 18.Goodlad JR, Fletcher CD. Solitary fibrous tumor arising at unusual sites: analysis of a series. Histopathology 1991;19:515–522 [DOI] [PubMed] [Google Scholar]
- 19.Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol 1996;17:1365–1371 [PMC free article] [PubMed] [Google Scholar]
- 20.Karamitopoulou E, Perentes E, Diamantis I, et al. Ki-67 immunoreactivity in human central nervous system tumors: a study with MIB 1 monoclonal antibody on archival material. Acta Neuropathol (Berl) 1994;87:47–54 [DOI] [PubMed] [Google Scholar]